INTRODUCTION
The treatment of pain related to vertebral body fractures by vertebroplasty or kyphoplasty has become a widespread practice. The main reported advantages of Kyphoplasty vs vertebroplasty are restoration of vertebral body height and a lower rate of cement extravasation. Although both techniques would appear effective in achieving pain relief, the impact on functional outcome is not sufficiently proved.
The choice of vertebroplasty or kyphoplasty and the selection of patients for one or other procedure remain unresolved questions. These issues are complicated by the considerable competition between the procedures and the conflicting claims made for each[1]. The degree of pain relief and functional improvement obtained must play an important role in the choice between these techniques. Previous studies reported good outcomes that remained stable during long follow-up periods for both vertebroplasty and kyphoplasty[2,3]. However, comparative studies are scarce. At present, different devices claiming advantages over more traditional methods are being developed.
Due to the existing controversies regarding the treatment of vertebral fractures, the objective of this review is to analyze the evidence of current literature supported by our own experience.
EPIDEMIOLOGY AND TYPES OF VERTEBRAL FRACTURES
Vertebral fractures can be secondary to high or low energy trauma. With population ageing most of the fractures (85%) are due to low energy trauma. These are more frequent in women and its prevalence increase with age in both sexes. High energy fractures are more frequent in men and are not related with older age. The incidence of clinically diagnosed vertebral fractures was assessed in a population-based study in Rochester, Minnesota, 1985-1989. The overall age- and sex-adjusted incidence rate was 117 per 100000 person-years (95%CI: 105 to 130). The age-adjusted rate in women (145 per 100000 person-years) was almost twice that in men (73 per 100000 person-years). Of all fractures, 47 (14%) followed severe trauma, 282 (83%) followed moderate or no trauma, and 12 (3%) were pathologic[4].
Vertebral compression fractures (VCF) are the most common fracture in osteoporotic patients, followed by hip, wrist or ankle fractures. These are known as low energy fractures or insufficiency fractures because the main cause is the fragility of bone that make it prone to injury with minimal or not trauma. After suffering the first vertebral fracture, the risk of developing new vertebral fractures increases 5-10 times[5].
Pathologic vertebral fractures are secondary to osseous involvement by a localized debilitating condition, mainly tumors. Most are due to malignancies such as metastases, myeloma and primary bone tumors, although benign tumors like haemangioma can also lead to a vertebral fracture. The spine is the musculoskeletal target most affected by metastases, mainly secondary to breast, lung, prostate, kidney and thyroid tumors[6]. Nevertheless, even in oncologic patients, a third of the vertebral fractures are due to coexisting osteoporosis[7]. Imaging techniques and biopsy help to differentiate the underlying cause.
Vertebral fractures happen in 50%-70% of patients with multiple myeloma. It may lead to spinal cord compression until 15% of the patients. On imaging its appearance may be misleading, simulating an osteoporotic vertebral compression fracture[8].
IMAGING OF VERTEBRAL FRACTURES
Conventional Radiographs are usually the first technique used to study patients with suspected vertebral fracture. While its ability to diagnose high energy posttraumatic fractures is high, its findings may be misleading diagnosing pathologic or insufficiency fractures.
When a high energy fracture is detected radiographically, many authors suggest the addition of computed tomography (CT) to the study, as it allows for better definition of fracture anatomy. When the clinical presentation suggests a fracture and conventional radiography is not diagnostic, a multi-slice CT or magnetic resonance imaging (MRI) help to clear out traumatisms of spine. A prospective study found a CT sensitivity of 99% in detecting fractures compared to 87% with plain-film radiography[9].
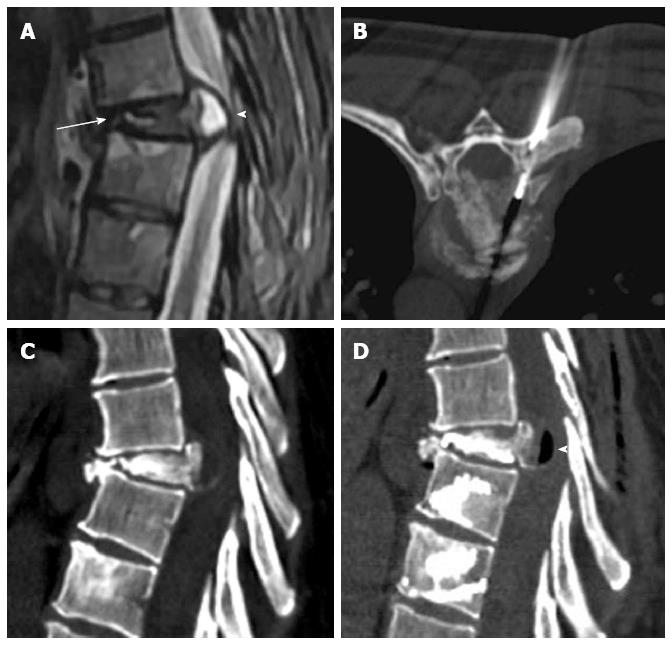
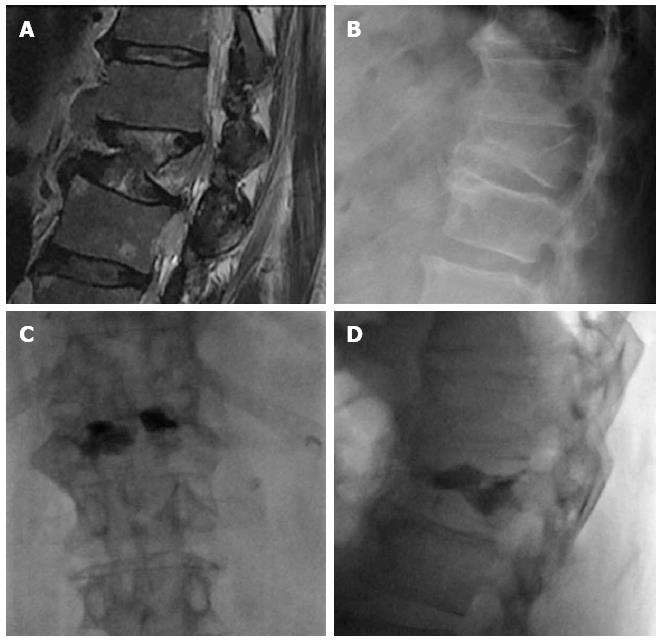
Osteoporotic vertebral fractures deformity usually is presented in two ways, wedge and biconcave (fish vertebra or diabolo-shaped vertebra) types, while pathological fractures often demonstrate predominately osteolytic changes. The presence of air collections within a vertebral body is considered a sign of vertebral cleft that may be due to fracture instability and/or necrosis (Kummel’s disease) and is more frequently associated to benign osteoporotic fractures[10]. Nevertheless cases associated to metastasis and myeloma has been described[11] (Figure 1).
