Published online Jun 28, 2016. doi: 10.4329/wjr.v8.i6.600
Peer-review started: November 6, 2015
First decision: November 29, 2015
Revised: March 1, 2016
Accepted: March 17, 2016
Article in press: March 18, 2016
Published online: June 28, 2016
Processing time: 226 Days and 0.9 Hours
AIM: To build and evaluate predictive models for contrast-enhanced ultrasound (CEUS) of the breast to distinguish between benign and malignant lesions.
METHODS: A total of 235 breast imaging reporting and data system (BI-RADS) 4 solid breast lesions were imaged via CEUS before core needle biopsy or surgical resection. CEUS results were analyzed on 10 enhancing patterns to evaluate diagnostic performance of three benign and three malignant CEUS models, with pathological results used as the gold standard. A logistic regression model was developed basing on the CEUS results, and then evaluated with receiver operating curve (ROC).
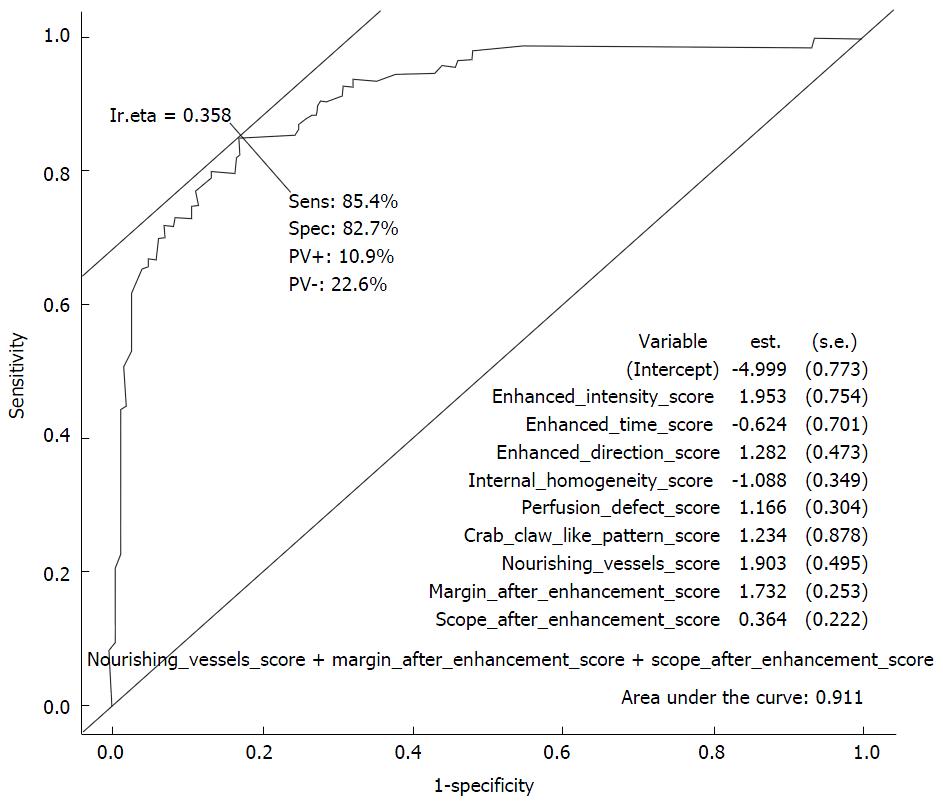
RESULTS: Except in cases of enhanced homogeneity, the rest of the 9 enhancement appearances were statistically significant (P < 0.05). These 9 enhancement patterns were selected in the final step of the logistic regression analysis, with diagnostic sensitivity and specificity of 84.4% and 82.7%, respectively, and the area under the ROC curve of 0.911. Diagnostic sensitivity, specificity, and accuracy of the malignant vs benign CEUS models were 84.38%, 87.77%, 86.38% and 86.46%, 81.29% and 83.40%, respectively.
CONCLUSION: The breast CEUS models can predict risk of malignant breast lesions more accurately, decrease false-positive biopsy, and provide accurate BI-RADS classification.
Core tip: Many studies published show that there are some enhanced patterns such as rapid, hyper-enhancement or enlarged size after contrast may predict malignant, but none of them reliably differentiates malignant from benign nodules. We try to build 6 predictive models (3 malignant and 3 benign) using a qualitative analysis of enhancement patterns, and get diagnostic sensitivity, specificity, and accuracy of the malignant vs benign contrast-enhanced ultrasound (CEUS) models were 84.38%, 87.77%, 86.38% and 86.46%, 81.29% and 83.40%, respectively. It shows that the breast CEUS models can predict risk of malignant breast lesions more accurately, decrease false-positive biopsy, and provide accurate breast imaging reporting and data system classification.
