Published online Aug 28, 2016. doi: 10.4329/wjr.v8.i8.750
Peer-review started: January 27, 2016
First decision: March 23, 2016
Revised: April 10, 2016
Accepted: May 31, 2016
Article in press: June 2, 2016
Published online: August 28, 2016
Processing time: 212 Days and 12.5 Hours
To assess the potential value of femoral head (FH) volume measurements to predict joint collapse, as compared to articular surface involvement, in post-treatment osteonecrosis (ON) in pediatric patients affected by lymphoproliferative diseases.
Considering 114 young patients with lymphoproliferative diseases undergone a lower-limbs magnetic resonance imaging (MRI) examination between November 2006 and August 2012 for a suspected post-treatment ON, we finally considered a total of 13 cases (7 males, mean age 15.2 ± 4.8 years), which developed a FH ON lesions (n = 23). The MRI protocol included coronal short tau inversion recovery and T1-weighted sequences, from the hips to the ankles. During the follow-up (elapsed time: 9.2 ± 2 mo), 13/23 FH articular surface (FHS) developed articular deformity. The first MRI studies with diagnosis of ON were retrospectively analyzed, measuring FH volume (FHV), FHS, ON volume (ONV) and the articular surface involved by ON (ONS). The relative involvement of FHS, in terms of volume [relative volume (RV): ONV/FHV] and articular surface [relative surface (RS): ONS/FHS], was then calculated.
By using receiver operating characteristic curve analysis (threshold of 23% of volume involvement), RV predicted articular deformity in 13/13 FHS [sensitivity 100%, specificity 90%, accuracy 95%, positive predictive value (PPV) 93%, negative predictive value (NPV) 100%]. Considering a threshold of 50% of articular involvement, RS predicted articular deformity in 10/13 femoral heads (sensitivity 77%, specificity 100%, accuracy 87%, PPV 100%, NPV 77%).
RV might be a more reliable parameter than RS in predicting FH deformity and could represent a potential complementary diagnostic tool in the follow-up of femoral heads ON lesions.
Core tip: Osteonecrosis can affect different bone segments but the most common sites are the weight-bearing joints of the lower limbs (hips and knees), with potential evolution to disability. To date magnetic resonance imaging represents the standard imaging method in the assessment of bone necrotic lesions [osteonecrosis (ON)], replacing other techniques in diagnostic work-up of initial ON, allowing also the detection of early bone marrow changes. Our preliminary data show that the volume of the necrotic portion of the femoral head might be a parameter highly predictive of future collapse of femoral head affected by osteonecrosis also in young patients treated for haematological malignancies.
- Citation: Ippolito D, Masetto A, Talei Franzesi C, Bonaffini PA, Casiraghi A, Sironi S. Relative volume measured with magnetic resonance imaging is an articular collapse predictor in hematological pediatric patients with femoral head osteonecrosis. World J Radiol 2016; 8(8): 750-756
- URL: https://www.wjgnet.com/1949-8470/full/v8/i8/750.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i8.750
The optimization of treatment strategies for haematological pediatric malignancies has led to a significant improvement of overall survival[1]; however, therapy has also turned out to determine several complications, particularly osteonecrosis (ON)[2]. Several risk factors might play a role in the development of bone tissue necrosis, both individual and related to treatment itself (glucocorticoids, chemotherapics, total body irradiation)[3-6]. ON can affect different bone segments but the most common sites are the weight-bearing joints of the lower limbs (hips and knees), with potential evolution to disability[7]. Therapeutic solutions and clinical management of post-treatment osteonecrosis depends on the joint affected, the stage of both osteonecrosis and primary disease and on symptoms. Core decompression is the most frequently therapeutic procedure in early femoral head ON, while joint replacement is performed in cases of collapse[8]. However, even minimally invasive surgery may be life threatening and septic complications may be severe[9].
Imaging may play a crucial role for prompt diagnosis and proper staging, above all in patients with no specific symptoms[10]. Magnetic resonance imaging (MRI) is the technique that demonstrated the highest sensitivity and specificity in the early diagnosis of ON: It allows detecting initial typical signal intensity alterations of the bone marrow, when other examinations show nonspecific findings or even no alterations at all[11]. Some studies reported that MRI is accurate also for the assessment of the size of femoral head osteonecrotic lesion[12]. This parameter seems to be one of the main determinants of collapse in adults[13,14] but to our knowledge there are only a few studies in young patients.