Figure 1 Sagittal T2 weighted (A) image shows metastatic compression fracture with intravertebral cleft (arrow) and epidural cyst (arrowhead), computed tomography guided biopsy (B), Sagittal computed tomography before (C) and after (D) vertebroplasty showing air filling of the cyst (arrowhead).
A 15% vertebral body height loss constitutes a vertebral compression fracture. The leading cause of vertebral compression fractures is osteoporosis. Morphologic changes that allow for the diagnosis of an osteoporotic fracture may require time for their development. Therefore, the absence of a fracture on plain-film radiography in an osteoporotic patient does not rule it out, and when symptoms persist, a MRI should be performed. MRI can detect fractures without vertebral deformities and can better discriminate between benign and malignant fractures[12]. Additionally, MRI provides valuable information on factors such as the degree of edema, vertebral deformities, and invasion of the spinal canal, all of which are useful data for planning medical, percutaneous (kyphoplasty, vertebroplasty) or surgical treatment[13].
Plain-film radiography is somewhat insensitive when it comes to the visualization of the bone destruction or marrow replacement, requiring, depending on the size of the lesion, between 30%-50% of bone density loss before the lesions become visible[14]. The detection of blastic lesions may also be delayed with conventional radiography. One study reported that in the case of breast cancer, plain film detection of blastic lesion may be delayed by 3-6 mo[15].
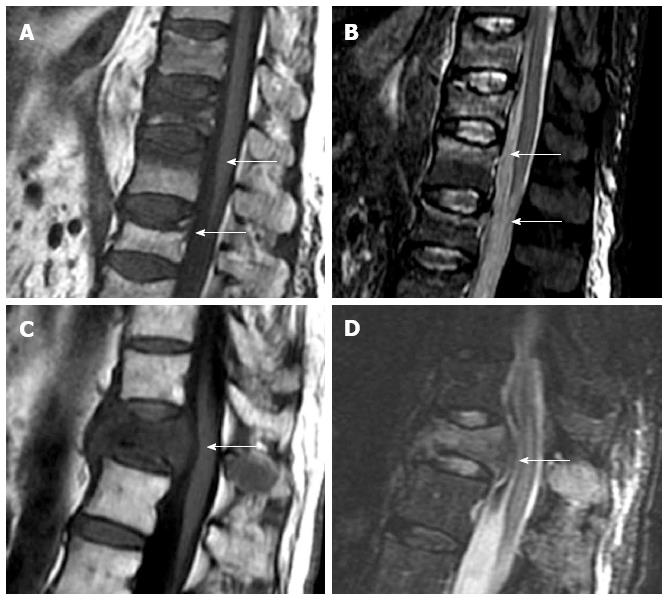
Based on vertebral morphology and bone marrow signal, MRI can differentiate between osteoporotic and pathologic fractures with high confidence. Pathologic fractures may show complete substitution of normal bone marrow or, when incomplete, tend to show and nodular or patchy pattern. Morphologic signs are a convex vertebral border, due to expansion by growing tumor, and the presence of asymmetric paravertebral mass. Acute osteoporotic vertebral fractures tend to show a band-like pattern of subchondral edema and, quite often, the lineal pattern of the vertebral fracture can be depicted inside the edema[13]. A retropulsed bone fragment and the presence of intra vertebral cleft are characteristic of benign compression fractures[11]. Chronic vertebral compression fractures are characterized by morphologic changes with recovery of normal signal of the bone marrow (Figure 2).
Figure 2 Sagittal T1 weighted (A) and STIR (B) images of osteoporotic fractures with typical band-like subchondral edema (arrows), sagittal T1 weighted (C) and STIR images (D) of a pathologic fracture, due to vertebral metastases, with typical convex border (arrows).
MEDICAL TREATMENT OF VERTEBRAL FRACTURES
Non-operative treatment of traumatic vertebral compression fractures is usually appropriate for patients with normal neurological status without suspected radiological instability, with an anterior vertebral body height > 50% of the posterior height and a Kyphotic angulation < 25º; or incomplete injury of the posterior column of the vertebral body[16,17]. Although in some cases early closed reduction and casting can be performed, bracing alone and physiotherapy in younger patients (< 65 years) is usually the best treatment option. A short period of bed rest (less than 1 wk) avoids complications caused by immobilization. In older patients, percutaneous vertebral augmentation may promote early mobilization and reduce analgesic intake[17].
