Published online Apr 28, 2016. doi: 10.4329/wjr.v8.i4.410
Peer-review started: September 6, 2015
First decision: October 27, 2015
Revised: November 16, 2015
Accepted: January 21, 2016
Article in press: January 22, 2016
Published online: April 28, 2016
Processing time: 234 Days and 17.3 Hours
AIM: To examine whether addition of 3T multiparametric magnetic resonance imaging (mpMRI) to an active surveillance protocol could detect aggressive or progressive prostate cancer.
METHODS: Twenty-three patients with low risk disease were enrolled on this active surveillance study, all of which had Gleason score 6 or less disease. All patients had clinical assessments, including digital rectal examination and prostate specific antigen (PSA) testing, every 6 mo with annual 3T mpMRI scans with gadolinium contrast and minimum sextant prostate biopsies. The MRI images were anonymized of patient identifiers and clinical information and each scan underwent radiological review without the other results known. Descriptive statistics for demographics and follow-up as well as the sensitivity and specificity of mpMRI to identify prostate cancer and progressive disease were calculated.
RESULTS: During follow-up (median 24.8 mo) 11 of 23 patients with low-risk prostate cancer had disease progression and were taken off study to receive definitive treatment. Disease progression was identified through upstaging of Gleason score on subsequent biopsies for all 11 patients with only 2 patients also having a PSA doubling time of less than 2 years. All 23 patients had biopsy confirmed prostate cancer but only 10 had a positive index of suspicion on mpMRI scans at baseline (43.5% sensitivity). Aggressive disease prediction from baseline mpMRI scans had satisfactory specificity (81.8%) but low sensitivity (58.3%). Twenty-two patients had serial mpMRI scans and evidence of disease progression was seen for 3 patients all of whom had upstaging of Gleason score on biopsy (30% specificity and 100% sensitivity).
CONCLUSION: Addition of mpMRI imaging in active surveillance decision making may help in identifying aggressive disease amongst men with indolent prostate cancer earlier than traditional methods.
Core tip: Multiparametric magnetic resonance imaging (mpMRI) has the potential to distinguish more aggressive prostate cancer even when indolent prostate cancer is the biopsy diagnosis. Addition of mpMRI to active surveillance protocols would benefit decision making.
- Citation: Vos LJ, Janoski M, Wachowicz K, Yahya A, Boychak O, Amanie J, Pervez N, Parliament MB, Pituskin E, Fallone BG, Usmani N. Role of serial multiparametric magnetic resonance imaging in prostate cancer active surveillance. World J Radiol 2016; 8(4): 410-418
- URL: https://www.wjgnet.com/1949-8470/full/v8/i4/410.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i4.410
Prostate cancer is one of the most common solid organ cancers and the third leading cause of cancer-related death in men[1]. Introduction of and widespread utilization of serum prostate specific antigen (PSA) screening has increased the incidence of low-risk prostate cancer with about 25%-50% of newly diagnosed patients having low-risk disease[2]. For the majority of these patients, the prostate cancer will behave as an indolent disease and only have clinically significant effects or cause death in a relatively small proportion of men[3-5]. This subgroup of patients often does not require immediate treatment and can be managed with an active surveillance protocol. This has become an increasingly popular strategy for men with low-risk prostate cancer, as it allows up to 65% of patients to avoid unnecessary treatments and their inherent risk of toxicities[6]. Patients on active surveillance are monitored regularly for signs of disease progression[4,7,8]. A dilemma currently encountered by physicians that counsel patients with prostate cancer is differentiating indolent malignancies from aggressive disease. Current assessments are based on clinical stage, the Gleason score of prostate biopsies, and serum PSA levels but these assays have been shown to have an accuracy of less than 75% in predicting indolent disease confined to the prostate[9-11]. This means that there is a subpopulation of patients within the low-risk category who will have disease progression during active surveillance and better methods of identifying early progression on active surveillance are needed.
Magnetic resonance imaging (MRI) is increasingly demonstrating its effectiveness in determining the location, size and grade of prostate cancer[12,13]. Higher field strength MRI can be advantageous due to an increased signal to noise ratio which has led some preliminary investigations to identify extracapsular disease in up to 88% of cases[14]. In addition it has been demonstrated that combining the information from multiple MRI sequences [i.e., T2 weighted imaging and diffusion weighted imaging (DWI)] can increase the accuracy of detecting prostate cancers by a magnitude of 10%, presumably due to the complementary information provided by the different sequences[15]. This has resulted in the addition of baseline multiparametric MRI (mpMRI) to some of the recommendation guidelines for stratification of risk[16,17].