On these bases, the purpose of our study was to evaluate if the volumetric measurement of post-treatment osteonecrotic lesions on MRI could be a predictor of femoral head collapse also in paediatric patients with haematological malignancies. We also compared volume with articular surfaces of affected femoral heads as an alternative parameter for prediction of collapse.
We retrospectively evaluated as a start 114 peadiatric and young patients (64 males, 50 females, mean age 14.8 years, range 3-23 years), affected by proven lymphoproliferative diseases and treated with chemotherapy and steroids and/or bone marrow transplantation. All these patients underwent at least one lower limb MRI study between November 2006 and March 2012 (80/114 because of symptoms suspicious for ON, 34/114 for screening purposes), while follow-up examinations were performed in 72/114 cases.
Among these patients, we selected only those who showed at follow-up osteonecrosis of one or both femoral heads, regardless symptoms and with the following exclusion criteria: (1) patients with suspected osteonecrosis but affected by non-lymphoproliferative diseases (e.g., thrombotic thrombocytopenic purpura) or those who underwent MRI study for different purposes (lymphoproliferative disease localization, inflammatory complications such as fasciitis, osteomyelitis or soft tissues abscess); (2) patients who did not perform follow-up studies in our Institution; (3) patients with osteonecrosis of the femoral head and evidence of joint deformity or collapse at the first MRI examination.
As a result, a total of 13 patients (7 males, 6 females; mean age 15.2 years, range 9-23 years), met the above-mentioned inclusion and exclusion criteria.
The MRI studies were performed either on a 1.5 T magnet (Achieva, Philips) using a built-in body coil (Q-Body) and the stepping table technique or a 1 T scanner (Panorama, Philips), with a three-channel surface body coil (extra large body coil). The acquisition protocol included: long echo time (TE) short time inversion recovery (STIR) (TE = 80 ms; repetition time (TR)/TE = 4935/150 ms; slice thickness = 5 mm; acquisition matrix MxP = 352 × 351; acquisition voxel measurement, phase and slice encoding (MPS) = 1.51/1.51/5.00 mm; reconstruction voxel MPS = 1.04/1.04/5.00; min. slice gap = 1 mm) and T1-weighted sequences (TE = 15 ms; TR = 225 ms; acquisition matrix MxP = 400 × 259; acquisition voxel MPS = 1.33/1.33/5.00 mm; reconstruction voxel MPS = 1.04/1.04/5.00; min. slice gap = 5 mm; act. slice gap 0.5 mm). Images were acquired coronal, from the hips to the ankle, with an average acquisition time of about 15-20 min (depending on patient’s height).
The diagnosis of osteonecrotic involvement of femoral heads was established when typical morphological alterations were present[15]: Sharply defined areas with geographical appearance affecting subchondral bone marrow, characterized by peripheral rim of low signal intensity on T1-weighted sequences and high signal intensity on STIR images.
