©2014 Baishideng Publishing Group Inc.
World J Gastrointest Pathophysiol. Aug 15, 2014; 5(3): 252-270
Published online Aug 15, 2014. doi: 10.4291/wjgp.v5.i3.252
Published online Aug 15, 2014. doi: 10.4291/wjgp.v5.i3.252
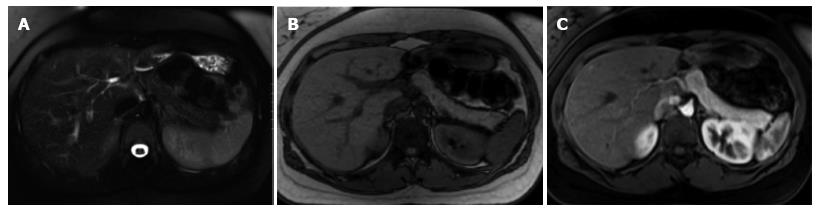
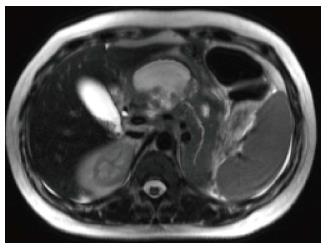
Figure 1 Normal pancreatic appearance on magnetic resonance imaging.
A: Axial T2-weighted image with fat-suppression; B: Axial GRE out-of-phase T1-weighted image; C: Axial post-contrast 3D-GRE T1-weighted image with fat-suppression during the late arterial phase. The pancreas demonstrates low T2 signal intensity (A) and high T1 signal intensity on pre-contrast images (B), reflecting high protein content of the exocrine gland. The pancreas demonstrates avid homogenous enhancement on immediate post-contrast images (C), reflecting a normal capillary blush.
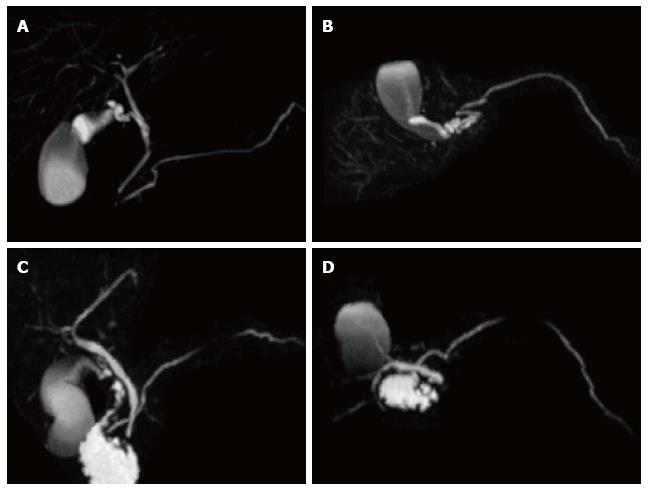
Figure 2 Normal pancreatic duct anatomy and pancreatic divisum.
(A and C) Coronal and (B and D) axial post-processed maximum intensity projection 3D-MRCP images from two different patients. In the first patient, the main pancreatic duct courses inferiorly (A) and posteriorly (B), joins the CBD and opens in the major papilla in keeping with normal pancreatic duct anatomy. In the second patient, the main pancreatic duct continues its course superiorly (C) and anteriorly (D), crosses the CBD and opens in the minor papilla in keeping with pancreatic divisum.
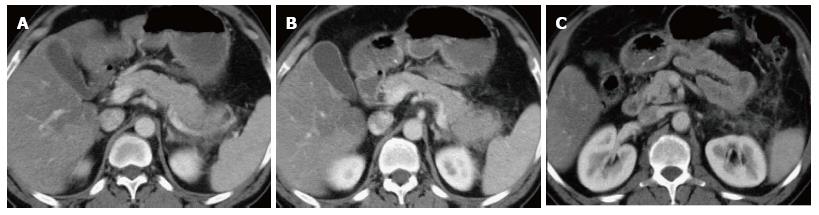
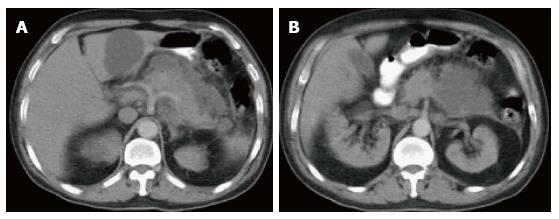
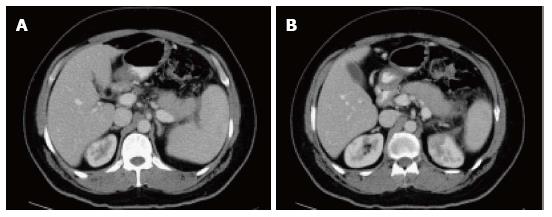
Figure 3 Focal acute edematous pancreatic tail pancreatitis.
