Published online Nov 28, 2016. doi: 10.4329/wjr.v8.i11.851
Peer-review started: August 22, 2016
First decision: September 6, 2016
Revised: September 20, 2016
Accepted: October 17, 2016
Article in press: October 18, 2016
Published online: November 28, 2016
Processing time: 95 Days and 1.4 Hours
The limitations of fluorine-18 fluorodeoxy-D-glucose (FDG) in detecting some cancers has prompted a longstanding search for other positron emission tomography (PET) tracers to complement the imaging of glycolysis in oncology, with much attention paid to lipogenesis based on observations that the production of various lipid and lipid-containing compounds is increased in most cancers. Radiolabeled analogs of choline and acetate have now been used as oncologic PET probes for over a decade, showing convincingly improved detection sensitivity over FDG for certain cancers. However, neither choline nor acetate have been thoroughly validated as lipogenic biomarkers, and while acetyl-CoA produced from acetate is used in de-novo lipogenesis to synthesize fatty acids, acetate is also consumed by various other synthetic and metabolic pathways, with recent experimental observations challenging the assumption that lipogenesis is its predominant role in all cancers. Since tumors detected by acetate PET are also frequently detected by choline PET, imaging of choline metabolism might serve as an alternative albeit indirect marker of lipogenesis, particularly if the fatty acids produced in cancer cells are mainly destined for membrane synthesis through incorporation into phosphatidylcholines. Aerobic glycolysis may or may not coincide with changes in lipid metabolism, resulting in combinatorial metabolic phenotypes that may have different prognostic or therapeutic implications. Consequently, PET imaging using dual metabolic tracers, in addition to being diagnostically superior to imaging with individual tracers, could eventually play a greater role in supporting precision medicine, as efforts to develop small-molecule metabolic pathway inhibitors are coming to fruition. To prepare for this advent, clinical and translational studies of metabolic PET tracers must go beyond simply estimating tracer diagnostic utility, and aim to uncover potential therapeutic avenues associated with these metabolic alterations.
Core tip: Positron emission tomography (PET) imaging using multiple distinct metabolic tracers could eventually play a greater role in supporting precision medicine as efforts to develop small-molecule metabolic pathway inhibitors are coming to fruition. To prepare for this advent, clinical and translational studies of metabolic PET tracers must go beyond simply estimating tracer diagnostic utility, and aim to uncover potential therapeutic avenues associated with metabolic alterations in cancer.
- Citation: Kwee SA, Lim J. Metabolic positron emission tomography imaging of cancer: Pairing lipid metabolism with glycolysis. World J Radiol 2016; 8(11): 851-856
- URL: https://www.wjgnet.com/1949-8470/full/v8/i11/851.htm
- DOI: https://dx.doi.org/10.4329/wjr.v8.i11.851
The clinical success of positron emission tomography (PET) can be attributed largely to the broad and robust diagnostic utility of the imaging radiopharmaceutical fluorine-18 fluorodeoxy-D-glucose (FDG). In oncology, FDG PET/CT has no less than revolutionized cancer diagnosis, staging, and assessment of inter-treatment response[1]. As a fluorinated analog of glucose, FDG behaves as a terminal substrate for hexokinase (HK), the initiating enzyme of glycolysis. The observation that glycolytic metabolism is frequently activated in malignant tumors under conditions otherwise suitable for oxidative metabolism, a phenomenon known as the Warburg effect[1], forms the primary rationale for imaging cancer with FDG. However, aerobic glycolysis is not a universal feature of all malignancies, and low FDG avidity may predominate in common malignancies such as hepatocellular carcinoma (HCC) and prostate cancer, with rates for detecting the primary tumor with FDG PET/CT falling as low as 55% and 4%, respectively[2,3].
