INTRODUCTION
Various gastrointestinal (GI) diseases such as acute GI bleeding, esophageal strictures, strictures associated with inflammatory bowel disease and enteral feedings were traditionally managed by the surgeons alone. However, surgery has been associated with high morbidity and mortality rates, thus leading on to a search for other modalities that were less invasive and equally or better efficacious. Though the first endoluminal visualization of the stomach was performed by Kussmaul in 1868, it was not until 1958 that the first fiberscope was introduced by Hirschowitz et al[1]. From then, the field of endoscopy has evolved rapidly with various innovations such as charged couple devices, video chip to hemostatic clips, biopsy forceps, snares, banding kit, etc. These innovations have expanded the horizons of endoscopy, changing it from a mere diagnostic tool to one of therapeutics. Endoscopists are now able to treat GI bleeding, perform biopsies, remove polyps, dilate strictures, place stents and feeding tubes. Similar to gastroenterology, the field of interventional radiology (IR) has had its share of technological advances. Fluoroscopy advanced during the early 1900s. The first angioplasty by Dotter in 1964 was a landmark in vascular interventions[2]. Embolization, angioplasty, and other fluoroscopic guided techniques significantly advanced have also decreased the need for first line surgery in many patients[2,3].
Interventional endoscopy and radiology are two minimally invasive disciplines that overlap and complement one another in the care of multiple complex GI disease processes. Acute GI bleeding is a common presentation to the emergency room which can be life threatening. Management of this often times requires a collaboration between a gastroenterologist, radiologist, and a surgeon. However, with the advent of therapeutic endoscopy and interventional radiology, in many cases, the role of surgery is now limited to technically challenging cases not amenable to endoscopic or radiological intervention. Though few articles addressing the need for multidisciplinary approach in treating GI bleeding have been published, there is a paucity of literature for other above mentioned conditions. Thus, in this article we hope to not only outline the role of endoscopists and radiologists in managing various GI conditions but also their complementary roles to overcome their individual short comings. Since this is an expansive topic we will be only focusing on endoluminal conditions such as GI bleeding, access for enteral nutrition, cecostomy tube placement and strictures. Hepatobiliary pathology including variceal bleeding, portal hypertensive gastropathy, biliary drainage, endoscopic ultrasound (EUS) guided internal drainage, EUS guided celiac block and tissue biopsy will be described elsewhere.
LITERATURE RESEARCH
We conducted an English literature review of the various GI topics. Searches were performed for GI hemorrhage with respect to management, endoscopy and interventional radiology. Searches for hemorrhage were further subdivided into upper and lower GI bleeding. Similar review was performed for enteral feeding, cecostomy tubes, and stricture management. Further literature was reviewed by evaluating references. Also, since many patients are complex and require the opinion of several specialists in the outpatient and emergent setting, the authors added the opinion of our institution when appropriate.
Acute upper GI bleed
Acute life threatening GI bleeding once considered a surgical emergency with significant mortality continues to have a high mortality rate despite tremendous advances made in endoscopic and radiographic techniques. The incidence of GI bleeding tends to increase with age and ranges between 37 and 172/100000 adults[4,5]. It has been reported to account for approximately 350000 hospital admissions per year in the United States alone[6]. Rebleeding following interventions remains relatively high at reported rates of 7%-16%[4]. It is a frequent presenting symptom to the hospital and requires management by a multidisciplinary team comprising of gastroenterologists, surgeons, interventional radiologists, and anesthesiologists[7].
GI bleeding is usually arbitrarily divided between upper and lower bleeds. Upper GI bleed constitutes any bleed that originates in the GI tract proximal to ligament of Treitz while anything distal constitutes a lower GI bleed. Upper GI hemorrhage may manifest as hematemesis, coffee ground emesis, bloody return through nasogastric tube or feeding tube, melena or as brisk hematochezia with hemodynamic compromise. Lower GI bleeding usually presents as melena (if from the right colon or distal small bowel) or hematochezia. The most common cause of nonvariceal upper GI bleed is peptic ulcer disease[8]. Other etiologies include neoplasms, inflammation, iatrogenic, trauma, ischemia, and vascular malformations (such as Dieulafoy’s lesions and angioectasis) with more than one diagnosis noted in 16%-20% of cases[4].
When a patient presents to the emergency room with GI bleeding, initial assessment must be made to ensure hemodynamic stability of the patient and determine the need for urgent intervention. Resuscitation with crystalloids and blood transfusion must be performed. In patients suspected with nonvariceal upper GI bleed, proton pump inhibitors must be initiated as they reduce the chances of finding high risk stigmata during endoscopy[9]. If the patient is stable enough to undergo upper endoscopy, then it must be performed next as it can be both diagnostic and therapeutic. The patient is placed in a left lateral position with head bend forward to facilitate the insertion of the endoscope. At the time of upper endoscopy, there are various endoscopic treatment modalities available to help achieve hemostasis. Traditionally, endoscopic therapy has been broadly categorized into injection, thermal and mechanical methods.
Injection therapy
Injection therapy includes administration of epinephrine (1:10000) around the bleeding vessel. This was first described by Soehendra et al[10]. In 1988, Chung et al[11] presented the first randomized trial comparing injection therapy to medical therapy in 68 patients and reported reduced surgery, transfusion requirements and shorter hospital stay in the group with injection therapy. This is performed by placing multiple aliquots of 0.5 to 1 mL of diluted epinephrine (1:10000) 1 to 2 mm away from the bleeding vessel. This technique works by a combination of tamponade and transient vasoconstriction. Typically, 5 mL can be administered in one setting but on occasion as high as 25 mL have also been administered with no significant side effects except transient tachycardia. However, it should be avoided in patients with active ongoing cardiac ischemia. After injection of epinephrine blanching of the surrounding mucosa is noticed. Studies[12,13] have demonstrated that epinephrine alone is effective, but epinephrine in combination with another endoscopic modality is superior to epinephrine alone. This is most likely due to its transient duration of action.