- Citation: Luo J, Chen JD, Chen Q, Yue LX, Zhou G, Lan C, Li Y, Wu CH, Lu JQ. Predictive model for contrast-enhanced ultrasound of the breast: Is it feasible in malignant risk assessment of breast imaging reporting and data system 4 lesions? World J Radiol 2016; 8(6): 600-609
- URL: https://www.wjgnet.com/1949-8470/full/v8/i6/600.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i6.600
Contrast-enhanced ultrasound (CEUS) was first utilized for the examination of breast lesions in the early 1990s. Most early studies focused on the enhancement of color doplor flow image using a contrast agent, and demonstrated that it could provide more information for differential diagnosis of breast lesions[1-4]. However, contrast enhanced power Doppler ultrasound diagnosis ability is limited, because of respiratory or cardiac motion artifacts of patient and low speed of Doppler[5], as well as its inability to delineate microvasculature and microcirculation features that would help distinguish between benign and malignant lesions. Over the past 10 years, CEUS with quantitative and qualitative studies have been greatly developed for breast lesions. Enhancement patterns will differ between benign and malignant lesions on quantitative analysis, with malignant lesions demonstrating rapid wash-in and sustained hyper-enhancement compared with benign lesions. Breast CEUS also demonstrates good correlation with magnetic resonance imaging (MRI) results[6-10], however, the enhancement patterns of lesions are influenced by multiple factors, including microvascular density, structure and permeability, as well as intercellular substance structure and baseline enhancement patterns[11-13]. For example, increased microvascular density and vascular permeability are not unique to malignant lesions, with almost all benign neoplasms, and a large number of benign non-neoplastic lesions, demonstrating varying degrees of hyper-enhancement. Conversely, some malignant lesions may be hypo-vascular. These may be the reasons that some studies showed enhanced MRI had a high sensitivity (80%-95%) but less specificity, with high false-positive rate (20%-80%)[14-16]. Since enhancement patterns cannot accurately predict malignant risk, the lack of clear diagnostic criteria of breast CEUS remains unclear and has limited its wider application[17-20]. In our study, we attempt to build breast CEUS predictive models based on the qualitative analysis of enhancement patterns, further explore the most valuable predictive patterns in order to enhance the value of CEUS in the risk assessment of breast lesions and optimize breast imaging reporting and data system (BI-RADS) classification to reduce the false-positive biopsy rate, and over-diagnosis.
From January 2013 to July 2014, a total of 230 patients (mean age 44 years, range 11-84 years) with 235 solid breast lesions were included in this study. The maximum diameters of the lesions ranged from 10.3 mm to 50.9 mm, with a mean of 18.1 mm ± 9.3 mm. Ultrasound examinations were performed within one week before surgery or core needle biopsy. The inclusion criterion was the presence of solid breast lesions on conventional ultrasound which were classified in BI-RADS 4. Patients were excluded from the study for any of the following reasons: Pregnancy or breastfeeding, lesions reclassified as BI-RADS 3 after reassessment, and any previous treatment or interventional diagnosis (BI-RADS 6). All of the 230 patients met the above criteria. The study was approved by the institutional ethics committee of Sichuan Provincial People’s Hospital, and written informed consent was obtained from each patient prior to study initiation.
All examinations were performed by the same sonographer, who had 10 years of experience with breast ultrasound and 2 years of experience with CEUS. CEUS was performed with the aforementioned unit with a 4.5-7.5 MHz linear transducer (LA522). Breast contrast-enhanced imaging was incorporated in the system and used. The machine parameters were adjusted so that mechanical index was less than 0.1 and gain was 100-120 dB. Parameters were not changed during the examination.
The contrast agent used in the study was SonoVue (Bracco Imaging S.P.A., Milan, Italy). For contrast-enhanced imaging, 4.8 mL of SonoVue was administrated via a peripheral vein in a bolus fashion, followed by injection of 5-10 mL of normal saline. Continuous imaging was performed immediately after injection of the contrast agent and lasted for 2 min. US images and video clips were stored on a hard disk for subsequent analysis. Dual image mode was applied to locate the lesion accurately during the procedure, especially for small lesions. The selected plane remained unchanged during the examination. The probe was placed gently on the skin to avoid exerting pressure on the lesion, particularly when the lesion was superficial. The patients were instructed to remain still and attempt to maintain eupnea during the examination to minimize motional artifacts.
All images were read by two sonographers with a minimum of 10 years of experience with breast ultrasound and 2 years of experience with breast CEUS. Both sonographers were blinded to patients’ clinical data and final pathological results.
Following shows the qualitative analysis of 10 enhanced patterns: (1) enhanced time (compare with the surrounding breast tissue, slow, synchronous, rapid); (2) enhanced intensity (compare with the surrounding breast tissue, hypo, iso, hyper); (3) enhanced direction (diffuse, centripetal, centrifugal); (4) internal homogeneity (homogeneous, heterogeneous); (5) perfusion defect (absent, present); (6) scope after enhancement [compare the maximal diameter of the lesion seen on CEUS image with the image in two-dimensional (2D) image, cannot distinguish, smaller, equal, larger]; (7) shape after enhancement (regular, cannot distinguish, irregular); (8) margin after enhancement (clear, cannot distinguish, unclear); (9) crab claw-like pattern (absent, present); and (10) nourishing (or penetrating) vessels (absent, present). Both doctors provided their opinions and consensus was reached through discussion if there was any controversy.
