Published online Dec 28, 2015. doi: 10.4329/wjr.v7.i12.521
Peer-review started: February 8, 2015
First decision: May 19, 2015
Revised: October 19, 2015
Accepted: November 24, 2015
Article in press: November 25, 2015
Published online: December 28, 2015
Processing time: 324 Days and 0.7 Hours
AIM: To evaluate the feasibility of using therapeutic ultrasound as an alternative treatment option for organ-confined prostate cancer.
METHODS: In this study, a trans-urethral therapeutic ultrasound applicator in combination with 3T magnetic resonance imaging (MRI) guidance was used for real-time multi-planar MRI-based temperature monitoring and temperature feedback control of prostatic tissue thermal ablation in vivo. We evaluated the feasibility and safety of MRI-guided trans-urethral ultrasound to effectively and accurately ablate prostate tissue while minimizing the damage to surrounding tissues in eight canine prostates. MRI was used to plan sonications, monitor temperature changes during therapy, and to evaluate treatment outcome. Real-time temperature and thermal dose maps were calculated using the proton resonance frequency shift technique and were displayed as two-dimensional color-coded overlays on top of the anatomical images. After ultrasound treatment, an evaluation of the integrity of cavernosal nerves was performed during prostatectomy with a nerve stimulator that measured tumescence response quantitatively and indicated intact cavernous nerve functionality. Planned sonication volumes were visually correlated to MRI ablation volumes and corresponding histo-pathological sections after prostatectomy.
RESULTS: A total of 16 sonications were performed in 8 canines. MR images acquired before ultrasound treatment were used to localize the prostate and to prescribe sonication targets in all canines. Temperature elevations corresponded within 1 degree of the targeted sonication angle, as well as with the width and length of the active transducer elements. The ultrasound treatment procedures were automatically interrupted when the temperature in the target zone reached 56 °C. In all canines erectile responses were evaluated with a cavernous nerve stimulator post-treatment and showed a tumescence response after stimulation with an electric current. These results indicated intact cavernous nerve functionality. In all specimens, regions of thermal ablation were limited to areas within the prostate capsule and no damage was observed in periprostatic tissues. Additionally, a visual analysis of the ablation zones on contrast-enhanced MR images acquired post ultrasound treatment correlated excellent with the ablation zones on thermal dose maps. All of the ablation zones received a consensus score of 3 (excellent) for the location and size of the correlation between the histologic ablation zone and MRI based ablation zone. During the prostatectomy and histologic examination, no damage was noted in the bladder or rectum.
CONCLUSION: Trans-urethral ultrasound treatment of the prostate with MRI guidance has potential to safely, reliably, and accurately ablate prostatic regions, while minimizing the morbidities associated with conventional whole-gland resection or therapy.
Core tip: Therapeutic ultrasound is a promising treatment modality for minimally invasive thermal ablation of tissue. This study assessed a novel trans-urethral ultrasound therapy device with magnetic resonance imaging (MRI) guidance to ablate canine prostate tissue in vivo. Real-time temperature monitoring and thermotherapy feedback control was performed in a clinical 3T whole-body MR scanner. Post-treatment evaluation of cavernous nerve functionality was performed with a nerve stimulator. Treatment accuracy was assessed by correlation of treatment planning, thermal dose maps, and histo-pathological results. Regions of thermal ablation were limited to areas within the prostate capsule and no damage was observed in adjacent anatomical structures. These results indicate that MRI-guided transurethral ultrasound therapy can accurately ablate prostatic regions with minimal damage to surrounding tissue.
- Citation: Sammet S, Partanen A, Yousuf A, Sammet CL, Ward EV, Wardrip C, Niekrasz M, Antic T, Razmaria A, Farahani K, Sokka S, Karczmar G, Oto A. Cavernosal nerve functionality evaluation after magnetic resonance imaging-guided transurethral ultrasound treatment of the prostate. World J Radiol 2015; 7(12): 521-530
- URL: https://www.wjgnet.com/1949-8470/full/v7/i12/521.htm
- DOI: https://dx.doi.org/10.4329/wjr.v7.i12.521
Cancer of the prostate is one of the most frequent malignant diseases and among the primary reasons of male cancer deaths in the United States[1]. Overtreatment is described as an unnecessary aggressive treatment of prostate cancer (Pca) including prostatectomy and radiation therapy and can lead to complications. The overtreatment of Pca is an important public health problem occurring in about 30%-40% of the cases[2]. Therefore, there is a need for the development of precise focal Pca treatment approaches to preserve continence and potency[3,4].