In this study, we performed serial mpMRI scans on patients with indolent prostate cancer who were enrolled in an active surveillance protocol. The objectives of the study were to examine the accuracy of mpMRI in detecting and localizing low-risk prostate cancer and to determine the ability of mpMRI to differentiate indolent prostate cancer from aggressive disease at baseline. The serial scans were also examined to determine if mpMRI was able to identify disease progression earlier than standard methodology.
Patients eligible for enrollment were men aged 18 years or older with histologically proven low-risk adenocarcinoma of the prostate. Low-risk classification was composite Gleason score ≤ 6, and either clinical stage ≤ T2a or PSA ≤ 10 ng/mL. Additional inclusion criteria included: Adequate kidney function; no contraindications for MRI scans; no history of previous malignancies except non-melanoma skin tumors; and reliability for follow-up.
This single-institution, prospective, phase II clinical trial was designed and performed at the Cross Cancer Institute. This trial was registered at ClinicalTrials.gov with the registration identifier NCT00676286. Research ethics approval was obtained from the local Alberta Cancer Research Ethics Committee. All patients provided written informed consent prior to enrollment and participation in this trial.
Studies monitoring similar patient populations have demonstrated that 20%-30% of patients with indolent prostate cancer demonstrate disease progression within the first two years of follow-up[6,18,19]. For the sample size calculation we assumed that there would be a 25% rate of disease progression and a 50% rate of detecting disease progression utilizing mpMRI[19,20]. This study was designed with a sample size of 24 to have a power of 80% and a significance level of 5% to detect at least one patient with disease progression.
All patients had clinical assessment, including digital rectal examination, and PSA testing performed at or within 8 wk of enrollment and then every 3 mo for the first year and biannually thereafter. Prostate biopsies were performed at baseline or within 16 wk of enrollment (minimum of sextant biopsies) and annually thereafter. MRI scans were performed at baseline and annually for the duration of the study, with caution taken to ensure that imaging was performed prior to biopsies to prevent artifact.
Evidence of disease progression was identified during follow-up by: Gleason pattern ≥ 4 (composite Gleason score ≥ 7); clinical stage ≥ T3a; or PSA doubling time less than 2 years. Patients with progressive disease were offered treatment with any one or a combination of standard treatment options.
Patients had a bowel preparation prior to the MRI scan and were asked to have an empty rectum and empty bladder for the MRI scan. Prior to imaging, Buscopan® 1 mL (20 mg/mL) was administered intramuscularly to decrease bowel peristalsis and allow for clear visualization of the prostate.
All measurements were performed with a 3T Philips Healthcare Intera whole body MRI scanner. The scanner’s built in body coil was employed for RF (radiofrequency) transmission while a Philips six element SENSE-TORSO coil was used for signal reception. It was ensured that the patient was positioned such that the prostate was at iso-centre and at the centre of the receiver RF coil. Axial, coronal and sagittal scout images were acquired. A set of sagittal T2-weighted images were acquired with parameters indicated on the first line of Table 1. The images were employed to align all the axial images perpendicular to the long axis of the prostate gland.
| Protocol | Sequence | Repetitiontime (ms) | Echo time(ms) | Flip angle | Slice thickness (mm) | Field of view | Matrixsize | Voxel size (mm × mm) | Reconstructed | Averages | Acquisition |
| (°) | (AP × RL) | voxel size | time | ||||||||
| (mm × mm) | (mm × mm) | (min:s) | |||||||||
| Sagittal T2-weighted | FSE | 3000 | 100 | 90 exc. | 5 | 160 × 160 | 224 × 216 | 0.71 × 0.74 | 0.63 × 0.63 | 6 | 3:42 |
| (18 echoes) | 135 refoc. | ||||||||||
| T1-weighted | FSE | 700 | 11 | 90 exc. | 5 | 160 × 160 | 400 × 319 | 0.4 × 0.5 | 0.4 × 0.4 | 3 | 4:54 |
| (7 echoes) | 145 refoc. | ||||||||||
| T2-weighted | FSE | 3000 | 100 | 90 exc. | 5 | 160 × 160 | 400 × 317 | 0.4 × 0.5 | 0.4 × 0.4 | 3 | 5:48 |
| (17 echoes) | 145 refoc. | ||||||||||
| Diffusion weighted | Single-shot EPI | 2500 | 64 | 90 exc. | 4 | 160 × 144 | 80 × 59 | 2.00 × 2.42 | 1.25 × 1.24 | 61 | 4:02 |
| 1 mm gap | |||||||||||
| dynamic contrast-enhanced | 3D-FFE | 4.4 | 2.1 | 15 exc. | 5 | 260 × 260 | 128 × 102 | 2.03 × 2.52 | 1.02 × 1.02 | 2 | 6:37 |
| T2 map | TSE | 1300 | N × 30 | 90 exc. | 5 | 160 × 1602 | 160 × 160 | 1.00 × 1.25 | 0.63 × 0.63 | 2 | 10:323 |
| (6 echoes) | 180 refoc. |
Table 1 shows the parameters used for the axial T1-weighted, T2-weighted, diffusion weighted, dynamic contrast-enhanced (DCE) and T2 map pulse sequences. All axial sets consist of 12 slices each 5 mm thick (or as with the diffusion weighted images 4 mm with a gap of 1 mm) so that the images could be registered with each other. The diffusion weighted sequence acquisition time was reduced by using a 0.667 half scan factor and a sense factor of 2 in the RL direction. Acquisitions with five b-values were acquired, namely, 0, 125, 251, 376 and 501 s/mm2. The diffusion weighted sequence also employed SPAIR fat suppression with an inversion delay of 90 ms. The DCE pulse sequence used a key-hole factor of 58.75%. ProHance gadolinium contrast (0.2 mL/kg) was administered to the patient over 20 s and acquisition of data began after approximately four dynamics. A total of 50 dynamic scans were acquired. The entire MRI session lasted approximately 45 min. T2 maps and apparent diffusion coefficient (ADC) maps were calculated on the console using Philips analysis software.