More recently hemostatic powders have gained popularity. These are designed to be delivered via a catheter passed through the accessory channel of the endoscope. Hemospray is an inorganic powder that is metabolically inert and nontoxic. This acts in two ways; the first is upon coming in contact with water it forms a stable mechanical barrier over the vessel and stops the bleeding. Secondly, it acts by increasing the local concentration of clotting factors and promoting clot formation[14]. The adherent clot that it forms sloughs off within 24-72 h and is eliminated from the GI tract[15]. In 2011, Sung et al[15] conducted a pilot study in 20 patients with active peptic ulcer bleeding. Hemostasis was achieved in all but one patient (95%). It has also proven to be efficacious in tumor related bleeding[16] given its ease of application to large surfaces even in difficult positions. In a small study, Holster et al[17] evaluated the efficacy of this novel technique in patients on antithrombotic agents and concluded that endoscopic hemostasis by Hemospray is not decreased by systemic antithrombotic effects such as Plavix, aspirin, or vitamin K antagonists. Thus, though initial reports are fascinating, further trials with larger populations are needed.
THERMAL METHODS
Thermal devices can be divided into contact devices such as heater probe and bipolar probe and noncontact devices such as argon plasma coagulation (APC). Contact probes are ideal for bleeding vessels that are less than 2 to 3 mm in size. The goal of a contact probe is to apply firm pressure on the visible vessel to interrupt the blood flow and then to apply enough heat to weld the walls of the vessel together[18]. Heater probes contain a nonstick Teflon coated heating element directly delivering heat to the vessel. It also contains three irrigation ports on the sides to wash out the clots and allow better visualization of the vessel. The heat is then delivered for a preset amount of time by tapping the coagulation pedal. For the treatment of actively bleeding ulcer four pulses of 30 Joules must be applied[18].
Bipolar probes work by delivering electrical current from an electrosurgical generator to electrodes situated at the tip of the probe. Tissue coagulation is obtained indirectly by conversion of electrical energy to heat energy. Similar to heater probes they also contain a water channel which is, however, centrally located. Unlike the heater probe coagulation time is determined by the amount of time the endoscopist presses the coagulation foot pedal. For bleeding peptic ulcers, a setting of 20 watts for a contact period of 7 to 10 s is suggested[19]. APC is a non-contact monopolar thermal method which acts by delivering high frequency electrical current conducted via argon gas (that has been ionized) to the tissue. This method, however, produces superficial coagulation only, and once the tissue gets desiccated, it loses its electrical conductivity. Hence, the maximum depth is about 3 mm to 4 mm which is a safety feature to prevent deep tissue injury. The probe can be circumferential, end or side fearing, and should be held 1-2 mm away from the target. However, owing to its superficial effect it is not routinely used for peptic ulcer disease.
MECHANICAL METHODS
Mechanical hemostasis can be achieved by causing a physical tamponade of the bleeding site. Currently two types of instruments are widely used: Clips and banding kits. The use of through-the-scope clips was first reported in 1975 by Hayashi et al[20] for endoscopic hemostasis. Since then, tremendous improvements have been made in both the clip designs and their deployment devices. They are either single use clips or reusable clips which can be rotated, closed and reopened multiple times. They are deployed over the bleeding vessel and act by clamping the bleeding point. They slough off within few days to weeks. They are most beneficial for accessible lesions that do not have a hard fibrotic base. Based on historical data, the vessel should be ≤ 2 mm in size. Recently, over-the-scope clipping devices have become available and can be applied to larger vessels. Banding devices are mostly used for esophageal varices, which will not be discussed in this review.
Etiologies
The two most common etiologies for peptic ulcers include non-steroidal anti-inflammatory drugs and helicobacter pylori infection. These are easily visualized at the time of endoscopy, and certain endoscopic features such as active bleeding, spurting arterial vessel, adherent clot and non-bleeding visible vessel, predict high rate of rebleeding and hence require endoscopic therapy and/or interventional embolization therapy[21]. While treating a high risk stigmata ulcer, it is recommended that injection therapy should not be used alone as studies[12,13] demonstrated that the combination therapy of epinephrine with clips or thermal devices was superior to injection alone. APC have not been demonstrated to be useful in peptic ulcer bleeding. Through-the-scope Hemoclips and contact thermal devices have found to be equally efficacious in treating vessels less than 2 mm in size[22]. Placing a clip may be challenging in difficult to access locations such as the posterior wall of the duodenal bulb where contact thermocoagulation should be attempted. In cases with oozing without a visible vessel monotherapy is adequate. Treating ulcers with adherent clots is challenging as meta-analysis[22] has shown conflicting results regarding endoscopic treatment vs medical management.
Dieulafoy’s lesions which are characterized by a large submucosal vessel eroding through the mucosa and then rupturing were first described almost a hundred years ago. Endoscopically it is identified when there is visible or an active bleeding vessel with no ulcer. Treatment is usually similar to actively bleeding vessel in peptic ulcer disease and includes injection therapy, clips, banding devices, heater probe and bipolar probe. Studies have shown that monotherapy with injection should not be attempted. Bipolar probes should be set at 20 watts and applied for 10 to 12 s, and heater probes should be set at 30 joules and 4 pulse should be administered.
Mallory Weiss tears are usually self-limited bleeds and do not need endoscopic therapy. However, in the presence of ongoing active bleeding clips are preferred, though other devices such as band devices and electrocautery have also been reported[23,24]. The settings for bipolar and heater probes include 15-20 watts for 4 s and 15-20 joules for 3 pulses respectively. However, there are no trials comparing the various treatment modalities.
Angioectasias and gastric antral vascular ectasias (GAVE) usually cause chronic and obscure GI bleeding. These are usually treated with APC. The probe should be set at 45 watts with 1 L/min argon flow rate for vascular ectasia; whereas for GAVE, 60 watts with 1 L/min is applied for deeper tissue penetration. Though other previously mentioned methods have been used, there are again no prospective comparison trials.
However, despite the advances made in therapeutic endoscopies there are still certain instances where we fail to achieve hemostasis endoscopically. Thus, it is important to realize the limitations of various modalities and be aware of other options that we may have. Large bleeding vessels more than 2 mm to 3 mm in size, or high stigmata ulcer in posterior wall of the duodenal bulb may not be amenable to endoscopic intervention. Rarely interventions in such instances may fuel a massive GI bleed requiring IR intervention (Figures 1-3).
