INTRODUCTION
Head and neck cancers (HNC) account for approximately 650000 new cancers each year across the world and result in about 350000 deaths, representing 6% of all cancer cases[1,2]. In the United States, approximately 52000 new cases of oral cavity, pharyngeal and laryngeal cancers are diagnosed every year with approximately 11000 deaths[3]. Approximately 95% of these are squamous cell carcinomas (HNSCC) and they often present in locally advanced stages. The treatment of head and neck cancers involves a multi-disciplinary approach and includes surgery, radiation therapy and chemotherapy. Traditional staging approaches for head and neck cancers include clinical examination and surgical pathologic staging. The advent of concurrent radiation and chemotherapy for organ preservation in head and neck cancers has reduced the incidence of surgical resection especially in locally advanced larynx cancers and oropharynx cancers[4,5]. However, this has also brought forth the need to have detailed non-invasive imaging techniques to accurately identify tumor size and location, cervical lymph node involvement and presence or absence of distant metastases. The use of computed tomography (CT) scans and magnetic resonance imaging (MRI) scans allowed structural and anatomical information to be obtained and vastly improved the ability of oncologists to clinically stage these patients appropriately. However, the use of fluorine-18-fluorodeoxyglucose positron-emission tomography (FDG-PET) has added a new biologic and functional end-point to these imaging techniques and opened a whole new arena for research and development in management of head and neck cancers. Additionally, PET/CT and PET/MRI combinations are now able to provide both anatomic and functional information in co-registered images.
The review will focus on the current applications of FDG PET/CT scans in management of squamous cell cancers of the head and neck. The use of PET/MRI and FDG PET/CT for other head and neck cancers (e.g., salivary gland, thyroid cancers etc.) is beyond the scope of this review.
BIOLOGY OF FDG-UPTAKE
Rapidly proliferating cancers cells utilize glucose as a source of energy and metabolism. Glucose undergoes glycolysis after intracellular transportation. This transportation is mediated by a family of glucose transporter proteins (GLUTs)[6-8]. These trans-membrane proteins allow energy independent transport of glucose across the hydrophobic cell membrane. Thirteen GLUTs have been identified of which GLUT1, GLUT3, and GLUT4 have high affinity for glucose. The expression of GLUTs is induced by hypoxia-inducible factor, growth factors and various oncogenes[9]. Increased expression of GLUT1 has been found in many cancers, including head and neck cancer[9]. The degree of expression of GLUT1 has also been shown to be associated with aggressiveness of the cancer. Elevated glycolytic activity and increased expression of GLUT1 are found in advanced cancer stages and predict significantly poorer treatment outcomes[10-13].
Fluorine18-FDG (18F-FDG) is an analog of glucose with 18F occupying the position of oxygen on carbon-2. Similar to glucose, GLUTs facilitate the transport of 18F-FDG into the cell. In the next step, both glucose and FDG are phosphorylated by the hexokinase enzyme. Glucose, upon phosphorylation, enters the glycolytic pathway for energy production. FDG, on the other hand, cannot undergo glycolysis and is trapped as FDG-6-phosphate in the intracellular environment. This trapped FDG can then be imaged to spatially locate the metabolically active cancer cells. FDG uptake in cancer cells of HNSCC was shown to be significantly correlated with cell proliferation by flow cytometry[14,15].
PHYSICS OF PET IMAGING
A detailed description of the physics of FDG-PET scanning is beyond the scope of this review. However, in brief, at the heart of this imaging technique is the radioisotope 18F. It is produced using a cyclotron and has a half-life of 110 min allowing it to be transported for use in PET scanner facilities.
18F decays by positron (β+) emission 97% of the time and is converted to oxygen-18. The emitted positron travels a short distance in soft tissue, decelerates rapidly and interacts with an electron near the end of its track. This interaction is called the annihilation reaction and mass is converted to energy with the release of two 0.511MeV photons which travel outwards at 180° to each other. These annihilation photons are detected by scintillators and a simultaneous or coincident detection of these photons makes it possible to spatially localize the point of origin. The information is collected on a multitude of such coincident events and processed to generate a PET image.
