Published online Dec 28, 2016. doi: 10.4329/wjr.v8.i12.916
Peer-review started: June 3, 2016
First decision: July 5, 2016
Revised: September 29, 2016
Accepted: October 17, 2016
Article in press: October 18, 2016
Published online: December 28, 2016
Processing time: 202 Days and 19.3 Hours
To evaluate brain metastases volume control capabilities of stereotactic radiosurgery (SRS) through serial magnetic resonance (MR) imaging follow-up.
MR examinations of 54 brain metastases in 31 patients before and after SRS were reviewed. Patients were included in this study if they had a pre-treatment MR examination and serial follow-up MR examinations at 6 wk, 9 wk, 12 wk, and 12 mo after SRS. The metastasis volume change was categorized at each follow-up as increased (> 20% of the initial volume), stable (± 20% of the initial volume) or decreased (< 20% of the initial volume).
A local tumor control with a significant (P < 0.05) volume decrease was observed in 25 metastases at 6-wk follow-up. Not significant volume change was observed in 23 metastases and a significant volume increase was observed in 6 metastases. At 9-wk follow-up, 15 out of 25 metastases that decreased in size at 6 wk had a transient tumor volume increase, followed by tumor regression at 12 wk. At 12-wk follow-up there was a significant reduction in volume in 45 metastases, and a significant volume increase in 4 metastases. At 12-mo follow-up, 19 metastases increased significantly in size (up to 41% of the initial volume). Volume tumor reduction was correlated to histopathologic subtype.
SRS provided an effective local brain metastases volume control that was demonstrated at follow-up MR imaging.
Core tip: Stereotactic radiosurgery (SRS) provided an effective long-term local volume control of brain metastases during 12-mo magnetic resonance (MR) imaging follow-up. A significant reduction of the tumor volume by 6 wk post-SRS was associated with long-term volume control suggesting that the timing for MR imaging follow-up at 6 wk, 9 wk, 12 wk and 12 mo after SRS, could be considered the most effective to provide useful information to make the best treatment decisions.
- Citation: Sparacia G, Agnello F, Banco A, Bencivinni F, Anastasi A, Giordano G, Taibbi A, Galia M, Bartolotta TV. Value of serial magnetic resonance imaging in the assessment of brain metastases volume control during stereotactic radiosurgery. World J Radiol 2016; 8(12): 916-921
- URL: https://www.wjgnet.com/1949-8470/full/v8/i12/916.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i12.916
Brain metastases account for 20%-40% of adult cancer and affect survival and quality of life[1]. The two most commonly used treatments for brain metastases are whole-brain radiation therapy and stereotactic radiosurgery (SRS), which extend survival from 3 mo to 5 mo and from 7 mo to 13 mo, respectively, depending on tumor type. Surgical resection remains a valuable approach for patients with larger symptomatic metastatic tumors[2].
SRS is an increasingly used procedure for the treatment of primary and metastatic intracranial brain tumors. Indications include patients with few, well-defined, and small intracranial brain tumors. In SRS, radiations are directly delivered into a brain tumor, thus reducing radiation dose of surrounding normal brain tissue and side effects such as neurotoxicity, skin damage, nausea and vomiting[3-5]. The damage to the peritumoral brain is further reduced by a step dose gradient at the target periphery of the tumor[3].
The objectives of SRS include local tumor control, defined as the absence of a substantial (< 25%) increase in tumor volume at follow-up magnetic resonance (MR) imaging, improved quality of life, and prolonged survival[6,8]. Metastatic lesions are particularly well-suited for the treatment with SRS because they are usually small (< 3 cm), well-circumscribed, and have well-defined margins[6].
Studies have demonstrated that SRS is an effective alternative to traditional surgical resection and whole brain radiotherapy in patients with single or few well-defined brain metastases[1,7-9].
Knowledge of natural history of brain metastases treated with SRS is crucial to prevent management dilemmas, and reduce patient anxiety. For instance, radiation toxicity can sometimes cause a pseudo-progression of brain metastases, which usually resolves with time[1,7,9,10].
The purpose of this study is to evaluate volume tumor control capabilities of SRS in the treatment of brain metastases trough serial MR imaging follow-up.