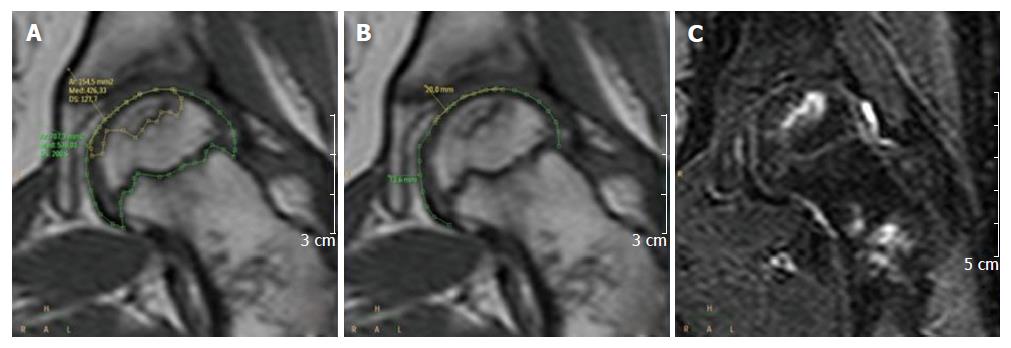
Considering the first MRI study that showed the presence of osteonecrosis, a radiologist measured on dedicated software (Brilliance Workspace Portal, V 2.6.1.5, Philips): The volume [femoral head volume (FHV)] and the articular surface area of the affected epiphysis [femoral head surface (FHS)], the osteonecrotic lesion volume (ONV) and the articular surface area affected by osteonecrosis (ONS). As shown in Figure 1, to determine the FHV in each slide of the T1 weighted images the corresponding epiphysis was contoured along its edge, using the cartilaginous physeal line as the caudal limit of the epiphysis itself. The procedure was performed for each slice and the values obtained (expressed in square millimeter) were added up together and then multiplied by section thickness (5 mm), obtaining the corresponding epiphyseal volumes (expressed in cubic millimeter). The same steps were followed for the assessment of the ONV, by contouring on T1 images the edge of the bone involved by necrosis. STIR sequence was contextually taken into account as reference to better assess lesion’s boundaries. Similarly, to determine the FHS (Figure 1), the convex articular edge was contoured in each slice of the T1 weighted sequence, obtaining linear values (expressed in millimeter) that were added up together; the resulting number was multiplied by the slice thickness (5 mm), obtaining surface values (expressed in square millimeter). The same procedure was performed for the ONS, considering only the surface of the femoral head with subchondral bone necrosis.
Following these measurements, the necrotic portions of the femoral heads involved were then calculated as ratio. First of all, the involvement of the femoral heads in terms of volume was defined as relative volume (RV) and calculated with the following formula: ONV/FHV. The articular surface involvement was defined as relative surface (RS) and assessed with the following formula: ONS/FHS (Table 1).
| RV (ONV/FHV) | RS (ONS/FHS) | |
| Threshold | 0.23 | 0.5 |
| Sensitivity | 100% | 77% |
| Specificity | 90% | 100% |
| Accuracy | 95% | 87% |
| PPV | 93% | 100% |
| NPV | 100% | 77% |
We employed the receiver-operator characteristic (ROC) curve analysis in order to determine the best thresholds of both RV and RS to predict joint deformity. Statistical analysis was performed with MedCalc software (version 12.4.0.0).
Twenty-three femoral heads were affected, since 10/13 patients had bilateral involvement. In the follow-up studies (elapsed time: 9.2 ± 2 mo), 13/23 (56%) femoral heads developed articular deformity or complete collapse, within a mean time of 10.2 mo (range 2-32 mo). The average follow-up period of the femoral heads that did not collapse was 12.5 mo (range 4-32 mo). The required time for postprocessing, volumes and surfaces measurements was about 10 min per lesion.
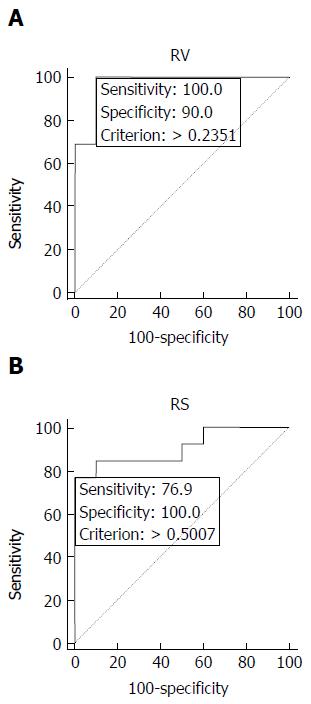
Applying the thresholds suggested by the ROC analysis (0.23), RV predicted correctly articular deformity in 13/13 (100%) of femoral heads affected by ON and erroneously in 1/10 that did not collapse (Figure 2), with sensitivity of 100%, specificity of 90%, accuracy of 95%, positive predictive value (PPV) of 93% and negative predictive value (NPV) of 100% (Table 1).