All processing of the DCE data was performed using in-house software written in Matlab. In conjunction, a graphical user interface was developed to handle the DCE data flow. After data for one patient and time-point was loaded, tissue of interest was contoured in the view window on one of the dynamic images. These pixels were then targeted for analysis. Each pixel was analyzed independently. First, an enhancement curve was generated for the pixel in question by normalizing the signal level through-time to the average of the first five pre-contrast dynamics. This curve was then fit to an enhancement model (modified Brix model[21]) whose shape is controlled by four parameters: A, kep, kel, and tarr. The A is an amplitude factor, which can be related to the size of the extravascular extracellular space (EES)[22]. The parameters kep and kel are rate constants which describe the transfer of contrast from the EES to plasma (kep), and the elimination of contrast from the plasma (kel). The tarr parameter is simply the time at which the contrast is seen to arrive at the pixel. This model is described in the equation:
{[S(t - tarr)]/[S(0)]} = 1 + A × kep× {exp[-kep(t - tarr)] - exp[-kel(t - tarr)]}/(kel - kep)
For each data set, one or more of the pixels were selected for initial analysis to better adjust the seed parameters used in the optimization routine. This optimization routine determined the four parameters described above that lead to the best fit of the model to the data. Changing the seed parameters was not found to affect the final result in this context, but it did increase the efficiency of the solver. The optimization code utilized the Matlab function “fminsearch”, which uses a Nelder-Mead method appropriate for multi-dimensional nonlinear optimizations.
The MRI images were anonymized of patient identifiers and clinical information to ensure that there was an unbiased interpretation of each investigation. Each scan underwent radiological review and was reported upon without the other results known. The radiologist reported the presence of characteristics consistent with malignancy (Table 2) and defined the index of suspicion (IOS) for each lesion using a score of 1-5 (1: most probably benign; 2: probably benign; 3: indeterminate; 4: probably malignant; 5: highly suspicious of malignancy). IOS of 3 or higher was used as the cut-off for identification of malignant disease[23].
| Procedure step | Structure examined | Index of suspicion assessment | |
| 1 | Assign region | Base | Superior most margin of prostate to the widest transverse diameter |
| Midgland | Widest transverse diameter to ejaculatory ducts at verumontanum | ||
| Apex | Inferior to midgland | ||
| 2 | Peripheral zone | Consider T2 features | 4: Moderate low signal with mass like appearance |
| 3: Mild low signal with mass like appearance | |||
| 2: Mild low signal which is focal but not clearly mass like, moderate diffuse low signal | |||
| 1: Mild low signal, diffuse and/or feathered, linear low signal | |||
| 0: Normal signal | |||
| Consider DWI features | 4: Definite abnormality (high DWI and low ADC relative to background) | ||
| 3: Probable abnormality (low ADC) | |||
| 2: Possible abnormality (mild decrease ADC or increase DWI) | |||
| 1: Mottled | |||
| 0: Homogeneous ADC or low DWI | |||
| Consider DCE features | 4: Rapid early enhancement, wash out | ||
| 3: Rapid early enhancement, remaining strong and prolonged | |||
| 2: Mild early enhancement, plateau or progressive | |||
| 1: No early upstroke, progressive enhancement | |||
| 0: No enhancement | |||
| Assign combined score | |||
| 3 | Central gland | Consider T2 features | 4: Mass like low T2 signal with invasion into AFMS or peripheral zone/disrupted surgical capsule, irregularly or poorly marginated mass like low T2 signal without a capsule |
| 3: Mass like homogeneous low T2 signal with no capsule, preserved surgical capsule | |||
| 2: Diffuse heterogeneous signal with intact surgical capsule | |||
| 1: Encapsulated nodules | |||
| 0: Normal | |||
| 4 | Fibromuscular stroma | Assess for presence of disease | |
| 5 | Extracapsular extension | Assess for presence of disease | |
| 6 | Seminal vesicles | Assess for presence of disease | |
The radiologist reviewed the serial scans for each patient to examine if there were signs of disease progression over time including imaging characteristics (increased exchange on DCE, decreases in the ADC on DWI), growth of lesions, new lesions identified or other changes suggestive of disease progression.