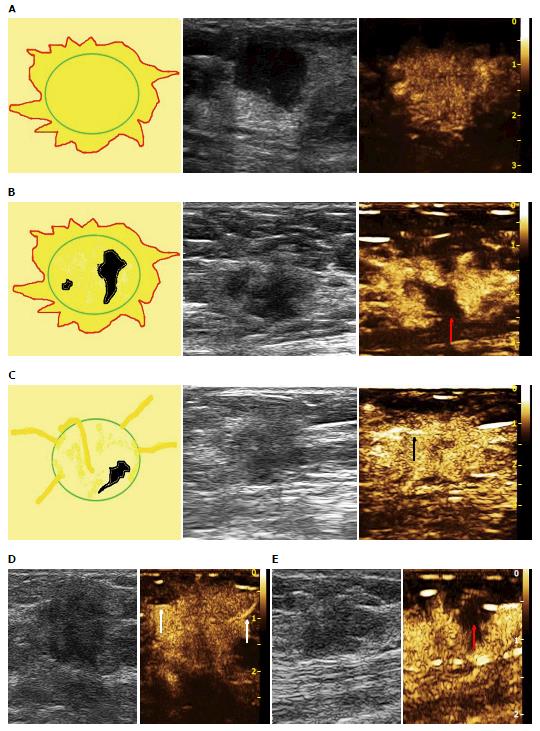
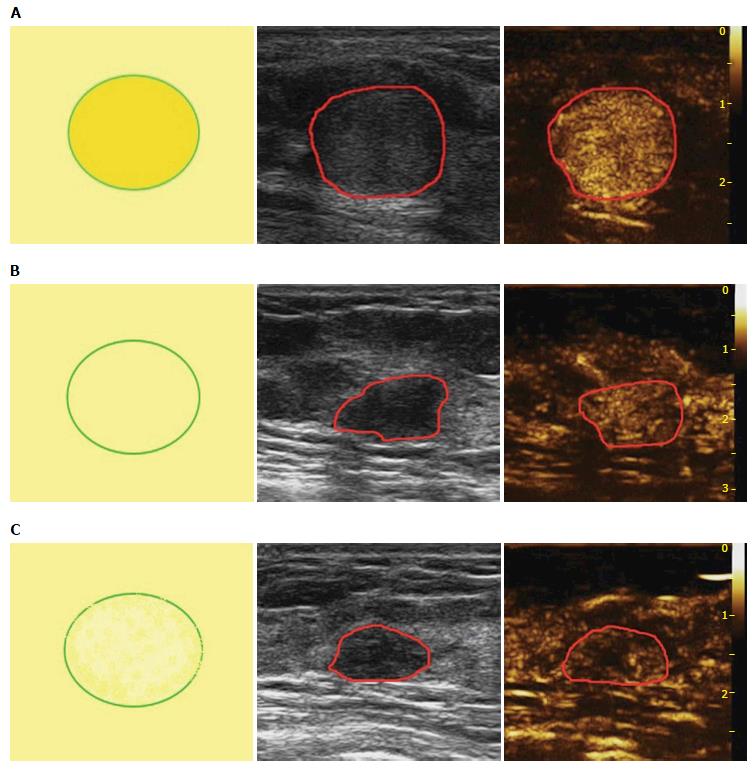
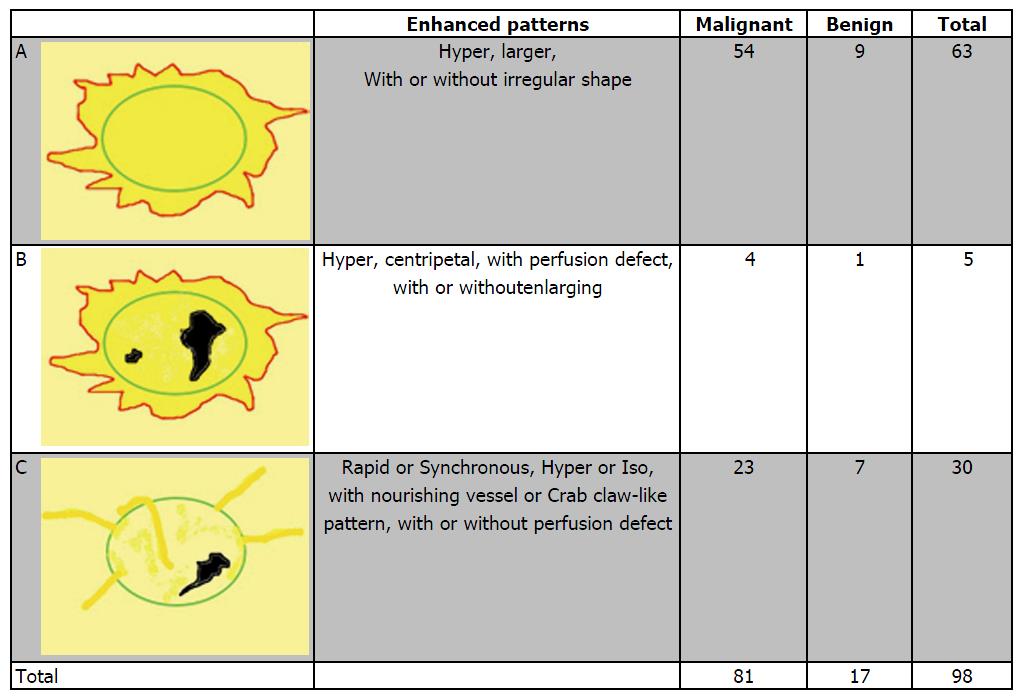
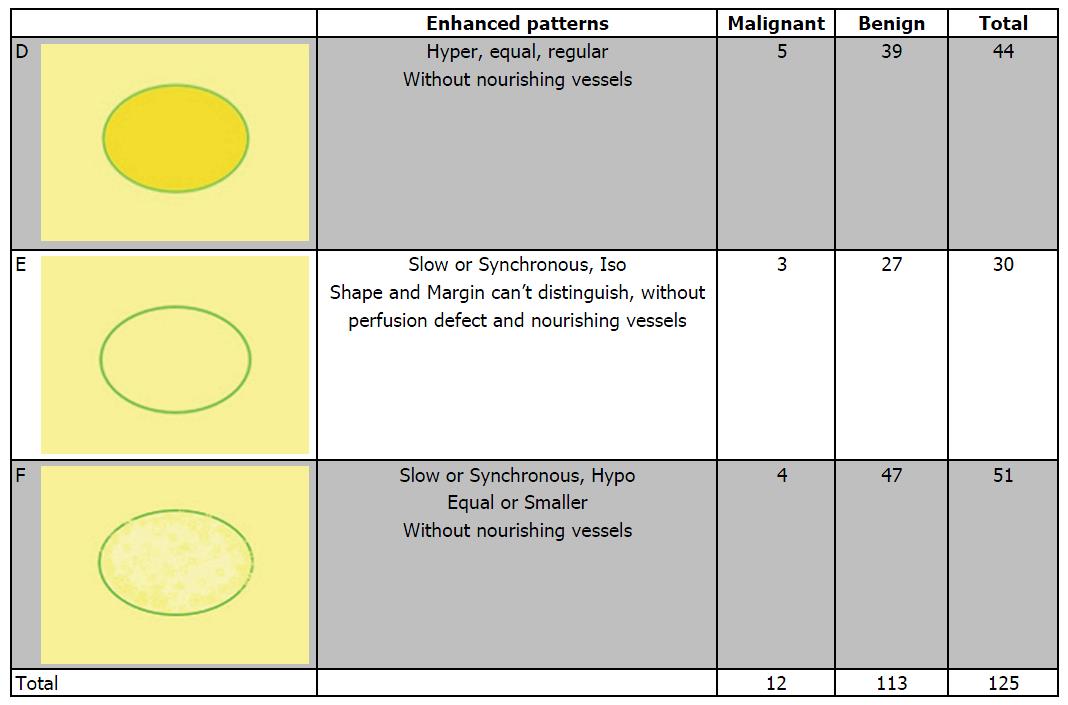
Based on the analysis of 10 patterns described above, review of published research literature and our clinical experience, we built predictive models of CEUS for malignant and benign breast lesions. Figure 1 shows three different malignant predictive models as follows: (1) hyper-enhancement with enlarged size, with or without irregular shape; (2) hyper-centripetal enhancement with perfusion defect, with or without enlarged size; (3) rapid or synchronous wash-in with hyper- or iso-enhancement, present penetrating vessels or crab claw-like pattern, with or without perfusion defect. Figure 2 shows three different benign predictive models as follows: (4) rapid wash-in with hyper-enhancement, clear margin after enhancement without enlarged size; (5) synchronous or slow wash-in with iso-enhancement, and cannot distinguish margin and shape after enhancement; and (6) synchronous or slow wash-in with hypo-enhancement, with equal or smaller size after enhancement.
All patients underwent surgery or core biopsy 1-2 d after the CEUS examination. The pathology findings were used as the final diagnostic standard.
Mean and standard deviation were calculated for con–tinuous variables. Dichotomous variables were evaluated with the Pearson χ2 test. A P value of less than 0.05 was considered indicative of a significant difference.
To assess the discriminative ability of the different enhancement patterns and parameters with respect to the prognostic factors, binary logistic regression analysis with stepwise forward variable selection was used (P values for entry and removal were 0.05 and 0.10, respectively).
All statistical evaluations were performed with statistical software (SPSS for Windows, version 13.0; SPSS, Chicago, Ill).