Traditional treatment for osteoporotic fractures has been medical, including lifestyle changes (diet, smoking and exercise), pain management with rest, analgesic and anti-inflammatory drugs and external brace. Medical treatment for osteoporosis includes calcium supplements, vitamin D, hormone replacement and bisphosphonates. These treatments have strong effects on pain, but minimal effect on vertebral stability, vertebral height restoration or reduction of kyphotic deformity. Side effects of chronic medication and rest are increased demineralization of bone and greater risk of developing new fractures. Surgery is left for fractures with vertebral instability or neurological compression.
Traditional treatment for painful vertebral metastases is based on rest, braces, analgesic drugs, radiotherapy and chemotherapy. External beam radiation therapy is the current gold standard treatment for cancer patients with localized bone pain. Nevertheless, 20%-30% of patients do not experience pain relief with this approach. Radiation treatment can also result in additional early bone loss due to inflammation, and limited weight-bearing should be recommended during radiation to prevent pathological fractures. Radiotherapy control of pain is achieved in approximately 70%-80% of the cases, but its maximum effects usually takes place 1 mo after the beginning of treatment and osseous reinforcement up to 2 to 4 mo after, increasing the risk of vertebral collapse with its biomechanical consequences[18].
PERCUTANEOUS AND SURGICAL TREATMENT OF VERTEBRAL FRACTURES
Criteria for surgical indication of high-energy fractures are variable[19]. In our center, surgery is mainly indicated when the body fracture is associated to injury of the posterior column or in cases with neurologic deficit (deterioration of the initial neurologic status constitutes an emergency. It can also be indicated in cases without neurological deficit when there are other radiological signs of instability: central canal narrowing > 50%, vertebral height loss > 50%, fracture-dislocation, local vertebral kyphosis > 25º-30º, regional traumatic angle of kyphosis > 20% and sagittal index (SI) > 15º.
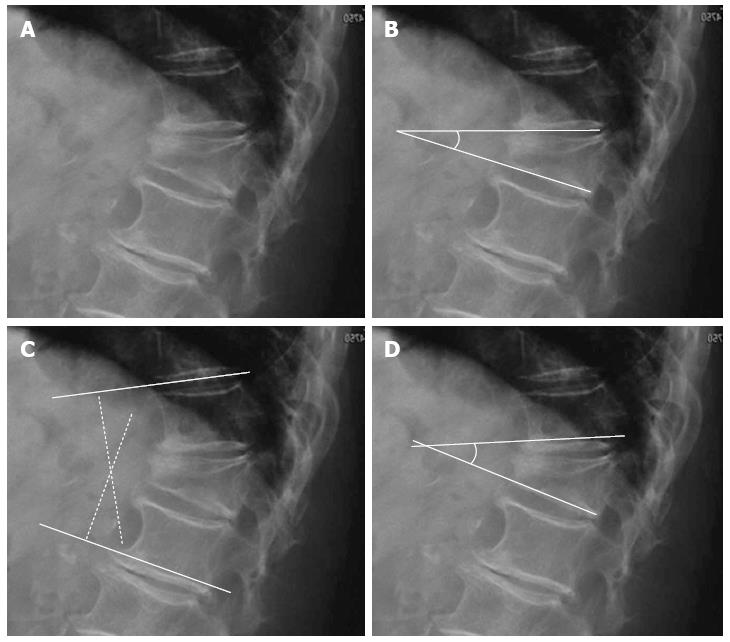
Local vertebral kyphosis angle is measured between the tangent to the upper endplate and the lower endplate of the injured vertebra. Regional kyphosis is the angle defined by the tangent to the upper endplate of the vertebra overlying the fracture and the tangent to the lower endplate of the vertebra underlying the injured vertebra. The SI is defined as segmental kyphotic deformity minus baseline sagittal contour in the segment with the fractured vertebral body. The segmental kyphotic deformity is the angle between the inferior endplate of the injured vertebra and the inferior endplate of the overlying vertebra. The baseline sagittal contour in each vertebral segment arbitrarily amounts to +5° for the thoracic region, 0° T12-L1 and -10° for the lumbar spine segments (Figure 3). The normal index is 0[20].
Figure 3 Segmental kyphotic deformity.
A: Wedge fracture of T12; B: Local vertebral kyphosis angle; C: Regional kyphosis; D: Segmental kyphosis (SK). One vertebra, one disc=one segment. Sagittal index (SI): SI = SK-X (X = +5 in the thoracic spine, X = -10 in the lumbar spine, X = 0 in T12-L1).
Accepted methods for surgical decompression and stabilization include anterior or posterior approaches and a combination of both procedures. Instrumented fusion is better than laminectomy alone because it does not restore neurological function and it’s associated with significant complications, such as persistent spinal instability and progressive kyphosis, mechanical pain and worsening of neurological injury[19].
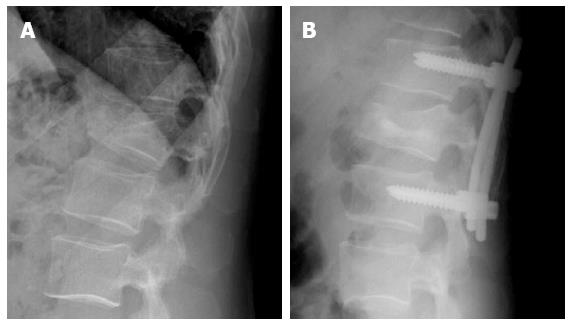
Sometimes, instrumentation is complemented with VT or KP. Transpedicular vertebral augmentation for the direct restoration of burst fractures in combination with posterior instrumentation may avoid the surgical anterior reconstruction. The aim is to reinforce the anterior column and prevent anterior vertebral body height loss[21] (Figure 4).
Figure 4 Forty-five years old man with acute Wedge impaction fracture of L1 (A) treated by posterior instrumentation and vertebral body kyphoplasty using biological cement (B).