Over 40 mo (January 2009-May 2012), 24 active surveillance patients consented to and were enrolled on the study. One patient was not evaluable as they did not have evaluable MRI scans. The baseline characteristics of the 23 evaluable patients are described in Table 3. All patients enrolled had low-risk Gleason 3 + 3 = 6 disease and the majority were clinical stage T1c (87%). Three patients had no positive cores on their enrollment biopsy but had at least one positive biopsy previously. Nine patients on this active surveillance study had ≥ 3 cores positive (or ≥ 25% positive cores) from their baseline biopsy. Six of these patients had subsequent disease progression during the course of follow-up.
| Characteristic | Value |
| Age (yr), median (range) | 65 (51-75) |
| PSA (ng/mL), median (range) | 6.4 (1.4-14.3) |
| Clinical stage, n (% of total) | |
| cT1c | 20 (87.0) |
| cT2a | 2 (8.7) |
| cT2b | 1 (4.3) |
| Gleason score 6 (3 + 3), n (% of total) | 23 (100%) |
| % of cores positive per patient, median (range) | 8.3 (01-58.3) |
| Density of tumor in positive biopsy cores (% of core), median (range) | 10 (1-60) |
| HGPIN, n (% of total) | 6 (26.1) |
| PNI, n (% of total) | 4 (17.4) |
| LVI, n (% of total) | 0 (0.0) |
Amongst the study patients, 67 sets of biopsies were analyzed and the median number of biopsies was 3 per patient. The median number of cores per biopsy at baseline and during active surveillance was 12. In total there were 809 cores [median 12 cores per biopsy set (7-17)], with 143 cores positive for prostate cancer [median 1 positive core per biopsy set (range: 0-9)] with 5 sets of biopsies having greater than 50% positive cores over the course of the study. During the study 21 biopsies in 8 patients had biopsies with no positive cores. Of these only one patient had subsequent disease progression during the course of follow-up.
The median follow-up time was 24.8 mo (6.2-50.8 mo). During the study 11 patients had disease progression and the median time from enrollment to progression was 21.7 mo (6.2-37.9 mo).
The primary cause for patients being categorized as having disease progression was upstaging to a higher Gleason score on biopsy (all 11 patients). In combination with the higher Gleason score, 5 of the 11 patients also had ≥ 50% of cores involved and 2 of these additionally had PSA doubling time less than 2 years. All of the patients with progressive disease were offered treatment. Two patients declined therapy and choose to remain on active surveillance, 5 had low dose rate prostate brachytherapy, 2 had external beam radiation treatment, and 2 underwent radical prostatectomies.
