Published online Nov 28, 2016. doi: 10.4329/wjr.v8.i11.895
Peer-review started: June 13, 2016
First decision: July 11, 2016
Revised: July 23, 2016
Accepted: September 6, 2016
Article in press: September 8, 2016
Published online: November 28, 2016
Processing time: 166 Days and 14.1 Hours
To find accompanying anomalies of typical and atypical Scheuermann’s disease (SD) is reported in the present study.
Study included 20 patients (16 men and 4 women) who had radiological imaging radiography, magnetic resonance imaging (MRI) and computed tomography, if available, due to back pain, curved back and low back pain in November 2011-February 2016 period. Patients were categorized into typical and atypical patterns based on the region involved. Thoracic kyphosis values were measured using real Cobb angle. Accompanying disc degeneration, herniations and spinal cord pathologies were studied using MRI.
Age of the patients ranged from 11.0 to 23.0 (mean 17.2 ± 3.0). Typical pattern of SD were detected in 15 patients while atypical pattern were detected in 5 patients. Cobb angle range was 40.2-67.2 (mean 55.5 ± 8.7) in typical Scheuermann’s patients and 24.7-49.9 (mean 36.7 ± 10.8) in atypical ones. Intervertebral level was affected and had the measures of 3-8 (mean 5.3 ± 1.6) and 7-9 (mean 8.2 ± 0.8) in typical and atypical Scheuermann’s patients, respectively. Level of degenerative disc disease in MRI was 1-7 discs (mean 4.1 ± 1.7) in typical patients and 5-10 discs (mean 7.6 ± 1.9) in atypical patients.
SD can be seen in typical and atypical patterns, typical being more frequent. Because degenerative disc diseases, herniations and cord pathologies such as syringomyelia can accompany SD (albeit more common in atypical pattern), it is necessary to evaluate these patients with plain radiography and MRI together.
Core tip: Scheuermann’s disease (SD) is the most common cause of degenerative structural thoracic or thoracolumbar hyperkyphosis associated with back pain in adolescents and could be observed in typical and atypical patterns. It manifests itself with successive endplate irregularities and anterior vertebral wedging in radiography, and additionally as disc degenerations, herniations and syringomyelia in spinal cord in magnetic resonance imaging (MRI). Impairment in intervertebral distance and disc degeneration are more evident in SD with atypical pattern. When multiple endplate irregularities and anterior vertebral wedging are observed in MRI of patients thought to have thoracolumbar disc pathology, SD should be considered.
- Citation: Gokce E, Beyhan M. Radiological imaging findings of scheuermann disease. World J Radiol 2016; 8(11): 895-901
- URL: https://www.wjgnet.com/1949-8470/full/v8/i11/895.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i11.895
Scheuermann’s disease (SD) or juvenile kyphosis is the most common cause of progressive, structural thoracic or thoracolumbar hyperkyphosis associated with back pain in adolescents. It has a prevalence of 0.4%-8.0%[1-3]. Typical patients are 13-16 years old. Progression is slow in most cases[1-5]. Reports about their morbidity frequencies in different genders are varied[1,5,6]. In terms of radiological examinations, some authors consider the presence of wedging in at least three successive vertebra at an angle of over 5° as diagnosis criterion for SD[1], while others[7] consider wedging in a vertebra along with irregular endplates enough as diagnosis criterion[8]. Radiological findings of SD are reported in the present study.