Ultrasound therapy is a novel, minimally invasive treatment option where an ultrasound transducer emits ultrasound waves with high acoustic intensities into the target regions. The deposited acoustic energy leads to temperature elevation and tissue destruction when the temperature exceeds ≥ 56 °C within the focal area; a phenomenon defined as thermo-ablation[4-8]. The ultrasound treatment can be guided with diagnostic ultrasound (US) or magnetic resonance imaging (MRI). MRI is superior to diagnostic US due to its ability to better detect real-time temperature changes in multiple planes. High resolution anatomical imaging sequences with superior soft tissues contrast and physiological protocols such as diffusion and perfusion MRI allow to evaluate the extent of tissue destruction post-ablation[9-11]. A combination of therapeutic ultrasound and MR guidance is particularly beneficial for focal and regional therapy of Pca[8]. Preliminary clinical trials utilizing trans-rectal and trans-urethral ultrasound for focal ablation of Pca have reported feasibility of these techniques[6,12]. One of the important unwanted side effects of whole gland treatment is erectile dysfunction. Even though focal therapy is expected to be safer in this regard, there is limited data in the literature to support this hypothesis.
This study utilized a novel transurethral ultrasound therapy system to ablate canine prostate tissue in vivo while simultaneously monitoring tissue temperature with multi-planar MRI. This dual modality system can assess temperature in real-time and is equipped with temperature feedback control[13]. The goal was in vivo evaluation of possible side-effects of this transurethral ultrasound therapy device. In addition to rectal damage, we specifically assessed the post-treatment functionality of the cavernosal nerves by analyzing the tumescence response qualitatively and quantitatively with a nerve stimulator during prostatectomy.
In this Institutional Animal Care and Use Committee (IACUC) approved (University of Chicago, IACUC protocol number: 72317) MRI guided ultrasound treatment study, 8 canines (age range: 6 to 57 mo, average age: 26 mo; weight range 24 to 35.8 kg average weight: 27.9 kg) were treated with therapeutic ultrasound. All procedures took place in facilities that are United States Department of Agriculture registered, and AAALAC International (Association for Assessment and Accreditation of Laboratory Animal Care) accredited.
A perineal urethrostomy was performed at least one week before the ultrasound treatment in all canines to better accommodate the ultrasound applicator in the incurvated penile urethra of the canines. A cephalic IV catheter was placed to induce anesthesia with either Propofol (5.5 mg/kg) or a combination of Buprenex (9 μg/kg), ketamine (3 mg/kg) and Dexdomitor (15 μg/kg) followed by an endotracheal intubation of the canines. A continuous inhalation of isoflurane (2%-4%) maintained the anesthesia during ultrasound treatment and subsequent MR imaging. A 6-French Foley catheter was inserted for bladder voiding until treatment. A 20-French rectal tube was placed before the ultrasound treatment to release gases from the rectum. The ultrasound applicator was manually carefully forwarded in the penile urethra to the prostate and the correct position within the prostate was verified with MR imaging. Experienced veterinarians and veterinary technologists monitored each canine throughout the procedure continuously. Monitoring included an electrocardiogram, blood pressure, blood oxygenation, in- and expired gases and physiological saline infusion.
A transurethral MR-guided ultrasound therapy prototype system (Philips, Vantaa, Finland) was utilized for administration of the ultrasound treatment on a clinical 3T MRI system (Achieva, Philips Healthcare, Best, The Netherlands). The ultrasound therapy system included a therapy workstation to plan and control the treatment, a radio-frequency generator, a water-cooled trans-urethral ultrasound system [5 mm (15 French) diameter] with eight transducer elements (4 mm × 5 mm/element) (Figure 1) and a motor to rotate the system (Figure 2). A clinical 8-channel cardiac receiver MR coil (Philips Healthcare, Best, The Netherlands) was used for MR imaging (Figure 3). The coil consisted of a 4-element anterior and a 4-element posterior part and was immobilized with straps around the canine and on the patient table (Figure 4).
Ultrasound treatment was performed at a frequency of 6.0 MHz in continuous wave mode. The motor rotated the applicator around its long axis and the ultrasound propagated through a 25 μm polyester membrane into the prostate tissue. Aqueous ultrasound gel was used to improve acoustic coupling. The urethras of the canines were cooled by pumping degassed water that circulated along the membrane and the transducer elements. Ablation depths from the urethra and ablation volumes were controlled by regulating the number of active transducer elements, output power of each element and the duration of sonication.