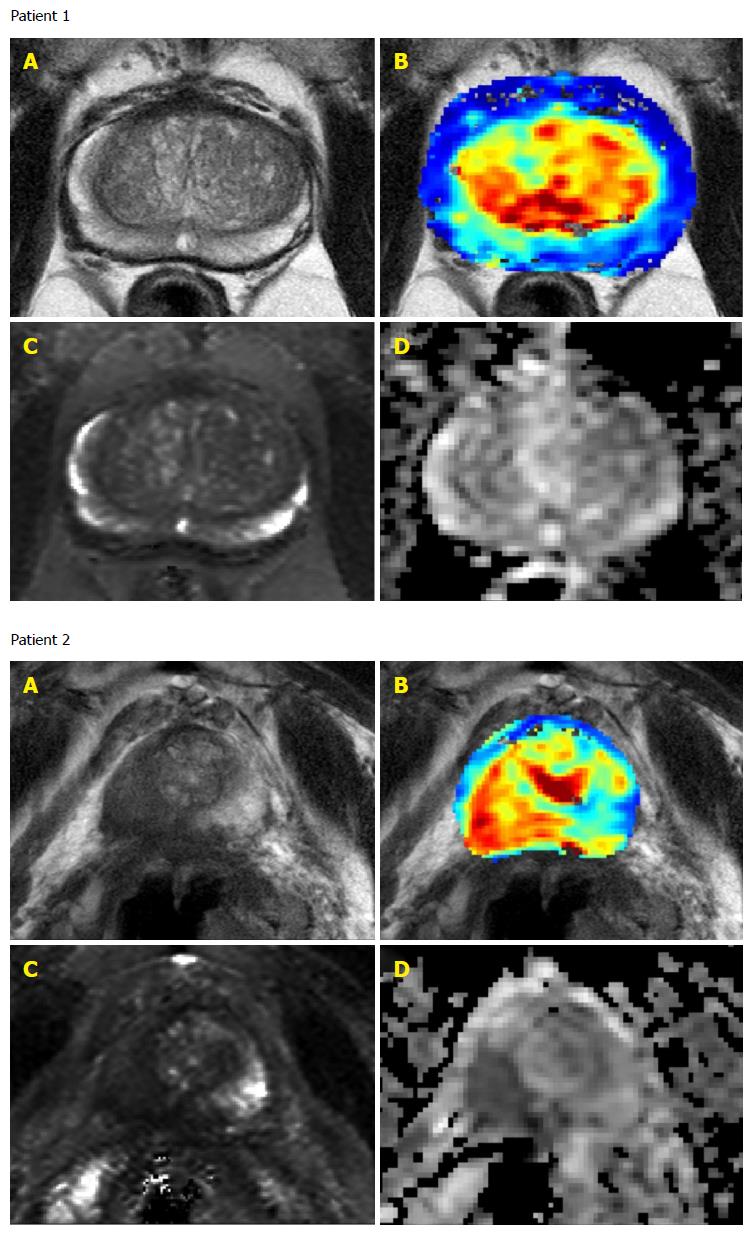
Although all patients had previously positive biopsies of Gleason score 6 prostate cancer only 10 of the 23 patients had positive identification of malignancy (IOS 3-5) on mpMRI at baseline (Table 4 and Figure 1). The sensitivity of prostate cancer detection at baseline was 43.5%.
| n = 23 | |
| Number of scans with baseline IOS of: | |
| 1 | 8 |
| 2 | 5 |
| 3 | 3 |
| 4 | 2 |
| 5 | 5 |
| Number of follow-up MRI scans, median (range) | 3 (0-4) |
| Number of follow-up scans with signs of disease progression | 3 |
| Number of follow-up scans with changed IOS | 1 |
Seven of the ten patients with IOS greater than or equal to 3 at baseline had disease progression during the study. The aggressive disease prediction from baseline mpMRI scans had specificity of 81.8% and sensitivity of 58.3%.
Twenty-two patients had serial mpMRI scans and the median number of scans was 3 scans per patient (range 1-5). One patient had only a baseline scan with an IOS of 5 before disease progression was detected on biopsy. The IOS of mpMRI scans remained the same for all but one patient whose fourth scan had an IOS of 2 rather than 1 but remained below the threshold of 3 and this patient did not have disease progression on biopsy. Additionally during follow-up only 3 patients had scans with signs of disease progression and all 3 had baseline IOS of 4 or 5. The sensitivity to detect disease progression was 100% but the specificity was only 30% as 7 patients did not exhibit signs of disease progression on mpMRI but had Gleason score 7 disease on biopsy.
Low-risk prostate cancer can behave like an indolent disease and causes death in only a small subpopulation of patients[3-5]. Extensive work is being done to distinguish aggressive low-risk prostate cancer from indolent disease that doesn’t require treatment but current surveillance protocols lack accurate tools. In addition to low accuracy due to undersampling, prostate biopsies cause discomfort and carry a risk of infection (including rare cases of sepsis leading to death). Addition of a sensitive and specific non-invasive method for monitoring patients would allow active surveillance to be a more attractive management approach for men with prostate cancer. To the best of our knowledge, this is the first study that utilized serial mpMRI scans to predict disease aggressiveness.
We examined whether mpMRI IOS was related to larger lesions and found that 75% of the patients with IOS 3-5 had 25% or greater positive cores on baseline biopsy. This was regardless of future disease progression status although higher positive cores were also predictive of more aggressive disease. Six of the 11 patients that had subsequent progressive disease had 25% or higher positive cores at baseline compared with only 3 of 12 patients without signs of disease progression.