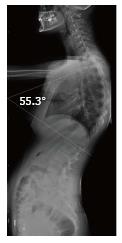
The present retrospective study included 20 patients (16 male and 4 female) who had radiological imaging taken at Radiology Department of Gaziosmanpaşa University Medical School due to back pain, curved back and low back pain complaints in November 2011-February 2016 period. After taking the approval of local ethic committee (No. 15-KAEK-156), radiological images of the patients were obtained from Picture Archiving and Communication System (PACS, GE). All patients had direct radiographies and eight of them had cervical, thoracic and lumbar magnetic resonance imaging (MRI), seven had thoracic MRI, two of which were contrasted, three had thoracic and lumbar MRI, one had cervical and lumbar MRI and one had thoracic MRI and lumbar CT. Based on Sørenson’s definition in radiological examinations, wedging over an angle of 5° in three or more vertebrae, kyphosis over 40° in sagittal plane and irregularities in vertebra endplates were considered typical SD[1]. Level of thoracic curve was determined based on measurement of Cobb angle (real Cobb angle is the angle between upper and plate of the most curved vertebra at the top and lower endplates of the most curved vertebra at the bottom) (Figure 1). The cases with thoracic involvement only (including T12-L1 level) were considered typical, whereas ones with thoracolumbar or lumbar involvement were considered atypical. Level where the apex of kyphosis was exactly located was examined. MRI was used to study accompanying degenerative disc disease, herniations and spinal cord pathologies (syringomyelia, etc.). Signal reduction of more than 50% in T2 series of intervertebral discs in MRI was considered in favor of degenerative disc disease.
The statistics were reviewed and analyzed by an author of this article. Descriptive analyses were made to gain information about general features of working groups. Data regarding continuous variable were given as mean (±) SD but data regarding categorical variables were given as n (%). Statistical software was used in statistical analyses (IBM PASW Statistics 18, SPSS Inc., IBM Co., Somers, NY).
Age of the patients ranged from 11.0 to 23.0 (mean 17.2 ± 3.0). Typical pattern of SD were detected in 15 patients while atypical pattern were detected in 5 patients. Cobb angle varied from 24.7 to 67.2 (mean 50.8 ± 12.7) in all patient population, while typical cases had Cobb angles from 40.2 to 67.2 (mean 55.5 ± 8.7) and atypical ones from 24.7 to 49.9 (mean 36.7 ± 10.8). Number of affected intervertebral levels in whole patient group varied from three to nine. In typical SD cases, range of affected levels was 3-8 (mean 5.3 ± 1.6) and in atypical ones 7-9 (mean 8.2 ± 0.8). Degenerative disc disease was at 1-7 level in typical SD cases (mean 4.1 ± 1.7) and 5-10 (mean 7.6 ± 1.9) in atypical ones. Demographic features of the patients, involvement levels and numbers, Cobb angles, degenerative disc disease and accompanying MRI findings were given in Table 1. Radiological images of SD patients with typical pattern were given in Figures 2-5, and those of SD patients with atypical pattern were given in Figures 6-10.
| Patient | Type | Gender | Age | Symptoms | No. of affected levels | Involved levels | Cobb Angle | Disc degeneration levels | Accompanying findings |
| 1 | T | Male | 16 | Back pain | 3 | T9-T12 | 44.1 | T9-T12 | T11-T12 diffuse bulging |
| 2 | T | Male | 16 | Low back pain | 5 | T7-T12 | 40.2 | T7-T12 | thoracic scoliosis |