This was a retrospective study approved by the Institutional Review Board of our institution. All patients were referenced with the diagnosis of brain metastases and were treated with Gamma Knife-SRS (Leksell Gamma Knife, model 4C, GammaPlan 5.3; Elekta Instruments, Stockholm, Sweden) at a single academic medical center from January 2015 to January 2016. All patients had given written consent for this retrospective study. Patients were included in this study if they had a pre-treatment MR examination and serial follow-up MR examinations within 6 wk, 9 wk, 12 wk, and 12 mo post-SRS.
Patients were excluded if SRS was performed for consolidation to a surgical resection bed only. Additionally, patients in whom lesions required salvage surgery due to symptomatic local failure, were excluded.
The SRS dose delivered to the tumor margins ranged from 18 to 24 Gy prescribed to the 40%-70% isodose surface. Radioresistant tumors (melanoma, renal cancer) received a median marginal dose of 23.7 Gy (range, 20-24 Gy), and radiosensitive tumors (lung and, breast cancer) received a median marginal dose of 21.3 Gy (range, 18-24 Gy).
There was a total of 31 patients (14 men, 17 women; age: 32-77 years; mean age, 51, 5 years) that underwent serial MR imaging examinations at 6 wk, 9 wk, 12 wk, and 12 mo after SRS.
Brain metastases were confirmed by pathology. There were 54 brain metastases: Non-small cell lung carcinoma n = 19 (36%), breast carcinoma n = 16 (29%), renal cell carcinoma n = 9 (16%), and melanoma n = 10 (19%). Patient population and primary cancer types are summarized in Table 1.
| No. ofpatients | Gender | Age (yr) | Primary cancer type | No. of lesions, (%) |
| 11 | 7 men - 4 women | 50-70 | Non-small cell lung carcinoma | 19 (36) |
| 9 | 1 men - 8 women | 32-60 | Breast carcinoma | 16 (29) |
| 7 | 5 men - 2 women | 55-77 | Renal cell carcinoma | 9 (16) |
| 4 | 1 men - 3 women | 32-65 | Melanoma | 10 (19) |
All MR examinations were performed with a 1.5T MR scanner (Signa Excite, GE Medical Systems, Milwaukee, United States). MR imaging protocol included axial and sagittal fast spin-echo (FSE) T2W [5100/110 (TR/TE)] images, axial fluid-attenuated inversion-recovery (FLAIR) [8000/140/2400 (TR/TE/TI)] images, along with axial, sagittal, and coronal non-enhanced and contrast-enhanced (0.1 mmol/Kg gadobutrol - Gadovist, Bayer, Germany) FSE T1W [650/15 (TR/TE)] images with a FOV of 22 cm, matrix 512 × 512, slice thickness 5 mm, intersection gap 1 mm, number of excitations 2. Follow-up MR examinations were performed at 6 wk, 9 wk, 12 wk, and 12 mo post-SRS.
Two experienced neuroradiologists evaluated in consensus the maximum enhancing metastasis volume measured in 3 orthogonal planes at initial MR examinations and at each follow-up. Tumor volume was calculated according to the following formula: Volume = length × width × height/2 as reported in other studies[7]. Metastases of at least 0.5 cm3 were included. Metastasis volume change was categorized at each follow-up as increased (> 20% of the initial volume), stable (± 20% of the initial volume) or decreased (< 20% of the initial volume). This criteria was chosen taking in account a measurement error of 20%, as there are no validated categorization schemes for tumor response.
Statistical analysis was performed using the statistical software package SPSS (SPSS, Chicago, Ill). The Wilcoxon signed-rank test for continuous variables was used to evaluate the significance of volume change. A multivariate logistic regression analysis was used to determine the correlation between histopathology and volume changes. A P value of < 0.05 was considered statistically significant.
Primary cancer types and effective time point for MR imaging follow-up to identify significant phases of the response to the SRS therapy are summarized in Table 2. At 6-wk follow-up, a local tumor control with a significant volume decrease up of 63% was observed in 25 brain metastases (46%) (12 non-small cell lung carcinoma, 11 breast carcinoma, 1 renal cell carcinoma, 1 melanoma). No significant volume change was observed in 23 metastases (43%) (6 non-small cell lung carcinoma, 5 breast carcinoma, 7 renal cell carcinoma, 5 melanoma), and a significant volume increase was observed in 6 metastases (11%) (1 non-small cell lung carcinoma, 1 renal cell carcinoma, 4 melanoma).