Gleason score remains the best available predictive factor for prostate cancer mortality and the presence of Gleason pattern 4 is often an indication that a patient should receive intervention[5]. mpMRI has been shown to have high detection accuracy for Gleason pattern 4 disease (97.4%)[24] and some of our patients with positive baseline scans may have had higher Gleason score disease that was missed on biopsy due to sampling inadequacy[11]. We performed sextant biopsies which, although standard practice, are subject to inaccuracy and inconsistency. This would be consistent with studies that showed better detection of Gleason score 7 disease and increased incidence of upstaging on MRI guided biopsies[24-27]. In one study 41% of Gleason score 6 patients were reclassified to higher Gleason score based on mpMRI targeted biopsy[28] and in another study 20% of patients were reclassified to a higher Gleason score upon repeat biopsy focused on MRI results[23].
This study was a pilot, feasibility study and as such was limited to a small sample size which resulted in limited power to correlate quantitative MRI parameters with disease progression. The standard b-value for the diffusion weighted MRI protocol changed from 500 to 1000 to 1400 s/mm2 during the course of this work. For consistency, we kept it at 500 s/mm2, which is the value we used at initiation. Also since study initiation, mpMRI technology and analyses have improved as well as increased experience by radiologists. Combined, these factors may have resulted in lower sensitivity for detection than desired at baseline and during follow-up.
In this study we only examined whether or not a prostate cancer lesion was identified on MRI. However, extracapsular extension, SV involvement, and LN involvement can also be examined through mpMRI. Utilization of imaging techniques may also improve detection of prostate cancer in the central or anterior aspect of gland which can often be missed on biopsy because of anatomical and technical limitations[9,29].
In this pilot study we found that baseline mpMRI was able to predict disease aggressiveness in 7 of 11 patients who had biopsy proven disease progression while on study. Three patients who did not have disease progression while on study were also predicted to have aggressive disease. If the mpMRI protocol utilized in this study were added to decision making in active surveillance protocols this would have resulted in 7 patients receiving earlier therapy which could benefit clinical outcome. There would however be 3 patients treated without disease progression and 4 patients who would have missed earlier therapy and not be treated until after biopsy proven disease progression.
Active surveillance protocols are still evolving and there is currently no gold standard. Follow-up with PSA serum levels and repeat biopsies requires improvement and imaging modalities are being examined. The use of fluoro-deoxyglucose and choline positron emission tomography has been examined and they appear to have moderate ability to distinguish aggressive from indolent disease[30-32]. Here we demonstrate the potential of mpMRI to detect prostate cancer with increased risk of progression although alone it has limited sensitivity and specificity. The future of active surveillance will likely include molecular imaging in combination with less frequent biopsies and novel serum or urine biomarkers of disease progression[33].
We would like to thank our clinical research nurse, Michelle Encarnacao, and our clinical trials coordinator, Juliette Jordan, for their help with this study.
Prostate cancer is one of the most common solid organ cancers and the third leading cause of cancer-related death in men. Introduction of and widespread utilization of serum prostate specific antigen (PSA) screening has increased the incidence of low-risk prostate cancer with about 25%-50% of newly diagnosed patients having low-risk disease. For the majority of these patients, the prostate cancer will behave as an indolent disease and only have clinically significant effects or cause death in a relatively small proportion of men. Many patients with low-risk indolent prostate cancer are choosing to delay treatment and as a result many are managed with active surveillance. This management strategy involves the monitoring of PSA values and prostate biopsies, but these tests lack accuracy for predicting aggressive or progressive disease. In this study the authors examined whether the addition of 3T multiparametric magnetic resonance imaging (mpMRI) at 3T to an active surveillance protocol could increase the detection of aggressive or progressive prostate cancer.
Active surveillance protocols are still evolving and there is currently no gold standard. Follow-up with PSA serum levels and repeat biopsies requires improvement and imaging modalities including positron emission tomography and MRI are being examined. Novel serum or urine biomarkers of disease progression are being examined to supplement PSA monitoring.
Numerous studies with mpMRI of patients with prostate cancer have shown its utility to detect intermediate risk prostate cancer. To the best of the authors’ knowledge, this is the first prospective study that utilized serial mpMRI scans during active surveillance of patients with low risk prostate cancer to predict disease aggressiveness.
Although this study was a small pilot trial, it supports further examination of mpMRI during active surveillance decision making. With the recent improvements in technology and clinical experience it is likely that a larger trial could show the ability of mpMRI to predict indolent disease and thus stratify patients to active surveillance or definitive treatment.
mpMRI provides a non-invasive way of measuring a range of possible quantitative physiological parameters in addition to conventional anatomical information. With regards to this study into prostate cancer, the T2 weighted imaging provided detailed soft tissue anatomical features and dynamic contrast enhanced provided physiological exchange parameters through the rate of intake and washout of the gadolinium contrast agent which is different in prostate cancer than normal prostate tissue. The measurement of apparent diffusion coefficient provided yet another quantitative parameter. Gleason score is the sum of primary and secondary Gleason patterns. The Gleason patterns are assigned by a pathologist examining the cellular features on the biopsy. The range of scores is 1 for cancer that closely resembles normal prostate tissue and glands are present through 5 for prostate cancer in which there are sheets of cells and few or no glands visible.