The role of vertebroplasty and kyphoplasty in the treatment of high energy vertebral fractures is not still well defined, although good results with regard pain relief and quality of life have been reported using both procedures[22,23]. In young patients it has been suggested the use of biological cement, instead of PPMA, due to its capacity of integrating with bone[24]. Nevertheless, it has been reported that low resistance against flexural, tractive, and shear forces compared to PMMA, may lead to a higher risk of cement failure and subsequent loss of correction, mainly when fracture of the posterior wall of the vertebra is present[25]. The aim of treating percutaneously these fractures is to recovery vertebral height and to obtain early relieving of pain in order to reducing recovery time in young active population.
The principal surgical options for treatment of osteoporotic VCFs are decompression and fusion. The success of surgical instrumentation is compromised by poor bone quality[26]. As a result, interest in new and quick methods for pain relief and early functional restoration has increased. Percutaneous treatment of osteoporotic fractures is mainly indicated after failure of medical treatment, when the patient has a disabling pain or when there are severe side effects due to analgesic medication. In order to minimize these effects in patients with VCFs and reduce prolonged hospital resource utilization, VP and KP have been increasingly used with the expectation of a more rapid pain relief and earlier mobilization than that achieved with medical pain management[27]. Recently a randomized non-blinded trial appeared, strongly indicating that vertebroplasty is dramatically superior to conservative therapy[28].
Surgery in vertebral metastases is left for lesions affecting a unique vertebra or for treating neurological deficit secondary to compression or instability. Inconveniences of surgery are long recovery times and high morbidity and mortality[29].
Although external beam radiation therapy is the current gold standard treatment for cancer patients with localized bone pain we have to take into consideration that the life expectancy of most patients with bone metastases is limited, and that around 12-20 wk are usually required before maximum benefits are obtained from post-radiation therapy. In these cases vertebral augmentation techniques in isolation or combined with thermal ablation provide the earliest possible pain relief[30].
HISTORICAL NOTES
Deramon and Galibert performed the first vertebroplasty in France in 1984. It was performed in a patient with an aggressive haemangioma at the C2 level with resolution of pain. The results were so gratifying that cement injections were soon used in more patients with symptomatic hemangiomas and fractures due to tumors. The application of vertebroplasty in osteoporotic VCF was first published in 1989[31]. The hopeful analgesic effect led to the widespread use of augmentation for treating osteoporotic VCF[32]. Over the last years, osteoporotic fractures have become the main indication for vertebroplasty[33].
Kyphoplasty is the most widely used modification of vertebroplasty and was developed specifically for use in the osteoporotic vertebra. The basic idea behind this procedure was to raise the end plate of fractured vertebral body with an orthopedic balloon to achieve a more favorable angle of kyphosis before the cement augmentation. Therefore, a cavity is first created within the vertebral body before injecting the cement. The first kyphoplasty was performed by the orthopedic specialist Mark Reiley in California in 1998 with good results[34].
TECHNICAL ISSUES
Vertebroplasty involves the injection of polymethil-metacrhilathe (PMMA) cement into an injured vertebral body via a needle that is placed percutaneously either using a transpedicular or extrapedicular approach. The injection has to be forced to surpass the local pressure of the trabecular bone of treated vertebra increasing the risk of leakage through the cracks of the fractured vertebra. It may be performed under general anesthesia, although more commonly the patient is given a local anesthetic at the injection site and conscious sedation[35].
Kyphoplasty, a modification of vertebroplasty, involves the percutaneous insertion of an inflatable high-pressure bone tamp into the fractured vertebral body with the aim of elevate the end-plates by creating a cavity inside the vertebral body that filled with cement help to restore and stabilize the vertebral height. The cavity allows low pressure injection of more viscous cement, lowering the risk of extravasation[33].
PMMA is the most frequent cement used in these procedures. It is the result of the polymerization of methyl methacrylate monomers to PMMA polymers. It is cheap, easy to manipulate, allows combination with radiopaque materials and gives the appropriate stiffness and strength to the vertebral body. However, it does not have osteoinductive or osteoconductive properties and, therefore, it will not integrate itself to host bone over time. Its stiffness may promote mechanical overload to adjacent vertebral bodies[25].
New biological materials have been introduced as alternatives to PMMA, such as calcium phosphate and hydroxyapatite. These are not exothermic, allowing the deposition of new bone that, eventually, could replace the cement. These cements would in time become incorporated into the patient’s bone, therefore functioning in a more physiological and biomechanically compatible fashion. This requires the presence of trabecular bone and the haversian canal system, thus cavity creation with a balloon will probably never be a part of the future of biological cements and prophylactic vertebroplasty[33]. Nevertheless, biological cements are expensive; their manipulation is not easy due to their high viscosity that makes difficult the interstitial diffusion inside the vertebral body[36]. These materials have been recommended in high-energy fractures of young patients[37], although other authors find a high rate of mechanical failure with these materials, due to its lower resistance to shear, flexion and distraction forces[25].
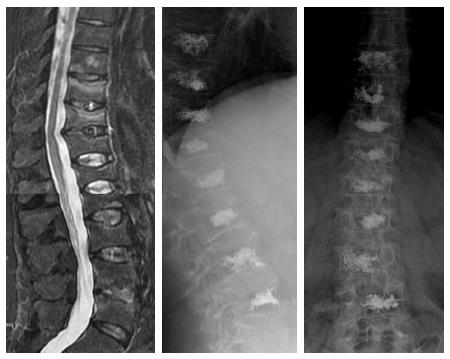
The number of vertebrae augmentable per session also remains unclear, although extensive augmentation to more than three vertebral levels per session has been shown as feasible[38]. However, it may lead to an increase of the amount of bone marrow floating to the pulmonary capillary system, increasing the risk of pulmonary fat embolism. Also, with PMMA there is some risk of incomplete polymerization, leading to increased residual toxic monomers that may result in adverse systemic effects, such as hypotension, bradycardia, asystole and bronchospasm. If the amount of cement injected in each vertebral body due to these reasons is reduced, it might result in incomplete stabilization of some vertebral body fractures leading to residual instability and pain. Notwithstanding, we have treated some cases of multilevel fractures in one single operative session with quite good results[35] (Figure 5). We recommend carefully planning the positioning of the needles and the amount of cement to be injected; taking into account the anatomical characteristics of each vertebral fracture, such as location of the fracture lines, integrity of vertebral walls and the presence of vertebral clefts.