There were a total of 235 lesions, of which 96 were benign and 139 were malignant. The lesion types are summarized in Table 1.
| Histopathologic diagnosis | Total |
| Benign lesions | 139 |
| Fibroadenoma | 53 |
| Fibrocystic mastopathy | 35 |
| Complex sclerosing adenosis | 5 |
| Hyperplasia | 4 |
| Chronic mastitis | 21 |
| Granulomatous mastitis | 4 |
| Intraductal papilloma | 9 |
| Benign phyllodes tumor | 3 |
| Hamartoma | 2 |
| Radial scar | 2 |
| Bolus material after operation | 1 |
| Malignant lesions | 96 |
| IDC | 78 |
| DCIS | 9 |
| Mucinous carcinoma | 3 |
| Infiltrating lobular carcinoma | 2 |
| Diffused large B-cell lymphoma | 2 |
| Malignant phyllodes tumor | 1 |
| Solid neuroendocrine carcinoma | 1 |
| Total | 235 |
In our study, 10 enhancement patterns were analyzed. χ2 tests indicated that the differences in the 9 enhancement patterns between malignant and benign lesions were statistically significant (P < 0.05), except internal homogeneity (P = 0.6539). Among them, the enhancement time, enhancement intensity, and margin after enhancement demonstrated good sensitivity, ranging from 68.8% to 81.2%. Scope after enhancement, enhanced direction, perfusion defect, nourishing vessels, crab claw-like pattern and shape after enhancement had specificity values of more than 80% (Table 2). Logistic regression was performed to identify patterns that were important in differentiating breast lesions. Nine independent patterns were identified in the final step of the logistic regression analysis forward model: Enhancement intensity, enhancement time, enhancement direction, internal homogeneity, perfusion defect, crab claw-like pattern, nourishing vessel, margin and scope after enhancement. These 9 variables for the model to predict malignant breast lesions showed sensitivity and specificity of 84.4% and 82.7%, respectively, and an area under the receiver operating curve curve of 0.911 (Figure 3).
