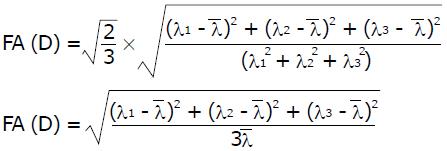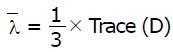Published online Apr 28, 2016. doi: 10.4329/wjr.v8.i4.397
Peer-review started: August 16, 2015
First decision: November 7, 2015
Revised: January 15, 2016
Accepted: February 14, 2016
Article in press: February 16, 2016
Published online: April 28, 2016
Processing time: 249 Days and 4.5 Hours
AIM: To investigate feasibility of a quantitative study of prostate cancer using three dimensional (3D) fiber tractography.
METHODS: In this institutional review board approved retrospective study, 24 men with biopsy proven prostate cancer underwent prostate magnetic resonance imaging (MRI) with an endorectal coil on a 1.5 T MRI scanner. Single shot echo-planar diffusion weighted images were acquired with b = 0.600 s/mm2, six gradient directions. Open-source available software TrackVis and its Diffusion Toolkit were used to generate diffusion tensor imaging (DTI) map and 3D fiber tracts. Multiple 3D spherical regions of interest were drawn over the areas of tumor and healthy prostatic parenchyma to measure tract density, apparent diffusion coefficient (ADC) and fractional anisotropy (FA), which were statistically analyzed.
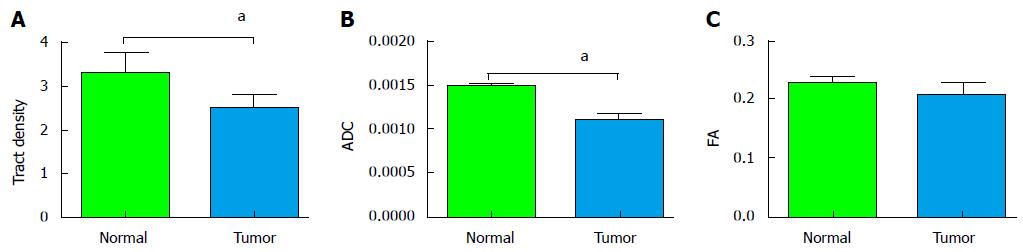
RESULTS: DTI tractography showed rich fiber tract anatomy with tract heterogeneity. Mean tumor region and normal parenchymal tract densities were 2.53 and 3.37 respectively (P < 0.001). In the tumor, mean ADC was 0.0011 × 10-3 mm2/s vs 0.0014 × 10-3 mm2/s in the normal parenchyma (P < 0.001). The FA values for tumor and normal parenchyma were 0.2047 and 0.2259 respectively (P = 0.3819).
CONCLUSION: DTI tractography of the prostate is feasible and depicts congregate fibers within the gland. Tract density may offer new biomarker to distinguish tumor from normal tissue.
Core tip: Our study identified 24 men with biopsy proven prostate cancer. These patients underwent prostate magnetic resonance imaging with an endorectal coil on a 1.5 T scanner. Software was used to generate a diffusion tensor imaging (DTI) map and three dimensional fiber tracts. DTI tractography demonstrated rich fiber tract anatomy with tract heterogeneity. Tract density may represent a new biomarker to distinguish tumor from normal tissue.
- Citation: Hedgire S, Tonyushkin A, Kilcoyne A, Efstathiou JA, Hahn PF, Harisinghani M. Quantitative study of prostate cancer using three dimensional fiber tractography. World J Radiol 2016; 8(4): 397-402
- URL: https://www.wjgnet.com/1949-8470/full/v8/i4/397.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i4.397
Despite recent advances in the magnetic resonance imaging (MRI), the extent and aggressiveness of prostate cancer remain difficult to predict by imaging[1]. To date there is no consensus as to what MRI sequence provides most reliable precise detection and characterization of prostate cancer[2]. As the reliance on diffusion weighted imaging (DWI) grows, development of newer quantitative parameters can provide added value besides currently used one such as apparent diffusion coefficient (ADC) and fractional anisotropy (FA). Diffusion tensor imaging (DTI) describes diffusion properties as a function of diffusion direction. It involves measurements of molecular diffusion into and out from the faces of an idealized infinitesimal cube oriented along the x, y and z imaging axes. A DTI acquisition consists of six separate point-wise measurements that comprise six of the nine tensor components. From these components the other three can be computed using the symmetry of the tensor. The nine components of the tensor compose a 3 × 3 matrix. When diffusion is anisotropic, the corresponding matrix differs from an identity matrix by a multiplier. The direction of a fiber tract is indicated by the tensor’s main eigenvector. DTI is commonly used to map neuronal fibers in the brain[3-6]. Although well described in the neuroradiology literature, this technique has seldom been used for other visceral organs. A few DTI prostate studies have been recently reported[7,8].