Figure 5 Sagittal STIR image (A) in a patient with multiple thoracolumbar compression fractures, eight vertebrae were treated in the same procedure (B and C).
CLINICAL SUCCESS OF VERTEBROPLASTY AND KYPHOPLASTY
Analgesic effect effects of these techniques can rely in many factors, such as ablation of C-nociceptive fibers by the thermal effect of the cement, mechanical stabilization of the fracture, height restoration of the vertebral body. The thermal effect also leads to tumor necrosis in patients with metastases[39].
Mechanical stabilization of the vertebral body relies on basal bone density, volume and localization of the injected cement. The filling of 14% to 30% of the volume is able to recover vertebral stiffness, although partial recovery of the stiffness, below the pre-fracture state, would be enough to obtain clinical healing[40].
Cadaveric studies have shown greater recovery of vertebral height with kyphoplasty (5.1 mm) than with vertebroplasty (2.3 mm)[41]. Yet, clinical studies are contradictory. While some authors found greater height restoration with kyphoplasty[42], others didn’t find differences between both techniques[43]. In comparison with cadaver studies, the disk and paravertebral soft tissues may hinder augmentation with more aggressive techniques in living patients.
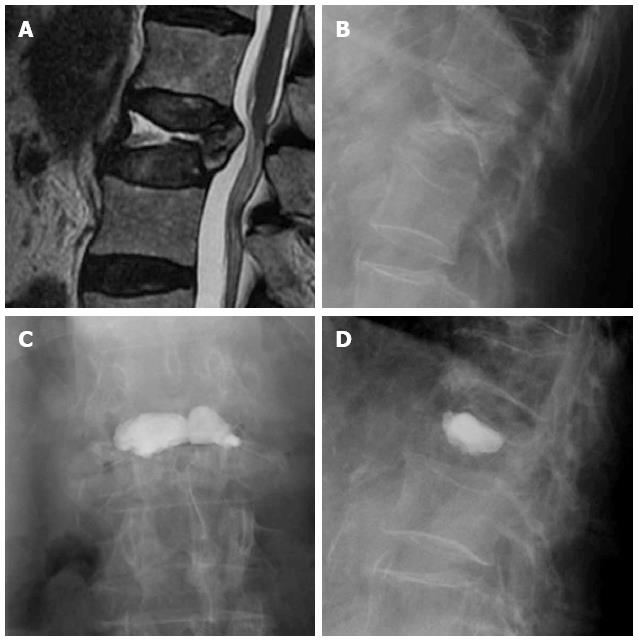
Vertebroplasty can mainly restore vertebral height when there is fragment instability with intravertebral clefts, indicating non-union of the fracture fragments. The cleft may be filled with greater amount of cement, mainly when there is integrity of the walls of the vertebral body, without extravasations leading loss of intravertebral pressure[41,44] (Figure 6).
Figure 6 Sagittal T2 weighted image (A) and lateral X-ray film (B) of a vertebral fracture with intravertebral cleft, AP (C) and lateral (D) view after vertebroplasty.
Relationship between vertebral height restoration and clinical evolution is not well established. Some studies found no better pain resolution with height restoration and don’t consider this factor mandatory in order to achieve pain control[45].
Another issue to consider is the stability over time of the height restoration. A follow up of height loss after performing these techniques shows the loss to be greater in kyphoplasty, due to less homogeneous distribution of cement, than in vertebroplasty, where the cement intermingles with host bone. Therefore, height recovery differences tend to vanish with time[46].
Short-term pain relief has been demonstrated in osteoporotic and tumor fractures treated with vertebroplaty and kyphoplasty[47,48]. Long-term outcomes have not been so well established, although some reports state that the beneficial effects of vertebroplasty remain after a follow-up period of several years[49,50].
Compared with medical treatments, pain relief after VP seems on the whole significantly superior. The follow-up point at which the difference becomes insignificant varies between studies at 3 mo[51], 6 mo[52] or 1 year[53]. Regarding Kyphoplasty, two prospective controlled studies evaluated and compared the efficacy and safety of this technique vs medical management and found better long-term pain relief and superior functional outcome with kyphoplasty, up to 3 years[54,55].
CLINICAL EVIDENCE OF AUGMENTATION EFFECTIVENESS
Two recent randomized works[56,57] stated that there is not better results between vertebroplasty and a sham treatment that only inject local anesthetic in the fractured area. Nevertheless, bias in both studies may invalidate their conclusions[58]. First of all, the small sample size avoid that a better evolution in the vertebroplastic group reaches statistical significance. Second at all, the percentage of control patients that chose to pass to the vertebroplasty group was high enough to invalidate the randomization of the studies. With regard selection of the patients, those with high pain scores were not included. These patients tend to show better results after vertebroplasty[59]. Another important factor was the inclusion of a high percentage of non-acute fractures; therefore, it is unclear if the origin of the back pain was the osteoporotic VCF or other common reasons for back pain in the elderly, such as arthritis of facet joint or disc pain. By nature of the patient population studied, “sham” facet injections may have led to decreased facet pain. Local anesthetic infiltration of the posterior longitudinal ligament is an established treatment for osteoarthritis back pain. Perhaps a sham procedure in which a dry needle was inserted might have been a more appropriate control. Other differences with previous studies are lower amount of injected PMMA, non-confirmation by imaging of vertebral fractures previous to procedure in patients with known fractures of less than 1 year and a lack of standardization of the medical treatment. The interpretation of the data is even more difficult due to the absence of a medical treatment group[60].