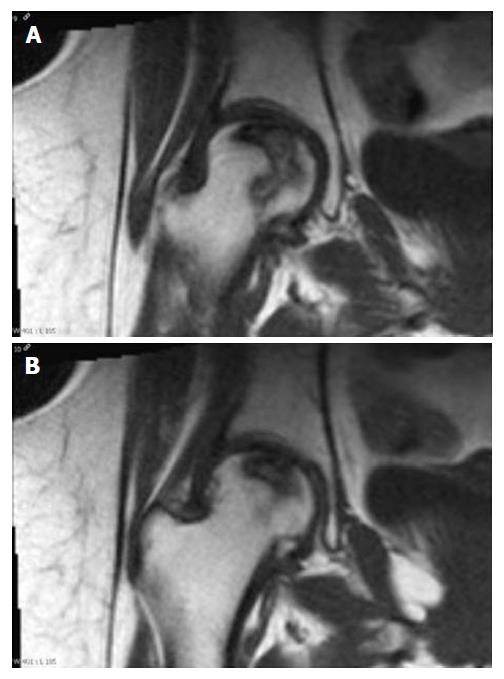
On the other hand, using a threshold of 0.50 (Figure 2), RS correctly predicted joint collapse in 10/13 (77%) femoral heads, without false positives; however, it missed 3/13 (23%) of them that developed deformity during follow-up, with corresponding sensitivity of 77%, specificity of 100%, accuracy of 87%, PPV of 100% and NPV of 77% (Table 1). In the 3 cases in which RS did not predict joint collapse and was not in agreement with RV, there was discrepancy between the overall size of the osteonecrotic lesion and its involvement of the corresponding articular surface of the femoral head affected (Figure 3).
Therapy for hematologic malignancies is a well-recognized cause of osteonecrosis in paediatric patients[16]. Femoral heads are the most critical sites affected, because of their high rate of progression towards collapse leading to surgical intervention[17]. To determine which patients with femoral head osteonecrosis will more likely develop collapse may be important in order to guide follow-up studies and to better address treatment strategies[10].
The main purpose of our study was to evaluate if the size of both the lesion and the joint surface involved by necrosis could be useful predictors of deformity in young patients affected by post-treatment femoral head ON. To this reason, we retrospectively selected in our database patients who underwent several lower-limbs MRI studies to reveal either stability of the osteonecrotic lesions or development of joint deformity. We measured the volume of the necrotic lesion related to the volume of the proximal epiphyseal region and we also assessed the extension of the lesion itself to the joint surface in relation to the whole articular surface of the femoral head. The two parameters were called RV and RS respectively and were compared each other in terms of prediction of joint collapse. In order to obtain a more reliable quantification of the necrotic lesion, without employing complex mathematical formulas, we measured epiphyseal volume only, considering the physeal line as the caudal limit of the epiphysis itself. In all our patients the physeal line was clearly evident or at least appreciable, particularly evaluating T1 and STIR images side by side, because of incomplete consolidation of the cartilaginous growth plate.
Several authors evaluated potential reliable parameters to predict collapse or outcome of core decompression in adult patients with femoral head osteonecrosis[18-20]. Most of these studies focused on the measurements of the necrotic portion of the femoral head or of the joint surface involved by necrosis. Morphologic evaluations were performed with radiographs in older works and more recently with MRI, employing several different techniques or formulas. Almost all the Authors reported the size of the necrotic lesion as the main predictor of progression towards deformity and collapse of the femoral head[21]. To our knowledge, only one study performed by Karimova et al[22] evaluated factors that could potentially forecast the outcome of post-treatment femoral head osteonecrosis in paediatric patients affected by haematological malignancies. Among several parameters (i.e., sex, age, stage disease), the necrotic portion of femoral head was considered. Radiological evaluation of involvement by ON was conducted with two approaches. The first one consisted of a quantitative assessment, according to the measurement technique described by Hernigou[12] and to the classification system of Steinberg et al[23] (mild: < 15% of femoral head affected; moderate: 15%-30%; severe: > 30%). The second approach relied on the method described by Sugano et al[24], where ON lesions are classified semi-quantitatively (A: Involvement of the medial 1/3 of femoral head or less; B: Medial 2/3 or less; C: More than 2/3). Similarly to the results reported for adults’ femoral head osteonecrosis, also in Karimova’s work the size of the necrotic lesion turned out to be the best predictor of future collapse. In particular, femoral heads with an involvement of more than 30% by osteonecrosis or type C lesions had higher rates of collapse. The involvement of the articular surface was also included among the potential factors for progression to deformity but it was considered only as a qualitative parameter, without measurements of the joint affected surface.