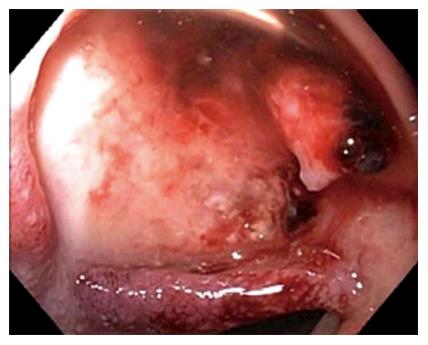
Figure 1 Endoscopic image showing a large ulcer in the superior wall of the bulb with a large visible vessel.
Attempted endoclip placement after epinephrine injection resulted in major bleeding and a loss of endoscopic view and patient was then emergently transferred to interventional radiology.
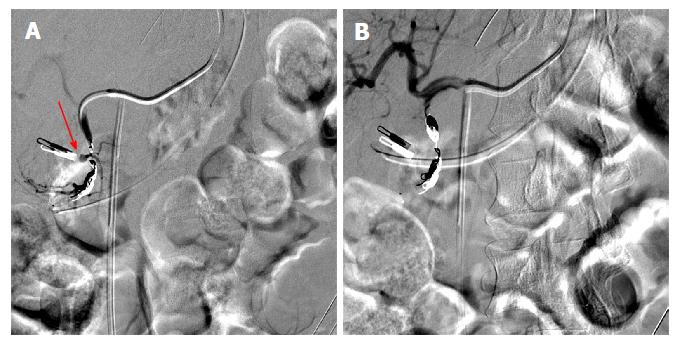
Figure 2 During interventional angiography, selected fluoroscopic images showing a pseudoaneurysm of the gastroduodenal artery (A, red arrow) that was successfully coiled with subsequent hemostasis via the sandwich technique (B).
Previously placed Endoclip is visible and can act as a fluoroscopic marker during angiography.
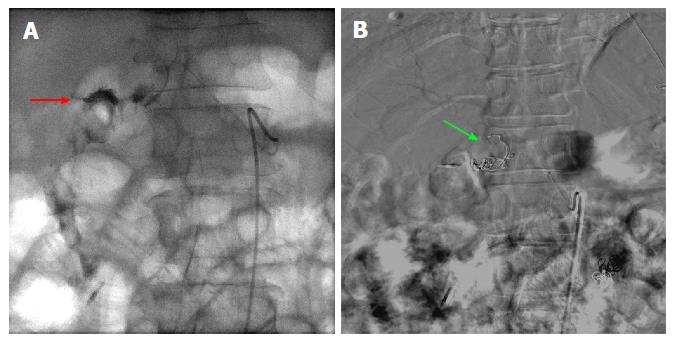
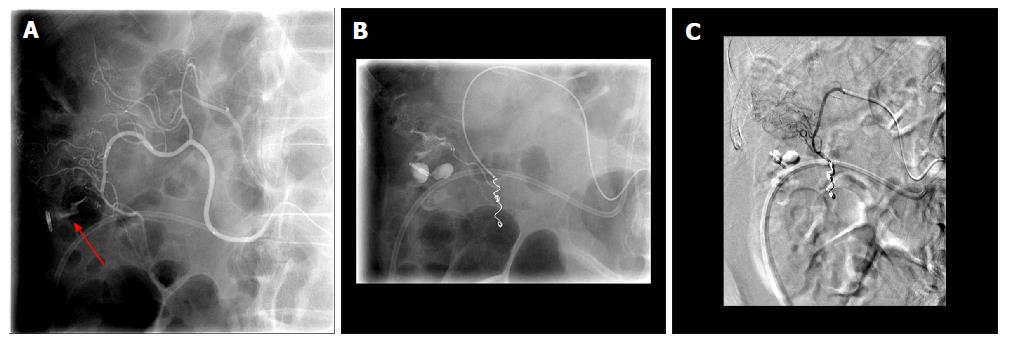
Figure 3 Fluoroscopic images of a case of 2-3 cm bleeding duodenal ulcer that failed endoscopic hemostasis with Endoclip application.
A: Fluoroscopic image following contrast injection to the right gastric artery showing active extravasation into the lumen. The bleeding vessel (red arrow) is identified which is near the endoscopically placed clip; B: Digital subtraction angiography post coiling of the right gastric artery (green arrow).
Pre-intervention imaging
If a patient is stable enough during presentation and plans are not made for immediate endoscopy, pre-procedure imaging can be performed to attempt localization of the culprit vessel or other underlying etiologies. Computerized tomography (CT) scanning is readily available in many centers and can tolerate patients with a tenuous clinical picture due to the speed of image acquisition. Multiphasic CT is usually performed without contrast followed by three contrasted series of images in the arterial, venous, and delayed phases to assist in localizing the bleed. A positive study occurs when there is contrast extravasation into the bowel lumen or identification of an abnormal vessel, mass, or other underlying etiology; the same is true for conventional catheter based angiography[8,25]. CT angiography can detect bleeds with a reported sensitivity of 0.5 cc/min of active extravasation which is compared to the sensitivity of catheter arteriography rate of detection of at least 1 cc/min[25].
Another imaging modality for patients is technetium labeled red blood cell scintigraphy. In this study, patient’s red blood cells are tagged with technetium and imaged for 60-90 min. Pooling of radiotracer is considered to be positive. Typical rates of bleeding required for detection of bleeding have been reported between 0.05-0.5 cc/min[25]. A benefit of this study is the ability to detect arterial or venous bleeding; a disadvantage, in turn, becomes the lack of precisely identifying the location of the bleed. Sensitivity and specificity of this study are 91% and 95% respectively and are improved with increasing volume of extravasation[25].
As mentioned earlier, if endoscopy is unsuccessful at either identifying or stopping the bleeding source, transcatheter arteriography is the next step at intervention. Typically, the femoral artery is used as the site for arterial access unless other factors prevent this approach; however, radial approach is an alternative which has been gaining interest at some centers[26]. If upper GI bleeding is suspected, the celiac artery is usually cannulated first[8]. Superselective evaluations are performed based on any prior studies used to help localize the location of the bleed. If a culprit vessel is identified, multiple methods of embolization have been described[8,27]. Patient breathing causes motion which can make visualization of bleeding difficult on angiography. Also, bowel gas and bowel movement can cause further limitations during catheter angiography.