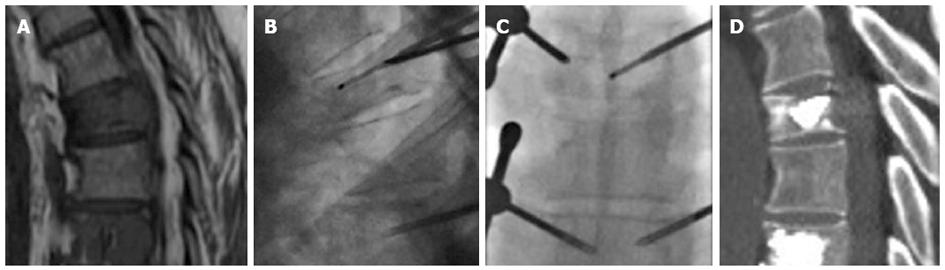
It has been stated that a percentage of patients with pain following VCF do not have pain arising from the fracture itself, but due to instability or overload on the facet joints produced by adjacent vertebral body deformity[61]. Since both causes of pain, VCF and osteoarthritis, may concur at the same time, we often combine vertebroplasty and spinal injection in the same session, mainly in older patients, relying on edema detected at the facet joints or on degenerative vertebral endplates changes, at the same or at a different level of the fractured vertebrae (Figure 7). This is supported by previous studies that found overall facet joint signal-change scores significantly higher at vertebral body levels affected by an acute/subacute compression fracture than in control levels with either normal bodies or chronic compression fractures[62]. This practice does not add too much time to vertebroplasty procedures, avoiding multiple scheduling of patients.
Figure 7 Sagittal T1 weighted image (A) showing vertebral compression fracture of L2 (arrow) and degenerative spondylolisthesis of L4 (arrowhead), computed tomography guided transforaminal epidural injection (B), Sagittal computed tomography after vertebroplasty (arrow) and epidural injection (arrowhead) (C).
It is highly recommended to perform a spine MRI close before any of these percutaneous procedures[63]. The presence of a pattern of bone marrow edema is associated with a good clinical short term success relieving pain[54]. Nevertheless, improvement has been also demonstrated in patients with vertebral fractures without bone marrow edema[64].
Grades of recommendation of these techniques are based on the clinical evidence of published papers as follow[65]: Good evidence [level I studies with consistent findings; e.g., high quality randomized controlled trials (RCT)], Fair evidence (level II or III studies with consistent findings; e.g., low quality RCT, case/control and cohorts studies), Poor quality evidence (level IV or V studies with consistent findings; e.g., case series and expert opinions), or Insufficient evidence (inconsistent findings or lack of investigation for or against recommending intervention).
Meta-Analysis of published papers show fair to good evidence that in patients with osteoporotic VCF outcomes on physical disability, general health and pain relief are better with VP and KP than with medical management within the first 3 to 6 mo after intervention. Nevertheless, there is fair evidence that by the first or second year after intervention, VP provides a similar degree of pain control and physical function as that attained with optimal medical management. There is insufficient evidence whether KP results in greater pain relief one and 2 years after intervention[27].
Although not assessed in comparative studies, the reported degree of acute pain improvement in tumor-associated vertebral compression fractures is far better than that typically reported with radiation and medical management. Nevertheless, studies yield poor-quality evidence[27].
COMPLICATION OF TREATMENT OF VERTEBRAL FRACTURES
The aggregate rates of complications of vertebroplasty and kyphoplasty are small; ranging from 2%, when treating osteoporotic compression fractures, to 10% in cases related to malignant tumors[46].
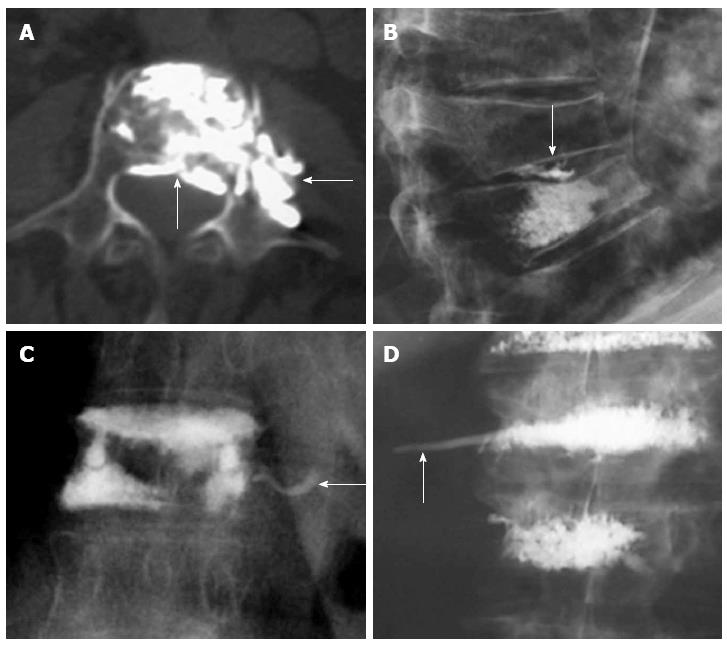
The main risk of these percutaneous procedures is the extravasation of PMMA. Investigations on cement leakage in vertebroplasty report a rate of 11%-76%. In investigations on kyphoplasty, cement leakage data ranges from 4.8% to 39%. Cement leakage is reported at a higher rate if CT scans are used[45]. There are many routes by which cement may leak from a vertebra: paravertebral leakage, venous leakage or leakage into the spinal canal and intervertebral foramen (Figure 8). Injury of the surrounding soft tissues is mainly due to the high temperature of polymerization of PMMA. The most sensitive structures are neural tissues, spinal cord and nerve roots. Fortunately, most of the extravasations are to the disk or paravertebral tissues, hence asymptomatic. Transient radicular symptoms have been described in up to 3%-4% of the patients[66] and only isolated cases of paraplegia after these procedures have been reported, most of them due to failure of technical issues[48].
