Published online Sep 28, 2015. doi: 10.4329/wjr.v7.i9.294
Peer-review started: January 23, 2015
First decision: March 6, 2015
Revised: June 30, 2015
Accepted: August 16, 2015
Article in press: August 17, 2015
Published online: September 28, 2015
Processing time: 264 Days and 4.4 Hours
AIM: To investigate whether the predominant emphysema type is associated with the high resolution computed tomography (HRCT) pattern of fibrosis in combined pulmonary fibrosis and emphysema (CPFE).
METHODS: Fifty-three smokers with upper lobe emphysema and lower lobe pulmonary fibrosis on - HRCT - were retrospectively evaluated. Patients were stratified into 3 groups according to the predominant type of emphysema: Centrilobular (CLE), paraseptal (PSE), CLE = PSE. Patients were also stratified into 3 other groups according to the predominant type of fibrosis on HRCT: Typical usual interstitial pneumonia (UIP), probable UIP and nonspecific interstitial pneumonia (NSIP). HRCTs were scored at 5 predetermined levels for the coarseness of fibrosis (Coarseness), extent of emphysema (emphysema), extent of interstitial lung disease (TotExtILD), extent of reticular pattern not otherwise specified (RetNOS), extent of ground glass opacity with traction bronchiectasis (extGGOBx), extent of pure ground glass opacity and extent of honeycombing. HRCT mean scores, pulmonary function tests, diffusion capacity (DLCO) and systolic pulmonary arterial pressure were compared among the groups.
RESULTS: The predominant type of emphysema was strongly correlated with the predominant type of fibrosis. The centrilobular emphysema group exhibited a significantly higher extent of emphysema (P < 0.001) and a lower extent of interstitial lung disease (P < 0.002), reticular pattern not otherwise specified (P < 0.023), extent of ground glass opacity with traction bronchiectasis (P < 0.002), extent of honeycombing (P < 0.001) and coarseness of fibrosis (P < 0.001) than the paraseptal group. The NSIP group exhibited a significantly higher extent of emphysema (P < 0.05), total lung capacity (P < 0.01) and diffusion capacity (DLCO) (P < 0.05) than the typical UIP group. The typical UIP group exhibited a significantly higher extent of interstitial lung disease, extent of reticular pattern not otherwise specified, extent of ground glass opacity with traction bronchiectasis, extent of honeycombing and coarseness of fibrosis (0.039 > P > 0.000). Although the pulmonary arterial pressure was higher in typical UIP group relative to the NSIP group, the difference was not statistically significant.
CONCLUSION: In CPFE patients, paraseptal emphysema is associated more with UIP-HRCT pattern and higher extent of fibrosis than centrilobular emphysema.
Core tip: This study aimed to investigate whether the predominant type of emphysema is associated with the pattern of fibrosis in combined pulmonary fibrosis and emphysema (CPFE). Patients were stratified into 3 groups according to predominant type of emphysema and fibrosis. High resolution computed tomography (HRCT) was retrospectively scored at 5 levels for various morphologic parameters characterizing emphysema and fibrosis. The predominant type of emphysema was strongly correlated with the predominant type of fibrosis. The centrilobular emphysema group exhibited a higher extent of emphysema and a lower extent of fibrosis. In CPFE, paraseptal emphysema was more associated with a UIP-HRCT pattern and a higher extent of fibrosis than was centrilobular emphysema.
- Citation: Oikonomou A, Mintzopoulou P, Tzouvelekis A, Zezos P, Zacharis G, Koutsopoulos A, Bouros D, Prassopoulos P. Pulmonary fibrosis and emphysema: Is the emphysema type associated with the pattern of fibrosis? World J Radiol 2015; 7(9): 294-305
- URL: https://www.wjgnet.com/1949-8470/full/v7/i9/294.htm
- DOI: https://dx.doi.org/10.4329/wjr.v7.i9.294
Wiggins et al[1] first described the coexistence of emphysema in the upper lobes and pulmonary fibrosis in the lower lobes on high-resolution computed tomography (HRCT), thus enhancing the clinical recognition of the simultaneous occurrence of these two separate entities. In 2005 with the publication of Cottin et al[2], a new syndrome based on clinical and radiological findings emerged under the name “combined pulmonary fibrosis and emphysema” (CPFE). This syndrome is characterized by the coexistence of distinct features, including tobacco smoking, severe dyspnea, pseudonormalization of the pulmonary function tests (PFTs) contrasting with significant impairment of diffusion capacity (DLCO) and hypoxemia during exercise[3]. CPFE is also associated with pulmonary hypertension, which predicts a dismal prognosis[4]. The syndrome of CPFE has been recently considered part of the spectrum of smoking-related interstitial lung diseases[5]. Imaging features are characteristic but various, with centrilobular emphysema (CLE) and/or paraseptal emphysema in the upper lobes and diffuse infiltrative opacities suggestive of pulmonary fibrosis predominating in the lower lobes[6]. In one study, although CLE was almost always present in the CPFE group, the coexistence of paraseptal emphysema was surprisingly high[2]; in another study, paraseptal emphysema was more common in the CPFE population compared with the COPD group[7]. CPFE was previously considered to be characterized more commonly by a usual interstitial pneumonia (UIP) pattern of fibrosis on HRCT, but more recently, CPFE is considered a heterogeneous disease on HRCT that may present with a UIP, a nonspecific interstitial pneumonia (NSIP) pattern or a complex pattern with predominantly reticular opacities[2,3]. Moreover, the ground glass opacity (GGO) pattern may occasionally be the only HRCT pattern of fibrosis[8].
Furthermore, the presence of concurrent emphysema and fibrosis may influence the distinction of UIP from nonspecific interstitial pneumonia, and emphysematous cysts surrounded by ground glass opacity may erroneously be mistaken for honeycombing[9,10].
The purpose of this study was to investigate whether a specific predominant type of emphysema is associated with a specific HRCT pattern of fibrosis in CPFE.
This retrospective study recruited patients from the outpatient clinic of the Pulmonology Department of our university hospital. HRCT and functional data were retrospectively collected and analyzed for the cases included in the study between January 2003 and March 2013. Data collection ended on March 31, 2013. According to our country’s legislation, informed consent is waived for retrospective analyses of data. Approval was obtained by the Research Ethics Board of our university hospital (530:2/15-2-2011).
Cases were included in the study according to the following criteria: (1) presence of emphysema on HRCT scan defined as well-demarcated areas of decreased attenuation relative to contiguous normal lung and marginated by a very thin (< 1 mm) or no wall and/or multiple bullae (> 1 cm) with upper zone predominance; and (2) presence of a diffuse parenchymal lung disease with pulmonary fibrosis on HRCT scan, characterized by reticular opacities with peripheral, subpleural and basal predominance, architectural distortion and/or traction bronchiectasis and bronchiolectasis that might be associated with ground-glass opacity, with or without honeycombing[2,11]. Only cases that had undergone HRCT and were available for reading were included in the study.
Patients with a known history of connective tissue disease or other known diagnoses of interstitial lung disease, such as sarcoidosis, histiocytosis, lymphangioleiomyomatosis, pneumoconiosis, drug-induced pneumonitis, hypersensitivity pneumonitis or eosinophilic pneumonia were excluded from the study.