Because FDG has these limitations, there is demand for other PET tracers to complement the imaging of glycolysis in oncology, with a significant amount of attention paid to lipogenesis given that lipid-related pathways are also frequently upregulated in cancer[4]. Radiolabeled acetate and choline are two prototypes for imaging lipid synthesis using small molecule PET radiopharmaceuticals. Labeled with carbon-11, both have shown clinical utility for detecting tumors elusive to detection with FDG[5-7]. Fluorine-18 labeled analogs of choline and acetate have also been developed in an attempt to take clinical advantage of a longer-lived positron-emitting isotope label. However, while fluorine-18 labeled choline analogs have proven useful as biomarkers of choline metabolism[8,9], 18F-fluoroacetate does not appear to behave as a functional analog of acetate for the purpose of cancer imaging[10]. A list of published clinical studies involving 15 or more patients comparing radiolabeled choline and/or acetate to FDG for specific cancer applications is shown in Table 1.
| Disease | Clinical role | First author | Publish year | No. of patients | Tracer(s) compared to FDG | Primary results |
| Prostate cancer | Diagnosis | Liu | 2016 | Meta-analysis 56 trials 3586 patients | 11C-choline 18F-choline, 11C-acetate | 18F-choline AUC 0.94, 95%CI: 0.92-0.96; 11C-choline AUC 0.89, 95%CI: 0.86-0.91; 11C-acetate AUC 0.78, 95%CI: 0.74-0.81; FDG AUC 0.73, 95%CI: 0.69-0.77 |
| Detection | Wata-nabe | 2010 | 43 | 11C-choline | Sensitivity 73%, 88%, and 31% for 11C-choline, MRI, and FDG, respectively | |
| Re-staging | Richter | 2010 | 73 | 11C-choline | Sensitivity was 60.6% for 11C-choline and 31% for FDG | |
| Hepato-cellular carcioma | Detection, metastatic | Ho | 2007 | 121 | 11C-acetate | Patient-based sensitivity for metastasis was 64% and 79% for 11C-cetate and FDG, respectively |
| Detection, primary | Ho | 2003 | 57 | 11C-acetate | Sensitivity was 87% and 47% for 11C-acetate and FDG respectively | |
| Detection, primary/metastatic | Park | 2008 | 112 | 11C-acetate | Sensitivity was 75%, 61%, and 83% for 11C-acetate, FDG, and dual-tracer PET.CT, respectively | |
| Detection, primary | Larsson | 2012 | 44 | 11C-acetate | Detection rate was 34/44 for 11C-acetate and 13/44 for FDG (P < 0.001) | |
| Detection, primary | Talbot | 2010 | 81 | 18F-choline | Sensitivity was 88% for 18F-choline vs 68% for FDG | |
| Detection | Wu | 2011 | 76 | 11C-choline | 11C-choline PET was positive in 28 patients with negative FDG PET | |
| Diagnosis | Castilla-Lievre | 2016 | 28 | 11C-choline | Sensitivity was 75%, 36%, and 93% for 11C-holine, FDG, and dual tracers, respectively | |
| Malignant glioma | Diagnosis | Yama-moto | 2008 | 15 | 11C-acetate, 11C-methi-onine | Sensitivity was 90%, 100%, and 40% for 11C-acetate, 11C-methionine, and FDG, respectively |
| Re-staging | Tan | 2011 | 55 | 11C-choline | Sensitivity/specificity was 92%/88%, 87%/81%, and 77%/63% for 11C-choline, MRI, and FDG, respectively | |
| Nasopha-ryngeal cancer | Staging | Wu | 2011 | 15 | 11C-choline | Sensitivity for detecting locally advanced nasopharyngeal cancer was 100% vs 86% percent for 11C-choline and FDG, respectively |
| Multiple myeloma | Staging | Lin | 2014 | 15 | 11C-acetate | Diffuse infiltration was detected in 100% of patients with 11C-acetate vs 40% with FDG |
| Re-staging | Cassou-Mounat | 2016 | 21 | 18F-choline | 18F-fluorocholine detected 75% more lesions, and with higher intra-observer agreement than FDG (kappa score 0.89 vs 0.81) | |
| Renal cell carcinoma | Diagnosis | Oyama | 2014 | 29 | 11C-acetate | Detection rate was 72% for 11C-acetate vs 22% for FDG |
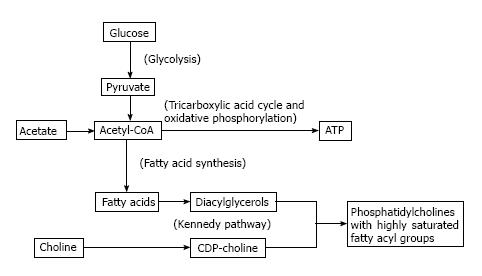
At face value, acetate metabolism may be viewed as a more direct biomarker of de-novo lipogenesis compared to choline metabolism. The authentic tracer 11C-acetate is a substrate for acetyl CoA synthetase to produce acetyl-CoA which is then carboxylated to form malonyl-CoA as the first committed step in de-novo lipogenesis mediated by fatty acid synthase (FAS). Upregulated FAS expression is a frequently observed phenomenon in many tumor types[4]. However, while the constitutive role of FAS in liver and adipose tissue is to create stored energy in the form of triglycerides, the primary role for FAS in cancer cells appears to be to supply fatty acids for phospholipid membrane synthesis[11,12]. Glycolysis, which is also frequently upregulated in cancer[1], can not only fuel this process by providing ATP, but also contribute substrate for de-novo lipogenesis by producing acetyl-CoA. This underscores a close biochemical relationship between lipogenesis, phospholipid synthesis, and glycolysis, as illustrated in Figure 1, with fatty acids produced by FAS undergoing esterification with glycerol to produce diglycerides which then react with CDP-choline to produce phosphatidylcholine (PtC) for cell membrane synthesis ostensibly in support of tumor cell proliferation.