Prostate gland consists of various vascular, neural, and other anisotropic water paths that can be treated as tracts and thus make the DTI potentially applicable to the prostate gland. Recently, a few researchers have used DTI of the prostate gland to assess FA in the central gland and peripheral zone, especially for mapping periprostatic fiber tracts[9-13]. Our study was based on the hypothesis that the high cellularity of the tumors that reduce ADC values implies restrictive isotropic intratumoral diffusion[14]. With the tumor cells altering the cellular matrix relative to the parenchyma, the diffusion through the tumor region would be isotropic and would show fewer numbers of tracts passing through the tumor region as a result of destruction of anisotropic water paths. With this premise, the purpose of this study was to apply diffusion tensor magnetic resonance tractography to the prostate gland and investigate feasibility of a quantitative study of prostate cancer using three dimensional (3D) fiber tracts. Following section elaborates on the theory of DTI in brief.
The diffusion tensor D (3 × 3 matrix) in each obtained voxel can be visualized as a diffusion ellipsoid. This ellipsoid is characterized by eigenvectors, which indicate the direction of the principal axes, and the eigenvalues. The square root of the eigenvalues defines the ellipsoidal radii. The eigenvalues λ1, λ2, λ3 define scalar properties of the tensor, i.e., scalar maps: FA, RA, Trace (D), that characterize the size and shape of the diffusion tensor:
Math 1
where:
Math 2
is a mean diffusivity. FA is a scalar index, independent on fiber orientation and gradient directions, is a characteristic of degree of anisotropy from 0 (isotropic) to 1 (anisotropic); RA is a relative anisotropy. For isotropic diffusion all three eigenvalues are comparable and for anisotropic diffusion one of the eigenvalues has to be much larger than the other two.
The eigenvectors define vector properties of the tensor. DTI tractography is based on the assumption that the fiber tracts are collinear with the direction of principal eigenvector.
When tensors have been determined for a number of voxels in region of interest (ROI), 3D tractography is used to create fiber tracts within such ROI. To date several tractography algorithms are available[3,14]. One of the most popular algorithms is deterministic or streamline tractography[3,15]. Streamline tractography connects voxels by following the fiber tracts from defined seed voxels until certain termination criteria are met, such as critical angle of the fiber tracts or threshold voxel FA value. One of the common streamline methods of propagating tracts is traversing between voxel boundaries in a single step, called FACT (fiber assessment by continuous tracking)[15].
The software TrackVis allows user defined hand-drawn ROIs as well as ROIs with any of several predefined shapes to create seed voxels[15]. The number of tracts is calculated by the software within each seed voxel and summed up for all voxels inside the ROI.
In this Health Insurance Portability and Accountability Act compliant, institutional review board approved retrospective study, 24 men with biopsy proven prostate cancer were included. Our institutional review board waived the informed consent requirement.
All patients underwent prostate MRI with an endorectal coil on a 1.5 T MRI scanner Signa Excite HD (GE Healthcare Waukesha, WI) equipped with 40 mT/m maximum strength and dual slew rates of 80 and 150 mT/m per second. Diffusion weighted images were acquired with TE/TR = 90.5/5000 ms, 8 nex, bw = 1.95 kHz, 128 × 128 matrix, slice thickness = 5 mm, FOV = 22 cm × 22 cm, b = 0.600 s/mm2, and 6 gradient directions used to generate DTI maps. Open-source software TrackVis and its Diffusion Toolkit (Wang R, Wedeen VJ, Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Boston, MA)[15] was used for image processing. Tract reconstruction was performed using a FACT propagation algorithm, with optimal parameter found to be: Angle threshold 35, spline-filtered, DWI mask image, FA threshold 0.1.