There are many different techniques for embolization and controlling active hemorrhage. These include placing covered stents, endovascular coils/plugs, and embolic glue. In some instances, the microcatheter used to evaluate the culprit vessel will occlude and stop the hemorrhage temporarily. This can be utilized in temporary situations to spasm an artery to achieve hemostasis without permanently occluding an artery. Care must be taken to evaluate the vasculature in the region of bleeding as many sites in the GI tract have collateral blood flow. In sites that have collateral flow, a sandwich technique can be utilized; this requires identifying the bleeding site and embolizing the distal and proximal side branches to provide occlusion ensuring no distal reconstitution and decreasing chances of re-bleeding[8].
In some instances, patients are too hemodynamically unstable to obtain imaging and may need to go directly to the angiography suite or the endoscopy lab. Close communication between the emergency room physicians, anesthesiologists, surgeons, gastroenterologists, and interventional radiologists must be encouraged in order to provide optimal care for these critically ill patients. One important caveat to consider regarding angiography over endoscopy as a first line intervention is that angiography will only be positive if there is active bleeding, an abnormal vessel, or tumor blush. Also, active bleeding with high clinical suspicion of the approximate location of a bleed can be an appropriate indication of taking the patient directly to angiography in order to identify and treat the site of bleeding as active bleeding may terminate during the time taken to obtain imaging[28]. Hyperemia can be identified by angiography but subtle mucosal abnormalities will be more readily identified with endoscopic management. Additionally, in cases of high risk ulcers which have had either successful or unsuccessful endoscopy, catheter arteriography has been shown to play a key role in preventing rebleeding by performing prophylactic embolization[29]. Surgical consultation is always recommended and performed at our hospital.
ACUTE LOWER GI BLEEDING
Acute lower gastrointestinal bleeding is defined as bleeding of recent duration (< 3 d), hemodynamic instability, anemia or requirement of blood transfusion[30]. Though most lower GI bleeds resolve spontaneously, mortality and morbidity is increased in elderly patients and those with comorbid medical conditions[31]. Bleeding rate and total blood loss become a critical factor in determining correct patient management. Initial management and assessment is similar to upper GI bleeds. A multidisciplinary approach is crucial for providing the best care for these critically ill patients.
Lower GI bleeding accounts for approximately 30% of all GI hemorrhage and has many etiologies[32]. The most common causes of lower GI bleeding are diverticula, angiodysplasia, anorectal neoplasm, and colitis[32,33]. The incidence increases with age with mean age of presentation ranging from 63 to 77 years of age. It has been estimated that lower GI bleeding is 200 times more likely in an 80 years old than a 20 years old[32]. Although bleeding can be life threatening, unlike upper GI bleeding, most cases of lower bleeding tend to be self-limited. Of the cases considered a lower bleed, the colon is the source in approximately 80% of cases[28]. Many patients with bleeding associated with diverticulosis can stop spontaneously in up to 80% of patients[32,33]. Mortality rates have been reported at less than 5%[34].
Initial management includes determination of the need for urgent evaluation and resuscitation with crystalloids and blood products and correction of coagulation factors in applicable. If stable, imaging plays a key role in identifying the source and etiology of the bleeding. As stated previously, CT and tagged red blood cell scintigraphy are excellent non-invasive options to assist with acute management decisions (Figures 4 and 5). Other useful tools in the management of patients with small bowel bleeding distal to the ligament of Treitz include capsule endoscopy and CT enterography to evaluate for a specific lesion or site of bleeding. Though diagnostic testing helps to localize the lesion, studies have shown that the diagnostic yield of colonoscopy ranges from 45% to 100%[34] which is higher than radiological evaluation. If stable enough, patients should undergo urgent colonoscopy within 8 to 24 h of admission as that improves diagnostic yield and likelihood of therapeutic intervention. This was also demonstrated by Strate and Syngal[35] in 2003 where they studied 144 patients and concluded that endoscopic therapy was successful in 29% of colonoscopies performed within 12 h and this dropped to 4% when performed between 24 to 48 h. These patients need to undergo rapid purge prep which involves drinking 1L of Golytely every 30 to 45 min until no fecal matter is noted in the effluent[36]. However, performing a colonoscopy at the time of active significant bleeding is often not useful as the bleeding impairs visualization in the colon; this is in contrast to angiography, which usually requires active extravasation to detect the hemorrhage. The various hemostatic devices are similar to the one discussed in the upper GI bleeding section. In cases of intermittent scant hematochezia, if hemodynamically stable, healthy individual less than 40 years of age can be considered for flexible sigmoidoscopy[37].
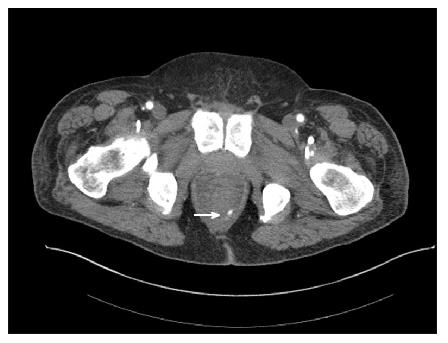
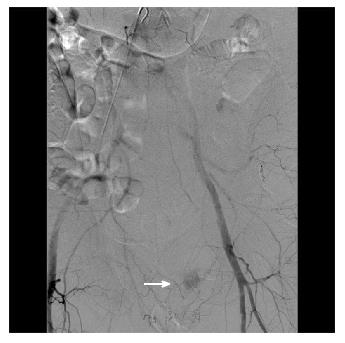
Figure 4 A male patient presented with massive bright red blood per rectum which was unresponsive to transfusions.
A representative computed tomography scan image demonstrates active contrast extravasation in the rectum (white arrow).
Figure 5 During interventional angiography, contrast extravasation is visualized into the colon via a distal branch artery from the internal iliac artery (white arrow).
The culprit vessel was occluded by spasm or dissection at the ostium with no residual active bleeding. During follow-up lower endoscopy, an endoscopic image showed no active bleeding with discrete large sized clean based ulcers, consistent with ischemic colitis.