MRI was used to localize the targets, plan ultrasound exposures, and to provide real-time intraprocedural temperature-monitoring during sonications. MRI images were also used to assess treatment outcome. Cumulative equivalent minutes at 43 °C (CEM43) were used as a metric to quantify thermal tissue damage. Thermal doses in excess of 240 CEM43 were defined as ablative exposures[14]. MRI treatment planning included two-dimensional multi-slice T2-weighted Turbo Spin Echo sequences in coronal, sagittal, and axial orientations. Seven slices were acquired in each dynamic in all orientations. Temporal resolution was 4.1 s/8 slices with spatial resolution of 1.5 mm × 1.5 mm in-plane and a 5 mm slice thickness. The MRI-guided ultrasound planning software allows the MRI slices to be automatically aligned with the ultrasound beam to monitor temperatures and to calculate thermal doses in the target region as well as in the peri-prostatic tissues. The MRI-based proton resonance frequency shift (PRFS = 0.0094 ppm/°C) method was used to calculate temperatures and thermal dose maps in real-time[15]. Color-coded temperature and thermal dose maps were displayed on top of anatomical MR images and updated in real time. Baseline temperature drift was accounted for by normalizing average apparent temperature changes in tissues outside the heated volumes.
Following ultrasound treatment, an axial T2-weighted sequence was acquired in the same locations and orientation as the pre-treatment sequence. Diffusion weighted MR images (DWI) were acquired before a T1-weighted fast-field echo sequence was used to monitor the inflow of an FDA approved gadolinium-chelate MRI contrast agent (Multihance, Bracco Diagnostics, 0.1 mmol/kg). The use of DWI and contrast-enhanced T1-weighted MRI to visualize necrotic tissue were validated in previous studies[16,17].
Before thermal ablations, a test-sonication (Pac = 1.1 W of one active element, t = 12 s) was performed to verify sufficient acoustic coupling, the correct angle of the ultrasound transducer and the location of the heated volume. The rotation angle of the ultrasound transducer was modified with the control software if necessary to reach the target volume precisely, and a sonication was performed. A cool down period (> 5 min) was applied to allow the sonicated prostatic tissue to return to its baseline temperature. In each canine prostate this procedure was repeated in two discrete locations.
Treatment volumes were selected in different locations of the prostate and MRI-based temperature monitoring was used in each ablation location. A temperature feedback control volume of 5 voxels was selected at radial distances of 1.3 to 1.9 cm from the applicator. The acoustic power level ranged from 1.1 to 1.8 W per transducer element and it was kept constant during all independent treatments. The software stopped the sonications automatically once the mean temperature reached 56 °C in the control volume[18].
Following the procedure, during the prostatectomy to harvest the prostate, CaverMap Surgical Aid (Blue Torch Corporation, Norwood, MA) was used intra-operatively to identify and map the integrity of cavernosal nerves responsible for potency (Figure 5)[19]. The CaverMap Surgical Aid includes a nerve stimulator and an erectile response detection system. The CaverMap Surgical Aid has three major components: (1) A control unit with the electronics, connectors for the probe handle and disposable kit and a user interface to control the system; (2) A sterile and reusable probe handle for controlling the device during surgeries; and (3) A disposable kit including a probe tip that can be attached to the probe handle and a tumescence sensor. The electrical current is emitted by the probe tip.
The system applies a mild electrical stimulation for a measured tumescence response. The probe tip was inserted in close proximity to the cavernous nerves (Figures 6 and 7). A biphasic current pulse train with pulse duration of 800 μs and a current of 8 mA to 20 mA was applied for stimulation. The current was automatically increased every 20 s from 8 to 20 mA. Stimulating the cavernous nerves with the CaverMap Surgical Aid leads to an erectile response that can be measured in penile circumference changes in the tumescence sensor loop that is placed around the canine’s penis. Stimulation with the probe tip produces a small erectile response that leads to an increased penile circumference. The integrity and functionality of the cavernous nerves after ultrasound therapy was evaluated by measuring erectile responses quantitatively by analyzing relative penile circumference changes with the tumescence sensor loop. Circumference changes of the sensor loop produced a change in the electric resistance in the mercury filled sensor. Tumescence changes of 0.5% produce a audible signal and a change of light-emitting diode scale (Figure 8).
Once an erectile response was measured, in a certain location of the neurovascular bundle, the position of stimulation was recorded and the probe tip was moved to another location[20].
Following treatment, post-treatment imaging, and caver-mapping, canines were euthanized by injecting 150 mg/kg sodium pentobarbital IV followed by a prostatectomy. Potential thermal damage to periprostatic anatomical structures (e.g., rectal wall, bladder wall) were also resected (Figure 9) for a detailed histological analysis (Figure 10).