All patients underwent HRCT of the thorax to determine the diagnosis of pulmonary fibrosis and emphysema. The thin-section CT studies were performed with 120 kVp, 160 mAs, and 1 mm collimation at 10-mm intervals from the apex of the lung to the diaphragm with a helical CT scanner (ProSpeed, SX Power, General Electric, Germany). The images were obtained with the patient in the supine position at full inspiration and were reconstructed using a high-spatial-frequency algorithm.
HRCT images were assessed independently by 2 observers, a senior chest radiologist with 13 years of experience in chest imaging (AO) and a junior radiologist with two years of training in chest imaging (PM), who were blinded to the clinical and functional information but who were aware that the patients had CPFE syndrome. HRCT studies were assessed using a modification of a previously described semiquantitative HRCT scoring system[12] at five predetermined levels: (1) origin of great vessels; (2) main carina; (3) pulmonary venous confluence; (4) halfway between the third and fifth section; and (5) immediately above the right hemidiaphragm. HRCT scans were assessed for the presence and extent of emphysema, the total extent of interstitial lung disease (including the presence of reticular pattern, ground glass pattern and honeycombing), the extent of reticular pattern not otherwise specified, the extent of ground glass opacity associated with bronchiectasis, the extent of pure ground glass opacity, the extent of honeycombing and the coarseness of fibrosis. The HRCT findings were assessed and analyzed according to the Fleischner glossary of terms[13]. The variables of the HRCT scoring system are described in detail.
Extent of emphysema (emphysema): Emphysema was defined as areas of decreased attenuation with no walls or discrete walls and was classified as centrilobular or paraseptal. Large air cysts with fibrotic thick walls with a diameter > 2 cm in areas of reticulation, which contained a centrilobular artery in their center, were considered to represent emphysema that was either evaluated as “centrilobular” (CLE) if they were in a non-subpleural location or “paraseptal” (PSE) if they were in a subpleural location. Similarly, emphysematous bullae were classified as “centrilobular” or “paraseptal” if they were located in a non-subpleural or subpleural location, respectively.
The total extent of emphysema was calculated to the nearest five percent in each of the five slices. The total extent of emphysema on HRCT was estimated as the mean of the scores. To establish the diagnosis of CPFE on HRCT, a total extent of emphysema higher than 5% was set as the threshold.
Total extent of interstitial lung disease: The total extent of interstitial lung disease was calculated to the nearest five percent in each of the five slices. The total extent of disease on HRCT was estimated as the mean of the scores.
Extent of reticular pattern not otherwise specified: The extent of reticular pattern non-otherwise specified was calculated to the nearest five percent in each of the five slices, with the total extent of the reticular pattern on HRCT estimated as the mean of the scores. From this total score, the contribution made by reticular pattern NOS to total extent of interstitial lung disease was calculated as the ratio of the reticular pattern to the total extent of interstitial lung disease (proportion of reticular pattern NOS - RetNOS prop).
Extent of ground-glass opacity with traction bronchiectasis: The extent of ground glass opacity with traction bronchiectasis (ExtGGOBx) was calculated to the nearest five percent in each of the five slices. The total extent was estimated as the mean of the scores. From this total score, the contribution made by GGO with traction bronchiectasis to the total extent of interstitial lung disease was calculated as the ratio of the extent of GGO with traction bronchiectasis to the total extent of interstitial lung disease (proportion of GGO with traction bronchiectasis - ExtGGOBx prop).
Extent of pure ground-glass opacity: The extent of pure GGO was calculated to the nearest five percent in each of the five slices. The total extent was estimated as the mean of the scores. From this total score, the contribution made by the extent of pure GGO to the total extent of interstitial lung disease was calculated as the ratio of the extent of pure GGO to the total extent of interstitial lung disease (proportion of pure GGO - GGO prop).
Extent of honeycombing: The extent of honeycombing (HC) was calculated to the nearest five percent in each of the five slices. The total extent was estimated as the mean of the scores. From this total score, the contribution made by the extent of HC to the total extent of interstitial lung disease was calculated as the ratio of the extent of HC to the total extent of interstitial lung disease (proportion of HC - HC prop).
Coarseness of fibrosis (Coarseness): The most severe disease in each section was quantified as follows: grade 0 = ground-glass attenuation alone; grade 1 = fine intralobular fibrosis; grade 2 = microcystic honeycombing (air spaces less than or equal to 4 mm in diameter); and grade 3 = macrocystic honeycombing (air spaces greater than 4 mm in diameter). The total coarseness score was the summed score for all five levels (range: 0-15).
After the initial assessment of HRCT images for the evaluation of HRCT scoring, each reader made a first-choice diagnosis for the predominant type of emphysema (paraseptal, centrilobular or equal extent of paraseptal and centrilobular). Accordingly, for each patient, the HRCT patterns of fibrosis were classified as typical for UIP, possible UIP and consistent with NSIP[14]. The HRCT pattern was considered typical for UIP if the following imaging findings were noted: Reticulation in all lobes with basal and peripheral predominance, high extent of honeycombing with or without traction bronchiectasis, and the absence or minimal ground-glass opacity. HRCT pattern consistent with possible diagnosis of UIP included bilateral reticular opacities with basal and peripheral predominance, minimal honeycombing and minimal to moderate ground glass opacity. The HRCT pattern consistent with NSIP included extensive or moderate ground-glass opacity, associated with mild reticulation and traction bronchiectasis or bronchiolectasis, no honeycombing, basal predominance and relative subpleural sparing[9].
All patients underwent functional assessment within 1 wk maximum from HRCT. Pulmonary function tests, which were all performed according to the current European Respiratory Society (ERS) guidelines, included forced vital capacity (FVC), diffusing capacity of the lung for carbon monoxide (DLCO), maximum midexepiratory flow (MMEF), forced expiratory volume at first second (FEV1), and the residual volume (RV)/total lung capacity (TLC) ratio[15]. The reversibility of airflow obstruction was not assessed. Airflow obstruction was defined as a post bronchodilator fixed ratio of FEV1/FVC < 0.70[16]. DLCO measurements were performed according to the American Thoracic Society/European Respiratory Society Task Force[15]. All patients underwent 6-min walking test and arterial blood gases measurements for alveolar-arterial gradient. Pulmonary artery pressure with transthoracic cardiac Doppler ultrasound was reviewed when available. The estimated systolic pulmonary artery pressure (eSPAP) was calculated as previously described[17].
Statistical analysis was performed with statistical software (SPSS software, version 18.0, Chicago, III). A P value of less than 0.05 was considered statistically significant.
The two observers stratified the patients into 3 groups according to the predominant type of emphysema on HRCT: group E1: Paraseptal predominant, group E2: Centrilobular predominant and group E3: Paraseptal = centrilobular. Subsequently, the patients were stratified in 3 other groups according to the predominant type of fibrosis on HRCT: group F1: Consistent with “typical UIP pattern”, group F2: “Possible UIP pattern” and group F3: “NSIP pattern”.
Interobserver agreement for the predominant type of emphysema and the predominant type of fibrosis, as well as for the extent and severity of the HRCT variables (as defined by the HRCT scoring system) were estimated using the Kappa statistic[18]. Crosstabs analysis was used to demonstrate the association of the predominant type of emphysema with the predominant type of fibrosis for both observers.