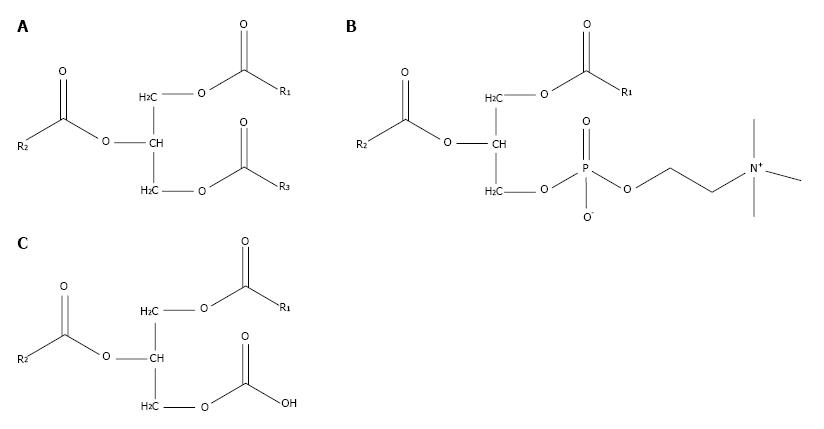
PtC is composed of a glycerol backbone esterified with two fatty acids and phosphocholine. PtC structurally resembles triglycerides, which are composed of a glycerol backbone esterified to three fatty acids (Figure 2). The major synthetic route for PtC in most cells follows the Kennedy pathway, starting with the production of phosphocholine by choline kinase (CK). The activity of CK is upregulated in many types of cancer[13], to the point that increased choline metabolism has been considered a metabolic hallmark of cancer[14].
While there are subtle biochemical differences between carbon-11 and fluorine-18 labeled cholines, both are avid substrates for CK[15]. On the basis of this mechanism, fluorine-18 fluorocholine PET/CT was tested in HCC, and found to be significantly more sensitive than FDG PET/CT, with a sensitivity of 84% vs 67%, respectively (P = 0.01)[16]. As the most abundant membrane phospholipid, PtC is believed to be the primary metabolic destination of most fatty acids synthesized de-novo by cancer cells[11,12]. Fatty acids produced by FAS may be distinguished by their relatively high saturation, and while humans lack the desaturase enzymes required to produce certain unsaturated fatty acids (i.e., essential fatty acids), de-novo synthesized fatty acids undergo sufficient modification by elongase and desaturase enzymes to still give rise to broad structural and functional variations among phospholipids such as phosphatidylcholine (PtC)[17].
Upregulated PtC synthesis may be coupled to lipogenesis in cancer, since treatment by the FAS inhibitor Orlistat can reduce the activity of CK and lower PtC levels in breast, prostate, and ovarian cancer cells[18]. This and other observations support speculation that imaging of choline metabolism can be used to monitor tumor lipogenesis[12,18]. The reason why choline-based tracers may be needed to assess tumor lipogenic activity despite 11C-acetate being available is that acetyl-CoA formed from acetate can also serve as a metabolic substrate for a variety of metabolic processes, including oxidative metabolism, histone modification, and cholesterol biosynthesis[19]. Thus, uncertainty is raised about the biochemical specificity of acetate as a PET imaging biomarker of lipogenesis. Case in point, uptake of 11C-acetate by xenograft human prostate cancers was found to be correlated with FAS expression, but FAS inhibition did not completely abrogate acetate uptake, raising the possibility of acetate participating in multiple metabolic pathways[5]. Furthermore, low 11C-acetate uptake has been observed in the PI3K/Met overexpressing mouse model of HCC, despite elevated expression of FAS in the tumors[19]. Therefore, acetate and choline-based tracers do need to be further validated as PET biomarkers of lipogenesis before they are deployed to the clinic for this purpose.