Even according to our results, RV performed better than RS, with an overall accuracy of 95% vs 87% and with a cut-off value of 23%, as reported by the cited papers also in adult patients. Particularly, RS did not properly forecast the progression to collapse in 3/13 cases, where the necrotic process had a minor involvement of the joint surface as compared to the underlying deeper portions of the epiphysis affected. Karimova’s results may seem quite similar to our in terms of cut-off value of necrotic lesion volume (30% vs 23%, respectively) but the differences between the two approaches should be take into account. We considered only the proximal femoral epiphysis involvement, while in Karimova’s study the reported percentage was related to the entire femoral head, including also the physeal and metaphyseal regions. Moreover, also measurements methods are different (semiquantitative evaluation of femoral head involvement vs quantitative assessment of proximal epiphysis). Basing on these observations, we presume that in our population collapse occurred with smaller lesions or with minor femoral head involvement than in Karimova’s patients.
The main limitations of our study were the small number of patients considered and the short non-standardized follow-up time; however it has to be considered that, according to the literature, our population study was quite in line with those reported in other studies. Inter- and intra-observer agreement analysis might have strengthened the reported results but it was out of the aims of this study. Furthermore, referring to physeal line as caudal limit of femoral epiphysis might be considered a less reproducible method for volume measurements in the clinical practice. Therefore, our data and the proposed technique for volume analysis have to be confirmed and validated by a wider study population. Another remark is that the majority of our patients were still being administered corticosteroids between the different MRI studies, that were either performed during the different phases of the therapy or after treatment for a graft vs host disease. As known, therapy itself along with the size of the lesion may determine the progression towards deformity. However, the main purpose of our study was to assess the potential value of volume and surface measurements in the prediction of joint collapse, regardless patients’ symptoms and related risk factors (i.e., therapy).
In conclusion, our preliminary data show that the volume of the necrotic portion of the femoral head might be a parameter highly predictive of future collapse of femoral head affected by osteonecrosis also in young patients treated for haematological malignancies. Since the management of osteonecrosis is often challenging, the quick and reproducible measurements of this parameter could properly guide follow-up studies and, therefore, help better address diagnostic resources, avoiding unnecessary examinations in small lesions or in those less prone to collapse. However, further prospective studies with a larger population study are fundamental to confirm these results.
The treatment strategies for haematological pediatric malignancies has improved the overall survival, but has also determined several complications, in particular osteonecrosis (ON), related to several risk factors, individual and related to treatment (glucocorticoids, chemotherapics, total body irradiation). The most common sites involved in ON are the weight-bearing joints of the lower limbs (hips and knees), with potential evolution to disability. Imaging may play a crucial role for prompt diagnosis and proper staging, and magnetic resonance imaging (MRI) is the technique that demonstrated the highest sensitivity and specificity in the early diagnosis of ON and in an accurate assessment of the size of osteonecrotic lesion. This parameter seems to be one of the main determinants of collapse in adults, but there are only a few studies in young patients.
MRI, with the advantages of no ionizing radiation, permits to safely evaluated young patients. Therefore the MRI study may be useful in the evaluation and follow-up of post-treatment femoral head osteonecrosis, in paediatric patients affected by haematological malignancies.
Almost all the authors reported the size of the necrotic lesion as the main predictor of progression towards deformity and collapse of the femoral head in adult patients. The measurement of the volume of the necrotic portion of the femoral head, with MRI, could potentially forecast the outcome of post-treatment femoral head osteonecrosis in paediatric patients affected by haematological malignancies.
The relevance of this work relies on the possibility, by measuring the volume of the necrotic portion of the femoral head, to obtain a new diagnostic parameter that might be highly predictive of future collapse of femoral head affected by osteonecrosis also in young patients treated for haematological malignancies. Furthermore the quick and reproducible measurements of this parameter could properly guide follow-up studies, improving the better address diagnostic resources and avoiding unnecessary examinations in small lesions or in those less prone to collapse.