Subsequently, the readings (expressed in percentages) of the two observers concerning the extent and presence of the various HRCT findings were combined by calculating the average percentage, which was then used in further analyses for the comparison of the HRCT variables between the groups of emphysema and fibrosis.
Continuous data are presented as the means (95%CI). Categorical data are presented as numbers or proportions (%). Data from each HRCT variable in the different groups are presented as the mean percentages (95%CI).
Group comparisons for HRCT variables and functional parameters were performed using the following: (1) Independent samples t test for group E1 (paraseptal) and group E2 (centrilobular) (group E3 included only 2 patients and therefore was excluded from the comparisons); and (2) one-way ANOVA for group F1 (typical UIP pattern), group F2 (possible UIP pattern) and group F3 (NSIP pattern).
CT features predictive of a predominant type of emphysema and of fibrosis were identified by means of multinomial logistic regression (OR, 95%CI).
Fifty-three patients with emphysema in the upper lobes and pulmonary fibrosis in the lower lobes on HRCT scans were included in the study. All patients were men with a mean age of 62 years (range 37-84 years). All patients were smokers, including 38 current smokers with 68 mean pack-years (range 30-110 years) and 15 ex-smokers with 47 mean pack-years (range 10-135) and mean period of cessation of smoking 19 years (range 3-30).
The interobserver agreement of the severity of the different parameters of the HRCT scoring system was moderate to excellent, with the K values ranging between 0.45 and 0.97 (data not shown).
Predominant type of emphysema: The incidence of CLE compared with the incidence of PSE was similar. Observer 1 identified 28 (52.8%) cases as CLE and 23 (45%) cases as PSE (Table 1). Observer 2 identified 26 (49%) cases as CLE and 25 (45%) cases as PSE (Table 1). Both observers evaluated 2 cases with equal extents of CLE and PSE.
The interrater reliability for the 2 observers was kappa = 0.929 (P < 0.001), 95%CI: 0.757-1.000.
Predominant type of pulmonary fibrosis: The incidence of typical - possible UIP compared with the incidence of NSIP type of fibrosis on HRCT was similar. As shown in Table 1, observer 1 evaluated 19 (35.8%) cases as predominant typical UIP pattern plus 10 (18.8%) cases as possible UIP pattern and 24 (45.3%) cases with NSIP pattern on HRCT, whereas observer 2 evaluated 24 (45.3%) cases as predominant typical UIP pattern plus 5 (9.4%) cases as possible UIP pattern and 24 (45.3%) cases as NSIP pattern on HRCT.
The interrater reliability for the observers was kappa = 0.785 (P < 0.001), 95%CI (0.646, 0.924).
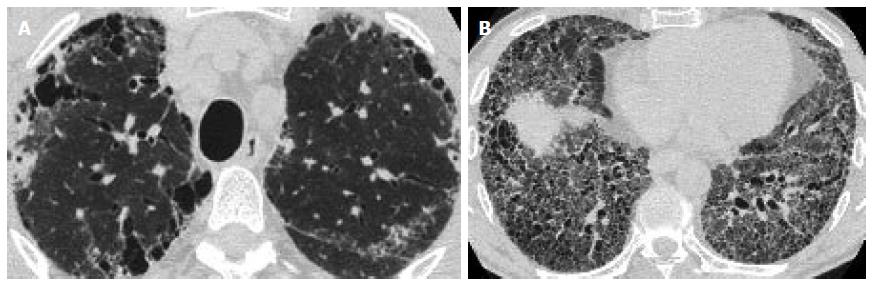
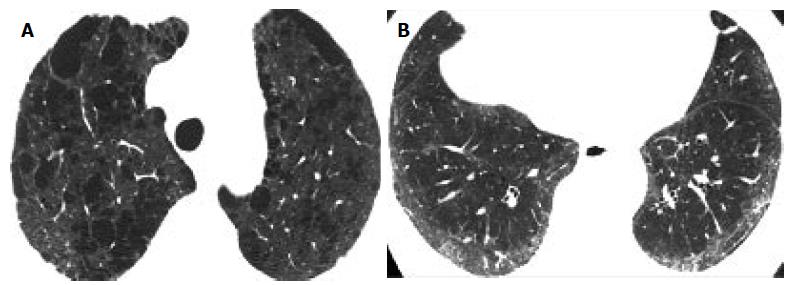
Correlation comparison of the pattern of fibrosis with the type of emphysema: According to both observers’ readings, there was a strong correlation between the predominant type of emphysema and the predominant type of fibrosis (observer 1: Spearman r = 0.862 and observer 2: Spearman r = 0.827). More specifically, PSE was more frequently associated with a UIP pattern of pulmonary fibrosis (Figure 1A and 1B), while CLE was associated with an NSIP pattern of fibrosis (Figure 2).
Observer 1 evaluated 19 patients with a predominant type of PSE to have an HRCT pattern of fibrosis consistent with typical UIP and 24 patients with a predominant type of CLE to have an HRCT pattern of fibrosis consistent with NSIP (Table 2).
| Predominant type ILD | |||||
| Typical UIP | Possible UIP | NSIP | |||
| Observer 1 | |||||
| Predominant emphysema type | CLE = PSE | 0 | 2 | 0 | 2 |
| Paraseptal | 19 | 4 | 0 | 23 | |
| Centrilobular | 0 | 4 | 24 | 28 | |
| 19 | 10 | 24 | |||
| Observer 2 | |||||
| Predominant emphysema type | CLE = PSE | 2 | 0 | 0 | 2 |
| Paraseptal | 21 | 2 | 2 | 25 | |
| Centrilobular | 1 | 3 | 22 | 26 | |
| 24 | 5 | 24 | |||
Similarly, observer 2 evaluated 21 patients with a predominant type of PSE to have an HRCT pattern of fibrosis consistent with typical UIP and 22 patients with a predominant type of CLE to have an HRCT pattern of fibrosis consistent with NSIP (Table 2).
Comparison of HRCT parameters and PFTs between patient groups according to the predominant type of emphysema (Table 3): (1) HRCT parameters. Only the groups E1 and E2 were included in the analyses of data. Group E3 included only 2 patients and therefore was excluded from the comparisons.