| 3 | A | Male | 18 | Low back pain | 9 | T9-S1 | 49.9 | T7-8,T9-S1 | Bulging at all levels of lumbar spine |
| 4 | T | Male | 17 | Low back pain | 5 | T7-T12 | 63 | T7-T11 | Diffused bulging in T6-7, 8-9, 9-10, 11-12 |
| 5 | T | Male | 20 | Curved back, low back pain | 7 | T5-T12 | 66.2 | T5-T12 | Bulging in T7-12, increase in lumbar lordosis |
| 6 | A | Male | 14 | Curved back | 9 | T5-L2 | 42.3 | T5-L2 | Thoracic syringomyelia, thoracic levoscoliosis, diffuse bulging in T11-T12 |
| 7 | T | Male | 23 | Back pain | 8 | T5-L1 | 60.1 | T5-T10 and T12-L1 | Bulging in T5-6, T7-T10; syringomyelia from T2 to L1, lumbar levoscoliosis |
| 8 | T | Male | 19 | Low back pain | 6 | T7-L1 | 52 | T7-T11 and T12-L1 | Bulging in T8-9 and T10-11 |
| 9 | T | Male | 17 | Back pain | 6 | T6-T12 | 61.7 | T6-T12 | Bulging in T10-11, L4-S1, syringomyelia at thoracic T9-10 |
| 10 | T | Male | 17 | Curved back, kyphosis | 7 | T6-L1 | 62.1 | T7-8 single level | Paramedian bulging in T7-8 |
| 11 | T | Male | 17 | Scoliosis | 7 | T6-L1 | 55 | None | None |
| 12 | T | Female | 11 | Kyphosis | 4 | T5-T9 | 67.2 | T5-6, 7-8, 8-9, 11-12 | Posterocentral protrusion in T11-12 |
| 13 | A | Male | 16 | Low back pain | 7 | T7-L2 | 40.6 | T4-5, T7-L1 | Posterocentral protrusion in T4-5, syringomyelia at T3-T5 levels |
| 14 | T | Female | 23 | Back pain | 3 | T7-T10 | 50.5 | T7-T10 | Multiple bulging |
| 15 | A | Male | 20 | Low back pain | 8 | T7-L3 | 26.3 | T3-4, 6-7-8-9, 11-12 | Bulging in T7-8; Posterocentral protrusion in T8-9, 11-12 |
| 16 | A | Male | 14 | Low back pain | 8 | T10-S1 | 24.7 | T10-L5 | None |
| 17 | T | Female | 14 | Low back pain, scoliosis | 5 | T6-T11 | 55.3 | T6-9, L1-2 | Diffused bulging in T12-L1, L1-L2 |
| 18 | T | Male | 16 | Low back pain | 4 | T5-T9 | 43.3 | T5-9 | Posterocentral bulging in T9-10 |
| 19 | T | Female | 20 | Back pain, scoliosis | 3 | T7-10 | 63.3 | T7-10 | Lumbar levoscoliosis |
| 20 | T | Female | 16 | Low back pain, scoliosis | 7 | T5-T12 | 48.5 | T7-9 | Posterior bulging in T7-8, T8-9 |
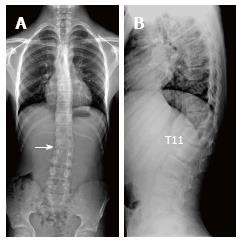
SD has been defined by Holger Werfel Scheuermann, a radiologist, in 1920 and has also been known as osteochondritis juvenile dorsi or kyphosis dorsalis juvenilis[4,9]. In SD, which leads to rigid kyphosis of vertebra, lower thoracic and upper lumbar regions are affected. It could involve a few vertebra segments or the whole vertebra[4]. Two patterns have been defined in SD based on affected area of vertebra[10]. In more typical pattern, thoracic region is frequently affected and is characterized by thoracic kyphosis increase and wedging in vertebra corpus. This pattern is accompanied by nonstructural hyperlordosis of cervical and lumbar spine[4,6,10]. SD of atypical pattern (thoracolumbar or lumbar) has been defined later and is distinguished from typical one by lack of thoracic kyphosis and evident wedging in corpus of vertebrae[11] which is considered to be more progressive in adulthood[6,10,12,13]. Apex of the kyphosis is lowered at the thoracolumbar junction (T11–T12) in atypical pattern of disease[8,12]. Such pathologies account for 25% to 80% of SD[8]. In the present study, 75% of the cases were of typical pattern and 25 were atypical. No cervical form of SD has been described so far, which could be due to immobile nature of uncus during puberty and thus could prevent vertebral endplate from mechanical stress[8]. SD of cervical form was not observed in the present study.