The authors evaluated the role of multiparametric MRI in detecting aggressive tumors during active surveillance in 23 low-risk prostate cancer patients.
| 1. | Canadian Cancer Society. Canadian cancer statistics 2014. Available from: http: //www.cancer.ca/~/media/cancer.ca/CW/publications/Canadian Cancer Statistics 2014/Canadian-Cancer-Statistics-2014-EN.pdf. |
| 2. | Draisma G, Boer R, Otto SJ, van der Cruijsen IW, Damhuis RA, Schröder FH, de Koning HJ. Lead times and overdetection due to prostate-specific antigen screening: estimates from the European Randomized Study of Screening for Prostate Cancer. J Natl Cancer Inst. 2003;95:868-878. [PubMed] |
| 3. | Chen J, Zhao Y, Li X, Sun P, Wang M, Wang R, Jin X. Imaging primary prostate cancer with 11C-Choline PET/CT: relation to tumour stage, Gleason score and biomarkers of biologic aggressiveness. Radiol Oncol. 2012;46:179-188. [PubMed] |
| 4. | Klotz L. Active surveillance for favorable risk prostate cancer: what are the results, and how safe is it? Semin Radiat Oncol. 2008;18:2-6. [PubMed] |
| 5. | Klotz L. Active surveillance for prostate cancer: overview and update. Curr Treat Options Oncol. 2013;14:97-108. [PubMed] |
| 6. | Choo R, Klotz L, Danjoux C, Morton GC, DeBoer G, Szumacher E, Fleshner N, Bunting P, Hruby G. Feasibility study: watchful waiting for localized low to intermediate grade prostate carcinoma with selective delayed intervention based on prostate specific antigen, histological and/or clinical progression. J Urol. 2002;167:1664-1669. [PubMed] |
| 7. | Bangma CH, Bul M, van der Kwast TH, Pickles T, Korfage IJ, Hoeks CM, Steyerberg EW, Jenster G, Kattan MW, Bellardita L. Active surveillance for low-risk prostate cancer. Crit Rev Oncol Hematol. 2013;85:295-302. [PubMed] |
| 8. | Parker C. Active surveillance: towards a new paradigm in the management of early prostate cancer. Lancet Oncol. 2004;5:101-106. [PubMed] |
| 9. | Bhojani N, Salomon L, Capitanio U, Suardi N, Shariat SF, Jeldres C, Zini L, Pharand D, Péloquin F, Arjane P. External validation of the updated partin tables in a cohort of French and Italian men. Int J Radiat Oncol Biol Phys. 2009;73:347-352. [PubMed] |
| 11. | Serefoglu EC, Altinova S, Ugras NS, Akincioglu E, Asil E, Balbay MD. How reliable is 12-core prostate biopsy procedure in the detection of prostate cancer? Can Urol Assoc J. 2013;7:E293-E298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 107] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 12. | Heenan SD. Magnetic resonance imaging in prostate cancer. Prostate Cancer Prostatic Dis. 2004;7:282-288. [PubMed] |
| 13. | Kirkham AP, Emberton M, Allen C. How good is MRI at detecting and characterising cancer within the prostate? Eur Urol. 2006;50:1163-1174; discussion 1175. [PubMed] |
| 14. | Rouvière O, Hartman RP, Lyonnet D. Prostate MR imaging at high-field strength: evolution or revolution? Eur Radiol. 2006;16:276-284. [PubMed] |
| 15. | Haider MA, van der Kwast TH, Tanguay J, Evans AJ, Hashmi AT, Lockwood G, Trachtenberg J. Combined T2-weighted and diffusion-weighted MRI for localization of prostate cancer. AJR Am J Roentgenol. 2007;189:323-328. [PubMed] |
| 16. | The National Institute for Health and Care Excellence. Nice guidelines. Available from: http: //www.nice.org.uk/guidance/cg175/chapter/key-priorities-for-implementation. |
| 17. | Turkbey B, Mani H, Aras O, Ho J, Hoang A, Rastinehad AR, Agarwal H, Shah V, Bernardo M, Pang Y. Prostate cancer: can multiparametric MR imaging help identify patients who are candidates for active surveillance? Radiology. 2013;268:144-152. [PubMed] |
| 18. | Carter HB, Walsh PC, Landis P, Epstein JI. Expectant management of nonpalpable prostate cancer with curative intent: preliminary results. J Urol. 2002;167:1231-1234. [PubMed] |