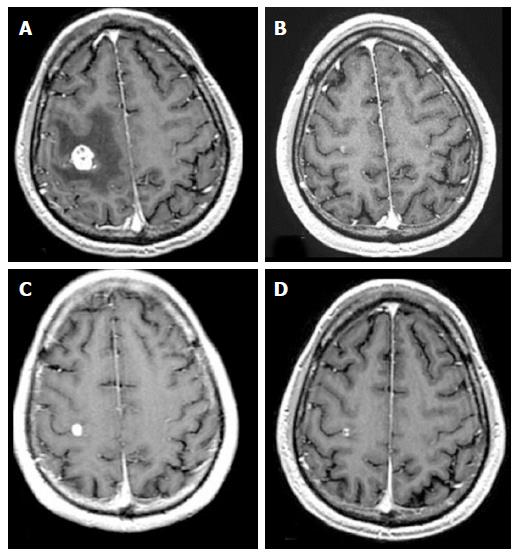
At 9-wk follow-up, 15 out of 25 radiosensitive brain metastases (28% of the total lesions) (8 non-small cell lung cancer, 7 breast metastases) that decreased in size at 6 wk had a transient tumor volume increase, followed by tumor regression at 12 wk with no clinical symptoms (pseudo-progression) (Figure 1).
At 12-wk follow-up, there was a significant reduction in volume in 45 metastases (18 non-small cell lung carcinoma, 14 breast carcinoma, 7 renal cell carcinoma, 6 melanoma), no significant volume change in 5 metastases (1 non-small cell lung carcinoma, 1 breast carcinoma, 1 renal cell carcinoma, 2 melanoma), and a significant volume increase in 4 metastases (1 breast carcinoma, 1 renal cell carcinoma, 2 melanoma).
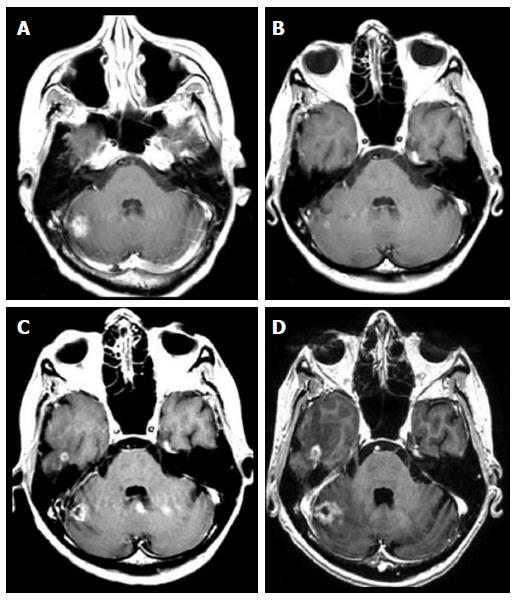
At 12-mo follow-up, 19 (35%) metastases increased (true-progression) significantly in size (up to 41% of the initial volume) (1 non-small cell lung cancer, 4 breast cancer, 6 renal cell carcinoma, 8 melanoma) (Figure 2).
The logistic regression analysis showed that volume tumor reduction correlates to histopathologic subtype: non-small cell lung carcinoma had a significant reduction of 38% of its initial volume; breast carcinoma had a significant reduction of 41% of its initial volume; renal cell carcinoma had a significant reduction of 14% of its initial volume; melanoma had a significant reduction of 8% of its initial volume. Thus, higher tumor reduction was observed in the radiation sensitive carcinomas (breast and non-small cell lung carcinomas).
Moreover, we evaluated the volume tumor variation of breast, non-small cell lung cancer, melanoma, and renal cell carcinoma metastases at 6 wk, 9 wk, 12 wk, and 12 mo post-SRS. Our results show that response categorization differences among these 4 primary types were not statistically significant, however melanoma and renal cell carcinoma metastases had less robust volume reduction than non-small cell lung cancer or breast metastases.
Temporary or permanent clinical complications were evaluated during 12 mo follow-up of these patients. Transient headache related to intracranial edema was noted in 10 patients, with nausea (5 patients) and arm or leg weakness (2 patients). Permanent neurologic deficits were noted in 6 patients.