Figure 8 Axial computed tomography shows extravasations to epidural space and paravertebral area (arrows) (A), leakage to the intervertebral disc (arrow) (B), venous leakage (arrow) (C), tail of cement in the path of the needle (arrow) (D).
The monomers that don’t contribute to the polymerization have systemic cardio-pulmonary effects. Pulmonary embolism can be due not only to the cement but also to the fat from the bone marrow extruded into the venous system by the high pressure injected cement or by inflating the balloons[67].
The relationship between percutaneous vertebral augmentation and the development of new fractures it is not well stated. Previous studies have found a greater rate of new fractures in these patients than in the osteoporotic population, but the same as in those osteoporotic patients that have already developed a fracture[68]. Another article found fewer incidences of new fractures in patients treated with kyphoplasty than in patients managed with medical therapy. This was attributed to improvement of mechanical conditions due to vertebral height restoration and kyphotic correction[36]. The higher incidence of fractures in the early postoperative period could potentially be explained by increased patient activity and higher stress secondary to a diminished level of pain (Figure 9).
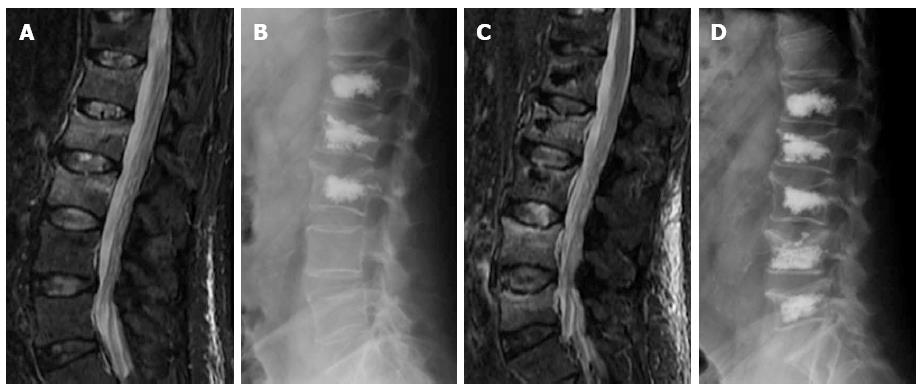
Figure 9 Sagittal STIR magnetic resonance image (A) shows fractures with edema of L1 to L3.
Kyphoplasty was performed in these vertebrae (B), 3 wk later back pain returned and magnetic resonance imaging showed development of new fractures in L4 and L5 (C), vertebroplasty was performed at these levels (D).
The possibility of treating adjacent vertebrae to the fractured vertebra is not supported by evidence studies[69], but we think that it is highly recommended treating a non-fractured vertebra when both, the upper and lower adjacent vertebrae, have been cemented.
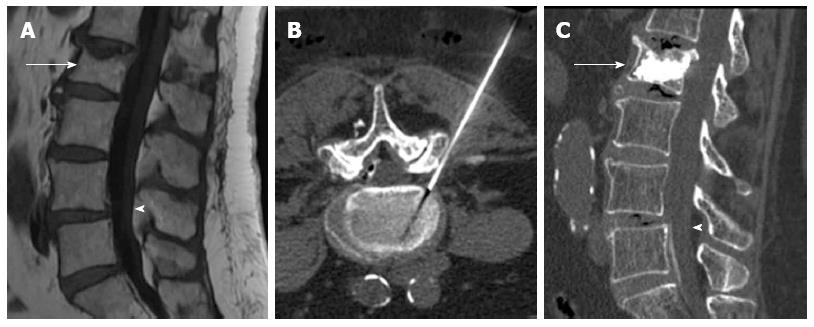
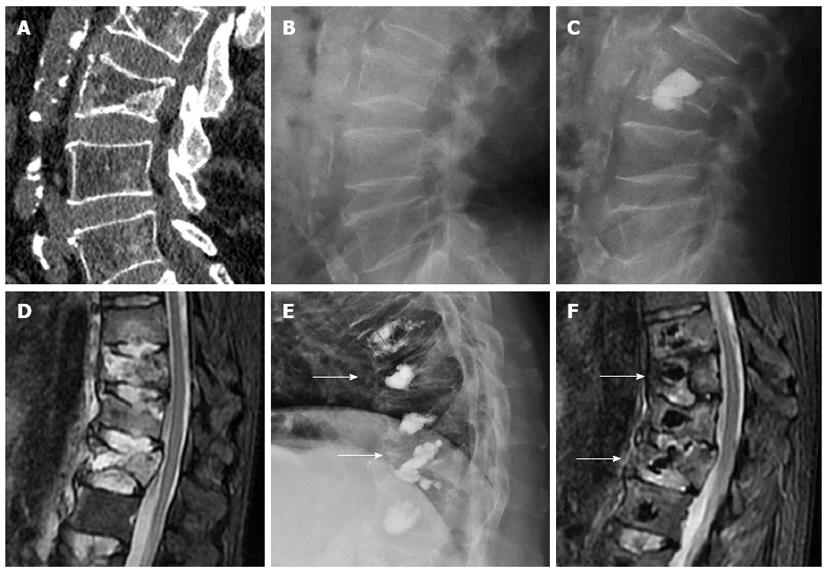
Another complication is incomplete stabilization or residual instability of treated vertebral bodies. Due to the lack of data in spine literature regarding this issue as a cause of procedural failure, there are no figures. Relapse of vertebral instability may be due to mechanical failure of the cement, mainly when biological substitutes are used in fractures with loss of integrity of the posterior vertebral wall[25]. Incomplete filling of vertebral fracture lines may be followed by persistent instability with residual edema. When a vertebral cleft or cyst exists, kyphoplasty might be more prone to cause instability than vertebroplasty because the cement ball does not intermingle with vertebral trabeculae (Figure 10).