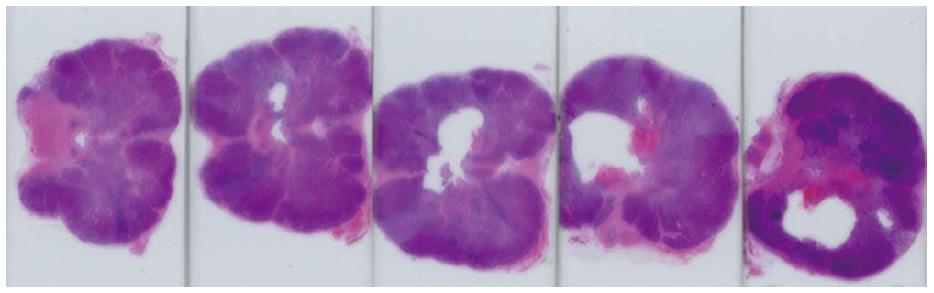
Following prostatectomies, the specimens were fixed in 10% formalin. The prostates were then sliced at 5 mm thickness parallel to the MRI axial slices and submitted for further processing. The processed paraffin blocks were cut at 4 μm and stained with haematoxylin and eosin stain. The immunohistochemical stain cytokeratin 8 (CK8) was used to analyze cell viability[21,22]. Photographs of the histological sections show visible lesions from the ultrasound treatment (Figure 10).
The ultrasound therapy console allows the measurement of the mean temperature, the maximum temperature, and a calculation of thermal dose (> 240 CEM43) volumes by using the Sapareto-Dewey equation[23].
An experienced genitourinary pathologist and a radiologist then visually correlated thermal dose (> 240 CEM43) volumes, non-perfused volumes on immediate post-ablation contrast-enhanced MR images with whole-mount sections of the prostate. In each case, the correlation between MRI and pathology for location and size of the ablation zone was separately scored on a consensus scale of 3 (no correlation: 1; modest correlation: 2; and excellent correlation: 3).
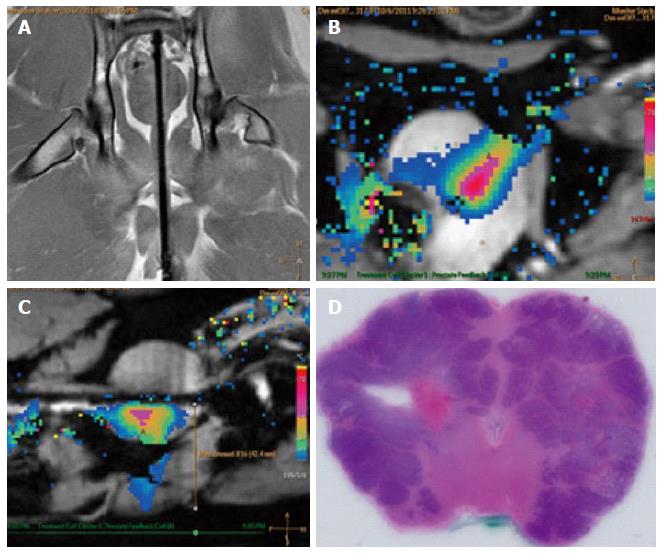
MRI was performed in this study to plan the ultrasound treatment and to monitor temperature changes (Figure 11). The canine prostate was identified in the pre-treatment MR images and target locations were prescribed (Figure 11A, Figure 12A and B). Temperature elevations (Figure 11B and C) were in alignment of the direction of the transducer elements and corresponded to the element length and width. Figure 11D shows a corresponding histological slide of the canine prostate in haematoxylin and eosin staining to evaluate ultrasound treatment effects.
A total of 16 sonications were performed in 8 canines. The ultrasound treatment procedures were automatically interrupted when the temperature in the target zone reached 56 °C. The MRI-guided trans-urethral ultrasound therapy system provided multi-planar thermal maps of the prostate and periprostatic tissues, allowing for well-prescribed and controlled ablation of the selected targets.
Example temperature elevations in direction of the ultrasound beam propagation at the end of a sonication are shown in Figure 11B and C. The time period to reach the target mean temperature of 56 °C within the control volume was 1-3 min, and depended on the local tissue characteristics, control point distance, number of active elements, and acoustic power. The ultrasound treatment was stopped after reaching the target temperature. Tissue temperature dropped to their baseline values after approximately 5 min.