Two radiologists with 5 and 15 years of experience in reading prostate MRI examinations reviewed the images on a clinical dual monitor picture archiving and communication system viewing workstation (Agfa, version 5.3, Richmond, VA) and in consensus, identified the location of the tumor in the peripheral zone based on conventional T2 weighted and DWI images. The readers were given the freedom to change the window level for optimum visualization. Multiple 3-dimensional spherical regions of interest (ROI) were drawn over the tumor and healthy tissue in the peripheral zone at the same slice level to generate multiple fiber tracts. We defined tract number by counting a number of fibers (that fit the reconstruction threshold) passing through the specific ROI volume. To measure the disruption of tracts, we introduced a tract density parameter, which is a tract number divided by the ROI volume (r3, which is proportional to the physical volume of a sphere), as a normalized measure of the number of tracts passing through the given ROI. The use of tract density as a quantitative parameter vs absolute number of tracts through ROIs was influenced by the large variation in the number of tracts across different ROI sizes. In addition, tract density would allow comparing ROIs with different volumes within a patient, although in our study we managed to keep all such ROIs the same. Mean ADC and FA values for the ROIs were also recorded (available for a random subset of 15 subjects). The tract densities in the tumor and normal parenchymal regions in the same patient were compared using a paired t-test (SOFA statistics version 1.3.2.) and P values of < 0.05 was considered statistically significant.
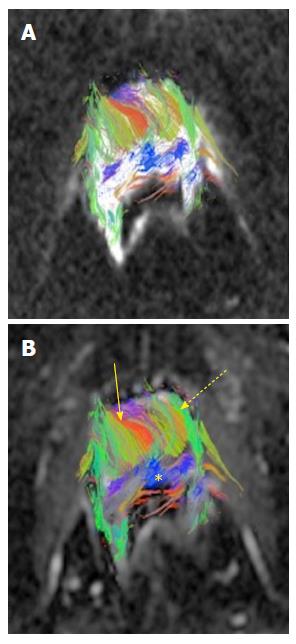
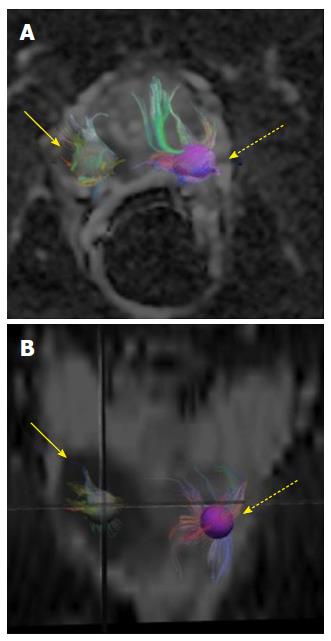
The average age of the study population was 63.2 years, with an average Gleason’s score of 7.3. The average tumor size was 8 mm (diameter recorded on T2 axial images). The average radius of 3D spherical ROI across the study population was 4.5 mm. The depiction of fiber tracts morphology and complex prostate gland architecture with tract heterogeneity was feasible using preexisting DWI data post-processed with TrackVis software (Figure 1). The tract density in tumors was lower than in normal peripheral zone tissue (Figure 2). Mean tumor and normal parenchymal tract densities were 2.53 and 3.37 respectively (P = 0.0009). ADC and FA values were available for the same ROIs as used for tract densities in a (random) subset of 15 MRI exams, in which the tumor region mean ADC was 0.0011 mm2/s vs 0.0014 mm2/s in the normal region (P = 0.0001). The FA values for tumor and normal regions were 0.2047 and 0.2259 respectively with a P value of 0.3819 (Figure 3).
Tractography can be conceptually defined as the virtual reconstruction of the trajectory of water molecules along water pathways. DTI tractography is an imaging tool that has been previously introduced for neuroimaging. One of its major limitations has been quantification[16,17]. DTI tractography can be applied to organs with fibrous structures with well-ordered microstructural composition[18,19]. In this regard, it has been tested for its utility for skeletal muscle, myocardium, tongue, uterus and kidneys[20-26]. Experimental usage of fiber tractography to visualize periprostatic neurovascular bundles for precise surgical planning and thereby improved outcome has recently been proposed[10,12]. The earliest attempts to use DTI in the prostate were by Sinha et al[27] who studied DTI in healthy volunteers. They suggested that anisotropy has the potential for early disease detection at the microstructural level, even before the morphological changes become apparent[27].