Figure 10 Sagittal computed tomography (A) a lateral radiography (B) showing fracture of L3 with intravertebral cyst (arrow), after Kyphoplasty (C) the ball of cement is not incorporated to the instable vertebral fracture, sagittal STIR image showing multiple thoracolumbar fractures with edema (D), treated with vertebroplasty, the cement do not stabilized the fracture of T10 and T12 (arrows) with persistent edema 6 mo after the procedure (E, F).
WHAT TREATMENT DO I CHOOSE?
Percutaneous vertebral augmentation has been shown to be more effective than prolonged non-operative medical treatment in patients with painful VCFs when adequate analgesia and improved functional status has not been achieved by nonoperative therapy[70]. The choice of vertebroplasty or kyphoplasty and the selection of patients for one or other procedure remain unresolved questions. These issues are complicated by the considerable competition between the procedures and the conflicting claims made for each[1]. Choice may well be influenced by degree of pain relief, functional improvement and anatomic and technical factors; including operator’s experience or preference. Previous studies reported good outcomes that remained stable during long follow-up periods for both vertebroplasty[71,72] and kyphoplasty[73,74]. However, comparative studies are scarce and acknowledge the need of more high quality randomized controlled trials[75]. A recent meta-analysis found significant differences regarding anatomical restoration by kyphoplasty versus vertebroplasty, such as a mean long term kyphotic correction of 2.64º, mean anterior vertebral height recovery of 3.67 mm and lower risk of cement extravasation (risk ratio of 0.7)[76]. Nevertheless, these anatomic differences were not clinically relevant because most of the studies comparing both techniques found no differences in clinical outcome in osteoporotic patients[77-79]. Although a systematic review found greater quality of life and disability improvement in kyphoplasty over vertebroplasty, the need to define confounding variables was pointed out, because the selection of patients may depend on different indications for KP or VP in non-randomized studies, thus leading to misleading conclusions[80]. An example could be a recent prospective study, included in this systematic review, that found better results in kyphoplasty patients but that was clearly biased because two of the oldest patients were arbitrarily reallocated in the vertebroplasty group[81].
Based on these data, we recommend vertebroplasty as the primary treatment for osteoporotic VCF that do not respond to medical treatment. Kyphoplasty, which is a much more costly procedure, should only be used as an alternative approach in selected patients, such as those with a recent fracture affecting one or two vertebrae.
FUTURE DEVELOPMENTS
A variety of modifications of these techniques with variable success is being used. The commercial success of Kyphoplasty led to the development of several similar devices, but none have shown any benefit over vertebroplasty in an independent head-to-head trial[33].
Now that the Kyphon device is off patent, there are legions of copycat devices available from most manufacturers. A modified procedure employs reusable hinged-tip curet to manually create a cavity in fractured vertebral bodies under fluoroscopy guidance, allowing low resistance injection of more viscous cement[82]. We have often used a similar curet to create space for the balloon when the hardness of the vertebral body prevented the balloon from creating a cavity of the appropriate size.
Vesselplasty has been introduced as a new alternative to vertebroplasty and kyphoplasty. It has been devised to obtain control of the volume of injected cement and restoration of the vertebral body height. It was first performed in 2004 by Darwono. Instead of using a balloon to create a cavity, vesselplasty uses a polyethylene terephthalate balloon container (vessel) that serves as both, a vertebral body expander and a bone cement container. Introduced into the collapsed vertebral body, it is expanded by the injection of PMMA. Due to the porous structure of the vessel, a small amount of bone cement interdigitates with the trabecular bone around the vessel increasing its stability[83]. We are now introducing this technique in our hospital and we have found it very useful in cases of severe vertebral collapse, where the vessel acts as a prosthetic material that minimally restores the crushed vertebral body (Figure 11).
Figure 11 Sagittal T2 magnetic resonance weighted image (A) and lateral X-ray film (B) showing a severe collapse of L1, AP (C) and lateral (D) view after vesselplasty.
Another issue is the combination of percutaneous cementoplasty using polymethyl methacrylate with other techniques, such as radiofrequency thermal ablation (RFTA). It is sometimes indicated to reinforce bone structures and stabilize bones with high risk of pathologic fractures resulting from metastatic disease, and it is especially indicated for weight bearing bones. The combined use of RF ablation and cementoplasty appears to be useful in order to achieve tumor necrosis and stabilize the fractured vertebrae. The coagulation necrosis produced by RFTA may promote the homogenous distribution of the bone cement within the ablated lesion. The clinical use of this combined therapy has been reported in several studies, but experience with this approach in vertebral fractures is still limited. Bone cement heats up to 80 °C, which may help to strengthen the anticancer effects of RFTA[84] (Figure 12). Radiofrequency assistance and heating the needle tip constantly can also be used to increase cement viscosity, lowering the extravasation.
Figure 12 Sagittal magnetic resonance imaging T1 weighted image of a patient with vertebral metastases (A), radiofrequency thermal ablation was performed before cementation (B, C), post procedure computed tomography (D).
CONCLUSION
This extended review try to update the knowledge about vertebral augmentation based in current literature and our own experience. However, operator’s experience or preference is also important clues in deciding appropriate treatment of vertebral fracture. Nevertheless, we hope this review will be helpful to clinician dealing with spinal diseases.