| Benign | Malignant | Sensitivity (%) | Specificity (%) | pearson χ2 | P | Cut-off | |
| Enhanced time | |||||||
| Slow | 49 | 11 | 76 | 56.1 | 25.1645 | < 0.0001 | Synchronous |
| Synchronous | 29 | 12 | |||||
| Rapid | 61 | 73 | |||||
| Enhanced intensity | |||||||
| Hypo | 49 | 9 | 81.2 | 56.8 | 34.5297 | < 0.0001 | Iso |
| Iso | 30 | 9 | |||||
| Hyper | 60 | 78 | |||||
| Scope of lesion | |||||||
| Can’t distinguish | 33 | 5 | 66.7 | 86.3 | 73.0619 | < 0.0001 | Equal |
| Smaller | 4 | 5 | |||||
| Equal | 87 | 27 | |||||
| Larger | 15 | 59 | |||||
| Enhanced direction | |||||||
| Diffuse | 114 | 63 | 34.4 | 82 | 10.1407 | 0.0063 | Diffuse |
| Centripetal | 24 | 1 | |||||
| Centrifugal | 28 | 5 | |||||
| Internal homogeneity | |||||||
| Homogeneous | 76 | 47 | 51 | 54.7 | 1.6242 | 0.6539 | |
| Heterogeneous | 63 | 49 | |||||
| Perfusion defect | |||||||
| Absent | 104 | 52 | 68.8 | 54.7 | 15.6391 | 0.0013 | Absent |
| Present | 35 | 44 | |||||
| Crab claw like pattern | |||||||
| Absent | 137 | 73 | 24 | 98.6 | 27.9683 | < 0.0001 | Absent |
| Present | 2 | 23 | |||||
| Nourishing vessels | |||||||
| Absent | 128 | 55 | 42.7 | 92.1 | 37.9019 | < 0.0001 | Absent |
| Present | 11 | 41 | |||||
| Margin after enhancement | |||||||
| Clear | 12 | 1 | 78.1 | 64.7 | 42.9752 | < 0.0001 | Can’t distinguish |
| Almost clear | 48 | 14 | |||||
| Can’t distinguish | 30 | 6 | |||||
| Unclear | 49 | 75 | |||||
| Shape after enhancement | |||||||
| Regular | 28 | 1 | 59.4 | 82.7 | 56.0275 | < 0.0001 | Can’t distinguish |
| Almost regular | 56 | 32 | |||||
| Can’t distinguish | 31 | 6 | |||||
| Irregular | 24 | 57 |
Figure 4 shows the distribution of pathologic results in different malignant predictive models. In all 96 malignant lesions, there were 54 in model A, 4 in model B and 23 in model C, with another 15 lesions didn’t fit any model. The diagnostic sensitivity, specificity and accuracy values were 84.38%, 87.77% and 86.38%, respectively. Otherwise, there were 9 benign lesions showed model A (including 8 inflammation lesions and 1 adenosis), 1 intraductal papilloma with apocrine metaplasia of surrounding tissue fit model B and 7 in model C (including 4 adenomas, 1 intraductal papilloma, 1 benign phyllodes tumor and 1 inflammation lesion). All of the 7 benign lesions in model C were without perfusion defect compared with 23 malignant lesions in model C which there were 7 lesions with perfusion defect [5 invasive ductal carcinomas (IDCs) and 2 ductal carcinoma in suit (DCIS)].
Figure 5 shows the distribution of pathologic results in different benign predictive models. In all 139 benign lesions, there were 39 in model D, 27 in model E and 47 in model F, with another 26 lesions didn’t fit any model. The diagnostic sensitivity, specificity and accuracy values were 86.46%, 81.29% and 83.40%, respectively. Otherwise, there were 5 IDCs showed model D, 3 fit model E (2 IDCs and 1 DCIS) and 4 in model F (including 2 DCIS, 1 Mucinous Carcinoma, 1 Diffused Large B-cell Lymphoma).
CEUS has been used in the clinical setting for years. Its liver tumor diagnostic efficiency with enhanced computed tomography (CT) and MRI is similarity[21]. However, the value of CEUS in the diagnosis of breast lesions remains controversial because diagnostic criteria is not clear at present. We analyzed the enhancement patterns of breast lesions, and attempts to develop predictive models for CEUS of malignant and benign breast lesions, in order to further assess the value of CEUS in breast lesion diagnosis.