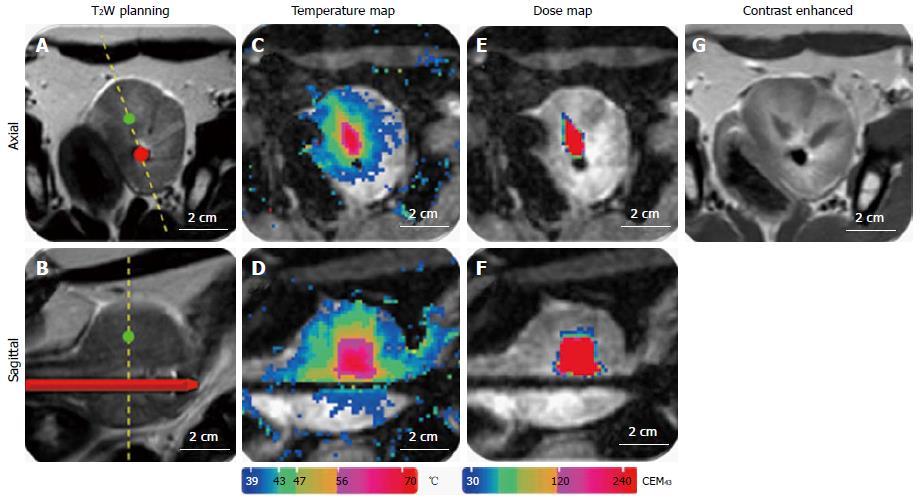
An exemplary T2-weighted planning image, contrast-enhanced MR-image, temperature maps, and thermal dose maps are displayed in Figure 12. Contrast-enhanced-images were acquired in all canines after ultrasound treatment for visualization of non-perfused tissue (Figure 12G). Thermal dose assessments in the ablation zones are displayed in Figure 12E and F. In all specimens, regions of thermal ablation were limited to areas within the prostate capsule and no damage was observed in peri-prostatic tissues (Table 1).
| Canine | Tumescence response | Inspection of peri-prostatic tissues | Correlation between MRI, thermal dose (> 240 CEM43) volumes and pathology for location and size of the ablation zone (no correlation: 1; modest correlation: 2; and excellent correlation: 3) |
| 1 | 3 | No damage | 3 |
| 2 | 4 | No damage | 3 |
| 3 | 3 | No damage | 3 |
| 4 | 2 | No damage | 3 |
| 5 | 4 | No damage | 3 |
| 6 | 2 | No damage | 3 |
| 7 | 3 | No damage | 3 |
| 8 | 3 | No damage | 3 |
In all canines erectile responses were evaluated with a cavernous nerve stimulator in average 5 d (range 0 to 15 d) post-treatment and showed tumescence responses ≥ 2 on an ordinal scale ranging from + 2 to + 4 after a stimulation with a maximum current of 14 mA (Table 1). These results indicated intact cavernous nerve functionality.
Additionally, a visual analysis of the ablation zones on contrast-enhanced MR images acquired post ultrasound treatment correlated excellent with the ablation zones on thermal dose maps. All of the ablation zones received a consensus score of 3 (excellent) for the location and size of the correlation between the histologic ablation zone and MRI based ablation zone during the review of the lesions by a genitourinary pathologist and radiologist (Table 1). During the prostatectomy and histologic examination, no damage was noted in the bladder or rectum.
Image guided focal therapy of prostate cancer is a promising new technology that may provide high efficacy with reduced treatment-related morbidity. Focal therapy options to treat prostate cancer are laser ablation, electroporation, cryotherapy and ultrasound therapy[4]. Focal ultrasound therapy delivers ablative hyperthermia to the prostate and may be efficacious in the treatment of prostate cancer[4]. Multiple clinical trials have demonstrated the value of high intensity focused ultrasound to treat cancer[6,12,24,25].
Trans-rectal ultrasound therapy has been successfully applied as a focal prostate cancer treatment before[4,6,12], but limitations of trans-rectal ultrasound, imaging and trans-rectal biopsies have also been demonstrated[26]. It has been documented that multi-parametric MRI is superior to trans-rectal biopsy for detection of prostate carcinoma[27] with high specificity and sensitivity[28]. The combined use of multi-parametric MRI with trans-urethral ultrasound therapy could improve diagnosis and clinical outcomes in prostate cancer[13].