MRI: MRI is the technique that demonstrated the highest sensitivity and specificity in the early diagnosis of ON; ON: ON is a bone disease, that affect the joints, caused by reduced blood flow. The typical aspect is a “geographic pattern” area, surrounded by low signal intensity serpentine rim and a high signal intensity line on T2 weighted images inside the rim; FHV: Femoral head volume, calculated by contouring in each slide of the T1 weighted images the corresponding epiphysis. The values obtained (expressed in square millimeter) were added up together and then multiplied by section thickness (5 mm), obtaining the corresponding epiphyseal volumes (expressed in cubic millimeter); ONV: Osteonecrotic volume, by contouring for each slide on T1 images, the edge of the bone involved by necrosis. The values obtained (expressed in square millimeter) were added up together and then multiplied by section thickness (5 mm), obtaining the corresponding epiphyseal volumes (expressed in cubic millimeter); FHS: Femoral head surface, assessed by contouring the convex articular edge in each slice of the T1 weighted sequence, obtaining linear values (expressed in millimeter) that were added up together; the resulting number was multiplied by the slice thickness (5 mm), obtaining surface values (expressed in square millimeter); ONS: Osteonecrotic surface, measured with the same procedure of FHS, considering only the surface of the femoral head with subchondral bone necrosis; RV: Relative volume, defines the articular volume involved on ON, calculated with the following formula: ONV/FHV; RS: Relative surface, defines the articular surface involved on ON, assessed with the following formula: ONS/FHS.
This is a technical and interdisciplinary study conducted in the assessment of receiver-operator characteristic. This preliminary data show that the volume of the necrotic portion of the femoral head might be a parameter highly predictive of future collapse of femoral head affected by osteonecrosis also in young patients treated for haematological malignancies.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Casciaro S, Gao BL, Li YZ, Shen J S- Editor: Gong XM L- Editor: A E- Editor: Zhang FF
| 1. | Hunger SP, Lu X, Devidas M, Camitta BM, Gaynon PS, Winick NJ, Reaman GH, Carroll WL. Improved survival for children and adolescents with acute lymphoblastic leukemia between 1990 and 2005: a report from the children’s oncology group. J Clin Oncol. 2012;30:1663-1669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 723] [Cited by in RCA: 905] [Article Influence: 64.6] [Reference Citation Analysis (0)] |
| 2. | Barr RD, Sala A. Osteonecrosis in children and adolescents with cancer. Pediatr Blood Cancer. 2008;50:483-485; discussion 486. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 27] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 3. | Harper PG, Trask C, Souhami RL. Avascular necrosis of bone caused by combination chemotherapy without corticosteroids. Br Med J (Clin Res Ed). 1984;288:267-268. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 69] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 4. | Jagasia S, Misfeldt A, Griffith M, Jagasia M. Age and total-body irradiation in addition to corticosteroid dose are important risk factors for avascular necrosis of the bone. Biol Blood Marrow Transplant. 2010;16:1750-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 5. | Kawedia JD, Kaste SC, Pei D, Panetta JC, Cai X, Cheng C, Neale G, Howard SC, Evans WE, Pui CH. Pharmacokinetic, pharmacodynamic, and pharmacogenetic determinants of osteonecrosis in children with acute lymphoblastic leukemia. Blood. 2011;117:2340-2347; quiz 2556. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 180] [Cited by in RCA: 199] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 6. | Niinimäki RA, Harila-Saari AH, Jartti AE, Seuri RM, Riikonen PV, Pääkkö EL, Möttönen MI, Lanning M. High body mass index increases the risk for osteonecrosis in children with acute lymphoblastic leukemia. J Clin Oncol. 2007;25:1498-1504. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 84] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 7. | Min BW, Song KS, Cho CH, Lee SM, Lee KJ. Untreated asymptomatic hips in patients with osteonecrosis of the femoral head. Clin Orthop Relat Res. 2008;466:1087-1092. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 8. | Vora A. Management of osteonecrosis in children and young adults with acute lymphoblastic leukaemia. Br J Haematol. 2011;155:549-560. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 36] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 9. | Singer K, Subbaiah P, Hutchinson R, Odetola F, Shanley TP. Clinical course of sepsis in children with acute leukemia admitted to the pediatric intensive care unit. Pediatr Crit Care Med. 2011;12:649-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 14] [Article Influence: 0.9] [Reference Citation Analysis (1)] |