A-C: Axial CT scan of the pancreas during the late arterial phase. There is evidence of ill-definition and reduced enhancement of the pancreatic tail (A and B), associated with mild peripancreatic fatty stranding extending to the anterior left perinephric space in keeping with focal acute edematous pancreatitis.
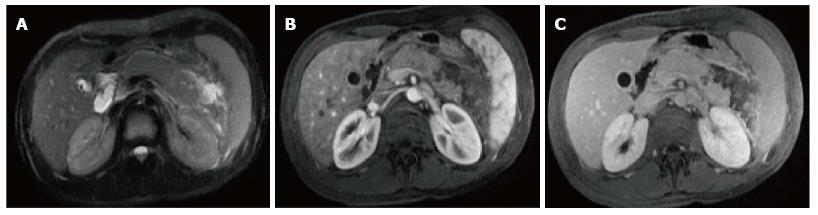
Figure 4 Gallstone acute edematous pancreatic tail pancreatitis.
A: Axial fast spin-echo (FSE) T2-weighted image with fat-suppression; B and C: Post-contrast 3D-GRE T1-weighted images with fat-suppression during the late arterial and portal venous phases. There is mild diffuse lace-like increased T2 signal involving the pancreatic parenchyma, associated with a small amount of peripancreatic fluid near the pancreatic tail (A). The pancreas demonstrates diffuse minimal decrease in T1 signal intensity (B) and minimally reduced enhancement on the late arterial phase (C) in keeping with diffuse edematous pancreatitis. There are also innumerable gallstones (A).
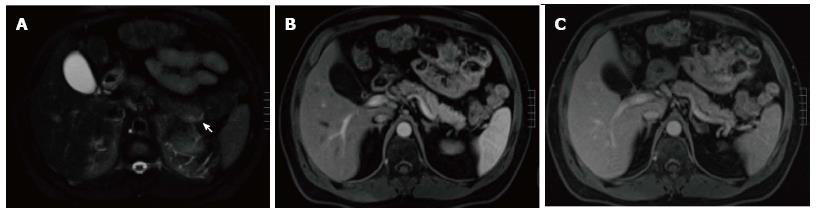
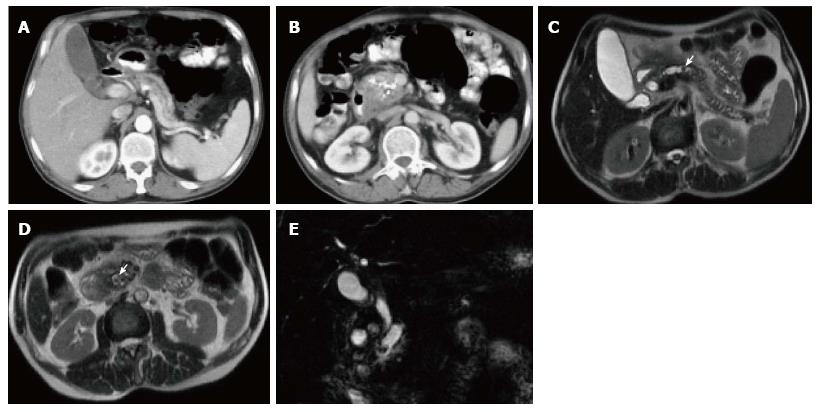
Figure 5 Subtle focal acute edematous pancreatic tail pancreatitis.