The results of this canine study demonstrate the feasibility of MR-guided trans-urethral ultrasound for the treatment of prostatic tissue. Our results suggest that focused ultrasound has sufficient spatial accuracy and precision to safely apply treatment of prostate tissue without damaging the neurovascular bundle or the surrounding organs. The nerves around the prostate responsible for erection remained functionally and anatomically intact following ultrasound treatment. We also demonstrated with MRI-based temperature monitoring that temperatures sufficient for ablation in target zones can be achieved while avoiding substantial increases in temperature in adjacent critical anatomical areas. Target locations in this study were located in different zones of the prostate and adequate depth of sonication could be achieved within the prostate. The ability to target diseased tissue in the prostate while avoiding thermal damage to periprostatic structure (especially to the neurovascular bundles) will be critical to the success of future clinical transurethral ultrasound ablation studies.
Although these pre-clinical results are promising, further investigations are needed to optimize trans-urethral MR-guided ultrasound therapy for use in humans with prostate cancer. In our feasibility study we chose not to rotate the applicator during ultrasound exposures. Future experiments could explore the system’s capability for regional or whole prostate treatments using this feature[29,30]. In particular, modifying the transducer orientation while sonicating may shorten the time it takes to treat the tissue[29,31]. Moreover, studies that employ a more flexible ultrasound applicator could facilitate urethral placement and improve tolerance of the therapy. Future research should also investigate the potential of this thermoablative therapy for the management of benign prostatic hypertrophy.
This study evaluated feasibility and precision of MRI-guided transurethral ultrasound in prostatic tissue in vivo and was therefore limited by not assessing the short-term or long-term outcomes of the therapy. As our canine subjects were cancer-free, we did not have a physiological target region. We instead defined a target region, applied only one ablation in this region, and assessed for correlation between planned targets and treatment. This may not adequately simulate clinical practice where several ablations may be required to treat the cancerous lesions. This study was also limited in its use of pre-pubertal canines. We recommend post-pubertal canines for future pre-clinical ultrasound therapy studies of the prostate to assure large enough prostate sizes for treatment of multiple locations.
In summary, a promising new dual-modality device which combines MRI guidance with a trans-urethral ultrasound therapy probe was able to precisely ablate canine prostate tissue while simultaneously providing accurate thermal maps of the prostate and periprostatic tissue. Real-time, multi-planar temperature mapping equipped with feed-back control allowed for well-targeted ablation of prescribed lesions. There was no impact of ablation on the function of the neurovascular bundle or surrounding organs such as rectum and bladder. Functionality of the cavernosal nerves after trans-urethral ultrasound therapy was confirmed with a nerve stimulator measuring the tumescence response. Results of the feasibility study reported here indicate that future research into the efficacy and functional outcomes of ultrasound therapy for prostate cancer should be explored.
We would like to thank our MRI technologists Peters S and Jamison E as well as the certified veterinary technicians Vosicky J, Bruner M, Peterson K, McGrath J, and Zamora M for their outstanding support of this study and Dr. Mustafi D for providing degassed water.
Cancer of the prostate is one of the most common malignant diseases and among the leading reasons of male cancer deaths in the United States. There is a need for the development of precise focal Pca treatment approaches to preserve continence and potency. Ultrasound therapy is a novel, minimally invasive treatment option where an ultrasound probe emits a high intensity beam that can be used to destroy or “ablate” cancerous tissue. This study evaluated possible side-effects of prostate tissue ablation in vivo with an magnetic resonance imaging (MRI) guided transurethral ultrasound therapy system.
In this study, an MRI guided transurethral ultrasound therapy system was tested in dogs. The authors evaluated the feasibility and safety of MRI-guided trans-urethral ultrasound to effectively and accurately ablate prostate tissue while minimizing the damage to surrounding tissues in canine prostates. In addition to assessing rectal and bladder damage, the authors specifically assessed the post-treatment functionality of the nerves by analyzing their response qualitatively and quantitatively with a nerve stimulator.
In this study, MRI was performed for treatment planning, ultrasound guidance and post-treatment evaluation in all canines. Prostate tissue was successfully ablated with the MRI-guided ultrasound therapy unit and regions of thermal ablation were limited to areas within the prostate capsule and no damage was observed in tissues surrounding the prostate. In all canines erectile responses were evaluated with a nerve stimulator post-treatment and indicated intact nerve functionality. No damage was noted in the bladder and rectum.
Image guided focal ultrasound therapy could help to treat prostate cancer with fewer side effects than standard treatment methods. The authors’ results in a canine model suggest that MRI-guided trans-urethral focal ultrasound treatment is a safe and accurate approach to ablate prostatic tissue without damaging the neurovascular bundle and surrounding organs.