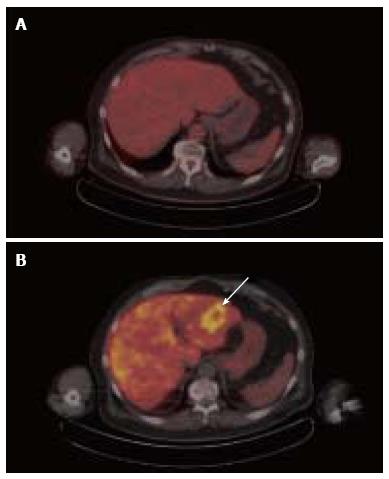
Increases in lipid metabolism and glucose metabolism can be observed to coincide in the same tumor. Among several clinical comparisons between acetate PET and FDG PET, over 90% of poorly differentiated or metastatic HCC tumors demonstrated increased uptake of both 11C-acetate and FDG, leading to the conclusion that the diagnostic advantage of acetate PET over FDG PET stems from its ability to detect more well-differentiated tumors[20-22]. In a study involving earlier stage HCC, the overall sensitivity of 11C-acetate PET for all grades of tumor was 87.3%, while the sensitivity of 18F-FDG was 47.3% in the same group of patients[6]. A similar pattern of dual tracer uptake in well- vs poorly-differentiated tumors was noted in a clinical trial that compared 18F-fluorocholine against FDG, with 18F-fluorocholine showing higher sensitivity for well-differentiated HCC, and both tracers showing similar sensitivity for less-differentiated HCC[16]. The detection by 18F-fluorocholine PET/CT of a well-differentiated HCC tumor that was not found by FDG PET is illustrated in Figure 3. Along these lines, in a tissue microarray of 157 HCC tumors, we found associations with overall survival for both CK expression and HK expression, but only HK expression correlated with tumor differentiation[23]. Thus, while choline and acetate affords PET with better overall sensitivity for HCC, only FDG PET shows promise for assessing tumor differentiation in HCC.
Biochemical insight on the relationship between choline metabolism and glycolysis can be garnered from studies involving metabolic profiling. In one pre-clinical study of ICL-CCIC-0019, a novel small molecule CK inhibitor, inhibition of choline metabolism resulted in increased glucose and acetate metabolism (without reactive oxygen species formation), ostensibly as a response to metabolic stress induced by the CK inhibitor[9]. There is also the possibility that glycolysis increases in cancer cells to sustain the metabolic demands of lipogenesis. By producing acetyl-CoA and ATP, glycolysis produces both the substrate (i.e., acetyl-CoA) and energy (i.e., ATP) required for de-novo lipogenesis. However, it does not appear that glycolysis directly drives lipogenesis, as anoxia-induced increases in glycolysis have not been shown to increase the rate of lipogenesis in cultured human breast cancer cells[24]. In cholangiocarcinoma, the second most common form of liver cancer, tumors may demonstrate high uptake on FDG PET/CT and low uptake on 18F-choline PET/CT, a metabolic phenotype that may differ from both well- and poorly-differentiated HCC[16,25]. We globally analyzed the gene expression from such tumors, predicting increased glucose transport and inhibited lipid metabolism based on a gene expression signature that distinguishes ICC from HCC[25]. Consistent with these findings, cholangiocarciomas have also been shown to uniformly demonstrate low 11C-acetate uptake[6]. The application of both FDG and choline/acetate-based PET imaging in liver tumors may therefore inform on both potential metabolic vulnerabilities as well as the tumorigenic pathways driving an individual patient’s cancer.