Diverticular bleeding accounts for 20% to 65% of acute lower GI bleeds[32] and causes significant bleeding in 3% to 15%[38] of the cases (Figure 6). The bleeding is characterized as painless hematochezia which stops simultaneously in 75% to 80% of the cases but recurs in about 25% to 40% of the cases within 4 years[38]. Endoscopic management involves using clipping or thermal contact modalities either alone or in conjugation with injection technique. Due to thinner walls of the right sided colon, perforation is a concern. Endoclip placement is often preferred to treat the bleeding or visible vessel at the neck or bottom of the diverticulum. If thermal methods are used care should be taken to apply lower setting for short periods of time only. Typically 10-15 joules (heater probe) or 10 to 16 watts (bipolar) should be applied for 2 to 3 second pulse contacts and mild pressure[39,40]. Endoscopic clips can be either deployed over the bleeding vessel or use to oppose the walls to act as a tamponade effect and prevent bleeding[41]. Kaltenbach et al[42] described using EndoCap to evert the diverticulum and placing the clip.
Figure 6 Fluoroscopic images of a case of colon diverticular bleeding that failed endoscopic hemostasis with Endoclip application.
A: Active extravasation at the site of the clip (red arrow); B: Ongoing extravasation superior to the clip after initial coils placed in the inferior branch of the middle colic artery; C: Digital subtraction angiography following additional coils with no extravasation.
Ischemic colitis affects about 1% to 19% of the cases[35] and typically presents as painful hematochezia[43] affecting the water shed areas of the colon: Splenic flexure and rectosigmoid junction. The majority of patients respond to conservative management and treatment of the underlying condition. Angiography is recommended in patients with severe ischemic colitis, right sided colitis or suspicion of underlying thromboembolism or concurrent mesenteric ischemia[44]. Radiation proctitis is caused by radiation induced endarteritis obliterans with resultant telangiectasia and neovascularization in the rectum[45]. Effective treatment includes serial management with APC.
The other etiologies for colitis such as Clostridium difficile colitis, inflammatory bowel disease, viral, bacterial and parasitic infections, diversion colitis can also present with hematochezia and usually managed by treating the underlying conditions.
Angioectasias account for 3% to 15% of cases with lower GI bleeding[30], and their incidence also increase with age[46]. They range from 2 mm to several centimeters and are characterized by ectatic blood vessels radiating from a central feeding vessel[46]. Though both contact and non-contact thermal methods are used for its treatment, APC is the preferred treatment modality[47]. Owing to thin walls of the right side of the colon power settings of 15 W to 30 W at 1 L/min argon flow rate is utilized. Short pulses of 1 to 2 s are applied and the probe is held 1 to 3 mm away from the mucosa[40]. Lee et al[48] in 2010 described application of Hemoclips in conjugation with APC to control bleeding.
Hemorrhoids are present in 75% of colonoscopies[46] but are indicated in only 2% to 10% of acute lower GI bleeds[49]. Bleeding hemorrhoids are usually managed with banding devices. Like esophageal varices they are also conducted in series with no more than 3 bands placed in one setting.
Management of rectal ulcers and Dieulafoy’s lesion are similar to upper GI bleeds and achieved by thermal or mechanical methods or dual therapy, including injection technique. Hence, as stated above most cases of lower GI bleeding are self-limited, but occasionally patients may present with massive GI bleeding where they are too unstable to undergo colonoscopy or despite drinking the prep the colon is still filled with blood obscuring endoscopic evaluation. In such cases, it is IR that can prove invaluable. Surgery is typically reserved as a last resort since even with location identified mortality is high, and mortality increases when the bleed cannot be localized.
Angiography is similar regarding lower GI bleeds compared to upper bleeds. A major advantage compared to colonoscopy is that no bowel prep is needed. Typically, the interventional radiologist will begin with the superior mesenteric artery (SMA) as this will be the major supply to the distal small bowel, ascending and transverse colon. The inferior mesenteric artery (IMA) supplies the descending sigmoid colon as well as the rectum and anus. Branches of the internal iliac artery also supply the rectosigmoid colon and anus and become the main supply in cases of an occluded or diminutive IMA. Many collaterals and normal anatomic variants exist which must be evaluated prior to any interventions[28]. For instance, the SMA may provide arterial supply to the entire colon in the event the IMA is occluded via the arc of Riolan or marginal artery of Drummond which are arterio-arterio anastomoses between the superior and inferior mesenteric arteries[50].
Ideally, a selective embolization is performed if the etiology is discovered by angiography. When distal branches are able to be cannulated, the risk of developing colonic ischemia is low and perhaps subclinical. Other methods such as vasospasm can prove useful to maintain normal blood flow while clot and hemostasis develop as demonstrated in Figures 4 and 5.
GASTROSTOMY TUBE
Though feeding tubes have been in place for over 400 years it was not until 1980 that the first description of percutaneous endoscopic gastrostomy tube was attempted by Gauderer et al[51]. Subsequently, in 1981 the first percutaneous gastrostomy tube was placed under radiological guidance[52]. This was initially developed for cases where endoscopy could not be performed or was too risky to be attempted[52,53]. Since then, these two approaches have widely replaced surgical open gastrostomy owing to its minimally invasive nature, reduced cost and ease of tube placement[54]. These tubes are not only used for feeding but can also be used for decompression. Typical indications for gastrostomy tube are for providing nutrition in patients with an inability to obtain adequate nutrition but with intact and functional GI tract. Impaired swallowing mechanism associated with neurological conditions and neoplastic conditions of the oropharynx, larynx and esophagus are some of the common indications[55,56]. It can also be performed to attain gastric decompression in patients with gastroparesis or obstruction such as peritoneal carcinomatosis.
Absolute contraindications to this procedure are an uncorrectable coagulopathy, thrombocytopenia, peritonitis, or bowel ischemia. Large gastric varices, if known, can pose an increased risk of internal hemorrhage. Ascites or peritoneal dialysis is a relative contraindication given potential for pericatheter leakage and life threatening peritonitis respectively. In these patients, paracentesis can provide assistance to make the procedure safe[57]. In peritoneal dialysis patients, the procedure should be discussed with patient’s nephrologist. Contraindications specific for endoscopic placement include inability to bring the gastric wall in apposition with the anterior abdominal wall, facial fractures, skull fractures and upper GI obstruction[58]. In these cases, radiologically placed gastrostomy tube is preferred. Specific contraindications to radiologically placed feeding tubes include inability to travel to the radiology suite in patients with hemodynamic instability[58]. Also, prior gastric surgery can make the anatomical window smaller and more challenging suggesting the need for CT guidance[55,56].