Thermo-ablation: Energy absorption to increase in temperature for tissue destruction when the temperature exceeds ≥ 56 °C within the focal area. Thermocoagulation: The use of heat to bring about localized destruction and congealing of tissue. Transurethral: A medical procedure performed via the urethra. Neurovascular bundle: The nerves, arteries, veins and lymphatics that travel together in the body, specifically in this article, around the prostate.Cavernous Nerve: The nerves that facilitate penile erection.
This article is very good.
| 1. | American Cancer Society. Cancer Facts & Figures 2014. Atlanta: American Cancer Society, 2014. accessed 2014; Jun 12 Available from: http://www.cancer.org/acs/groups/content/@research/documents/webcontent/acspc-042151.pdf. |
| 2. | Klotz L. Prostate cancer overdiagnosis and overtreatment. Curr Opin Endocrinol Diabetes Obes. 2013;20:204-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 99] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 3. | Hoang AN, Volkin D, Yerram NK, Vourganti S, Nix J, Linehan WM, Wood B, Pinto PA. Image guidance in the focal treatment of prostate cancer. Curr Opin Urol. 2012;22:328-335. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 4. | Lindner U, Trachtenberg J, Lawrentschuk N. Focal therapy in prostate cancer: modalities, findings and future considerations. Nat Rev Urol. 2010;7:562-571. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 77] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 5. | Hildebrandt B, Wust P, Ahlers O, Dieing A, Sreenivasa G, Kerner T, Felix R, Riess H. The cellular and molecular basis of hyperthermia. Crit Rev Oncol Hematol. 2002;43:33-56. [PubMed] |
| 6. | Ahmed HU, Freeman A, Kirkham A, Sahu M, Scott R, Allen C, Van der Meulen J, Emberton M. Focal therapy for localized prostate cancer: a phase I/II trial. J Urol. 2011;185:1246-1254. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 159] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 7. | Lindner U, Lawrentschuk N, Weersink RA, Davidson SR, Raz O, Hlasny E, Langer DL, Gertner MR, Van der Kwast T, Haider MA. Focal laser ablation for prostate cancer followed by radical prostatectomy: validation of focal therapy and imaging accuracy. Eur Urol. 2010;57:1111-1114. [PubMed] |
| 8. | Lukka H, Waldron T, Chin J, Mayhew L, Warde P, Winquist E, Rodrigues G, Shayegan B. High-intensity focused ultrasound for prostate cancer: a systematic review. Clin Oncol (R Coll Radiol). 2011;23:117-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 55] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 9. | Soylu FN, Eggener S, Oto A. Local staging of prostate cancer with MRI. Diagn Interv Radiol. 2012;18:365-373. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 12] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 10. | Jia G, Abaza R, Williams JD, Zynger DL, Zhou J, Shah ZK, Patel M, Sammet S, Wei L, Bahnson RR. Amide proton transfer MR imaging of prostate cancer: a preliminary study. J Magn Reson Imaging. 2011;33:647-654. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 158] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 11. | Wang S, Peng Y, Medved M, Yousuf AN, Ivancevic MK, Karademir I, Jiang Y, Antic T, Sammet S, Oto A. Hybrid multidimensional T(2) and diffusion-weighted MRI for prostate cancer detection. J Magn Reson Imaging. 2014;39:781-788. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 42] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 12. | Ahmed HU, Hindley RG, Dickinson L, Freeman A, Kirkham AP, Sahu M, Scott R, Allen C, Van der Meulen J, Emberton M. Focal therapy for localised unifocal and multifocal prostate cancer: a prospective development study. Lancet Oncol. 2012;13:622-632. [PubMed] |
| 13. | Partanen A, Yerram NK, Trivedi H, Dreher MR, Oila J, Hoang AN, Volkin D, Nix J, Turkbey B, Bernardo M. Magnetic resonance imaging (MRI)-guided transurethral ultrasound therapy of the prostate: a preclinical study with radiological and pathological correlation using customised MRI-based moulds. BJU Int. 2013;112:508-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 22] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 14. | Meshorer A, Prionas SD, Fajardo LF, Meyer JL, Hahn GM, Martinez AA. The effects of hyperthermia on normal mesenchymal tissues. Application of a histologic grading system. Arch Pathol Lab Med. 1983;107:328-334. [PubMed] |
| 15. | Ishihara Y, Calderon A, Watanabe H, Okamoto K, Suzuki Y, Kuroda K, Suzuki Y. A precise and fast temperature mapping using water proton chemical shift. Magn Reson Med. 1995;34:814-823. [PubMed] |