We found heterogeneity in the tracts within the prostate, which we attribute to the heterogeneous orientation of myocytes within the gland[9]. In our study, the tract density values were significantly lower in the tumor region implying interruption of the fiber tracts. This observation was correlated and found to be consistent with the well-known trend in scalar ADC values, which are lower in tumor than in normal peripheral zone.
The measured FA values, however, did not yield a significant difference between tumor and normal peripheral zone. This result differs from findings previously published[19]. We attribute that to noise that affects FA secondary to the attenuation of the DWI signal that can be variable throughout the gland[9,28]. However, the trend in tract density parameter was similar to the trend in ADC values in this study as well as data published elsewhere[29,30].
As the differences between the tract densities are statistically significant, we can try designing a new quantitative marker for evaluation of prostate cancer. Quantifying the tractographic data may provide insight into early detection of morphologically unapparent foci, although, that would require a more substantial study. Another potential problem tractography can attempt addressing is the clinical scenario of rising prostate specific antigen level with repeated negative biopsies. Additionally it remains to be investigated whether it can assess tumor aggressiveness or serve as a treatment response marker.
Our study certainly has a few limitations. It is a retrospective, preliminary observation with prior knowledge of tumor (confined to peripheral zone) location based on T2 and DWI weighted images. The prostate DTI tractography may be limited to an endorectal coil due to higher DWI resolution and SNR than the ones obtained with a pelvic array, although the latter could potentially produce fewer artifacts. Our data is also limited by the magnet strength of 1.5 T and related SNR thereby quality of the reconstructed fiber tracts. DWI was performed at b = 0 and 600, however a b = 1000 or higher is currently being found most useful[31] and our future study will use an accordingly modified protocol. Lastly, the DWI data was acquired with minimum required number of diffusion gradient directions which implies certain limitations[32,33]. It is known from brain tractography studies that a large number of directions are required for precise depiction of neuronal fiber tracts as smaller number of gradient directions create false positive tracts[5,6,28]. In prostate DTI, a study revealed differences in FA numbers when larger than the minimum number of diffusion gradients were used[34].
Although the relation between the scalar index such as FA and 3D fiber tracts is not clearly established, previous studies[31-33] imply that a smaller number of diffusion gradient directions could erroneously depict higher FA values and therefore false positive 3D fiber tracts.
In conclusion, tractography is a feasible; add on tool that has potential to improve performance of MRI in the characterization of prostate cancer by depicting disease processes at a microstructural level.
Despite recent advances in magnetic resonance imaging (MRI), the extent and aggressiveness of prostate malignancy remains difficult to predict by imaging. To date there is no consensus as to which MRI sequence provides the most reliable, precise detection and characterization of prostate cancer. Diffusion tensor imaging (DTI) describes diffusion properties as a function of diffusion direction. It involves measurements of molecular diffusion into and out from the faces of an idealized infinitesimal cube oriented along that x, y and z imaging axes. Although well described in the neuroradiology literature, this technique has seldom been used for other visceral organs. Some researchers have used DTI of the prostate gland to assess fractional anisotropy (FA) in the central gland and peripheral zone, especially for mapping periprostatic fiber tracts. The study was based on the hypothesis that the high cellularity of the tumors that reduce apparent diffusion coefficient (ADC) values implies restrictive isotropic intratumoral diffusion. With the tumor cells altering the cellular matrix relative to the parenchyma, the diffusion through the tumor region would be isotropic and would show fewer numbers of tracts passing through the tumor region as a result of destruction of anisotropic water paths.
The prostate gland consists of various vascular, neural and other anistropic water paths that can be treated as tracts and thus make the DTI potentially applicable to the prostate gland. DTI is commonly used to map neuronal fibers in the brain. A small number of DTI prostate studies have been reported.
DTI is an emerging tool in the evaluation of prostate malignancy. The retrospective study demonstrates that DTI has the potential to improve performance of MRI in the characterization of prostate cancer by depicting disease processes at a microstructural level.
DTI: Is a promising method for characterizing microstructural changes or differences with pathology and treatment. The diffusion tensor may be used to characterize the magnitude, anisotropy and orientation of the diffusion tensor. Tractography: A three dimensional modeling technique used to visually represent neural tracts using data collected by DTI. It uses special techniques of MRI, and computer-based image analysis.