After analyzing 10 enhancement patterns, we found that none demonstrated sufficient sensitivity and specificity at the same time. This means both benign and malignant breast lesions may have various microcirculation patterns, and a single pattern cannot provide all the information of microvasculature, nor lead to the correct diagnosis. For example, although enhancement intensity reached 81.20% sensitivity and showed that lesions with hyper-enhancement are more likely to be malignant, its specificity was only 56.80%, and 60/139 (43.16%) benign lesions still showed hyper-enhancement. At the same time, there were only 18/96 (18.75%) malignant lesions that showed hypo- or iso-enhancement. Therefore, even most malignant breast lesions show hyper-enhancement, but lesions with hyper-enhancement are not necessarily malignant. On the other hand, if a lesion shows hypo- or iso-enhancement, the risk to malignancy may be relatively low. It Similar results in enhancement time between benign and malignant lesions were observed. One possible explanation for this may be the pathologic constitution of malignant breast tumors, as non-specific invasive intraductal carcinoma with hyper-vascular comprises 60% to 70% of all malignant breast tumors. Enhancement time and intensity mainly rely on microvascular density of the lesion, not pathologic results. Some benign lesions such as intraductal papilloma, inflammatory lesion, adenoma and hyperplasia with active cell proliferation or infantile style are still with hyper-vascular, may lead to enhanced mode of malignant lesions overlap[22,23], with rapid wash-in and hyper-enhancement on CEUS. But, unlike malignant tumors which are nonencapsulated, adenomas or intraductal papillomas often have a true capsule or pseudocapsule, causing these benign lesions to show clear margins upon enhancement. Otherwise, adenosis is a frequently met disease in clinical practice. Although most of the adenosis will have clear diagnosis through patient’s history, physical examination and US or mammography, there is still a considerable number of solid-nodule adenosis that appear similar to breast cancer on 2D ultrasound, often classified in BI-RADS 4 category and recommended for biopsy. Due to a lack of pseudocapsule, adenosis often shows unclear margin with irregular shape, similar to malignant lesions But in contrast with malignant lesions,adenosis are more likely to have slow or synchronous wash-in with hypo- or iso-enchancement, equal or smaller size after enhancement or can’t distinguish, rarely has nourishing vessel or perfusion defect. These features will help us make a differential diagnosis.
In addition, our study shows enlarged size after enhancement is a strong predictive feature for malignancy, as confirmed in previous studies[20,24]. However, real boundary of lesions compared with pathological specimens are always underestimated in the gray scale ultrasound, and breast contrast ultrasound can evaluate breast lesion boundary more accurately[19,25]. In the enhanced mode, the range of malignant lesions was significantly larger than that of 2D gray-scale ultrasound. This phenomenon may be pathological and malignant lesions related. First of all, 60%-70% breast cancer is invasive ductal carcinoma, lack of capsule, and 85% invasive ductal carcinoma with carcinoma in situ and invasive carcinoma at the same time[20]. New microvasculature formation is an important factor in solid tumor development, invasion, and metastases. Carcinoma in situ is always located in the surrounding part of the lesion. In addition, surrounding malignant lesions are always atypical hyperplasia, adenosis or precarcinoma lesions with hyper-vascular findings. We need to pay attention to the inflammatory lesions, due to infiltration of inflammatory cells, in the enhanced mode, also showed a larger range[20]. In our study, there were 15 benign lesions with enlarged scope after enhancement, 9 of them were inflammation lesions, 3 adenoses with atypical hyperplasia, 2 intraductal papillomas and 1 adenoma. Depending on the analysis of different diagnostic cut-off points in 10 patterns, lesions showed slow or synchronous wash-in with hypo- or iso-enhancement, without perfusion defect, margin is clear or can’t be distinguished is more likely to be benign; lesions showed enlarged scope with centripetal or centrifugal enhanced direction, presents nourishing vessel or crab claw-like pattern, and with irregular shape is more likely to be malignant. Our findings are in-line with previous research conducted by other investigators[20,24,26-28]. Although, nourishing vessel, margin and shape were identified in the final step of the logistic regression analysis to be independent variables for diagnosis, other features such as crab claw-like pattern still has 98.6% specificity. Histological study showed that peripheral microvascular are richer than in lesions[29,30]. This may be the pathophysiological basis of crab claw-like performance. However, some inflammatory lesions may also display this pattern, such as granulomatous mastitis.
Through the above analysis we can see, whether a single pattern or the results of logistic regression analysis, cannot describe the overall enhanced features of benign and malignant lesions very well. In clinical practice, various combinations of different enhancement patterns in different lesions are seen. The key point is how to effectively analyze and use these different combinations for diagnosis. So we proposed the breast CEUS predictive models. From our results, model A has the best predictive value in three malignant predictive models. It coincides the type of pathology and pathological features of the most breast cancers. But cases distribute equally in different benign predictive models. One possible explanation is that adenoma and adenosis are the main pathological types in breast benign lesions, and different stages of adenoma or adenosis may exhibit different microcirculation status result in different enhanced patterns. However, the six predictive models showed good predictive accuracy, and further defined the diagnostic criteria for breast lesions. It also reduced the false-positive biopsy rate and optimized the BI-RADS categorization, especially for BI-RADS 4 lesions with these CEUS predictive models. Our study showed that there would be 113/235 (48.08%) of the BI-RADS 4 lesions where we could avoid unnecessary biopsies, while only 12/235 (5.10%) missing malignant lesions, depending on the CEUS predictive models.
Good quality of CEUS images is the key point to good results. Based on our experience, the following points are important for breast CEUS: (1) Specialized probes and breast contrast parameters is the basis of good performance, it is different from different US machines and need to be adjusted with engineers; (2) 4.8 mL dose of contrast agent would be enough for good performance; (3) Dual view is helpful to follow the lesions and keep the same scan during the contrast (especially for small nodules), and we should leave enough normal breast tissue around the lesion to be compared; (4) The scan selected for contrast should be hyper-vascular, with penetrating vessels or with irregular shape, avoiding thick calcification with shadow behind and fluid areas; (5) The probe should put on the skin near the lesion slightly without much pressure, bladder with water can be used if lesion is too close to the probe, for deep or large lesions we can choose abdomen probe with low frequency; (6) Sometimes, change from cross-sectional to sagittal or radial section for another more CEUS will bring you more information if you are not satisfied with the first examination; (7) Quantitative analysis can help us avoid subjective bias if it’s not easy to judge the enhanced patterns; and (8) CEUS may not be applicable for breast diffused lesions, and it’s still hard to identify different types of pathology.