A: Axial T2 weighted-image with fat-suppression; B and C: Axial post-contrast 3D-GRE T1- weighted images with fat-suppression during the late arterial and portal venous phases. There is a very subtle area of increased T2 signal seen around the pancreatic tail (arrow, A), with fairly normal enhancement of the pancreas on the post-contrast images (B and C) in keeping with subtle focal acute edematous pancreatic tail pancreatitis.
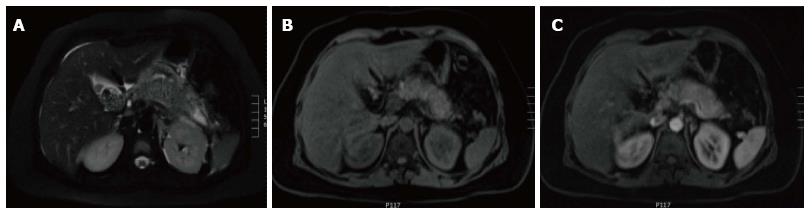
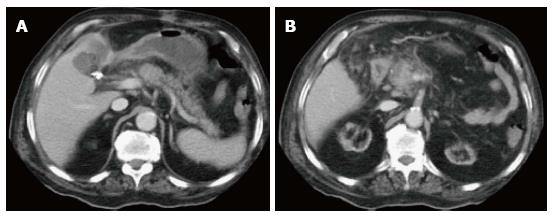
Figure 6 Focal pancreatic head necrotizing pancreatitis confined to the pancreatic parenchyma.
A and B: Axial CT scan during the portal venous phase. There is evidence of significantly reduced enhancement of the pancreatic head (B), without peripancreatic extension or necrosis in keeping with focal acute necrotizing pancreatitis.
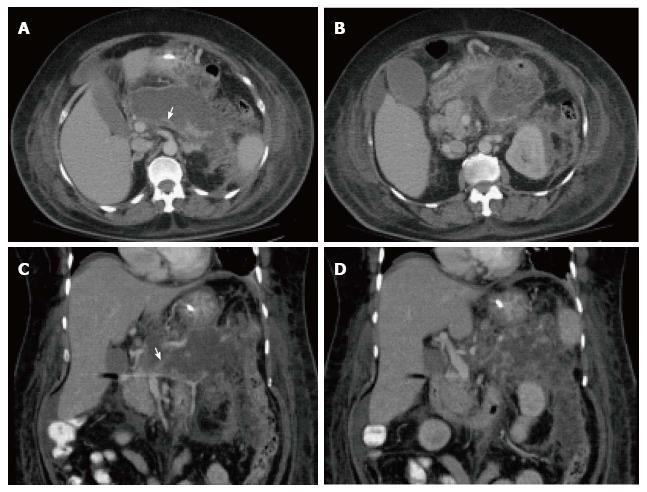
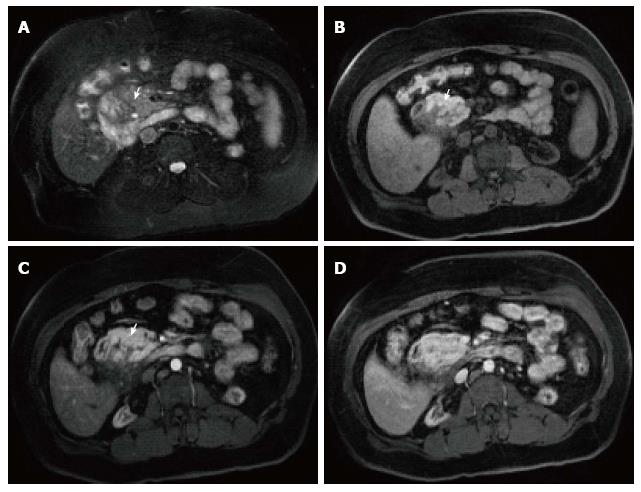
Figure 7 Severe acute necrotizing pancreatitis and peri pancreatitis.
A-B: Axial CT scan during the late arterial phase; C-D: Coronal reformatted CT images. There is evidence of lack of arterial enhancement involving the pancreatic body and tail, which are replaced by necrotic tissue, associated with heterogenous peripancreatic tissue inflammation and necrosis extending to left perinephric space (A-B) and paracolic gutter (C-D), in keeping with severe necrotizing pancreatitis and peripancreatitis. There is also evidence of splenic vein thrombosis (arrow, A, C), a known complication of acute pancreatitis.