Relationships between glycolysis, fatty acid synthesis, and choline metabolism, in terms of pathways and metabolic substrates, have been described biochemically, but this has not led to absolute clarity about what these pathways and substrates reflect as molecular imaging biomarkers in cancer. Acetate, as a precursor to acetyl-CoA, may have value as an imaging biomarker of cancer lipogenesis, however, choline biomarkers may complement or even supplant acetate as a cancer imaging biomarker for lipogenicity given that acetate is also a substrate for aerobic respiration and other pathways which may become abnormal in cancer. Phosphatidylcholine synthesis is reliant on de-novo fatty acid synthesis for its supply of diglycerides, and FAS inhibition may down-regulate CK activity and PtC production, further raising the possibility that radiolabeled cholines can indirectly serve as PET biomarkers of lipogenic activity. However, CK inhibition may cause concomitant increases in glucose and acetate metabolism, underscoring the need to further validate these metabolic PET biomarkers for specific clinical applications. With better understanding of these metabolic interactions, multi-tracer metabolic PET, in addition to affording higher sensitivity than single-tracer PET for cancer detection, is likely to provide valuable information on the metabolic vulnerabilities associated with cancer.
| 1. | Kelloff GJ, Hoffman JM, Johnson B, Scher HI, Siegel BA, Cheng EY, Cheson BD, O’shaughnessy J, Guyton KZ, Mankoff DA. Progress and promise of FDG-PET imaging for cancer patient management and oncologic drug development. Clin Cancer Res. 2005;11:2785-2808. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 490] [Cited by in RCA: 477] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 2. | Khan MA, Combs CS, Brunt EM, Lowe VJ, Wolverson MK, Solomon H, Collins BT, Di Bisceglie AM. Positron emission tomography scanning in the evaluation of hepatocellular carcinoma. J Hepatol. 2000;32:792-797. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 303] [Cited by in RCA: 295] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 3. | Liu IJ, Zafar MB, Lai YH, Segall GM, Terris MK. Fluorodeoxyglucose positron emission tomography studies in diagnosis and staging of clinically organ-confined prostate cancer. Urology. 2001;57:108-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 177] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 4. | Menendez JA, Lupu R. Fatty acid synthase and the lipogenic phenotype in cancer pathogenesis. Nat Rev Cancer. 2007;7:763-777. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1925] [Cited by in RCA: 2286] [Article Influence: 120.3] [Reference Citation Analysis (0)] |
| 5. | Vāvere AL, Kridel SJ, Wheeler FB, Lewis JS. 1-11C-acetate as a PET radiopharmaceutical for imaging fatty acid synthase expression in prostate cancer. J Nucl Med. 2008;49:327-334. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 129] [Cited by in RCA: 126] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 6. | Ho CL, Yu SC, Yeung DW. 11C-acetate PET imaging in hepatocellular carcinoma and other liver masses. J Nucl Med. 2003;44:213-221. [PubMed] |
| 7. | Kwee SA, DeGrado TR, Talbot JN, Gutman F, Coel MN. Cancer imaging with fluorine-18-labeled choline derivatives. Semin Nucl Med. 2007;37:420-428. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 83] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 8. | Salem N, Kuang Y, Wang F, Maclennan GT, Lee Z. PET imaging of hepatocellular carcinoma with 2-deoxy-2[18F]fluoro-D-glucose, 6-deoxy-6[18F] fluoro-D-glucose, [1-11C]-acetate and [N-methyl-11C]-choline. Q J Nucl Med Mol Imaging. 2009;53:144-156. [PubMed] |
| 9. | Trousil S, Kaliszczak M, Schug Z, Nguyen Q, Tomasi G, Favicchio R, Brickute D, Fortt R, Twyman FJ, Carroll L. The novel choline kinase inhibitor ICL-CCIC-0019 reprograms cellular metabolism and inhibits cancer cell growth. Oncotarget. 2016;7:37103-37120. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 10. | Lindhe O, Sun A, Ulin J, Rahman O, Långström B, Sörensen J. [(18)F]Fluoroacetate is not a functional analogue of [(11)C]acetate in normal physiology. Eur J Nucl Med Mol Imaging. 2009;36:1453-1459. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 11. | Swinnen JV, Van Veldhoven PP, Timmermans L, De Schrijver E, Brusselmans K, Vanderhoydonc F, Van de Sande T, Heemers H, Heyns W, Verhoeven G. Fatty acid synthase drives the synthesis of phospholipids partitioning into detergent-resistant membrane microdomains. Biochem Biophys Res Commun. 