This study had a limitation that should be noted. One limitation is that the number of patients enrolled was small, and further multi-center prospective studies with large sample sizes are needed to confirm our findings. In addition, qualitative analysis of enhancement patterns still have to face the problem of inter-observer agreement, selection of the region of interest and classification of the enhancement patterns were subjective, and contrast patterns in the differential diagnosis of benign and malignant breast lesions have not yet reached a consensus. Finally, in our study, we chose a rich blood supply or irregular sections for CEUS. The single plane may not be representative of the entire lesion, may cause a significant loss of important information.
In conclusion, CEUS is beneficial for differential diagnosis of breast tumors. The predictive models for CEUS f breast lesions were easy to use and displayed high diagnostic accuracy. It is a promising method for the early diagnosis of breast cancer, which merits further development and evaluation to avoid over-diagnosis and over-treatment. We look forward to optimizing BI-RADS category classification with such models but multicenter research will be needed to improve them.
Many studies published show that there are some enhanced patterns such as rapid, hyper-enhancement or enlarged size after contrast may predict malignant, but none of them reliably differentiates malignant from benign nodules. Since enhancement patterns cannot accurately predict malignant risk, the effectiveness of breast contrast-enhanced ultrasound (CEUS) remains unclear and the lack of clear diagnostic criteria has limited its wider application.
How to use those enhanced patterns to predict malignant accurately, is current hotpots in the research field, which our study try to solve this problem.
The study has first claimed that CEUS models can predict malignant lesions more accurately and decrease false-positive biopsy. It can offer new information or significant findings that enhance our knowledge of clinical aspects of breast diseases.
For those readers who want to do breast CEUS, the predict models we described in the authors’ study can be used in clinical practice. And the authors can still do further study to improve these models and make it more accuracy.
Predictive models: This means some models which combined 3-4 enhanced patterns that the authors can use them to predict those breast nodules are benign or malignant.
This is an interesting paper with a fairly large number of patients.
| 1. | Schröder RJ, Bostanjoglo M, Hidajat N, Rademaker J, Röttgen R, Mäurer J, Felix R. [Analysis of vascularity in breast tumors--comparison of high frequency ultrasound and contrast-enhanced color harmonic imaging]. Rofo. 2002;174:1132-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 2. | Algül A, Balci P, Seçil M, Canda T. [Contrast enhanced power Doppler and color Doppler ultrasound in breast masses: Efficiency in diagnosis and contributions to differential diagnosis]. Tani Girisim Radyol. 2003;9:199-206. [PubMed] |
| 3. | Kook SH, Kwag HJ. Value of contrast-enhanced power Doppler sonography using a microbubble echo-enhancing agent in evaluation of small breast lesions. J Clin Ultrasound. 2003;31:227-238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 4. | Kedar RP, Cosgrove D, McCready VR, Bamber JC, Carter ER. Microbubble contrast agent for color Doppler US: effect on breast masses. Work in progress. Radiology. 1996;198:679-686. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 138] [Cited by in RCA: 124] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Kettenbach J, Helbich TH, Huber S, Zuna I, Dock W. Computer-assisted quantitative assessment of power Doppler US: effects of microbubble contrast agent in the differentiation of breast tumors. Eur J Radiol. 2005;53:238-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 6. | Saracco A, Aspelin P, Leifland K, Themudo R, Wilczek B, Axelsson R. Bolus compared with continuous infusion of microbubble contrast agent using real-time contrast harmonic imaging ultrasound in breast tumors. Acta Radiol. 2009;50:854-859. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 7. | Balleyguier C, Opolon P, Mathieu MC, Athanasiou A, Garbay JR, Delaloge S, Dromain C. New potential and applications of contrast-enhanced ultrasound of the breast: Own investigations and review of the literature. Eur J Radiol. 2009;69:14-23. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 66] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 8. | Caproni N, Marchisio F, Pecchi A, Canossi B, Battista R, D’Alimonte P, Torricelli P. Contrast-enhanced ultrasound in the characterisation of breast masses: utility of quantitative analysis in comparison with MRI. Eur Radiol. 2010;20:1384-1395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 49] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 9. | Szabó BK, Aspelin P, Wiberg MK, Boné B. Dynamic MR imaging of the breast. Analysis of kinetic and morphologic diagnostic criteria. Acta Radiol. 2003;44:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 8] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 10. | Ricci P, Cantisani V, Ballesio L, Pagliara E, Sallusti E, Drudi FM, Trippa F, Calascibetta F, Erturk SM, Modesti M. Benign and malignant breast lesions: efficacy of real time contrast-enhanced ultrasound vs. magnetic resonance imaging. Ultraschall Med. 2007;28:57-62. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 53] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 11. | Veltman J, Stoutjesdijk M, Mann R, Huisman HJ, Barentsz JO, Blickman JG, Boetes C. Contrast-enhanced magnetic resonance imaging of the breast: the value of pharmacokinetic parameters derived from fast dynamic imaging during initial enhancement in classifying lesions. Eur Radiol. 2008;18:1123-1133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 47] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 12. | Choyke PL, Dwyer AJ, Knopp MV. Functional tumor imaging with dynamic contrast-enhanced magnetic resonance imaging. J Magn Reson Imaging. 2003;17:509-520. [PubMed] |