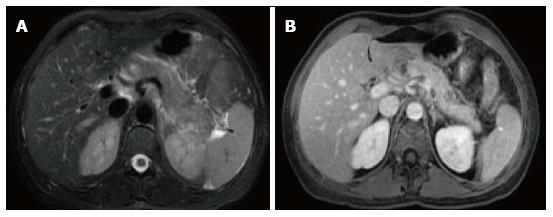
Figure 8 Focal acute necrotizing pancreatitis.
A: Axial fast spin-echo T2- weighted image with fat-suppression; B: Axial post-contrast 3D-GRE T1- weighted images with fat-suppression during the venous phase. There is a focal area of low T2 signal involving the proximal part of the pancreatic tail, associated with minimal peripancreatic fat stranding (A). This focal area demonstrates significantly reduced enhancement on the post-contrast images, in keeping with focal necrotizing pancreatitis.
Figure 9 Acute interstitial edematous pancreatitis and acute peripancreatic fluid collections.
A-B: Axial CT scan during the portal venous phase. The pancreas is mildly thickened and demonstrates mildly heterogenous enhancement, reflective of edema, in keeping with acute interstitial edematous pancreatitis. There is a peripancreatic fluid with imperceptible wall in keeping with acute peripancreatic fluid collections.
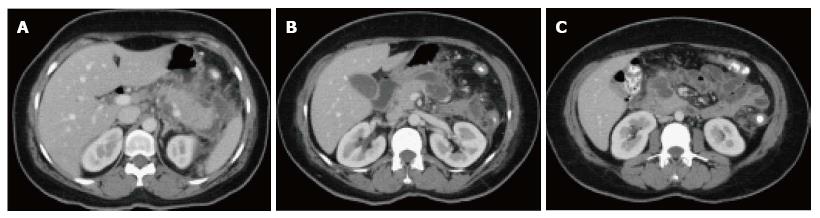
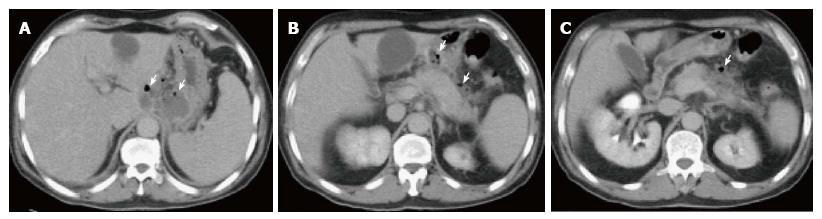
Figure 10 Peripancreatic fluid secondary to multifocal acute necrotizing pancreatitis.
A-C: Axial CT images during the late arterial phase. There are two areas of focal necrosis involving the pancreatic body (B) and pancreatic head/uncinate process (C), associated with loculated peripancreatic fluid collection (A).
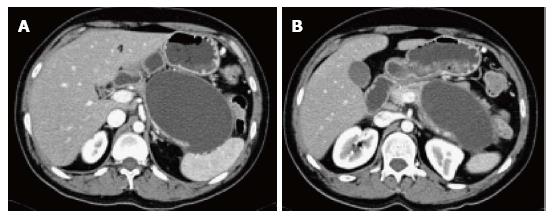
Figure 11 Large pancreatic pseudocyst.
A-B: Axial CT scan during the late arterial phase. There is a large oval shaped pancreatic pseudocyst located anterior to the pancreatic body and tail, associated with mass effect on the thinned out pancreatic tissue in keeping with a large pancreatic pseudocyst.
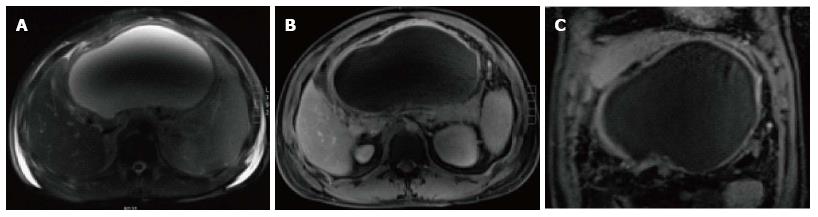
Figure 12 Large pancreatic pseudocyst.