This is an interesting study, three parameters were compared in in tumour region and normal tissue, including tract density, ADC and FA values. The results come out that tract density may offer new biomarker to distinguish tumour from normal tissue.
| 1. | Hricak H, Choyke PL, Eberhardt SC, Leibel SA, Scardino PT. Imaging prostate cancer: a multidisciplinary perspective. Radiology. 2007;243:28-53. [PubMed] |
| 2. | de Rooij M, Hamoen EH, Fütterer JJ, Barentsz JO, Rovers MM. Accuracy of multiparametric MRI for prostate cancer detection: a meta-analysis. AJR Am J Roentgenol. 2014;202:343-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 343] [Article Influence: 28.6] [Reference Citation Analysis (0)] |
| 3. | Basser PJ, Pajevic S, Pierpaoli C, Duda J, Aldroubi A. In vivo fiber tractography using DT-MRI data. Magn Reson Med. 2000;44:625-632. [PubMed] |
| 4. | O’Donnell LJ, Westin CF. An introduction to diffusion tensor image analysis. Neurosurg Clin N Am. 2011;22:185-196, viii. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 397] [Cited by in RCA: 369] [Article Influence: 24.6] [Reference Citation Analysis (0)] |
| 5. | Nucifora PG, Verma R, Lee SK, Melhem ER. Diffusion-tensor MR imaging and tractography: exploring brain microstructure and connectivity. Radiology. 2007;245:367-384. [PubMed] |
| 6. | Jones DK, Knösche TR, Turner R. White matter integrity, fiber count, and other fallacies: the do’s and don’ts of diffusion MRI. Neuroimage. 2013;73:239-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1637] [Cited by in RCA: 1857] [Article Influence: 132.6] [Reference Citation Analysis (0)] |
| 7. | Gürses B, Tasdelen N, Yencilek F, Kılıckesmez NO, Alp T, Fırat Z, Albayrak MS, Uluğ AM, Gürmen AN. Diagnostic utility of DTI in prostate cancer. Eur J Radiol. 2011;79:172-176. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 8. | Reischauer C, Wilm BJ, Froehlich JM, Gutzeit A, Prikler L, Gablinger R, Boesiger P, Wentz KU. High-resolution diffusion tensor imaging of prostate cancer using a reduced FOV technique. Eur J Radiol. 2011;80:e34-e41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 43] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 9. | Bourne RM, Kurniawan N, Cowin G, Sved P, Watson G. Microscopic diffusion anisotropy in formalin fixed prostate tissue: preliminary findings. Magn Reson Med. 2012;68:1943-1948. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 10. | Finley DS, Ellingson BM, Natarajan S, Zaw TM, Raman SS, Schulam P, Reiter RE, Margolis D. Diffusion tensor magnetic resonance tractography of the prostate: feasibility for mapping periprostatic fibers. Urology. 2012;80:219-223. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 33] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Panebianco V, Salciccia S, Cattarino S, Minisola F, Gentilucci A, Alfarone A, Ricciuti GP, Marcantonio A, Lisi D, Gentile V. Use of multiparametric MR with neurovascular bundle evaluation to optimize the oncological and functional management of patients considered for nerve-sparing radical prostatectomy. J Sex Med. 2012;9:2157-2166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 24] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 12. | Kitajima K, Takahashi S, Ueno Y, Miyake H, Fujisawa M, Sugimura K. Visualization of periprostatic nerve fibers before and after radical prostatectomy using diffusion tensor magnetic resonance imaging with tractography. Clin Imaging. 2014;38:302-306. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Zelhof B, Pickles M, Liney G, Gibbs P, Rodrigues G, Kraus S, Turnbull L. Correlation of diffusion-weighted magnetic resonance data with cellularity in prostate cancer. BJU Int. 2009;103:883-888. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 190] [Cited by in RCA: 190] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 14. | Mori S, Crain BJ, Chacko VP, van Zijl PC. Three-dimensional tracking of axonal projections in the brain by magnetic resonance imaging. Ann Neurol. 1999;45:265-269. [PubMed] |
| 15. | Xue R, van Zijl PC, Crain BJ, Solaiyappan M, Mori S. In vivo three-dimensional reconstruction of rat brain axonal projections by diffusion tensor imaging. Magn Reson Med. 1999;42:1123-1127. [PubMed] |
| 16. | Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4:316-329. [PubMed] |
| 17. | Jbabdi S, Johansen-Berg H. Tractography: where do we go from here? Brain Connect. 2011;1:169-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 442] [Cited by in RCA: 467] [Article Influence: 31.1] [Reference Citation Analysis (0)] |