2003;302:898-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 198] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 12. | Salem N, Kuang Y, Corn D, Erokwu B, Kolthammer JA, Tian H, Wu C, Wang F, Wang Y, Lee Z. [(Methyl)1-(11)c]-acetate metabolism in hepatocellular carcinoma. Mol Imaging Biol. 2011;13:140-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 13. | Ramírez de Molina A, Rodríguez-González A, Gutiérrez R, Martínez-Piñeiro L, Sánchez J, Bonilla F, Rosell R, Lacal J. Overexpression of choline kinase is a frequent feature in human tumor-derived cell lines and in lung, prostate, and colorectal human cancers. Biochem Biophys Res Commun. 2002;296:580-583. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 254] [Cited by in RCA: 266] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 14. | Glunde K, Bhujwalla ZM, Ronen SM. Choline metabolism in malignant transformation. Nat Rev Cancer. 2011;11:835-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 611] [Cited by in RCA: 624] [Article Influence: 41.6] [Reference Citation Analysis (0)] |
| 15. | Bansal A, Shuyan W, Hara T, Harris RA, Degrado TR. Biodisposition and metabolism of [(18)F]fluorocholine in 9L glioma cells and 9L glioma-bearing fisher rats. Eur J Nucl Med Mol Imaging. 2008;35:1192-1203. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 60] [Cited by in RCA: 56] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 16. | Talbot JN, Fartoux L, Balogova S, Nataf V, Kerrou K, Gutman F, Huchet V, Ancel D, Grange JD, Rosmorduc O. Detection of hepatocellular carcinoma with PET/CT: a prospective comparison of 18F-fluorocholine and 18F-FDG in patients with cirrhosis or chronic liver disease. J Nucl Med. 2010;51:1699-1706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 145] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 17. | Stubbs CD, Smith AD. The modification of mammalian membrane polyunsaturated fatty acid composition in relation to membrane fluidity and function. Biochim Biophys Acta. 1984;779:89-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1185] [Cited by in RCA: 1142] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 18. | Ross J, Najjar AM, Sankaranarayanapillai M, Tong WP, Kaluarachchi K, Ronen SM. Fatty acid synthase inhibition results in a magnetic resonance-detectable drop in phosphocholine. Mol Cancer Ther. 2008;7:2556-2565. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 19. | Li L, Che L, Wang C, Blecha JE, Li X, VanBrocklin HF, Calvisi DF, Puchowicz M, Chen X, Seo Y. [(11)C]acetate PET Imaging is not Always Associated with Increased Lipogenesis in Hepatocellular Carcinoma in Mice. Mol Imaging Biol. 2016;18:360-367. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 20. | Ho CL, Chen S, Yeung DW, Cheng TK. Dual-tracer PET/CT imaging in evaluation of metastatic hepatocellular carcinoma. J Nucl Med. 2007;48:902-909. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 143] [Cited by in RCA: 155] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 21. | Park JW, Kim JH, Kim SK, Kang KW, Park KW, Choi JI, Lee WJ, Kim CM, Nam BH. A prospective evaluation of 18F-FDG and 11C-acetate PET/CT for detection of primary and metastatic hepatocellular carcinoma. J Nucl Med. 2008;49:1912-1921. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 191] [Cited by in RCA: 219] [Article Influence: 12.2] [Reference Citation Analysis (0)] |
| 22. | Cheung TT, Chan SC, Ho CL, Chok KS, Chan AC, Sharr WW, Ng KK, Poon RT, Lo CM, Fan ST. Can positron emission tomography with the dual tracers [11 C]acetate and [18 F]fludeoxyglucose predict microvascular invasion in hepatocellular carcinoma? Liver Transpl. 2011;17:1218-1225. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 23. | Kwee SA, Hernandez B, Chan O, Wong L. Choline kinase alpha and hexokinase-2 protein expression in hepatocellular carcinoma: association with survival. PLoS One. 2012;7:e46591. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 94] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 24. | Hopperton KE, Duncan RE, Bazinet RP, Archer MC. Fatty acid synthase plays a role in cancer metabolism beyond providing fatty acids for phospholipid synthesis or sustaining elevations in glycolytic activity. Exp Cell Res. 2014;320:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 78] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 25. | Kwee SA, Okimoto GS, Chan OT, Tiirikainen M, Wong LL. Metabolic characteristics distinguishing intrahepatic cholangiocarcinoma: a negative pilot study of (18)F-fluorocholine PET/CT clarified by transcriptomic analysis. Am J Nucl Med Mol Imaging. 2016;6:73-83. [PubMed] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Kim SS, Rubello D, Soreide K S- Editor: Ji FF L- Editor: A E- Editor: Lu YJ