A: Axial fast spin-echo T2- weighted image with fat-suppression; B-C: Axial and coronal post-contrast 3D- GRE T1-weighted images with fat-suppression during the portal venous phase. There is a very large thin-walled cyst (A) within the lesser sac; which demonstrates mild uniform wall enhancement (B-C) in keeping with a large pancreatic pseudocyst. The central drop of signal on (A) is related to dielectric shading artifact.
Figure 13 Acute necrotic collection.
Axial T2 single-shot turbo spin-echo image. There is a well-defined fluid collection involving the pancreatic neck with peripancreatic extension and communication with the main pancreatic duct. This collection demonstrates well-defined outlines and heterogenous low T2 signal intensity debris within it in keeping with acute necrotic collection. Multiple gallstones are also noted.
Figure 14 Necrotizing pancreatitis, with peripancreatic walled-off necrosis.
A: Axial fast spin-echo T2-weighted image with fat-suppression; B-C: Axial post-contrast 3D-GRE T1-weighted images with fat-suppression during the late arterial and venous phase. There is a focal area of heterogeneous iso to slightly high T2 signal involving the pancreatic body-tail junction (A); which demonstrates lack of enhancement on the post-contrast images (B-C). There is associated sizable peripancreatic fluid collection; which demonstrates heterogeneous T2 signal intensity and thick enhancing wall post-contrast in keeping with walled-off necrosis.
Figure 15 Infected peripancreatic fluid in a patient with acute pancreatitis.
A-C: Axial CT scan during the portal venous phase. There are a few gas bubbles seen within a small peripancreatic fluid (arrows, A-C). In the absence of any intervention in keeping with infected peripancreatic fluid.
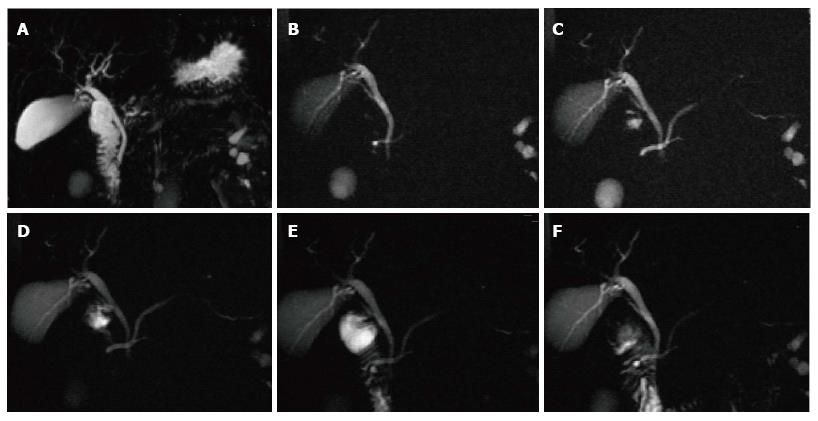
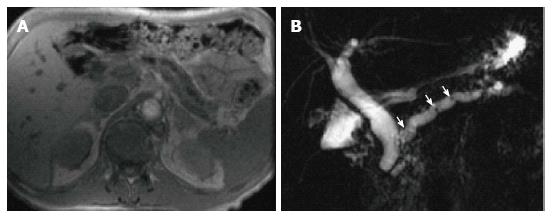
Figure 16 Pancreatic divisum, with a small Santorinicele.
A: Coronal 3D- maximum intensity projection MRCP image before administration of secretin; B-F: Selected dynamic secretin thick-slab MRCP images obtained at 30 s (B), 60 s (C), 120 s (D), 4 min (E) and 9 min (F). Prior to administration of secretin, it is difficult to identify the main pancreatic duct (A). After administration of secretin, there is better delineation of the main pancreatic duct (C), with demonstration of pancreatic divisum. There is also enlargement of the accessory pancreatic duct, with demonstration of a small santorinicele (B, F). S-MRCP allows qualitative and quantitative assessment of pancreatic exocrine secretions. In this case, the pancreatic flow output was considered within normal limits; excluding early chronic pancreatitis.