Thoracic kyphosis normally varies between 20 and 45° based on measurement by Cobb method on a standing lateral radiograph in which arms are kept in a position 60° below the horizontal[6,14]. Kyphosis normally progresses by age, and women have slightly higher kyphosis than men[6,14]. Thoracic kyphosis increase is one of the diagnostic criteria of typical SD[1]. In the present study, average value of thoracic kyphosis was 55.5° (range 40.2°-67.2°) in SD patients of typical pattern and it was elevated. Average thoracic kyphosis value in SD patients of atypical pattern, on the other hand, was 36.7° and were within normal limits except for one patient who had elevated level (49.9°). Tyrakowski et al[10] reported that apex of kyphosis was between T6 and T9 in SD patients of typical pattern while between T10 and L2 levels in patients of atypical pattern. Similar to the findings of Tyrakowski et al[10], apices of kyphosis in SD patients with typical pattern were between T6 and T9 in the present study. However, unlike their results, apices of kyphosis in SD patients of atypical pattern were between T9 and L1 levels. In typical pattern SD cases in the present study, it was found that three to eight involvement were detected between T5 (inferior endplate) and L1 vertebra (superior endplate). SD cases of atypical pattern, on the other hand, had endplate involvements varying from seven to nine levels between T5 (inferior endplate) and S1 (superior endplate) of vertebra. Therefore, although our population was relatively small, it could be stated that a higher number of levels in a larger interval are affected in SD patients with atypical pattern compared to the ones with typical pattern.
Most SD patients get diagnosis towards the end of juvenile stage, around 8-12 years of age, when the disease has already caused more rigid and severe deformities depending upon age[15]. In our patient population, the youngest age of presentation was 11 and the oldest was 23 years. Despite the presence of some studies reporting that prevalence of SD is about the same in both genders, there are also studies reporting higher incidence in males[1,5,6,16]. In the present study, male patients constituted the majority of the cases (80%).
Etiology of SD still remains largely unknown. Among the several theories proposed are elevated levels of growth hormone release, impaired collagen fibril formation and, as a consequence, weakening in vertebral endplates, juvenile osteoporosis, vitamin A deficiency, trauma, epiphysis and poliomyelitis[5,6,16,17]. Recent studies report major effects of genetic background for the disease[3,8,18,19]. Disorganized endochondral ossification, collagen decrease and mucopolisaccaride increase in vertebral endplates have been reported in histopathology of SD[6]. As secondary to these, intervertebral discs can be influenced due to low quality endplate development, which could in turn pave the way for the degenerative disease[4,8]. SD frequently has a benign prognosis and can lead to small deformities and symptoms. Back pain and fatigue are the most common complaints during the development, which generally clears after skeletal maturity[6]. Disc impairment or inflammatory lesions of SD can cause pain especially at the apices of kyphosis. Muscular tension after long-term sitting or movement often induces lumbar pain. Lumbar pain may also be result of spine damage due to disc herniation or spondylolysis[4,8,20]. Deformities are usually noticed during school age. An increase can be seen in lumbar lordosis and cervical lordosis to offset the kyphosis occurred[4]. Treatment of patients with Scheuermann’s kyphosis is decided based on the degree of kyphosis and maturity of the patient[4]. For patients with kyphosis between 55° and 80°, brace treatment is almost fully successful with diagnoses made before skeletal maturity. Symptomatic SD patients with kyphosis greater than 80° in the thoracic spine or 65° in the thoracolumbar spine cannot be treated successfully without surgery. Surgical treatment is needed in adolescents and young adults when there is deterioration, refractory pain, and loss of sagittal balance or neurologic involvement[6]. In adults, progressive kyphosis with pain has been reported to generally occur as a result of SD with thoracolumbar pattern rather than with thoracic pattern[16]. There are studies in the literature reporting increase in the prevalence of back pain in SD with classic thoracic pattern[5,21]. Ristolainen et al[21] found that low back pain increased by 2.5-fold in SD patients compared to healthy controls. Similarly, Murray et al[5] reported much higher frequency of thoracic back pain in SD patient population (28%) compared to healthy controls (3%). Both groups found no association between back pain and the degree or level of apex of kyphosis[5,21].