| 18. | Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, Chabriat H. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534-546. [PubMed] |
| 19. | Manenti G, Carlani M, Mancino S, Colangelo V, Di Roma M, Squillaci E, Simonetti G. Diffusion tensor magnetic resonance imaging of prostate cancer. Invest Radiol. 2007;42:412-419. [PubMed] |
| 20. | Damon BM, Ding Z, Anderson AW, Freyer AS, Gore JC. Validation of diffusion tensor MRI-based muscle fiber tracking. Magn Reson Med. 2002;48:97-104. [PubMed] |
| 21. | Zijta FM, Froeling M, Nederveen AJ, Stoker J. Diffusion tensor imaging and fiber tractography for the visualization of the female pelvic floor. Clin Anat. 2013;26:110-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 22. | Sosnovik DE, Wang R, Dai G, Reese TG, Wedeen VJ. Diffusion MR tractography of the heart. J Cardiovasc Magn Reson. 2009;11:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 126] [Cited by in RCA: 111] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Sosnovik DE, Mekkaoui C, Huang S, Chen HH, Dai G, Stoeck CT, Ngoy S, Guan J, Wang R, Kostis WJ. Microstructural impact of ischemia and bone marrow-derived cell therapy revealed with diffusion tensor magnetic resonance imaging tractography of the heart in vivo. Circulation. 2014;129:1731-1741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 73] [Cited by in RCA: 66] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 24. | Gaige TA, Benner T, Wang R, Wedeen VJ, Gilbert RJ. Three dimensional myoarchitecture of the human tongue determined in vivo by diffusion tensor imaging with tractography. J Magn Reson Imaging. 2007;26:654-661. [PubMed] |
| 25. | Fiocchi F, Nocetti L, Siopis E, Currà S, Costi T, Ligabue G, Torricelli P. In vivo 3 T MR diffusion tensor imaging for detection of the fibre architecture of the human uterus: a feasibility and quantitative study. Br J Radiol. 2012;85:e1009-e1017. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 29] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 26. | Notohamiprodjo M, Dietrich O, Horger W, Horng A, Helck AD, Herrmann KA, Reiser MF, Glaser C. Diffusion tensor imaging (DTI) of the kidney at 3 tesla-feasibility, protocol evaluation and comparison to 1.5 Tesla. Invest Radiol. 2010;45:245-254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 90] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 27. | Sinha S, Sinha U. In vivo diffusion tensor imaging of the human prostate. Magn Reson Med. 2004;52:530-537. [PubMed] |
| 28. | Mukherjee P, Chung SW, Berman JI, Hess CP, Henry RG. Diffusion tensor MR imaging and fiber tractography: technical considerations. AJNR Am J Neuroradiol. 2008;29:843-852. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 289] [Cited by in RCA: 287] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 29. | Murphy G, Haider M, Ghai S, Sreeharsha B. The expanding role of MRI in prostate cancer. AJR Am J Roentgenol. 2013;201:1229-1238. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 105] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 30. | Donati OF, Afaq A, Vargas HA, Mazaheri Y, Zheng J, Moskowitz CS, Hricak H, Akin O. Prostate MRI: evaluating tumor volume and apparent diffusion coefficient as surrogate biomarkers for predicting tumor Gleason score. Clin Cancer Res. 2014;20:3705-3711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 31. | Hasan KM, Parker DL, Alexander AL. Comparison of gradient encoding schemes for diffusion-tensor MRI. J Magn Reson Imaging. 2001;13:769-780. [PubMed] |
| 32. | Papadakis NG, Murrills CD, Hall LD, Huang CL, Adrian Carpenter T. Minimal gradient encoding for robust estimation of diffusion anisotropy. Magn Reson Imaging. 2000;18:671-679. [PubMed] |
| 33. | Kim CK, Jang SM, Park BK. Diffusion tensor imaging of normal prostate at 3 T: effect of number of diffusion-encoding directions on quantitation and image quality. Br J Radiol. 2012;85:e279-e283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 34. | Lazar M, Weinstein DM, Tsuruda JS, Hasan KM, Arfanakis K, Meyerand ME, Badie B, Rowley HA, Haughton V, Field A. White matter tractography using diffusion tensor deflection. Hum Brain Mapp. 2003;18:306-321. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 335] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Chu JP, Gao BL, Gumustas OG, Li YZ, Park H S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK