INTRODUCTION
Congenital hyperinsulinism (CHI) is a heterogeneous condition due to the dysregulated and inappropriate secretion of insulin from the β-cells of the pancreas leading to severe hypoglycaemia in infants. The incidence of CHI is 1 in 50000 in the general population and 1 in 2500 in the consanguineous cohorts[1]. Based on the clinical presentation-CHI can be classified into two major subgroups, transient or persistent and based on histology into three forms such as focal (40%), diffuse (50%) or atypical (10%)[2]. Transient CHI is observed in children who are born small for gestational age, those with intra-uterine growth restriction (IUGR), those subjected to birth asphyxia and those born to mothers with diabetes mellitus (pre or gestational diabetes) and can last up to a week[3]. However it can last longer up to a few months in some children with IUGR[4].
The insulin secreting β-cells of the pancreas consists of a potassium channel (KATP channel) which plays a crucial role in insulin secretion and glucose homeostasis[5]. The KATP channel proteins are encoded by two genes. These are “sulphonylurea receptor subunit” (SUR1 encoded by ABCC8) and the “inward rectifying potassium channel subunit” (Kir6.2 encoded by KCNJ11) genes[6]. Both these genes are localised to chromosome 11p15.1[7]. Nearly 90% of medically unresponsive persistent CHI is caused by loss of function (recessive inactivating) mutations in these two subunits of the KATP channel involved in regulating insulin secretion[8]. They are predominantly autosomal recessive (AR) and rarely autosomal dominant (AD) in inheritance.
Mutations in genes encoding enzymes involved in insulin secretion are rare causes of CHI. They are (1) glutamate dehydrogenase (GDH) encoded by GLUD1 gene (AD); (2) glucokinase (GCK) encoded by GCK gene (AD); (3) L-3-hydroxyacyl-coenzyme A dehydrogenase (HADH) encoded by HADH gene (AR); (4) hepatocyte nuclear factor 4-alpha (HNF4-a) encoded by HNF4A gene(AD); (5) monocarboxylate transporter (MCT1) encoded by SLC16A1 gene (AD); and (6) uncoupling protein 2 encoded by UCP2 gene (AD)[9]. No genetic aetiology has been identified in about 70%-80% of patients who are responsive to medical therapy with diazoxide.
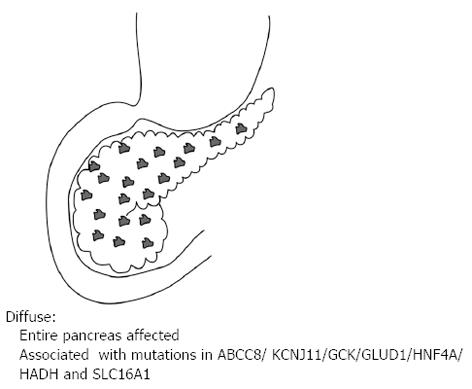
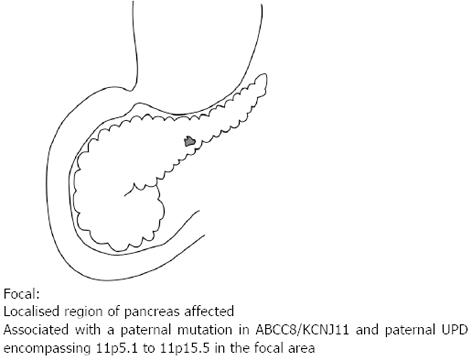
Of the three histological forms of CHI, the typical diffuse form is characterised by the abundant distribution of enlarged and hyperchromatic nuclei throughout the islets[2]. It is predominantly caused by recessive loss of function mutations in the KATP channel (ABCC8 and KCNJ11 genes) and when unresponsive to medical therapy requires a near total (≥ 95%) pancreatectomy[2] (Figure 1). The focal form is characterised by an isolated cluster of abnormal insulin producing cells with ‘normal ‘surrounding tissue within the pancreas (Figure 2). Histologically it involves focal adenomatous hyperplasia of the islet cells with ductuloinsular complexes and scattered giant β-cell nuclei surrounded by normal pancreatic parenchyma[2,10,11]. Focal CHI is always sporadic in inheritance and caused by the paternal heterozygous mutation in ABCC8/KCNJ11 genes along with somatic loss of maternal allele in the focal hyperplastic tissue requiring a focal lesionectomy[12].
Figure 1 Diffuse lesion where the entire pancreas is affected.
It is associated with recessive and dominant mutations in the ABCC8/KCNJ11/GCK/GLUD1/HNF4A/HADH and SLC16A1.
Figure 2 Focal lesion affecting only a single region of the pancreas.
It is associated with a paternal mutation in the ABCC8 or KCNJ11 and paternal uniparental disomy encompassing 11p5.1 to 11p5.5 in the focal area.
Nearly 10% of CHI is of atypical form, the molecular mechanism and histopathological differentiation of which is yet to be completely understood[13]. Children with persistent CHI often require high glucose concentrations (> 8mg/kg/min) to maintain euglycemia. The diagnosis of CHI is primarily made by biochemical investigations demonstrating detectable levels of insulin in relation to hypoglycaemia along with reduced/absent free fatty acids and ketone bodies[12]. Current medical management for CHI includes Diazoxide along with Chlorothiazide as the first line therapy and Octreotide as a second line drug[12].
Children who are not responding to medical management, and in whom the genetic testing is inconclusive or in favour of a focal lesion, should undergo 18F-DOPA-PET scan to ascertain the type of lesion whether it is focal or diffuse and provide guidance towards surgery[1].
Pre-operative delineation of the subtype of the lesion becomes extremely crucial as the management approach and the treatment modalities differ significantly based on the type of the lesion. Focal CHI typically does not respond to medical management and a focal lesionectomy provides a definite cure for the condition by avoiding further hypoglycaemic episodes and its neurological complications. A focal lesionectomy also minimises the risk of iatrogenic diabetes mellitus and exocrine pancreatic insufficiency. Children with diffuse CHI warrant a sub total or a near total pancreatectomy to prevent brain damage from neuroglycopaenia, however the risk of developing diabetes mellitus and exocrine insufficiency in later life is quite high[14].
DOPAMINE METABOLISM
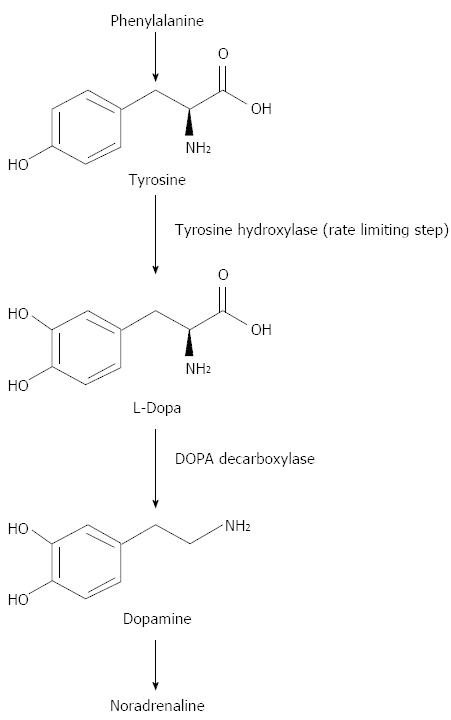
Dopamine is synthesised from a non-essential amino acid, tyrosine which is first converted to L-dopa by tyrosine hydroxylase. This is the rate limiting step in dopamine production. Then L-Dopa is decarboxylated to dopamine by DOPA decarboxylase. Dopamine is then oxidised to nor-adrenaline (Figure 3).
Figure 3 Dopamine biochemistry.
Phenylalanine is converted into L-Tyrosine. L-Tyrosine is then converted into L-Dopa by Tyrosine Hydroxylase. L-Dopa is then converted into Dopamine by DOPA decarboxylase.
Dopamine plays different roles in various internal organs. In the brain, it acts as a neurotransmitter and plays a major role in neuropsychiatric and movement disorders such as Schizophrenia and Parkinson’s disease[15]. It also acts as a local chemical messenger in other organs such as bloods vessels, kidney, gastrointestinal mucosa and including pancreas[16].
Dopamine exerts its effects by binding to and activating receptors on the surface of cells. In humans five subtypes of dopamine receptors have been identified, labelled D1 through D5[16]. All of them exert their effects via a complex second messenger system [e.g., cyclic AMP]. Dopamine signalling is mediated by five cloned receptors, grouped into D1-like (D1 and D5 receptors) and D2-like (D2, D3 and D4 receptors) families. The presence of dopamine receptors from both families has been identified in human isolated islets[16]. D2 receptor expression was confirmed by immunodetection revealing localization on insulin secretory granules of human β-cells[16].
Studies carried out in mouse pancreatic islets have shown pancreatic islets as the site for Dopamine synthesis and storage outside the central nervous system and both Dopamine and L-Dopa exerts a negative feedback action on insulin secretion in correlation with the reduction in intracellular [Ca2+] influx[17,18].
A study done recently has shown a negative feedback regulatory circuit for glucose-stimulated insulin secretion in purified human islets in vitro[19]. The release of dopamine and insulin together in response to the glucose load is demonstrated by the in-vitro infusion of dopamine into the insulin-containing secretory granules of human β-cells. Dopamine in turn exerts an antagonistic action on the D2 receptors that are also expressed on β-cells and thus inhibiting insulin secretion[19].
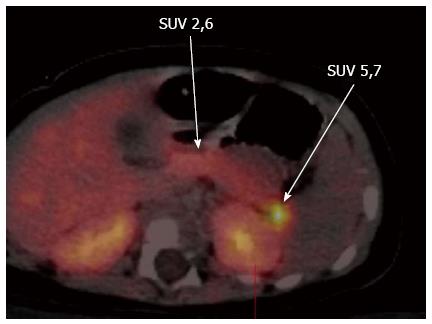
Neuroendocrine cells and pancreatic islets take up L-DOPA and convert it into Dopamine by the enzyme DOPA decarboxylase, which is expressed in β-cells of the pancreatic islets[20-23]. Thus decarboxylation of the L-DOPA to dopamine in the β-cells of the pancreatic islets allows localization of the lesion by means of PET scanning, using radioactive isomer 18F-L-DOPA[24]. 18F-DOPA is a radiotracer analogue of DOPA and this radioactive tracer is taken up, decarboxylated and stored in cytoplasmic secretory granules by both the endocrine and exocrine cells of the pancreas[23,25]. This mechanism acts as the principle behind the use of this non-invasive 18F-DOPA-PET imaging technique as the diagnostic tool of choice in localising the focal lesion. Both focal and diffuse forms have high DOPA decarboxylase activity and in focal lesions there is excessive tracer uptake in the lesion when compared to the rest of the pancreas (Figure 4).
Figure 4 Fluorine-18L-3, 4-hydroxyphenylalanine positron emission tomography scan showing the focal lesion in the tail of the pancreas.
In diffuse CHI there is generalised increased tracer uptake with a relatively higher uptake in the head when compared to the rest of the pancreas[14,26,27]. 18F-DOPA is excreted by kidneys hence normal bio-distribution is seen in kidneys, ureter and urinary bladder. An excessive uptake is also seen in gall bladder and biliary tract and a low uptake is seen in liver, heart and basal ganglia[26].
DIAGNOSTIC METHODS TO LOCALISE FOCAL LESION
Until recently the methods used to localise the focal lesion were (1) Hepatic portal venous sampling (PVS)[28,29]; (2) arterial calcium stimulation test[30,31]; and (3) tolbutamide response test[32]. PVS is invasive, time consuming, technically challenging and is associated with risk of severe hypoglycaemia and the accuracy is only about 70%[28,33]. Arterial calcium stimulation and tolbutamide response test are not found to be accurate in distinguishing between focal and diffuse CHI[30-32,34,35] Imaging modalities such as computed tomography (CT) and magnetic resonance imaging (MRI) has not been found very useful in localising the focal lesion[14].
18F-DOPA-PET/CT was first reported for the localisation of the focal lesion by Riberio et al[13] in 2005 and Otonkoski et al[36] in 2006. Since then several studies have shown that 18F-DOPA-PET/CT provides precise differentiation between focal and diffuse forms albeit exact localisation of the lesion may not be accurately attributed by the scan technique[13,21,37]. However, 18F-DOPA-PET/CT is non-invasive, relatively simple to use and more efficient than the invasive procedures such as PVS, and arterial calcium stimulation tests in differentiating and localising the pancreatic lesions[38]. The sensitivity and specificity of 18F-DOPA-PET/CT in detecting focal lesions measuring between 2 mm and 10 mm is approximately 90% and 100% respectively[39]. Thus the use of 18F-DOPA-PET/CT has revolutionised the surgical outcome in these children with CHI.
USE OF 18F-DOPA-PET/CT IN CHI
The use of 18F-DOPA-PET/CT in distinguishing between focal and diffuse CHI was first reported by Riberio et al[13], where the authors subjected 15 neonates to 18F-DOPA-PET scan. Abnormal focal uptake was observed in 5 children and diffuse uptake in the rest. Histopathology findings of all 5 patients with focal lesion and 4 patients with diffuse uptake who underwent surgery matched with their PET scan findings.
Otonkoski et al[36] used 18F-DOPA-PET/CT in 14 patients (ABCC8 mutation in 11/14 patients) and found focal uptake in 5 patients, diffuse uptake in the remaining 9 patients. 18F-DOPA-PET/CT findings were confirmed by histology in all 5 patients with focal uptake and 4 out of 9 in patients with diffuse uptake. This group also measured the standardized uptake value (SUV) of 18F-DOPA and found a SUV of > 50% higher uptake than the maximum SUV of the unaffected part of the pancreas in the focal group which corresponded with the histology findings. The remaining 9 patients with a diffuse uptake on the 18F-DOPA-PET/CT scan had a SUV ratio of < 1.5. Histology findings confirmed diffuse disease in 4 patients and pancreatic venous sampling in 4 patients. The authors concluded by proposing 18F-DOPA-PET/CT as the best modality of choice in locating a focal lesion[36].
Riberio et al[40] did a retrospective study in 2007 on forty nine children with CHI who had undergone 18F-DOPA-PET/CT scanning. They identified abnormal focal pancreatic uptake of 18F-DOPA in 15 children, where diffuse radio-tracer uptake was observed in the pancreatic area in the other 34 children. They also subjected 12 of the 49 children for pancreatic venous sampling (PVS) and 31 for MRI. In children who underwent both PET and PVS, the results were concordant in 11 out of 12. The authors concluded that PET scan with 18F-DOPA is an accurate non-invasive technique allowing differential diagnosis between focal and diffuse forms of CHI[40].
Arbizu Lostao et al[41] reported their first patient from Spain in whom the diagnosis of focal CHI was made in a 13 month old child using combined genetic analysis [paternal heterozygous mutation (G111R) in the ABCC8 gene] and 18F-DOPA-PET/CT imaging (focal uptake in the body of the pancreas) was successfully treated by surgery. This case report reiterates the importance of performing combined investigations towards the successful management of CHI.
Barthlen et al[22] in 2008 reported the correlation of 18F-DOPA-PET/CT scan findings with the intra-operative findings in 9 out of 10 children. A limited resection was found to be curative in 8 out of 9 children. They also reported their follow up data on these children with no evidence of diabetes or exocrine pancreatic insufficiency[22].
Cherubini et al[42] have reported that 18F-DOPA-PET/CT imaging can distinguish between focal and diffuse lesions in majority of the cases and 100% accurate in locating the focal lesion. The authors also highlighted the potentiating effects of non-invasive imaging technique using 18F-DOPA-PET/CT imaging combined with laparoscopic pancreatic surgery in the prompt localisation and excision of the focal lesion, preventing iatrogenic diabetes mellitus in later life[42].
Yorifuji et al[43] reported a boy with focal CHI whose disease activity was not consistent with the uptake of 18F DOPA. A diagnosis of CHI was made on day 2 of his life when he presented with hypoglycaemic seizures. A paternal heterozygous mutation c.4186G 1T (p.D1396Y) in the ABCC8 was identified followed by an uptake in the body of pancreas in the 18F-DOPA-PET/CT scan. A diagnosis of focal CHI was made and he was managed conservatively with frequent feeding regime. He achieved spontaneous remission at 1 year and 10 mo of age and a follow up 18F-DOPA-PET/CT scan revealed no difference in uptake between the two scans despite achieving clinical remission. Further to this an arterial stimulation venous sampling test was done which showed a low basal and stimulated insulin release illustrating that 18F-DOPA-PET/CT uptake may not always correlate with the insulin secreting capacity of the β-cells and the spontaneous remission of hypoglycaemia can be a functional process and not due to the apoptotic death of β abnormal cells[43].
Zani et al[44] evaluated the accuracy of 18F-DOPA-PET/CT imaging technique in differentiating between focal and diffuse lesions and localisation of the focal lesion. The authors reviewed the results of 18F-DOPA-PET/CT scan performed in 19 patients. Five of them had diffuse uptake and the same was confirmed by histology. The remaining 14 patients showed a focal uptake which was confirmed by histology however the localisation was not accurate in 5 children leading to incorrect surgical resection. The authors concluded that 18F-DOPA-PET/CT scan can distinguish between focal and diffuse CHI and the exact localisation in only 2/3 of patients with focal lesions. The authors also suggested undertaking intraoperative histological confirmation of the focal lesion prior to complete excision[44].
Masue et al[45] reported their experience of the use of 18F-DOPA-PET/CT in 17 Japanese children by assessing their results either by simple inspection or by calculating the pancreas percentage and compared those results with the genetic analysis and histology. Pancreas percentage is the expression of uptake of the head, body and tail of the pancreas as the total maximum SUV of the whole pancreas. They found the localisation and histology was consistent in all 17 children. However the overall results were consistent with the molecular diagnosis and histology in only 7/17 and 6/12 patients respectively. They also reported a substantial improvement in the accuracy of PET studies by using pancreatic percentage[45].
Ismail et al[12] reported the marked variation in the clinical, genetic, radiological and histopathological features of focal CHI in 3 of their patients. All 3 of them had paternal heterozygous mutation in ABCC8 gene (c.3992-9G → A in the first 2 patients and c.4477G → A in third patient). Of the 2 patients, first patient was responded to Diazoxide but not the second patient. The focal lesions in these 2 patients were accurately localised using 18F-DOPA-PET/CT imaging. Both of them underwent focal lesionectomy and were completely cured. Histology confirmed the presence of focal nodules with large nuclei along with the remaining normal pancreatic tissue in the first 2 patients. The authors proposed that unknown genetic or environmental factors may influence the phenotypic variation in response to treatment and some focal lesions can respond to medical management. The authors have also highlighted that a paternally inherited c.3992-9G → A mutation in the ABCC8 gene is associated with a mild focal phenotype responding to medical management in some patients of Ashkenazi Jewish origin[12]. This finding is based on previous reports that nearly 90% of CHI in Ashkenazi Jewish population is associated with c.3992-9G → A mutation and p.F1388del in the ABCC8 gene[46]. The third patient had undergone two 18F-DOPA-PET/CT imaging, with an unusually large focal lesion involving the whole pancreas. The first scan showed the tracer uptake in the body and tail and the second scan performed post lesionectomy showed a tracer uptake in the head of the pancreas and the patient underwent 2 pancreatic surgeries. Macroscopically this patient had a normal looking pancreas but microscopically islet cell nodules with large nuclei were found along with some normal pancreatic tissue. Despite undergoing the second surgery this patient was reported to be dependent on continuous gastrostomy feeds to maintain euglycemia[12].
Giurgea et al[47] reported that the size of the focal lesion is determined by the timing when the somatic loss of maternal allele occurs during the gestational age[47]. The earlier it occurs the larger the size of the focal lesion can be, as found in the 3 patient in this report. Also it has been suggested that an unknown mechanism might play a role in different rate of tracer uptake in different regions of pancreas in large focal lesions[47].
Meintjes et al[26] recently evaluated and reported the accuracy of delineating the focal and diffuse CHI using 18F fluoro-L-DOPA/CT and contrast enhanced CT. They performed 22 18F fluoro-L-DOPA/CT and contrast enhanced CT studies on 18 patients and assessed those results using visual assessment followed by quantitative comparison of SUVs measured by calculating the uptake on head, body and tail of the pancreas. They derived an SUV ratio using the formula-highest SUV (max)/next highest SUV (max). The authors also proposed a time activity curve which showed the focal pancreatic islet uptake relatively constant over time suggesting performing imaging at 20 min and 50 min after the radio tracer injection. The use of intravenous contrast agents during CT provides invaluable information for the surgeons in delineating the anatomical landmarks while performing surgery.
Of the 18 patients 13 showed diffuse with an SUV ratio of < 1.3 and five showed focal uptake with an SUV ratio of > 1.5 with an SUV (max) 50% higher than that of the unaffected area of the pancreas. Out of these five patients four of them had paternal ABCC8 mutation. All five patients were cured after limited focal resection with three of them requiring second 18F-L-DOPA/CT and contrast enhanced CT and surgery. Of the 13 patients who had diffuse disease, 9 of them were negative for ABCC8/KCNJ11 mutations and 3 had positive paternal ABCC8 mutation. Out of 13, 2 patients underwent surgery and 11 patients remain on high dose Diazoxide treatment one of them had 3 pancreatectomies without cure. The authors concluded by highlighting the importance of per-operative 18F-L-DOPA/CT and contrast enhanced CT studies in not only distinguishing between the focal and the diffuse disease but also the precise localisation of the anatomical landmarks[26].
Laje et al[48] did a retrospective review to determine the accuracy of 18F-DOPA-PET/CT scan in diagnosing focal CH on 105 children in whom a pre-operative 18F-DOPA-PET/CT scan was undertaken. Out of 105 patients, 53 patients had focal lesion and the remaining 52 patients had diffuse disease. Eight out of 53 patients with focal lesion were reported to have diffuse disease on their pre-operative 18F-DOPA-PET/CT scan. The location of the eight missed lesions was head (3), body (2) and tail (3) which showed a homo/heterogeneous tracer uptake throughout the pancreas. Thus the sensitivity of 18F-DOPA-PET/CT scan in diagnosing a focal lesion was 85% based on this study. Two out of 52 patients with diffuse disease were reported to have focal lesion on their pre-operative 18F-DOPA-PET/CT scan with a specificity of 96%. The positive predictive value of the study was 96% with 45 out of 47 patients having a true focal lesion. The authors concluded that the sensitivity and specificity of the 18F-DOPA-PET/CT scan varies based on the location of the lesion and the experience of the radiologists[48].
A meta-analysis was performed and published recently by Yang J and his colleagues reviewing the diagnostic role of 18F-DOPA PET and 18F-DOPA PET/CT imaging in CHI in 10 studies involving 181 children. The pooled sensitivity and the specificity in detecting focal CHI using 18F DOPA PET and PET/CT was reported to be 88% (95%CI: 80%-94%) and 79% (95%CI: 69%-87%) respectively on a per-patient-based analysis in this systematic review. The area under the summary receiver operating characteristic curve (SROC) was estimated to be 0.92% suggesting that 18F-DOPA PET and PET/CT imaging are accurate tools for distinguishing focal diagnosing CHI, although there is a minimal risk of both false positive and false negative results. The authors concluded that 18F-DOPA PET is very helpful in differentiating between focal and diffuse lesions, and should be the first investigation of choice when the genetic test results are inconclusive and 18F-DOPA PET/CT is very helpful in localising the lesion and thereby improving the treatment outcome in focal CHI[27].
Blomberg et al[38] conducted a systematic review and meta-analysis recently to quantify the diagnostic performance of pancreatic venous sampling (PVS), selective pancreatic arterial calcium stimulation with hepatic venous sampling (ASVS) and 18F-DOPA-PET/CT in diagnosing and localising focal CH. They reported that 18F-DOPA-PET/CT is superior in distinguishing focal from diffuse CH with a summary diagnostic odds ratio (DOR) of 73.2 when compared to PVS (summary DOR 23.5) and ASVS (summary DOR, 4.3). Also the pooled accuracy for localising a focal lesion by 18F-DOPA-PET/CT is higher (0.82) when compared to PVS (0.76) and ASVS (0.64). Thus this review concluded that 18F-DOPA-PET/CT is superior in diagnosing and localising focal CHI lesion in patients requiring surgery albeit the limitation of the review is the inclusion of small sample sizes and high probability of bias leading to the overestimation of diagnostic accuracy[38].
ECTOPIC PANCREAS AND THE ROLE OF 18F-DOPA-PET/CT
The pancreas begins to develop from the distal end of the foregut endoderm during the fourth week when both the dorsal and ventral pancreatic buds grow into the mesogastrium[49]. Thus the pancreatic acinar and the islet cells are derived from the endodermal cells lining the upper and duodenal region of the foregut[50]. During the course of this development an ectopic pancreatic tissue may occur in the stomach, duodenum, jejunum, ileum and rarely in Meckel’s diverticulum, appendix, biliary tract or lungs[49].
There have been reports of ectopic pancreatic tissue causing CHI in both adults and children. We reported a child with persistent and severe hyperinsulinaemic hypoglycaemia (HH) despite undergoing three pancreatectomies with a choledochoduodenostomy and a cholecystectomy. An 18F-DOPA-PET/CT scan localised the ectopic lesion in the vicinity of the former head of pancreas. The same lesion was found to be localized near the duodenum, either in the duodenal wall or cavity on the magnetic resonance scan (MRI). He is subsequently managed medically[33].
Peranteau et al[51] in 2007 reported another patient with persistent hypoglycaemia despite undergoing a near-total pancreatectomy. A subsequent 18F-DOPA-PET/CT scan demonstrated one focus in the remnant pancreatic head and 3 in the abdomen. The lesion in the pancreatic remnant was removed completing a total pancreatectomy and further abdominal exploration revealed 4 pancreatic ectopic rests in the jejunum. The lesions were surgically removed and the histopathology confirmed focal islet cell hyperplasia in all the lesions. This patient required insulin therapy for a short term post operatively.
In both of these patients 18F-DOPA-PET/CT scan was performed post near total pancreatectomy with the persistence of hypoglycaemic symptoms. However a preoperative localisation of the focal lesions would have led to the removal of local and ectopic lesions and preservation of the rest of the pancreas. Thus the use of 18F-DOPA-PET/CT scan in the management of CHI is justified in identification of both focal lesions within the pancreas and ectopic pancreatic tissue[51].
A standardised protocol for the use of 18F-DOPA-PET/CT was derived in 2005 and advocated to achieve maximum acquisition with minimum radiation. This guideline was derived based on the survey conducted in 2005 reviewing the experience of all the PET centres. The result of the survey showed that 18F-DOPA-PET/CT has 94% sensitivity and 100% specificity. Thus pre-operative performance of 18F-DOPA-PET/CT has been proposed as the most accurate way of localising the focus enabling limited resection and thereby preventing the risk of iatrogenic diabetes[14]. Studies recommended performing 18F-DOPA/CT studies only in tertiary endocrine centre equipped with necessary expertise to perform and interpret the results[14].
CONCLUSION
We conclude that 18F-DOPA-PET/CT is a safe, non-invasive and the most preferred investigation of choice (1) to distinguish between the focal and diffuse forms of CHI; and (2) to enable accurate localisation and enucleation of the focal lesion preventing the risk of developing iatrogenic diabetes mellitus and pancreatic insufficiency. However a multi-disciplinary team (MDT) approach is essential and has to be undertaken in a tertiary centre built-in with necessary expertise for the successful interpretation and management of CHI patients.
P- Reviewers: Das UN, Shyng SL S- Editor: Wen LL L- Editor: A E- Editor: Zhang DN