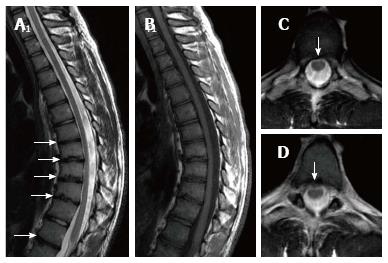
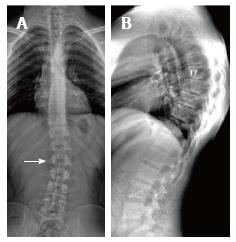
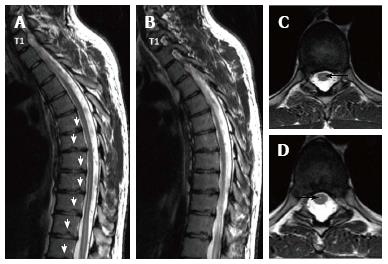
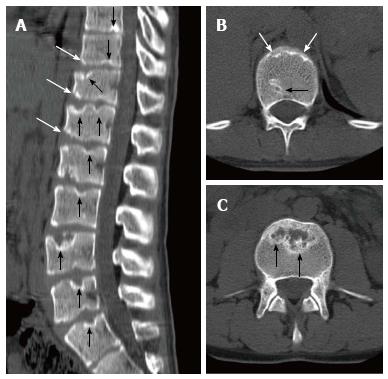
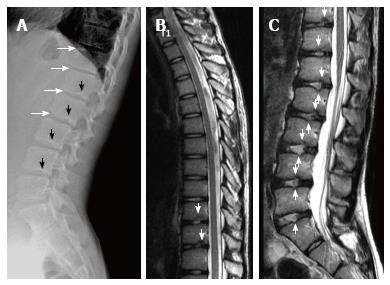
Sørensen[1] described radiographic criteria for typical SD including anterior wedging greater than 5° in at least three adjacent vertebral bodies. Schmorl’s nodes, irregularity and flattened vertebral endplates, narrowed intervertebral disc spaces and anteroposterior elongation of the apical vertebral bodies are other associated radiological features of SD. Blumenthal et al[13], on the other hand, described the criteria for atypical SD, including wedging in one or two vertebral bodies, changes in vertebral endplate, narrowed disc space and anterior Schmorl nodes. MRI features of atypical SD have been described by Heithoff et al[11], and they include narrowed disc space, disc dehydration, endplate irregularity, wedging in edges of anterior vertebral body and appearance of Schmorl nodes. The authors concluded that at least three of these criteria are needed to make SD diagnosis. Some studies reported associations between variations in SD types in vertebra and degenerative lumbar disease in younger patients[11,22,23]. In the study by Paajanen et al[22], 55% of the SD patients had thoracolumbar disc disease based on MRI findings. The finding in the present study that 93.3% of SD patients with typical pattern (Figures 2, 3 and 5) and 100% of patients with atypical pattern (Figures 7, 9 and 10) had degenerative disc disease suggests that SD paves the way for degenerative changes.
Disc degeneration was observed at all levels affected by the disease with both typical and atypical patterns in the present study. In addition, other levels also had disc degeneration to some extent. It seems that compared to SD with typical pattern, SD with atypical pattern affects more levels and facilitates degenerative disc disease to a higher degree. This fact implies that in younger patients for whom thoracolumbar MRI is required for degenerative disc disease especially when endplate irregularities are detected in a number of successive levels, SD should be strongly considered in differential diagnosis.