Our results suggest that a significant early reduction of tumor volume is associated with a good long-term volume tumor control as reported in previous studies[6,7,10-14]. Conversely, increased tumor volume at 6-wk follow-up has a higher probability of a final increase in lesion size, thus in a poor tumor volume control.
Transient volume growth at 9-wk follow-up occurred in 15 radiosensitive brain metastases (8 non-small cell lung cancer, 7 breast metastases) (28% of the total lesions), followed by tumor regression at 12 wk with no clinical symptoms (Figure 1 and Table 2). This transient growth must be careful interpreted as it could be misinterpreted as tumor recurrence, whereas it should be interpreted as a pseudo-progression[7].
The histopathology of pseudo-progression is probably related to treatment-induced tumor inflammation and necrosis[7,10-13]. Tumor volume variation trend in our series demonstrates that melanoma and renal cell carcinoma metastases showed less volume reduction than non-small cell lung cancer or breast metastases.
However, response categorization differences among these 4 primary types were not statistically significant, thus suggesting that the most effective timing for MR imaging follow-up, regardless the type of primary tumor, could be considered at 6 wk, 9 wk, 12 wk and 12 mo after SRS.
The observation that a small percentage of lesions may undergo a transient volume increase indicate that initial lesion growth does not necessary preclude local volume control. Conversely, there were a low number of metastases that exhibited initial volume growth and continued to grow with no volume control during SRS (Figure 2).
SRS has become the standard procedure for the treatment of brain metastases as it allows a longer survival and higher local control rates compared to whole-brain radiation therapy[6,7,9,14]. Compared to surgical resection, SRS is associated to lower morbidity and decrease cost[1].
To summarize, SRS is effective in treating brain metastases regardless of their histology, including those that are radio-resistant to conventional whole-brain radiation therapy, such as metastases that originates from melanoma and renal cell carcinoma.
Although initial consistent tumor volume reduction after SRS is predictive of long term volume control, initial tumor growth does not necessarily indicate tumor progression but radiation-induced inflammation and necrosis (pseudo-progression) and it should be taken into account to avoid to be misinterpreted as a recurrence.
This study was a retrospective, single-institution study with a relative small size population and these factors could be considered limitations.
To prevent potential inaccuracies in the volume measurement of the intracranial lesion, we excluded lesions with an initial tumor volume of less than 0.5 cm3 and a 20% cutoff for volume response categorization was chosen.
In conclusion, effective long-term SRS local volume control of brain metastases can be demonstrated at 12 mo follow-up. Significant tumor volume reduction by 6 wk post-SRS was associated with long-term volume control suggesting that the timing for MR imaging follow-up at 6 wk, 9 wk, 12 wk and 12 mo after SRS, could be considered the most effective to provide useful information to make the best treatment decisions. Although it is necessary to validate these results in a larger, prospective series, the results are encouraging that an early local volume reduction after SRS is associated with significant local control for metastatic brain lesions.
Brain metastases account for 20% to 40% of adult cancer and affect both survival and quality of life. Brain metastases volume reduction is associated with significant local control of the lesions and prolongation of patient’s survival. Stereotactic radiosurgery (SRS) is an increasingly used procedure for the treatment of primary and metastatic intracranial brain tumors to achieve local volume reduction.
Volume tumor control capabilities of SRS in the treatment of brain metastases is an important factor for post-treatment decision making and delivery salvage therapy.
Volume tumor control capabilities of SRS could be demonstrated trough serial magnetic resonance (MR) imaging follow-up. Accurate determination of the timing for MR imaging follow-up is crucial for decision making and delivery timely salvage therapy.
Serial MR imaging follow-up at 6 wk, 9 wk, 12 wk, and 12 mo is the most effective timing to demonstrate volume reduction of brain metastases after SRS. The information derived from serial MR imaging follow-up could affect clinical management and improve survival of these patients.
SRS is a procedure for the treatment of primary and metastatic intracranial brain tumors. Indications include patients with few, well-defined, and small intracranial brain tumors. In SRS, radiations are directly delivered into a brain tumor, thus reducing radiation dose of surrounding normal brain tissue and side effects such as neurotoxicity, skin damage, nausea and vomiting. The damage to the peritumoral brain is further reduced by a step dose gradient at the target periphery of the tumor. The objectives of SRS include local tumor control, defined as the absence of a substantial (< 25%) increase in tumor volume at follow-up MR imaging, improved quality of life, and prolonged survival.