| 10. | Marchese VG, Connolly BH, Able C, Booten AR, Bowen P, Porter BM, Rai SN, Hancock ML, Pui CH, Howard S. Relationships among severity of osteonecrosis, pain, range of motion, and functional mobility in children, adolescents, and young adults with acute lymphoblastic leukemia. Phys Ther. 2008;88:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Mitchell MD, Kundel HL, Steinberg ME, Kressel HY, Alavi A, Axel L. Avascular necrosis of the hip: comparison of MR, CT, and scintigraphy. AJR Am J Roentgenol. 1986;147:67-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 113] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 12. | Hernigou P, Lambotte JC. Volumetric analysis of osteonecrosis of the femur. Anatomical correlation using MRI. J Bone Joint Surg Br. 2001;83:672-675. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 37] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 13. | Hernigou P, Lambotte JC. Bilateral hip osteonecrosis: influence of hip size on outcome. Ann Rheum Dis. 2000;59:817-821. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 30] [Reference Citation Analysis (0)] |
| 14. | Koo KH, Kim R. Quantifying the extent of osteonecrosis of the femoral head. A new method using MRI. J Bone Joint Surg Br. 1995;77:875-880. [PubMed] |
| 15. | Kaplan P, Helms CA, Dussault R, Anderson MW, Major NM. Musculoskeletal MRI. W.B. Saunders, Philadelphia. 2001;46-48. |
| 16. | Mattano LA, Sather HN, Trigg ME, Nachman JB. Osteonecrosis as a complication of treating acute lymphoblastic leukemia in children: a report from the Children’s Cancer Group. J Clin Oncol. 2000;18:3262-3272. [PubMed] |
| 17. | Ribeiro RC, Fletcher BD, Kennedy W, Harrison PL, Neel MD, Kaste SC, Sandlund JT, Rubnitz JE, Razzouk BI, Relling MV. Magnetic resonance imaging detection of avascular necrosis of the bone in children receiving intensive prednisone therapy for acute lymphoblastic leukemia or non-Hodgkin lymphoma. Leukemia. 2001;15:891-897. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 84] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 18. | Kerboul M, Thomine J, Postel M, Merle d’Aubigné R. The conservative surgical treatment of idiopathic aseptic necrosis of the femoral head. J Bone Joint Surg Br. 1974;56:291-296. [PubMed] |
| 19. | Ohzono K, Saito M, Sugano N, Takaoka K, Ono K. The fate of nontraumatic avascular necrosis of the femoral head. A radiologic classification to formulate prognosis. Clin Orthop Relat Res. 1992;73-78. [PubMed] |
| 20. | Mont MA, Jones LC, Pacheco I, Hungerford DS. Radiographic predictors of outcome of core decompression for hips with osteonecrosis stage III. Clin Orthop Relat Res. 1998;159-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 58] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 21. | Lee GC, Steinberg ME. Are we evaluating osteonecrosis adequately? Int Orthop. 2012;36:2433-2439. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Karimova EJ, Rai SN, Howard SC, Neel M, Britton L, Pui CH, Kaste SC. Femoral head osteonecrosis in pediatric and young adult patients with leukemia or lymphoma. J Clin Oncol. 2007;25:1525-1531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 74] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 23. | Steinberg ME, Bands RE, Parry S, Hoffman E, Chan T, Hartman KM. Does lesion size affect the outcome in avascular necrosis? Clin Orthop Relat Res. 1999;262-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 99] [Cited by in RCA: 92] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 24. | Sugano N, Ohzono K, Masuhara K, Takaoka K, Ono K. Prognostication of osteonecrosis of the femoral head in patients with systemic lupus erythematosus by magnetic resonance imaging. Clin Orthop Relat Res. 1994;190-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 68] [Article Influence: 2.1] [Reference Citation Analysis (0)] |