| 16. | Chen J, Daniel BL, Diederich CJ, Bouley DM, van den Bosch MA, Kinsey AM, Sommer G, Pauly KB. Monitoring prostate thermal therapy with diffusion-weighted MRI. Magn Reson Med. 2008;59:1365-1372. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 46] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 17. | Jacobs MA, Herskovits EH, Kim HS. Uterine fibroids: diffusion-weighted MR imaging for monitoring therapy with focused ultrasound surgery--preliminary study. Radiology. 2005;236:196-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 89] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 18. | Chopra R, Tang K, Burtnyk M, Boyes A, Sugar L, Appu S, Klotz L, Bronskill M. Analysis of the spatial and temporal accuracy of heating in the prostate gland using transurethral ultrasound therapy and active MR temperature feedback. Phys Med Biol. 2009;54:2615-2633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 53] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 19. | Kim HL, Mhoon DA, Brendler CB. Does the CaverMap device help preserve potency? Curr Urol Rep. 2001;2:214-217. [PubMed] |
| 20. | Walsh PC, Marschke P, Catalona WJ, Lepor H, Martin S, Myers RP, Steiner MS. Efficacy of first-generation Cavermap to verify location and function of cavernous nerves during radical prostatectomy: a multi-institutional evaluation by experienced surgeons. Urology. 2001;57:491-494. [PubMed] |
| 21. | Makin CA, Bobrow LG, Bodmer WF. Monoclonal antibody to cytokeratin for use in routine histopathology. J Clin Pathol. 1984;37:975-983. [PubMed] |
| 22. | Van Leenders GJ, Beerlage HP, Ruijter ET, de la Rosette JJ, van de Kaa CA. Histopathological changes associated with high intensity focused ultrasound (HIFU) treatment for localised adenocarcinoma of the prostate. J Clin Pathol. 2000;53:391-394. [PubMed] |
| 23. | Sapareto SA, Dewey WC. Thermal dose determination in cancer therapy. Int J Radiat Oncol Biol Phys. 1984;10:787-800. [PubMed] |
| 24. | Foster RS, Bihrle R, Sanghvi N, Fry F, Kopecky K, Regan J, Eble J, Hennige C, Hennige LV, Donohue JP. Production of prostatic lesions in canines using transrectally administered high-intensity focused ultrasound. Eur Urol. 1993;23:330-336. [PubMed] |
| 25. | Gelet A, Chapelon JY, Margonari J, Theillere Y, Gorry F, Cathignol D, Blanc E. Prostatic tissue destruction by high-intensity focused ultrasound: experimentation on canine prostate. J Endourol. 1993;7:249-253. [PubMed] |
| 26. | Hwang SI, Lee HJ. The future perspectives in transrectal prostate ultrasound guided biopsy. Prostate Int. 2014;2:153-160. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 15] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 27. | Sciarra A, Barentsz J, Bjartell A, Eastham J, Hricak H, Panebianco V, Witjes JA. Advances in magnetic resonance imaging: how they are changing the management of prostate cancer. Eur Urol. 2011;59:962-977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 202] [Cited by in RCA: 185] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 28. | Turkbey B, Pinto PA, Mani H, Bernardo M, Pang Y, McKinney YL, Khurana K, Ravizzini GC, Albert PS, Merino MJ. Prostate cancer: value of multiparametric MR imaging at 3 T for detection--histopathologic correlation. Radiology. 2010;255:89-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 392] [Cited by in RCA: 413] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 29. | Chopra R, Colquhoun A, Burtnyk M, N’djin WA, Kobelevskiy I, Boyes A, Siddiqui K, Foster H, Sugar L, Haider MA. MR imaging-controlled transurethral ultrasound therapy for conformal treatment of prostate tissue: initial feasibility in humans. Radiology. 2012;265:303-313. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 81] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 30. | Diederich CJ, Stafford RJ, Nau WH, Burdette EC, Price RE, Hazle JD. Transurethral ultrasound applicators with directional heating patterns for prostate thermal therapy: in vivo evaluation using magnetic resonance thermometry. Med Phys. 2004;31:405-413. [PubMed] |
| 31. | Siddiqui K, Chopra R, Vedula S, Sugar L, Haider M, Boyes A, Musquera M, Bronskill M, Klotz L. MRI-guided transurethral ultrasound therapy of the prostate gland using real-time thermal mapping: initial studies. Urology. 2010;76:1506-1511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 54] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
P- Reviewer: Soria F S- Editor: Ji FF L- Editor: A E- Editor: Wu HL