| Parameters | Group E1Paraseptal emphyseman = 23Mean (95%CI) | Group E2Centrilobular emphyseman = 28Mean (95%CI) | Independent samplest testP value |
| Emphysema | 6.3 (2.9-9.7) | 21.82 (14.93-28.85) | < 0.001 |
| TotExtILD | 53.34 (41.05-65.63) | 29.82 (22.09-37.55) | 0.002 |
| RetNOS | 17.56 (12.45-22.67) | 10.75 (7.67-13.82) | 0.023 |
| ExtGGOBx | 16.73 (11.52-21.89) | 6.14 (1.65-10.62) | 0.002 |
| GGO | 7.86 (3.49-12.23) | 12.5 (8.06-16.93) | 0.136 |
| HC | 11.21 (6.76-15.67) | 0.42 (-0.31-1.17) | < 0.001 |
| Coarseness | 10.34 (9.36-11.32) | 5.42 (4.61-6.24) | < 0.001 |
| RetNOS prop | 32.62 (28.57-36.67) | 40.51 (34.03-46.99) | 0.039 |
| ExtGGOBx prop | 30.83 (23.71-37.95) | 15.62 (8.76-22.48) | 0.003 |
| GGO prop | 12.97 (6.64-19.31) | 41.70 (32.72-50.67) | < 0.001 |
| HC prop | 23.87 (14.80-32.94) | 2.14 (-1.56-5.82) | < 0.001 |
| FEV1 | 72.05 | 83.81 | 0.382 |
| FEV1/FVC | 81.82 | 75.79 | 0.109 |
| PEF | 89.47 | 76.17 | 0.436 |
| MMEF 75%-25% | 78.87 | 62.07 | 0.184 |
| FVC | 72.64 | 82.21 | 0.256 |
| TLC | 58.54 | 80.98 | 0.002 |
| RV | 53.3 | 88.54 | 0.022 |
| RVTLC | 89.92 | 108.3 | 0.276 |
| DLCOsb | 32.75 | 47.64 | 0.067 |
| DLCOva | 55.22 | 71.51 | 0.293 |
| eSPAP (n = 12) | 48.12 | 35.25 | 0.324 |
The mean HRCT score for the extent of emphysema (Emphysema) in patients with PSE (group E1) was significantly lower than that in patients with CLE (group E2) (6.3 vs 21.89, P < 0.001). However, the mean HRCT score for the total extent of interstitial lung disease (TotExtILD) was significantly higher in group E1 relative to group E2 (53.34 vs 29.82, P = 0.002). Similarly, the HRCT variables that represented coarser fibrosis, such as extent of reticular disease not otherwise specified (RetNOS), GGO with bronchiectasis (ExtGGOBx), extent of HC and coarseness of fibrosis (coarseness), were significantly higher in group E1 relative to group E2 (0.039 > P > 0.001). Similarly, the proportions of the HRCT variables (to the total extent of interstitial lung disease) that represented coarser fibrosis, such as proportion of GGO with bronchiectasis (ExtGGBx prop) and proportion of HC (HC prop), were significantly higher in group E1 compared with group E2 (P < 0.003 and P < 0.001, respectively). However, the proportion of pure GGO (GGO prop) (reflecting less coarse fibrosis) was significantly lower in group E1 relative to group E2 (P < 0.001).
(2) Pulmonary function tests. There were no statistically significant differences between the E1 (PSE) and E2 groups (CLE) with respect to pulmonary function parameters.
Comparison of HRCT parameters and PFTs amongst patient groups according to pulmonary fibrosis (Table 4): (1) HRCT parameters. With respect to the 3 different groups of pulmonary fibrosis (groups F1, F2 and F3), group F1 (typical UIP) exhibited a significantly lower extent of emphysema (emphysema) relative to group F3 (NSIP) (P < 0.002).
| Parameters | Group F1Typical UIPn = 19Mean (95%CI) | Group F2Possible UIPn = 10Mean (95%CI) | Group F3NSIPn = 24Mean (95%CI) |
| Emphysema | 7.05 (2.96-11.14)c | 9.6 (3.33-15.86)e | 23.08 (15.1-31.06) |
| TotExtILD | 56.84 (43.18-70.49)d | 39.6 (19.83-59.36) | 28.16 (20.94-35.38) |
| RetNOS | 18.68 (12.78-24.58)c | 9.9 (5.51-14.28) | 11.04 (7.48-14.6) |
| ExtGGOBx | 18.05 (12.02-24.08)c | 17.9 (2.84-32.95)e | 3.66 (1.84-5.48) |
| GGO | 7.36 (2.34-12.39) | 8 (2.98-13.01) | 13.45 (8.45-18.45) |
| HC | 12.78 (7.65-17.92)da | 3.8 (1.25-6.34) | - |
| Coarseness | 10.73 (9.67-11.80)da | 8.5 (7.22-9.77)f | 4.87 (4.24-5.50) |
| RetNOS prop | 32.44 (27.51-37.36) | 30.08 (21.35-38.80) | 42.63 (35.51- 49.76) |
| ExtGGOBx prop | 30.86 (22.36-39.35)d | 36.98 (21.53-52.42)f | 11.24 (5.63-16.85) |
| ExtGGO prop | 10.44 (4.07-16.81)d | 19.97 (10.11-29.82)f | 46.10 (36.95- 55.25) |
| HC prop | 26.63 (16.13-37.13)d | 12.95 (2.16-23.74) | - |
| FEV1 | 66.51 (41.27-91.75) | 76.82 (27.32-126.32) | 87.39 (70.19-104.58) |
| FEV1/FVC | 81.04 (75.47-86.61) | 84.83 (74.00-95.66) | 74.98 (68.95-81.00) |
| PEF | 82.80 (44.11-121.50) | 85.86 (-55.76-227.49) | 80.53 (61.05-100.01) |
| MMEF 75%-25% | 73.90 (47.97-99.84) | 75.76 (43.89-107.64) | 65.02 (47.55- 82.50) |
| FVC | 69.16 (55.02- 83.30) | 74.38 (51.03-97.73) | 85.66 (73.70-97.62) |
| TLC | 54.77 (41.67-67.87)d | 67.3 (52.53-82.06) | 84.09 (75.99-92.18) |
| RV | 54.69 (17.24-92.13) | 71.3 (36.58-106.01) | 87.80 (68.45-107.15) |
| RVTLC | 89.92 (47.00-132.84) | 97.89 (-64.43- 260.21) | 101.06 (88.98-113.14) |
| DLCOsb | 27.35 (19.85-34.85)ca | 54.44 (27.51- 81.36) | 47.73 (34.24-61.22) |
| SPAP (n = 12) | 48.12 (32.49-63.75) | - | 27.33 (10.42-44.24) |
However, group F1 (typical UIP) exhibited a significantly higher extent of interstitial lung disease (TotExtILD), extent of reticular NOS (Ret NOS), extent of GGO with bronchiectasis (ExtGGOBx), extent of HC (HC) and coarseness of fibrosis (Coarseness) (0.039 > P > 0.000), thus reflecting coarser fibrosis.
Similarly, group F1 exhibited a significantly higher proportion of GGO with bronchiectasis (ExtGGOBx prop) (P < 0.001) and honeycombing (HC prop) (P < 0.000) compared with Group F3 (NSIP).
The proportion of pure GGO (GGO prop), reflecting less coarse fibrosis, was significantly lower in group F1 relative to group F3 (P < 0.000).
(2) Pulmonary function tests. As expected, TLC (P < 0.001) and DLCO (P < 0.031) were significantly lower in group F1 (“typical UIP” pattern) compared with group F3 (NSIP pattern) (Table 4). DLCO was also significantly lower in group F1 compared with group F2 (“possible UIP” pattern) (P < 0.037) (Table 5).
| Patient (no) | Histology | HRCT - observer 1 | HRCT - observer 2 | |
| 1 | 7 | Possible UIP | NSIP | NSIP |
| 2 | 10 | Possible UIP | Possible UIP | Typical UIP |
| 3 | 11 | NSIP | NSIP | NSIP |
| 4 | 14 | Possible UIP | Typical UIP | Typical UIP |
| 5 | 15 | Definite UIP | Typical UIP | Typical UIP |
| 6 | 17 | Possible UIP | Typical UIP | Typical UIP |
| 7 | 19 | NSIP | NSIP | NSIP |
| 8 | 23 | Definite UIP | Typical UIP | Typical UIP |
| 9 | 25 | Definite UIP | Typical UIP | Typical UIP |
| 10 | 26 | Definite UIP | Possible UIP | Possible UIP |
| 11 | 31 | Definite UIP | Typical UIP | Typical UIP |
| 12 | 33 | Definite UIP | Typical UIP | Typical UIP |
| 13 | 34 | Definite UIP | Typical UIP | Typical UIP |
| 14 | 35 | Possible UIP | NSIP | NSIP |
| 15 | 41 | Definite UIP | Possible UIP | Possible UIP |
| 16 | 45 | Definite UIP | Typical UIP | Typical UIP |
| 17 | 46 | Definite UIP | Possible UIP | Typical UIP |
| 18 | 51 | Definite UIP | Typical UIP | Typical UIP |
| 19 | 53 | Definite UIP | Typical UIP | Typical UIP |
(3) Pulmonary hypertension results. Systolic pulmonary arterial pressure was measured in only 12 patients by ultrasound, and the average was 44.16 mm/Hg (range: 22-80). Pulmonary arterial pressure was higher in group F1 (typical UIP) (48.12 mm/Hg) than in group F3 (NSIP) (27.33 mm/Hg) (Table 4), although the difference was not statistically significant due to the small number of patients who underwent eSPAP evaluation (P < 0.063) (Table 4). No significant difference was observed between group F1 and group F2 (Table 4).
(4) Histopathology. Histological analysis of open lung or thoracoscopic lung biopsy was available for review in nineteen cases and confirmed the presence of emphysema in the upper lobes. Interstitial pneumonia was classified as definite UIP in twelve cases, possible UIP in 5 cases and NSIP in two cases. The pathology correlation of the HRCT readings of the 2 observers is presented in Table 5. The 2 cases of NSIP on pathology were evaluated as NSIP by both observers. Nine of the 12 cases of definite UIP on pathology were evaluated as typical UIP by both observers.
The correlation of histology and HRCT was Kappa = 0.338, P < 0.042 (95%CI: 0.692, -0.016) for observer 1 and kappa = 0.289, P < 0.069 (95%CI: 0.632, -0.054) for observer 2. When definite UIP and possible UIP were coupled as one diagnosis (UIP) for HRCT and for histologic diagnosis, the correlation between histology and HRCT for both observers improved: kappa = 0.612, P < 004 (95%CI: 0.14356-1.08044).
(5) Regression analysis. According to multinomial regression analysis, coarseness of fibrosis was the only HRCT feature that could differentiate typical UIP from NSIP (OR = 6.912; 95%CI: 2.493-19.167), possible UIP from NSIP (OR = 3.939; 95%CI: 1.595-9.726) and PSE from CLE (OR = 0.398; 95%CI: 0.248-0.638).
Patients with simultaneous emphysema and fibrosis present with dyspnea, pulmonary hypertension, hypoxemia, preserved lung volumes and normal spirometry in the setting of severely impaired diffusion lung capacity, a condition known as CPFE[3]. The coexistence of emphysema and pulmonary fibrosis may have been underappreciated clinically because the two entities present dissimilar/opposite physiologic effects; therefore, one entity may partially mask the other. HRCT has significantly contributed to the clinical recognition of the simultaneous occurrence of emphysema and pulmonary fibrosis[1,6,19].
In the present study, we analyzed the HRCT findings of 53 patients with coexistent emphysema and pulmonary fibrosis and found that PSE is associated with a UIP-HRCT pattern, whereas CLE is associated with an NSIP-HRCT pattern.
All patients were exclusively male (53/53), and all were current or ex-smokers. The sex predominance, the mean age (62 years) and the history of smoking were consistent with those reported in the literature for CPFE patients[2,3,6], where a male preponderance and a strong association with smoking history has been reported. The relationship between CPFE and smoking is not surprising given the association of both emphysema/COPD[20] and fibrosis with smoking[21,22]. Similarly, both emphysema and pulmonary fibrosis are associated with male sex; however, these associations alone are not sufficient to explain the significant male preponderance in CPFE patients[23,24]. It has been proposed that this preponderance may be explained by the greater vulnerability of men to lung aging[3].
There was good agreement between the 2 observers with respect to the severity of the different parameters of the HRCT scoring system. Interobserver agreement for the predominant type of emphysema and pulmonary fibrosis on HRCT was also satisfactory.
According to the results of the present study, the incidence of CLE (as the predominant type of emphysema) is similar to the incidence of PSE (CLE/PSE = observer 1: 28/23, observer 2: 26/25). A previous study reported that although CLE was almost always present in the CPFE group, the coexistence of PSE was surprisingly high[2]; in another study, PSE was more common in the CPFE population compared with the COPD group[7]. According to the results of our study, in CPFE patients, certain patterns of emphysema are more commonly associated with certain patterns of fibrosis, rather than certain patterns of emphysema being more prevalent than others. Bullous emphysema has also been reported to be common in CPFE patients, as was the case in our study[3,6,7]. However, we did not evaluate bullous emphysema as a separate category, as bullous emphysema may be the progression of CLE or PSE depending on the intraparenchymal or subpleural location of the bullae. Moreover, in the advanced stages of CLE, the centrilobular distribution of abnormalities is no longer recognizable on HRCT or on pathology, and this appearance can closely mimic the appearance of panlobular emphysema, rendering the distinction between these two entities very difficult on HRCT and of little clinical significance[25].
Similarly, there was no significant difference between the incidence of the UIP pattern and NSIP pattern on HRCT (as the predominant pattern of fibrosis on HRCT) in CPFE patients (typical UIP + possible UIP/NSIP = observer 1: 19 + 10/24, observer 2: 24 + 5/24). Cottin et al[2] reported that 51% of patients presented with HRCT findings consistent with typical IPF, whereas 34% of patients presented with a CT pattern consistent with probable IPF or fibrosing NSIP, and 15% of cases exhibited a complex pattern with predominant reticular opacities. Jankowich et al[8] reported that 8 of the 10 patients in their study exhibited honeycombing on CT, whereas in Kitaguchi et al[7]’s paper, 75.6% of patients presented with honeycombing. However, in all three previous studies, GGO was present in 66%, 20% and 62.2% accordingly. The discrepancy in the incidence (lower incidence) of the NSIP pattern in previous studies relative to ours may be explained by the fact that presence of GGO and fibrosis around emphysematous spaces and cysts may erroneously mimic and therefore lead to the overestimation of honeycombing[9,10]. In accordance with this finding, Akira et al[9] reported in their study that the coexistence of emphysema renders the distinction between UIP and NSIP more difficult; therefore, NSIP may mimic UIP when it coexists with emphysema. Similarly, Watadani et al[10] reported that the diagnosis of honeycombing is very variable even amongst experienced readers and may be misdiagnosed, especially when emphysema or traction bronchiolectasis coexist. In our study, we evaluated the subpleural thin-walled cysts (< 1 mm wall thickness) of varying sizes with a centrilobular artery within their center as PSE. On the other hand, the subpleural cluster of cysts with relatively thick walls (1-3 mm) and cysts of the same size that lacked a centrilobular artery at their center were evaluated as honeycombing.
Another interesting finding in our study was that CPFE patients with a predominant type of CLE exhibited a significantly higher extent of emphysema than patients with PSE. On the other hand, CPFE patients with a predominant type of PSE exhibited a higher extent of interstitial lung disease (fibrosis). This finding may be explained by the fact that by definition, in order for the “paraseptal” type to be the predominant type of emphysema, it must be located subpleurally around the periphery of the lungs or along the fissures, which is overall a smaller area than the “non-subpleural” part of the lungs (where CLE is located). Therefore, when PSE predominates, the non-subpleural part of the lungs is not affected by emphysema, and the fibrotic process has “free area” to expand. On the contrary, when CLE predominates, comparatively greater area is affected, and there is limited “free space” for fibrosis to develop. If this sequence of phenomena is correct, then we could speculate that emphysema chronologically precedes the development of fibrosis in CPFE patients. Another intriguing finding in our study was that in CPFE patients, a predominant type of “CLE” was associated with more GGO and less coarse fibrosis and honeycombing (resembling an NSIP pattern), whereas a predominant type of PSE was associated with coarser fibrosis, reticulation and honeycombing, resembling a UIP pattern. These findings were confirmed by the regression analysis results, which demonstrate that among HRCT features, the “coarseness of fibrosis” most strongly differentiated typical and probable UIP from NSIP and PSE from CLE. There have been no previous studies in the literature exploring the possible relationship between the predominant type of emphysema with the predominant type of fibrosis on HRCT. However, Marten et al[26] reported that the coexistence of CLE with NSIP patients provided evidence for smoking as a pathogenetic factor in a subset of NSIP patients. Longitudinal studies both in the development of CLE and NSIP have demonstrated that CLE replaces macrophage-related respiratory bronchiolitis[27,28] and that macrophage accumulation of DIP may recede leaving residual fibrotic NSIP[26,29]. In a more recent study, Antoniou et al[30] reported coarser fibrosis and notable honeycombing in smokers with IPF and rheumatoid lung relative to non-smoking counterparts. The latter finding suggests a pathogenetic link between smoking-related damage and lung fibrosis, although differentiation between CLE and PSE was not attempted. Tobacco smoking has been suggested to be a causative agent in both emphysema and lung fibrosis[11] because cigarette smoke may directly trigger pathways common to pulmonary fibrosis and emphysema, including excessive oxidative stress, induction of inflammation, protease-antiprotease imbalance[31], promotion of apoptosis, recruitment of macrophages or dysregulation of connective tissue matrix[30]. In addition to epigenetic modifications, genetic variants, including mutations of the surfactant protein C[32] and overexpression of platelet-derived growth factor and tumor necrosis factor α and β in animal models[33], have also been associated with the development of both emphysema and fibrosis. Recently it has been reported that specific genes of the protease-antiprotease balance pathway may be associated with specific emphysema types, particularly MMP9 and TGFB1 to CLE and TIMP2 and TNF to PSE[31]. Autoimmunity has also been implicated in the pathogenesis of CPFE[34]. Based on these findings, one could speculate that there may be a possible pathogenetic linkage in CPFE patients leading to combinations of specific patterns of lung fibrosis and emphysema. The above findings could have significant prognostic and therapeutic implications because a predominant type of CLE would be associated with a more reversible type of pulmonary fibrosis that would be more likely to respond to smoking cessation and immunosuppressive therapy[8], whereas a predominant PSE would be associated with a more irreversible type of pulmonary fibrosis with a worse prognosis[35]. In support of our results, a recent study by Todd et al[36] has reported that there is improved prognosis in CPFE patients presenting with advanced CLE compared with PSE. The authors hypothesized that this may be attributed to the more severe pulmonary inflammation present in CLE vs PSE that could be partially protective against the adverse effects of pulmonary fibrosis.
DLCo and TLC were significantly lower in the “typical UIP” group compared with the “NSIP group”. Systolic pulmonary arterial pressure was found to be higher in the “typical UIP” group (48.12 mm/Hg) compared with the “NSIP” group (27.33 mm/Hg), although the last finding did not exhibit statistical significance due to the small number of patients having undergone eSPAP evaluation. These findings were in accordance with previous studies in which DLCo was found to be a significant predictor of mortality in IPF patients[37,38] and in which the presence of pulmonary arterial hypertension was found to be associated with poor survival both in IPF[38] and CPFE patients[4,39].
There was fair HRCT-pathologic correlation in the 19 out of 53 cases where histology was available. Only 2 out of the 19 cases were consistent with non-UIP histologic diagnosis (2 NSIP). There may have been a bias towards the histologic diagnosis of UIP (definite UIP or possible UIP) because these cases were more likely to have undergone biopsy due to worse clinical symptoms that would require more aggressive treatment. On the other hand, patients with an NSIP pattern on HRCT were more likely to exhibit extensive emphysema (as shown in our study), therefore avoiding a lung biopsy[40].
There are several limitations in our study that are mainly caused by its observational and uncontrolled design, with retrospective collection of the data from a single center. Our results are also subject to selection bias, as only patients who had undertaken HRCT and patients with evidence of both emphysema and fibrosis on HRCT were included, creating bias towards patients with less typical findings. Another limitation may be the fact that the presence of only 3 considerations in the differential diagnosis of HRCT findings may have resulted in an artificial high interobserver agreement. A potentially important limitation in study design was that the 2 HRCT readers were aware of the fact that patients had coexistence of emphysema and fibrosis on HRCT and that the main goal was to identify any relationship between the predominant type of emphysema and the predominant type of fibrosis on HRCT, although the readers were unaware of the clinical findings and the histology of the cases at the time of evaluation. To overcome this problem, the HRCT readers evaluated the HRCT studies blindly in three different steps with a one month interval between each step: first, they evaluated the predominant type of emphysema for each study; second, they evaluated the predominant type of fibrosis; and last, they scored the different HRCT parameters of the HRCT scoring system. Another limitation of the study was the small number of patients who had undergone eSPAP measurements using cardiac ultrasound due to the poor echo window, which was mainly attributed to the presence of emphysema.
In conclusion, we have found that in CPFE patients, a predominance of PSE may be associated with a higher extent of fibrosis and with a UIP-HRCT pattern, whereas a predominance of CLE may be associated with a higher extent of emphysema and an NSIP-HRCT pattern of fibrosis that could be more reversible and responsive to smoking cessation and immunosuppression. Thus, although viewed with caution, our observations justify future studies to explore possible common pathogenetic mechanisms that may lead to the simultaneous development of specific types of emphysema and fibrosis affecting the prognosis of CPFE patients.
“Combined pulmonary fibrosis and emphysema” (CPFE) is a relatively newly reported syndrome in the literature based on clinical and computed tomography (CT) findings. Clinical findings include history of smoking, severe dyspnea, pseudonormalization of the pulmonary function tests contrasting with severe decrease on diffusion capacity and hypoxemia during exercise. Association with pulmonary hypertension significantly impairs prognosis. Imaging findings include presence of emphysema in the upper lobes and pulmonary fibrosis in the lower lobes. The predominant type of emphysema and pulmonary fibrosis when these two entities coexist and their possible association and possible relevant clinical implications has not been fully elucidated in the litterature.
The predominant type of emphysema varies in the few studies about CPFE reported in the literature, with some of them reporting centrilobular type to be more common, while others reporting paraseptal type to be the predominant one. As far as the fibrotic component is concerned, in the earlier days CPFE was considered to be characterized more commonly by a usual interstitial pneumonia (UIP) pattern of fibrosis on High resolution computed tomography (HRCT), however there is a more recent concept according to which CPFE is a heterogeneous disease on HRCT that may present with a UIP, a nonspecific interstitial pneumonia (NSIP) pattern or a complex pattern with predominantly reticular opacities. Moreover ground glass opacity pattern may occasionally be the only HRCT pattern of fibrosis.
In the present study the authors found that in patients with coexistent emphysema and pulmonary fibrosis paraseptal emphysema is associated more with a usual interstitial pneumonia-HRCT pattern, whereas centrilobular emphysema is associated more with a nonspecific interstitial pneumonia-HRCT pattern. The incidence of centrilobular emphysema is similar to the incidence of paraseptal emphysema and similarly there was no significant difference between the incidence of UIP pattern and NSIP pattern on HRCT. According to the results of this study it seems that in CPFE patients a certain pattern of emphysema is associated more often with a certain pattern of fibrosis, rather than a certain pattern of emphysema being more prevalent than the other one. Moreover CPFE patients with a predominant type of centrilobular emphysema demonstrated significantly higher extent of emphysema than patients with paraseptal emphysema. On the other hand, CPFE patients with a predominant type of paraseptal emphysema demonstrated higher extent of pulmonary fibrosis.
In CPFE patients predominant type of paraseptal emphysema may be associated with higher extent of fibrosis and with a UIP-HRCT pattern, while predominant type of centrilobular emphysema may be associated with higher extent of emphysema and an NSIP-HRCT pattern of fibrosis that coould be more reversible and responsive to smoking cessation and immunosuppression. The observations justify future studies in order to explore possible common pathogenetic mechanisms that may lead to the simultaneous development of specific types of emphysema and fibrosis affecting the prognosis in CPFE patients.
Centrilobular emphysema is the commonest form of emphysema in cigarette smokers characterized by destroyed centrilobular alveolar walls and enlargement of respiratory bronchioles and associated alveoli. On CT it presents as centrilobular areas of decreased attenuation, usually without visible walls, of nonuniform distribution and predominantly located in upper lung zones
Paraseptal emphysema is characterized by predominant involvement of the distal alveoli and their ducts and sacs. It borders any pleural surface and the interlobular septa. On CT it presents as subpleural and peribronchovascular regions of low attenuation separated by intact interlobular septa, sometimes associated with bullae. UIP is a histologic pattern of pulmonary fibrosis characterized by temporal and spatial heterogeneity, with established fibrosis and honeycombing interspersed among normal lung. Key findings include fibroblastic foci with fibrotic destruction of lung architecture, often with honeycombing. Honeycombing with a basal and subpleural distribution is regarded as pathognomonic, but not all cases of biopsy-proved UIP have this distinctive CT pattern. NSIP is characterized by a histologic pattern of uniform interstitial involvement by varying degrees of chronic inflammation or fibrosis. NSIP has variable thin-section CT appearances: the most frequent is ground-glass opacities with reticulation, traction bronchiectasis or bronchiolectasis, and little or no honeycombing with a predominantly basal and subpleural distribution.
This study investigated the relationship between specific predominant type of emphysema and the specific HRCT pattern of fibrosis in CPFE. The manuscript is well structured and provides strong data.
| 1. | Wiggins J, Strickland B, Turner-Warwick M. Combined cryptogenic fibrosing alveolitis and emphysema: the value of high resolution computed tomography in assessment. Respir Med. 1990;84:365-369. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 142] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 2. | Cottin V, Nunes H, Brillet PY, Delaval P, Devouassoux G, Tillie-Leblond I, Israel-Biet D, Court-Fortune I, Valeyre D, Cordier JF. Combined pulmonary fibrosis and emphysema: a distinct underrecognised entity. Eur Respir J. 2005;26:586-593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 614] [Cited by in RCA: 629] [Article Influence: 30.0] [Reference Citation Analysis (0)] |
| 3. | Jankowich MD, Rounds SI. Combined pulmonary fibrosis and emphysema syndrome: a review. Chest. 2012;141:222-231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 200] [Cited by in RCA: 211] [Article Influence: 15.1] [Reference Citation Analysis (0)] |
| 4. | Mejía M, Carrillo G, Rojas-Serrano J, Estrada A, Suárez T, Alonso D, Barrientos E, Gaxiola M, Navarro C, Selman M. Idiopathic pulmonary fibrosis and emphysema: decreased survival associated with severe pulmonary arterial hypertension. Chest. 2009;136:10-15. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 318] [Article Influence: 18.7] [Reference Citation Analysis (0)] |
| 5. | Balbi B, Cottin V, Singh S, De Wever W, Herth FJ, Robalo Cordeiro C. Smoking-related lung diseases: a clinical perspective. Eur Respir J. 2010;35:231-233. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 17] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 6. | Brillet PY, Cottin V, Letoumelin P, Landino F, Brauner MW, Valeyre D, Cordier JF, Nunes H. Combined apical emphysema and basal fibrosis syndrome (emphysema/fibrosis syndrome): CT imaging features and pulmonary function tests. J Radiol. 2009;90:43-51. [PubMed] |
| 7. | Kitaguchi Y, Fujimoto K, Hanaoka M, Kawakami S, Honda T, Kubo K. Clinical characteristics of combined pulmonary fibrosis and emphysema. Respirology. 2010;15:265-271. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 108] [Cited by in RCA: 121] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 8. | Jankowich MD, Polsky M, Klein M, Rounds S. Heterogeneity in combined pulmonary fibrosis and emphysema. Respiration. 2008;75:411-417. [PubMed] |
| 9. | Akira M, Inoue Y, Kitaichi M, Yamamoto S, Arai T, Toyokawa K. Usual interstitial pneumonia and nonspecific interstitial pneumonia with and without concurrent emphysema: thin-section CT findings. Radiology. 2009;251:271-279. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 94] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 10. | Watadani T, Sakai F, Johkoh T, Noma S, Akira M, Fujimoto K, Bankier AA, Lee KS, Müller NL, Song JW. Interobserver variability in the CT assessment of honeycombing in the lungs. Radiology. 2013;266:936-944. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 237] [Cited by in RCA: 260] [Article Influence: 20.0] [Reference Citation Analysis (0)] |
| 11. | Cottin V, Nunes H, Mouthon L, Gamondes D, Lazor R, Hachulla E, Revel D, Valeyre D, Cordier JF, Groupe d'Etudes et de Recherche sur les Maladies "Orphelines" Pulmonaires. Combined pulmonary fibrosis and emphysema syndrome in connective tissue disease. Arthritis Rheum. 2011;63:295-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 128] [Cited by in RCA: 127] [Article Influence: 8.5] [Reference Citation Analysis (0)] |
| 12. | Goh NS, Desai SR, Veeraraghavan S, Hansell DM, Copley SJ, Maher TM, Corte TJ, Sander CR, Ratoff J, Devaraj A. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med. 2008;177:1248-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 703] [Cited by in RCA: 855] [Article Influence: 47.5] [Reference Citation Analysis (0)] |
| 13. | Hansell DM, Bankier AA, MacMahon H, McLoud TC, Müller NL, Remy J. Fleischner Society: glossary of terms for thoracic imaging. Radiology. 2008;246:697-722. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2471] [Cited by in RCA: 2674] [Article Influence: 148.6] [Reference Citation Analysis (0)] |
| 14. | Travis WD, Costabel U, Hansell DM, King TE, Lynch DA, Nicholson AG, Ryerson CJ, Ryu JH, Selman M, Wells AU. An official American Thoracic Society/European Respiratory Society statement: Update of the international multidisciplinary classification of the idiopathic interstitial pneumonias. Am J Respir Crit Care Med. 2013;188:733-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2232] [Cited by in RCA: 3119] [Article Influence: 239.9] [Reference Citation Analysis (0)] |
| 15. | Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, Gustafsson P, Hankinson J, Jensen R, McKay R, Miller MR, Navajas D, Pedersen OF, Pellegrino R, Wanger J. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. Eur Respir J. 2005;26:720-735. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1454] [Cited by in RCA: 1680] [Article Influence: 84.0] [Reference Citation Analysis (0)] |
| 16. | Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, mamagement, and prevention of COPD (updated 2013). Available from: http://www.goldcopd.org/uploads/users/files/GOLD_Report_2013_Feb20.pdf. |
| 17. | Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70:657-662. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1186] [Cited by in RCA: 1143] [Article Influence: 27.2] [Reference Citation Analysis (0)] |
| 18. | Brennan P, Silman A. Statistical methods for assessing observer variability in clinical measures. BMJ. 1992;304:1491-1494. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 679] [Cited by in RCA: 739] [Article Influence: 21.7] [Reference Citation Analysis (2)] |
| 19. | Sakai F, Tominaga J, Kaga A, Usui Y, Kanazawa M, Ogura T, Yanagawa N, Takemura T. Imaging diagnosis of interstitial pneumonia with emphysema (combined pulmonary fibrosis and emphysema). Pulm Med. 2012;2012:816541. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 18] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 20. | Bauer CM, Morissette MC, Stämpfli MR. The influence of cigarette smoking on viral infections: translating bench science to impact COPD pathogenesis and acute exacerbations of COPD clinically. Chest. 2013;143:196-206. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 58] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Baumgartner KB, Samet JM, Stidley CA, Colby TV, Waldron JA. Cigarette smoking: a risk factor for idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 1997;155:242-248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 486] [Cited by in RCA: 504] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 22. | Flaherty KR, Martinez FJ. Cigarette smoking in interstitial lung disease: concepts for the internist. Med Clin North Am. 2004;88:1643-1653, xiii. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 23. | Dransfield MT, Washko GR, Foreman MG, Estepar RS, Reilly J, Bailey WC. Gender differences in the severity of CT emphysema in COPD. Chest. 2007;132:464-470. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 126] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 24. | Raghu G, Weycker D, Edelsberg J, Bradford WZ, Oster G. Incidence and prevalence of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2006;174:810-816. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 880] [Cited by in RCA: 938] [Article Influence: 46.9] [Reference Citation Analysis (0)] |
| 25. | Webb WR, Muller NL, Naidich DP. High-Resolution CT of the lung. 2nd ed. Philadelphia: Lippincott Williams & Wilkins 1996; . |
| 26. | Marten K, Milne D, Antoniou KM, Nicholson AG, Tennant RC, Hansel TT, Wells AU, Hansell DM. Non-specific interstitial pneumonia in cigarette smokers: a CT study. Eur Radiol. 2009;19:1679-1685. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 43] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 27. | Marten K, Hansell DM. Imaging of macrophage-related lung diseases. Eur Radiol. 2005;15:727-741. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 15] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 28. | Remy-Jardin M, Edme JL, Boulenguez C, Remy J, Mastora I, Sobaszek A. Longitudinal follow-up study of smoker’s lung with thin-section CT in correlation with pulmonary function tests. Radiology. 2002;222:261-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 82] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 29. | Craig PJ, Wells AU, Doffman S, Rassl D, Colby TV, Hansell DM, Du Bois RM, Nicholson AG. Desquamative interstitial pneumonia, respiratory bronchiolitis and their relationship to smoking. Histopathology. 2004;45:275-282. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 90] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 30. | Antoniou KM, Walsh SL, Hansell DM, Rubens MR, Marten K, Tennant R, Hansel T, Desai SR, Siafakas NM, du Bois RM. Smoking-related emphysema is associated with idiopathic pulmonary fibrosis and rheumatoid lung. Respirology. 2013;18:1191-1196. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 45] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 31. | Kukkonen MK, Tiili E, Vehmas T, Oksa P, Piirilä P, Hirvonen A. Association of genes of protease-antiprotease balance pathway to lung function and emphysema subtypes. BMC Pulm Med. 2013;13:36. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 26] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 32. | Cottin V, Reix P, Khouatra C, Thivolet-Béjui F, Feldmann D, Cordier JF. Combined pulmonary fibrosis and emphysema syndrome associated with familial SFTPC mutation. Thorax. 2011;66:918-919. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 69] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Lundblad LK, Thompson-Figueroa J, Leclair T, Sullivan MJ, Poynter ME, Irvin CG, Bates JH. Tumor necrosis factor-alpha overexpression in lung disease: a single cause behind a complex phenotype. Am J Respir Crit Care Med. 2005;171:1363-1370. [PubMed] |
| 34. | Tzouvelekis A, Zacharis G, Oikonomou A, Mikroulis D, Margaritopoulos G, Koutsopoulos A, Antoniadis A, Koulelidis A, Steiropoulos P, Boglou P. Increased incidence of autoimmune markers in patients with combined pulmonary fibrosis and emphysema. BMC Pulm Med. 2013;13:31. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 41] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 35. | Wells AU. The revised ATS/ERS/JRS/ALAT diagnostic criteria for idiopathic pulmonary fibrosis (IPF)--practical implications. Respir Res. 2013;14 Suppl 1:S2. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 36. | Todd NW, Jeudy J, Lavania S, Franks TJ, Galvin JR, Deepak J, Britt EJ, Atamas SP. Centrilobular emphysema combined with pulmonary fibrosis results in improved survival. Fibrogenesis Tissue Repair. 2011;4:6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 37. | Schmidt SL, Nambiar AM, Tayob N, Sundaram B, Han MK, Gross BH, Kazerooni EA, Chughtai AR, Lagstein A, Myers JL. Pulmonary function measures predict mortality differently in IPF versus combined pulmonary fibrosis and emphysema. Eur Respir J. 2011;38:176-183. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 38. | Hamada K, Nagai S, Tanaka S, Handa T, Shigematsu M, Nagao T, Mishima M, Kitaichi M, Izumi T. Significance of pulmonary arterial pressure and diffusion capacity of the lung as prognosticator in patients with idiopathic pulmonary fibrosis. Chest. 2007;131:650-656. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 261] [Cited by in RCA: 252] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 39. | Cottin V, Le Pavec J, Prévot G, Mal H, Humbert M, Simonneau G, Cordier JF, GERM"O"P . Pulmonary hypertension in patients with combined pulmonary fibrosis and emphysema syndrome. Eur Respir J. 2010;35:105-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 279] [Cited by in RCA: 296] [Article Influence: 17.4] [Reference Citation Analysis (0)] |
| 40. | Cottin V, Cordier JF. Combined pulmonary fibrosis and emphysema in connective tissue disease. Curr Opin Pulm Med. 2012;18:418-427. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 46] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Bernardin L, Li YZ S- Editor: Gong XM L- Editor: A E- Editor: Wu HL