About one third of SD patients have scoliosis at varying degrees. It is known that spondylolysis or spondylolisthesis are more frequent in SD patients, a fact which might account for their low back pain. Some degree of degenerative spondylosis is observed mainly in apex of kyphosis in adults and is the cause of most back pain in these patients[6,22]. In untreated Scheuermann’s kyphosis, secondary complications such as neurological problems, dural cysts or thoracic disc herniation have been described though in limited number of patients[6,16,24,25]. In the present study, on the other hand, only a quarter of the patients had scoliosis (Figures 4 and 8) and none had spondylolysis or spondylolisthesis. MRI had been requested in 80% of our cases due to upper or lower back pain. Disc herniation (mostly bulging) was detected at levels ranging from 1 to 5 in 80% of cases. Average number of herniations was roughly the same in SD patients of typical and atypical pattern (2.4 ± 1.3 and 2.5 ± 1.9, respectively). No neurological complications secondary to herniations were detected in any patients.
The major limitations of the present study were limited number of cases studied retrospectively, unequal number of typical and atypical cases, relatively limited length of study, and use of only radiological evaluations.
In conclusion, SD could be seen in typical and atypical patterns. Since degenerative diseases accompany SD, especially atypical pattern, when irregularities are detected in successive endplates in patients for whom spinal MRI is requested for disc pathology pre-diagnoses, radiologists should consider SD.
Scheuermann’s disease (SD) is a progressive disease associated with back pain or low back pain in adolescents. It is the most common cause of structural thoracic or thoracolumbar hyperkyphosis, and could be seen in typical or atypical patterns. Diagnosis of SD is made by radiological methods along with clinical findings. Magnetic resonance imaging (MRI), on the other hand, is useful to indicate accompanying disc and spinal cord pathologies. In the present study, radiological findings of typical and atypical pattern Scheuermann’s patients were reported.
Since SD is frequently accompanied by degenerative disc diseases especially in atypical pattern, SD should be considered when successive endplate irregularities are observed in patients for whom spinal MRI is requested with disc pathology pre-diagnosis.
The present study revealed that more intervertebral levels were affected in SD with atypical pattern compared to SD with typical pattern [7-9 (mean 8.2 ± 0.8) and 3-8 (mean 5.3 ± 1.6), respectively]. Similarly, degenerative disc disease also affected more levels in SD with atypical pattern [5-10 (mean 7.6 ± 1.9)] compared to SD with typical one [1-7 (mean 4.1 ± 1.7)].
In addition to radiography findings, spinal pathologies such as discal degenerations, herniations and syringomyelia could be evaluated using MRI.
Typical SD: It is a progressive disease of unknown cause accompanied by juvenile dorsal kyphosis, and is characterized by successive endplate irregularities in thoracic vertebrae and anterior vertebral wedging; atypical SD: It is the pattern of SD lacking evident thoracic kyphosis and vertebral wedging, characterized by successive endplate irregularities in lower thoracic and lumbar vertebrae, Schmorl nodules and disc degeneration.
This manuscript reported the imaging findings of patients having the Scheuermann’s diseases. There are 20 patients in the study and both MRI and CT images were investigated. This is a serious work with detailed description.