This study is interesting. However the manuscript would be of higher value to the reader if the manuscript focuses on the pseudo-progression period, that period is confusing for the practicing physician and can lead to misinterpretation and additional or changes in treatment strategies.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Italy
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Fatterpekar GM, Rodriguez GJ S- Editor: Kong JX L- Editor: A E- Editor: Wu HL
| 1. | Stafinski T, Jhangri GS, Yan E, Menon D. Effectiveness of stereotactic radiosurgery alone or in combination with whole brain radiotherapy compared to conventional surgery and/or whole brain radiotherapy for the treatment of one or more brain metastases: a systematic review and meta-analysis. Cancer Treat Rev. 2006;32:203-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 2. | Sharpton SR, Oermann EK, Moore DT, Schreiber E, Hoffman R, Morris DE, Ewend MG. The volumetric response of brain metastases after stereotactic radiosurgery and its post-treatment implications. Neurosurgery. 2014;74:9-15; discussion 16; quiz 16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 32] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 3. | Young RF. Radiosurgery for the treatment of brain metastases. Semin Surg Oncol. 1998;14:70-78. [PubMed] |
| 4. | Kaal EC, Niël CG, Vecht CJ. Therapeutic management of brain metastasis. Lancet Neurol. 2005;4:289-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 125] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 5. | Suh JH. Stereotactic radiosurgery for the management of brain metastases. N Engl J Med. 2010;362:1119-1127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 172] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 6. | Peterson AM, Meltzer CC, Evanson EJ, Flickinger JC, Kondziolka D. MR imaging response of brain metastases after gamma knife stereotactic radiosurgery. Radiology. 1999;211:807-814. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Patel TR, McHugh BJ, Bi WL, Minja FJ, Knisely JP, Chiang VL. A comprehensive review of MR imaging changes following radiosurgery to 500 brain metastases. AJNR Am J Neuroradiol. 2011;32:1885-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 133] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 8. | Chang WS, Kim HY, Chang JW, Park YG, Chang JH. Analysis of radiosurgical results in patients with brain metastases according to the number of brain lesions: is stereotactic radiosurgery effective for multiple brain metastases? J Neurosurg. 2010;113 Suppl:73-78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 102] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 9. | Kondziolka D, Martin JJ, Flickinger JC, Friedland DM, Brufsky AM, Baar J, Agarwala S, Kirkwood JM, Lunsford LD. Long-term survivors after gamma knife radiosurgery for brain metastases. Cancer. 2005;104:2784-2791. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 82] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 10. | Shah R, Vattoth S, Jacob R, Manzil FF, O’Malley JP, Borghei P, Patel BN, Curé JK. Radiation necrosis in the brain: imaging features and differentiation from tumor recurrence. Radiographics. 2012;32:1343-1359. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 152] [Cited by in RCA: 183] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 11. | Dequesada IM, Quisling RG, Yachnis A, Friedman WA. Can standard magnetic resonance imaging reliably distinguish recurrent tumor from radiation necrosis after radiosurgery for brain metastases? A radiographic-pathological study. Neurosurgery. 2008;63:898-903; discussion 904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 130] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 12. | Ruzevick J, Kleinberg L, Rigamonti D. Imaging changes following stereotactic radiosurgery for metastatic intracranial tumors: differentiating pseudoprogression from tumor progression and its effect on clinical practice. Neurosurg Rev. 2014;37:193-201; discussion 201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 30] [Cited by in RCA: 38] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Wang LL, Leach JL, Breneman JC, McPherson CM, Gaskill-Shipley MF. Critical role of imaging in the neurosurgical and radiotherapeutic management of brain tumors. Radiographics. 2014;34:702-721. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 14. | Linskey ME, Andrews DW, Asher AL, Burri SH, Kondziolka D, Robinson PD, Ammirati M, Cobbs CS, Gaspar LE, Loeffler JS. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:45-68. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 369] [Cited by in RCA: 358] [Article Influence: 22.4] [Reference Citation Analysis (0)] |