For percutaneous endoscopic gastrostomy tube placement, there are currently three techniques: (1) “pull” or Pomsky-Gauderer technique[51]: This involves insufflating the stomach and selecting an appropriate site by indenting the abdominal wall with a finger. Sterile precautions should then be followed and the site anesthetized with lidocaine. Subsequently the needle is advanced in to the stomach while withdrawing the plunger. The endoscopist confirms that the gastric puncture of the needle corresponds to the air in the syringe. This is essential to ensure the absence of any intervening bowel. A small skin incision is made and trocar is introduced into the stomach. A guidewire is then fed through the trocar and grasped endoscopically and pulled out through the mouth along with the endoscope. The feeding tube is then attached to the guidewire and pulled through the esophagus, stomach and abdominal wall and held in place by both internal and external bumper; (2) “push” or Sacks-vine technique[59]. This technique is similar to pull technique till the guidewire is placed. Then an introducer tube is threaded over the guidewire and pushed till it emerges through the abdominal wall and then is grasped manually and secured in position; and (3) introducer technique or Russell technique[60], this was developed in 1984 and has recently gained popularity to be used in cases with head and neck cancer to avoid seeding of the gastrostomy tract[61]. This uses a transabdominal approach. In this technique once the access to stomach is obtained endoscopically, gastropexy is performed next using either T fasteners[62] or gastropexy sutures[63]. Subsequently, the stomach is accessed with a needle and a guidewire. The tract is then dilated with a dilating catheter and finally a balloon tip gastrostomy catheter is placed into the stomach through the peel away sheath.
Interventional radiological gastrostomy tube can be placed with either fluoroscopic, CT, or ultrasound guidance. These procedures can be performed with excellent success rates[55]. Success typically depends on the appropriate anatomic window in order to make a percutaneous approach into the stomach. Previous cross sectional imaging is utilized to evaluate for appropriate anatomical window for gastrostomy tube placement. Patients are administered barium orally or via nasogastric/orogastric tube at least 12 h prior to the procedure in order to opacify the transverse colon. Upon the patient entering the IR suite, a nasogastric tube is inserted if not already present. At our institution, we then use ultrasound to evaluate the liver margin and outline prior to the procedure. The insertion site is chosen below the costal margin, above the transverse colon, and to the left of midline. Some institutions, including ours, will administer 0.5-1 mg of intravenous glucagon to inhibit gastric motility during the procedure. The stomach is then insufflated with air. Gastropexy is next performed with T-fasteners in order to apply the stomach to the abdominal wall. An incision is then made between the gastropexy sutures. A needle is inserted into the stomach which is confirmed by aspirating air at an angle directed towards the pylorus. Care must be taken during these next steps to ensure the stomach remains insufflated with air; typically, a technologist will assist with insufflating air as needed. A wire is then inserted and the tract is dilated to the appropriate tube size. The gastrostomy tube is then inserted by using a peel-away sheath. Gastrostomy tubes may have a pigtail or balloon to secure within the stomach; we use tubes with balloons for securing the tube location. Contrast is then injected and both AP and lateral fluoroscopic views obtained to ensure the tube has been placed into the stomach only and is secured to the abdominal wall. This procedure is illustrated in Figure 7. Occasionally, this method will be adjusted and performed under CT guidance for patients with a narrow anatomic window. Percutaneous sonographic gastrostomy which was initially described by Gebel et al[64] in 1991 for patients with upper GI tract obstruction is yet another technique. This procedure is currently not widely performed in United States, though is still popular in Europe. This process involves passage of a nasogastric tube into the stomach, followed by instillation 500 to 1500 cc of water. The stomach is then localized by ultrasound. The puncture site into the stomach is identified after establishing absence of vessels and colon or small intestine interposition with ultrasound and Doppler. A small skin incision is made, and a needle is introduced into the stomach. A wire is then passed through the stomach into the duodenum. The puncture site is then dilated with serial dilators and gastrostomy tube placed. In 1998 Bleck et al[65], reported successful placement of feeding tube in 38 patients via this method with no major complications reported in a 4 mo follow-up period. Major complications can include internal hemorrhage, catheter dislodgement, peritonitis/sepsis, or death. Minor complications are catheter leakage and clogging requiring exchange.
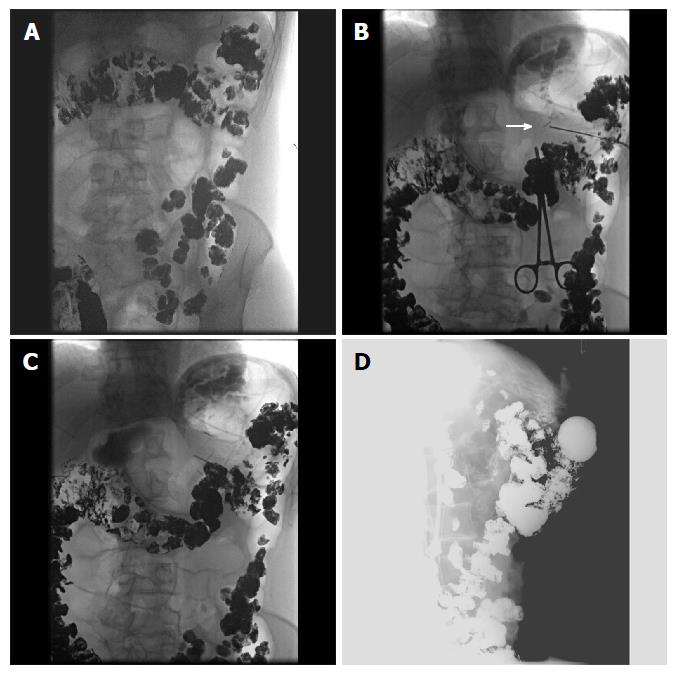
Figure 7 Selected fluoroscopic images demonstrating fluoroscopically guided gastrostomy placement.
A: Contrast seen throughout the transverse colon; B: The first T-tac (white arrow) is deployed following needle insertion into the gastric lumen; C: Two more T-tacs placed; D: Static lateral image of the gastrostomy tube against abdominal wall and not in the colon. The contrast is injected into the tube to demonstrate intraluminal placement.
Thus, though studies have proven them to have equal success rates with both endoscopic and radiological tube placement, each procedure has a distinct advantage over the other[66]. With endoscopic placement, the procedure can be performed at the bed side and have diagnostic capabilities. In a study published in 1990, 10%-71% of patients were found to have abnormal endoscopy findings out of which management had to be altered in 36% of patients[67]. Also the endoscopic procedure reduces the radiation exposure. Meanwhile, radiological placement is possible in patients who fail endoscopic management such as those who are morbidly obese or have severe upper GI luminal narrowing[68]. Hence it is of utmost importance that practitioners are aware of these indications so that the patient can be sent to appropriate discipline for gastrostomy tube placement.
CECOSTOMY TUBES
Cecostomy tubes can be placed surgically or percutaneously with endoscopic or image guidance[69]. These tubes are mainly indicated in patients with neurological disorder with resultant fecal incontinence to facilitate cleansing enemas[70], in the assistance of bowel training in the pediatric population, neurogenic bowel due to any issue, chronic colonic pseudo obstruction and colonic obstruction[71]. The contraindications are similar to gastrostomy tube[72,73].
Placement of a percutaneous endoscopic cecostomy tube is similar to endoscopic percutaneous gastrostomy tube placement[74]. The night before the procedure 4 liters of Golytely is administered to clean the colon. In patients with chronic constipation more prolonged prep may be needed[75]. The colonoscope is then advanced all the way to the cecum which is insufflated. An appropriate site is selected by finger indentation in the right lower quadrant and transillumination is performed. The rest of the procedure is similar to percutaneous endoscopic gastrostomy tube placement via the “pull” technique. The cecum needs to be fixed to the abdominal wall with the help of a “fixation” device to prevent leakage of the fecal content[69].
IR can perform these procedures either under fluoroscopic or CT guidance. The bowel will be prepped prior to the procedure similar to endoscopic procedures. Similar to gastrostomy tubes, the colon is insufflated with air and glucagon administered to decrease bowel contractions. A needle is inserted, T-tacks used to secure the position of the cecum, a wire inserted with subsequent dilation, and a tube is then inserted. This has been reported as a safe procedure although not commonly performed at our institution[72]. Though only small case series have been described, the procedure is easily performed and has a good success rate. However, the complication rate has been reported to be as high as 42% and includes wound infection, bleeding, perforation leading to peritonitis and buried bumper syndrome[76]. Buried bumper syndrome (BBS) which is a well-known complication of gastrostomy tube placement, occurs due to excessive outward traction on the tube. In 2011 Rao et al[77] described its occurrence in a patient who had undergone percutaneous endoscopic cecostomy tube placement about 1.5 years prior to the presentation. BBS usually presents with peristomal cellulitis owing to the presence of intraluminal stool. If the tract is immature, BBS can lead to fecal peritonitis and intra-abdominal sepsis. Management involves antibiotics and removal of the tube. Cecostomy tubes should not be removed by external traction as it can lead to colonic laceration. Instead, the tube should be cut externally and removed endoscopically with the help of a snare. This complication can potentially be prevented with the usage of balloon tube or by avoiding excessive traction. In 2006, Uno[78], described the introducer technique to reduce the incidence of wound infection. In 2015, Duchalais et al[79] published a prospective trial to evaluate the efficacy of constipation in patients undergoing percutaneous endoscopic cecostomy tube placement and reported successful results in three quarters of the patient population. However, chronic wound pain prompted the removal in the other one fourth of the patient population. Currently there are no trials comparing the efficacy of endoscopic and fluoroscopically placed percutaneous cecostomy tubes.
STRICTURES
Esophageal strictures are routinely seen in practice. The common causes include peptic strictures which develop as a sequel to GERD and account for almost 80% of benign strictures[80]. Their incidence seems to be decreasing in recent years with the more widespread use of proton pump inhibitors. Other causes include: Schatzki’s ring, radiation, caustic injury, anastomotic stricture, pill induced esophagitis, esophageal web, eosinophilic esophagitis and malignancy[81]. They can be further divided into simple and complex strictures[82]. Simple strictures are symmetric, < 2 cm in length, with a diameter of greater than equal to 12 mm, and allow easy passage of an upper endoscope. Complex strictures, however, are asymmetric, have a diameter < 12 mm, more than > 2 cm long, and do not allow the passage of the scope. Most of these strictures are amenable to treatment with dilation and stent placement which can be done both endoscopically and radiographically.
These patients present with dysphagia and should undergo initial endoscopic evaluation as that not only helps in diagnosis but can also aid in performing possible therapeutic intervention such as dilation[83]. Further, they can also assess to see if they are any predisposing factors such as angulated stricture, diverticula, and hiatal hernia which may increase the risk of complications. Most benign strictures respond to dilation unlike malignant strictures which have a greater risk of complications[84]. Active esophageal perforation is an absolute contraindication to dilation[85]. There are currently three types of dilators: Maloney bougie (without a guidewire), savory-Gilliard (passed over a guidewire) and through the scope (TTS) balloon dilators[86]. Prior to dilation, the endoscopist should consider the method of dilation, the diameter to which the obstruction should be dilated, need for wire guidance and need for radiographic screening[85]. Benign esophageal strictures are usually dilated to about 13 to 15 mm. However Schatzki’s ring can be dilated to about 16 to 20 mm[85]. Maloney dilators range from 16F to 60F and exert both longitudinal and radial force. This can be done blindly or under fluoroscopic guidance. These dilators can also be used for self-dilation without sedation[82]. Patients with benign esophageal stricture requiring frequent dilations are ideal candidates for self-dilation. The Maloney dilator is usually marked at two points: 20 cm from the entry site and 10 cm beyond the distal end of the stricture. The procedure is performed with the patient in sitting position. The dilator is lubricated with water and introduced over the tongue into the oropharynx. Once the patient visualizes the 20 cm marking, the tube is in the esophagus. The dilator is then advanced until the second marking is seen at the level of incisors, which confirms the passage of maximal diameter of the dilator across the stricture. Lastly, the dilator is carefully withdrawn.
Similarly, Savory dilators also range from 16F to 60F and have the same mechanism of action. The rigid tip is passed over a guidewire. They are also marked with radiopaque band at their maximal diameter. The guidewire is usually passed to the antrum and can be done endoscopically or fluoroscopically. Subsequently, the dilator is passed over the guidewire till the maximal diameter is beyond the stricture. If no force is experienced, then the dilator is slowly removed in one to one exchange manner to ensure the positioning of the guidewire. This uses tactile perception to determine the amount of resistance encountered. Sequential dilation is performed but usually in one setting no greater than 3 dilators are passed though studies have shown no increased risks with passing > 3 dilators in cases of a benign simple esophageal stricture[87]. TTS balloons work by exerting only radial force. They can usually increase up to 3 diameters and are useful for serial dilations with a single balloon. The endoscope is usually positioned proximal to the stricture and the balloon dilator is advanced through the stricture. The balloon is then inflated under direct endoscopic visualization and the pressure is held for 30 to 60 s.
Studies have shown similar efficacy in treating peptic strictures via any of the above mentioned methods[88,89]. In the treatment of postesophagectomy anastomotic strictures these methods show a similar efficacy of 93%; however, they have a high recurrence rate and need multiple sessions[90,91]. Though most procedures are performed under endoscopic guidance, fluoroscopy is important in the setting of complex strictures as it aids in dilation[85]. Further in cases where an endoscope is unable to cross proximal lesions, fluoroscopic dilation must be performed.
Fluoroscopic or other image guided balloon dilatation has been described in the literature as a safe method for stricture treatment of the esophagus in various disease states[92,93]. However, this has not been a common procedure in our IR department. In cases of refractory benign strictures, steroid injection into the stricture prior to dilation has shown to be effective in increasing post dilation diameter, reducing the need for repeat dilation and increasing the interval between dilations. Temporary esophageal stenting is also an option. A systemic review published regarding the use of plastic stents in benign esophageal strictures reported successful dilation in 52% of the cases. However, stent migration was reported in 24% of the population[94]. Song et al[95] in 2000 published a study where fully covered metal stents were placed in 25 patients, but migration was noted in 80% while 48% of them developed a new stricture. Few studies[96,97] have also been published regarding the use of biodegradable stents without promising results.
Similar to the upper GI tract, patients with inflammatory bowel disease are known to develop strictures. This is more common in Crohn’s disease where 40% of patients with ileal disease develop strictures[98]. Further studies demonstrate that 60% of patients with strictures would undergo surgery within the next 20 years[99]. Strictures in Crohn’s disease can occur de novo, at the site of bowel anastomosis, or at the ileal pouch. They can be divided into inflammatory vs fibrotic[100]. Inflammatory strictures can be treated with medical management. However, fibrotic strictures were traditionally treated with only surgical management[101]. Studies have shown that stricturoplasty tends to preserve bowel length and is associated with high recurrence rate leading to frequent operations. In younger adults, the course tends to be more aggressive leading to frequent operations and finally short bowel syndrome[102]. Endoscopic managements have been devised in an attempt to reduce this dreaded complication. Prior to any therapy endoscopic evaluation and biopsy, assessment of the stricture must be performed to rule out malignancy especially in the setting of ulcerative colitis. Endoscopic management includes dilation therapy, local injection of steroids, needle-knife stricturotomy and stent placement[103]. The technique of balloon dilation (TTS) is similar to the one described for esophageal stricture. Endoscopic balloon dilation has shown to both avoid or delay the need for surgery, though frequent dilations may be needed. In a systemic review published by Hassan et al[104] in patients with Crohn’s disease related strictures, endoscopic dilation achieved success in 86% of the patients with long term clinical efficacy obtained in 56% of them. In a multivariate analysis, stricture length of < 4 cm was the only predictor for surgery free follow up period. The mean adverse events (perforation and bleeding) were less than 2%. In 2010, Mueller et al[105] published a prospective single center study of 55 patients. They reported an initial success rate of 95% with 76% of those patients not requiring surgery during the follow-up period. Given the high relapsing rate, addition of steroid injection to the stricture at the time of dilation has been studied. However, currently the data is conflicting[106,107]. More recently stent placement at the time of endoscopy in this patient population has been evaluated. However, studies using self-expandable metallic stents (SEMS)[108] and biodegradable stents[109] have reported high rates of stent migration and other adverse events. In 2012 Loras et al[110] published a small series of 25 SEMS placement with technical success obtained in 92% of the stent placements that was maintained for a 4 wk follow-up period.
Similar to upper GI strictures, fluoroscopic guided balloon dilatation and/or stent placement has also been described for the lower GI tract for patients as a pre-surgical or palliative relief[111,112]. Typically, the procedure involves placing the patient supine on the fluoroscopy table. A wire is then inserted through the anus retrograde to the level of the obstruction. Once the wire passes the obstruction and is proximal, a catheter is inserted for the purpose of water soluble contrast injection. This step is critical to evaluate the dimensions of the stent and type of delivery system used. Appropriate stents should cover the lesion with 1-2 cm extension beyond the obstruction. Covered stents are not recommended due to risk of migration[111]. Water-soluble contrasted enema can be repeated immediately or any time after the procedure to evaluate placement. However, this is also another procedure not commonly performed in our IR department.
CONCLUSION
Advances in the medical field have been to the advantage of the patient in many disease processes as discussed above. In today’s era of minimally invasive procedures, surgery as a first choice of management is getting less popular. Therapeutic endoscopy and interventional radiology have come a long way from their initial inception to being main modalities of treatment. Treatment modality of choice is often based on availability of the services, clinical stability of the patients and their presentation. However, these are complex patients that often require close collaboration between gastroenterologists, radiologists and surgeons.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Andersen PE, Francica G, Lassandro F S- Editor: Kong JX L- Editor: A E- Editor: Li D