| 1. | Sørensen KH. Scheuermann’s juvenile kyphosis: clinical appearances, radiography, etiology and prognosis. Copenhagen: Munksgaard 1964; . |
| 2. | Gavin TM. The Etiology and Natural History of Scheuermann’s Kyphosis. Prost Orth. 2003;15:11-14. [RCA] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 4] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 3. | Damborg F, Engell V, Andersen M, Kyvik KO, Thomsen K. Prevalence, concordance, and heritability of Scheuermann kyphosis based on a study of twins. J Bone Joint Surg Am. 2006;88:2133-2136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 4. | Yaman O, Dalbayrak S. Kyphosis and review of the literature. Turk Neurosurg. 2014;24:455-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 5. | Murray PM, Weinstein SL, Spratt KF. The natural history and long-term follow-up of Scheuermann kyphosis. J Bone Joint Surg Am. 1993;75:236-248. [PubMed] |
| 6. | Lowe TG. Scheuermann’s kyphosis. Neurosurg Clin N Am. 2007;18:305-315. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 36] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 7. | Bradford DS. Vertebral osteochondrosis (Scheuermann’s kyphosis). Clin Orthop Relat Res. 1981;158:83-90. [PubMed] |
| 8. | Palazzo C, Sailhan F, Revel M. Scheuermann’s disease: an update. Joint Bone Spine. 2014;81:209-214. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 9. | Scheuermann HW. Kyphosis dorsalis juvenilis. Ugeskr Laeger. 1920;82:385-393. |
| 10. | Tyrakowski M, Mardjetko S, Siemionow K. Radiographic spinopelvic parameters in skeletally mature patients with Scheuermann disease. Spine (Phila Pa 1976). 2014;39:E1080-E1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 11. | Heithoff KB, Gundry CR, Burton CV, Winter RB. Juvenile discogenic disease. Spine (Phila Pa 1976). 1994;19:335-340. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 40] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 12. | Edgren W, Vainio S. Osteochondrosis juvenilis lumbalis. Acta Chir Scand Suppl. 1957;227:1-47. [PubMed] |
| 13. | Blumenthal SL, Roach J, Herring JA. Lumbar Scheuermann’s. A clinical series and classification. Spine (Phila Pa 1976). 1987;12:929-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 64] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Fon GT, Pitt MJ, Thies AC. Thoracic kyphosis: range in normal subjects. AJR Am J Roentgenol. 1980;134:979-983. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 283] [Cited by in RCA: 264] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 15. | Tomé-Bermejo F, Tsirikos AI. [Current concepts on Scheuermann kyphosis: clinical presentation, diagnosis and controversies around treatment]. Rev Esp Cir Ortop Traumatol. 2012;56:491-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 16. | Lowe TG, Line BG. Evidence based medicine: analysis of Scheuermann kyphosis. Spine (Phila Pa 1976). 2007;32:S115-S119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 76] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 17. | Fotiadis E, Kenanidis E, Samoladas E, Christodoulou A, Akritopoulos P, Akritopoulou K. Scheuermann’s disease: focus on weight and height role. Eur Spine J. 2008;17:673-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 28] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 18. | Damborg F, Engell V, Nielsen J, Kyvik KO, Andersen MØ, Thomsen K. Genetic epidemiology of Scheuermann’s disease. Acta Orthop. 2011;82:602-605. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 34] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 19. | McKenzie L, Sillence D. Familial Scheuermann disease: a genetic and linkage study. J Med Genet. 1992;29:41-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 36] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 20. | Ogilvie JW, Sherman J. Spondylolysis in Scheuermann’s disease. Spine (Phila Pa 1976). 1987;12:251-253. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 50] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 21. | Ristolainen L, Kettunen JA, Heliövaara M, Kujala UM, Heinonen A, Schlenzka D. Untreated Scheuermann’s disease: a 37-year follow-up study. Eur Spine J. 2012;21:819-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 22. | Paajanen H, Alanen A, Erkintalo M, Salminen JJ, Katevuo K. Disc degeneration in Scheuermann disease. Skeletal Radiol. 1989;18:523-526. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 63] [Cited by in RCA: 50] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 23. | Tertti MO, Salminen JJ, Paajanen HE, Terho PH, Kormano MJ. Low-back pain and disk degeneration in children: a case-control MR imaging study. Radiology. 1991;180:503-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 87] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 24. | Chiu KY, Luk KD. Cord compression caused by multiple disc herniations and intraspinal cyst in Scheuermann’s disease. Spine (Phila Pa 1976). 1995;20:1075-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 17] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Kapetanos GA, Hantzidis PT, Anagnostidis KS, Kirkos JM. Thoracic cord compression caused by disk herniation in Scheuermann’s disease: a case report and review of the literature. Eur Spine J. 2006;15 Suppl 5:553-558. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 27] [Cited by in RCA: 15] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: Turkey
Peer-review report classification
Grade A (Excellent): A
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Chen F, Chow J, Shen J S- Editor: Qiu S L- Editor: A E- Editor: Lu YJ