Figure 17 Chronic pancreatitis with pancreatic parenchymal calcifications and pancreatic duct stones.
A, B: Axial CT scan during the late arterial phase; C, D: Axial T2 single-shot fast spin-echo images; E: Coronal 3D- Cholangiopancreatogram (MRCP) image. CT shows a markedly dilated and tortuous main pancreatic duct (MPD) (A, B), with foci of thick calcification involving the pancreatic head and uncinate process parenchyma (B). Large the proximal MPD stone was suspected on CT (arrow, B). MRCP shows gross pancreatic ductal dilatation with confirmation of the distal intraductal calculus (arrow, D), and shows an additional mid-pancreatic duct stone, not clearly seen on CT (arrow, C). No pancreatic masses or ductal anomalies are identified.
Figure 18 Chronic pancreatitis.
A: Axial T1-weighted GRE MRI. B: Coronal-oblique thick-slab MRCP image. There is evidence of diffuse thinning of the pancreatic parenchyma with uniform dilatation of the pancreatic duct and prominence of the pancreatic duct side-branches (A-B), associated with multiple tiny stones at the proximal pancreatic duct (arrows, B) in keeping with chronic pancreatitis. There is also mild uniform dilatation of the CBD, which tapers down to the level of the pancreatic duct (B).
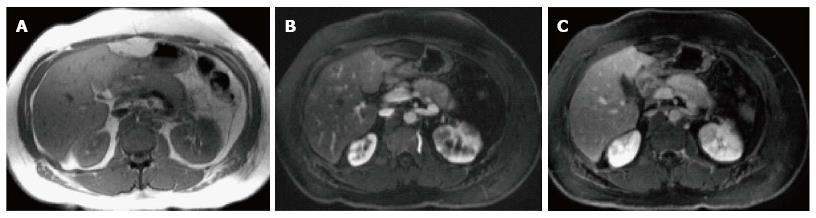
Figure 19 Autoimmune pancreatitis.
A, B: Axial CT scan during the late arterial phase. There is evidence of diffuse pancreatic swelling with loss of the normal pancreatic lobulation, obliteration of the pancreatic duct and subtle low attenuating peripancreatic rim (A, B) in keeping with autoimmune pancreatitis. Patient had high IgG4 level (> 0.500 g/L).
Figure 20 Autoimmune pancreatitis.
A: GRE T1-weighted image; B, C: Post- contrast 3D-GRE T1-weighted images with fat-suppression during the late arterial and portal venous phases. There is evidence of diffuse pancreatic swelling with reduced T1 signal, loss of the normal pancreatic lobulation and obliteration of the pancreatic duct, associated with a rim of low T1 signal (A). The pancreas demonstrates diffuse reduced enhancement on the late arterial phase and progression of enhancement on the portal venous phase in keeping with autoimmune pancreatitis. The patient had significant biliary tree irregularities in keeping with primary sclerosing cholangitis (not shown). Additionally, there are a few bilateral wedge-shaped areas of renal hypo- enhancement in keeping with segmental infarcts.
Figure 21 Groove pancreatitis.
A: Axial T2-weighted single-shot fast spin-echo (SS-FSE) images with fat-suppression; (B) Pre- and (C, D) Post-contrast 3D-GRE T1- weighted images with fat-suppression during the late arterial and portal venous phases. There is a slightly low T2 signal sheet-like mass in the pancreaticoduodenal groove, with tiny cystic changes (arrow, A). The mass shows low T1 signal with extension into the pancreatic head (arrow, B). Imperceptible enhancement is depicted on the immediate post-contrast image (arrow, C), with progressive enhancement on the subsequent delayed images (D) in keeping with groove pancreatitis.
- Citation: Busireddy KK, AlObaidy M, Ramalho M, Kalubowila J, Baodong L, Santagostino I, Semelka RC. Pancreatitis-imaging approach. World J Gastrointest Pathophysiol 2014; 5(3): 252-270
- URL: https://www.wjgnet.com/2150-5330/full/v5/i3/252.htm
- DOI: https://dx.doi.org/10.4291/wjgp.v5.i3.252