| 13. | Huang W, Fisher PR, Dulaimy K, Tudorica LA, O’Hea B, Button TM. Detection of breast malignancy: diagnostic MR protocol for improved specificity. Radiology. 2004;232:585-591. [PubMed] |
| 14. | Lord SJ, Lei W, Craft P, Cawson JN, Morris I, Walleser S, Griffiths A, Parker S, Houssami N. A systematic review of the effectiveness of magnetic resonance imaging (MRI) as an addition to mammography and ultrasound in screening young women at high risk of breast cancer. Eur J Cancer. 2007;43:1905-1917. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 173] [Cited by in RCA: 178] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 15. | Bluemke DA, Gatsonis CA, Chen MH, DeAngelis GA, DeBruhl N, Harms S, Heywang-Köbrunner SH, Hylton N, Kuhl CK, Lehman C. Magnetic resonance imaging of the breast prior to biopsy. JAMA. 2004;292:2735-2742. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 388] [Cited by in RCA: 361] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 16. | Peters NH, Borel Rinkes IH, Zuithoff NP, Mali WP, Moons KG, Peeters PH. Meta-analysis of MR imaging in the diagnosis of breast lesions. Radiology. 2008;246:116-124. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 381] [Cited by in RCA: 406] [Article Influence: 21.4] [Reference Citation Analysis (0)] |
| 17. | Liu H, Jiang YX, Liu JB, Zhu QL, Sun Q. Evaluation of breast lesions with contrast-enhanced ultrasound using the microvascular imaging technique: initial observations. Breast. 2008;17:532-539. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 60] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Zhao H, Xu R, Ouyang Q, Chen L, Dong B, Huihua Y. Contrast-enhanced ultrasound is helpful in the differentiation of malignant and benign breast lesions. Eur J Radiol. 2010;73:288-293. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 119] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 19. | Jiang YX, Liu H, Liu JB, Zhu QL, Sun Q, Chang XY. Breast tumor size assessment: comparison of conventional ultrasound and contrast-enhanced ultrasound. Ultrasound Med Biol. 2007;33:1873-1881. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 44] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 20. | Xiao X, Ou B, Yang H, Wu H, Luo B. Breast contrast-enhanced ultrasound: is a scoring system feasible? A preliminary study in China. PLoS One. 2014;9:e105517. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 53] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 21. | Xu HX. Contrast-enhanced ultrasound: The evolving applications. World J Radiol. 2009;1:15-24. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 53] [Cited by in RCA: 52] [Article Influence: 3.1] [Reference Citation Analysis (1)] |
| 22. | Weind KL, Maier CF, Rutt BK, Moussa M. Invasive carcinomas and fibroadenomas of the breast: comparison of microvessel distributions--implications for imaging modalities. Radiology. 1998;208:477-483. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 50] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 23. | Ellis RL. Differentiation of benign versus malignant breast disease. Radiology. 1999;210:878-880. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 24. | Liu J, Gao YH, Li DD, Gao YC, Hou LM, Xie T. Comparative study of contrast-enhanced ultrasound qualitative and quantitative analysis for identifying benign and malignant breast tumor lumps. Asian Pac J Cancer Prev. 2014;15:8149-8153. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 25. | van Esser S, Veldhuis WB, van Hillegersberg R, van Diest PJ, Stapper G, ElOuamari M, Borel Rinkes IH, Mali WP, van den Bosch MA. Accuracy of contrast-enhanced breast ultrasound for pre-operative tumor size assessment in patients diagnosed with invasive ductal carcinoma of the breast. Cancer Imaging. 2007;7:63-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 27] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 26. | Wang L, Du J, Li FH, Fang H, Hua J, Wan CF. Diagnostic efficacy of contrast-enhanced sonography by combined qualitative and quantitative analysis in breast lesions: a comparative study with magnetic resonance imaging. J Ultrasound Med. 2013;32:1805-1814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 8] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 27. | Du J, Li FH, Fang H, Xia JG, Zhu CX. Microvascular architecture of breast lesions: evaluation with contrast-enhanced ultrasonographic micro flow imaging. J Ultrasound Med. 2008;27:833-842; quiz 844. [PubMed] |
| 28. | Brown LF, Berse B, Jackman RW, Tognazzi K, Guidi AJ, Dvorak HF, Senger DR, Connolly JL, Schnitt SJ. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in breast cancer. Hum Pathol. 1995;26:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 421] [Cited by in RCA: 441] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 29. | Lichtenbeld HC, Barendsz-Janson AF, van Essen H, Struijker Boudier H, Griffioen AW, Hillen HF. Angiogenic potential of malignant and non-malignant human breast tissues in an in vivo angiogenesis model. Int J Cancer. 1998;77:455-459. [PubMed] |
| 30. | Wan CF, Du J, Fang H, Li FH, Zhu JS, Liu Q. Enhancement patterns and parameters of breast cancers at contrast-enhanced US: correlation with prognostic factors. Radiology. 2012;262:450-459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 62] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Sonoda K, Vinh-Hung V, Yokoyama Y S- Editor: Ji FF L- Editor: A E- Editor: Li D