| 19. | Coakley FV, Chen I, Qayyum A, Westphalen AC, Carroll PR, Hricak H, Chen MH, Kurhanewicz J. Validity of prostate-specific antigen as a tumour marker in men with prostate cancer managed by watchful-waiting: correlation with findings at serial endorectal magnetic resonance imaging and spectroscopic imaging. BJU Int. 2007;99:41-45. [PubMed] |
| 20. | Shukla-Dave A, Hricak H, Kattan MW, Pucar D, Kuroiwa K, Chen HN, Spector J, Koutcher JA, Zakian KL, Scardino PT. The utility of magnetic resonance imaging and spectroscopy for predicting insignificant prostate cancer: an initial analysis. BJU Int. 2007;99:786-793. [PubMed] |
| 21. | Hoffmann U, Brix G, Knopp MV, Hess T, Lorenz WJ. Pharmacokinetic mapping of the breast: a new method for dynamic MR mammography. Magn Reson Med. 1995;33:506-514. [PubMed] |
| 22. | Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging. 1997;7:91-101. [PubMed] |
| 23. | Vargas HA, Akin O, Afaq A, Goldman D, Zheng J, Moskowitz CS, Shukla-Dave A, Eastham J, Scardino P, Hricak H. Magnetic resonance imaging for predicting prostate biopsy findings in patients considered for active surveillance of clinically low risk prostate cancer. J Urol. 2012;188:1732-1738. [PubMed] |
| 24. | Porpiglia F, Russo F, Manfredi M, Mele F, Fiori C, Regge D. Preoperative prostate biopsy and multiparametric magnetic resonance imaging: reliability in detecting prostate cancer. Int Braz J Urol. 2015;41:124-133. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 25. | Panje C, Panje T, Putora PM, Kim SK, Haile S, Aebersold DM, Plasswilm L. Guidance of treatment decisions in risk-adapted primary radiotherapy for prostate cancer using multiparametric magnetic resonance imaging: a single center experience. Radiat Oncol. 2015;10:47. [PubMed] |
| 26. | Bonekamp D, Bonekamp S, Mullins JK, Epstein JI, Carter HB, Macura KJ. Multiparametric magnetic resonance imaging characterization of prostate lesions in the active surveillance population: incremental value of magnetic resonance imaging for prediction of disease reclassification. J Comput Assist Tomogr. 2013;37:948-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 27. | Schoots IG, Petrides N, Giganti F, Bokhorst LP, Rannikko A, Klotz L, Villers A, Hugosson J, Moore CM. Magnetic resonance imaging in active surveillance of prostate cancer: a systematic review. Eur Urol. 2015;67:627-636. [PubMed] |
| 28. | Marliere F, Puech P, Benkirane A, Villers A, Lemaitre L, Leroy X, Betrouni N, Ouzzane A. The role of MRI-targeted and confirmatory biopsies for cancer upstaging at selection in patients considered for active surveillance for clinically low-risk prostate cancer. World J Urol. 2014;32:951-958. [PubMed] |
| 29. | Damiano R, Autorino R, Perdonà S, De Sio M, Oliva A, Esposito C, Cantiello F, Di Lorenzo G, Sacco R, D’Armiento M. Are extended biopsies really necessary to improve prostate cancer detection? Prostate Cancer Prostatic Dis. 2003;6:250-255. [PubMed] |
| 30. | Boychak O, Vos L, Makis W, Buteau FA, Pervez N, Parliament M, McEwan AJ, Usmani N. Role for (11)C-choline PET in active surveillance of prostate cancer. Can Urol Assoc J. 2015;9:E98-E103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 31. | Scher B, Seitz M, Albinger W, Tiling R, Scherr M, Becker HC, Souvatzogluou M, Gildehaus FJ, Wester HJ, Dresel S. Value of 11C-choline PET and PET/CT in patients with suspected prostate cancer. Eur J Nucl Med Mol Imaging. 2007;34:45-53. [PubMed] |
| 32. | Richter JA, Rodríguez M, Rioja J, Peñuelas I, Martí-Climent J, Garrastachu P, Quincoces G, Zudaire J, García-Velloso MJ. Dual tracer 11C-choline and FDG-PET in the diagnosis of biochemical prostate cancer relapse after radical treatment. Mol Imaging Biol. 2010;12:210-217. [PubMed] |
| 33. | Dijkstra S, Mulders PF, Schalken JA. Clinical use of novel urine and blood based prostate cancer biomarkers: a review. Clin Biochem. 2014;47:889-896. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Onal C, Shen J S- Editor: Kong JX L- Editor: A E- Editor: Jiao XK