Nowadays, most PET scans are co-registered with simultaneously obtained CT scans to produce PET/CT images which give functional information along with anatomic co-localization. For areas like the head and neck a smaller area can be scanned with intravenous contrast administration to obtain further normal tissue anatomy and tumor extent.
QUANTITATIVE IMAGE INTERPRETATION
In order to be used as a valid imaging biomarker, accurate and reproducible quantification of FDG uptake is necessary. A simplified measurement using standardized uptake value (SUV), given by the following formula, is now the most widely used method for the quantification of FDG up take.
In this formula, tissue activity is the radioactivity measured by the PET scanner within a region of interest (ROI) or the maximal value; injected dose is the dose of 18F-FDG administered, corrected for physical decay. The SUV in this formula represents the activity of 18F-FDG within the tumor measured over a certain interval after 18F-FDG injection and normalized to the dose of 18F-FDG administered and to the body weight[16].
There are many different factors that can affect 18F-FDG uptake and its subsequent quantification. The biologic factors include blood glucose level, interval between injection and start of PET study, patient motion and breathing, patient comfort, and inflammatory process near or at the tumor. There are also many technical and physical factors such as attenuation correction, calibration, image reconstruction, data analysis, etc, which are beyond the scope of this review and have been discussed by others[17-20]. Despite these, it has been shown that there is a good correlation between SUV and glucose utilization rate in various cancers including HNSCC[21].
Various forms of SUV-based parameters have been described in literature. These include: (1) SUVmax- This measures the highest (maximal) SUV in a region of interest. This has been the most common used parameter in clinical practice as it is thought to be the most reproducible and independent of how the ROI is defined. However, it represents only a single point within the tumor/lesion and may not be representative of the entire tumor volume; (2) SUVmean or SUVaverage- This value may provide a more global picture of the tumor activity as it averages the intensity of uptake in a region of interest (ROI). This, however, suffers from the subjectivity and variability of the definition of this ROI which may differ between individuals and institutions; (3) Metabolic tumor volume (MTV)- This is measured in cubic centimeters and represents the tumor volume with active FDG uptake. There is no standard way for tumor segmentation from PET images (For further discussion, refer to Chapter 4 on PET in radiotherapy treatment planning). Threshold-based method is often used. Some authors used a threshold of SUV > 2.5. Others used 40% or 50% SUVmax as a threshold. The MTV is measured as the tumor volume with SUV above the threshold selected. However, the volumes vary significantly depending on the threshold selected; and (4) Total lesion glycolysis (TLG) - This is a product of the tumor volume, determined by CT - scan or MRI, and SUVmean.
USE OF PET/CT IN THE DIAGNOSIS AND STAGING OF HEAD AND NECK CANCERS
Primary cancers of the head and neck are mainly diagnosed by clinical examination in the office and supplemented by imaging studies such as CT scans and MRI. Staging of the primary tumor, i.e., T-stage in the American Joint Committee on Cancer (AJCC) staging system, mainly depends on the tumor size and invasion of the primary tumor, which is better assessed by CT and MRI imaging.
Occasionally, in about 2%-9% of cases, patients may present with a lymph node in the neck with no obvious primary site visible on clinical or routine diagnostic imaging tests[22]. Such cases are labeled as unknown or occult primary head and neck cancers. Pathologic evaluation of the nodes, usually by fine needle aspiration, may reveal a diagnosis of squamous cell carcinoma or adenocarcinoma. Squamous cell histology indicates that the primary site is likely in the head and neck while adenocarcinomas mainly arise below the clavicle (e.g., lung cancer, gastric cancer). The traditional method for detection of the primary site in cases of squamous cell histology involves examination under anesthesia and random biopsies from the nasopharynx, oropharynx, hypopharynx, larynx and any mucosal areas which appear abnormal. Tonsillectomy is often performed. These maneuvers are able to detect the primary site in about half of the cases initially labeled as unknown primary head and neck cancers[23].
FDG-PET scans have been proven to be an invaluable tool in these cases. Rusthoven et al[24] summarized the results of 16 published studies with a combined total of 302 patients to evaluate the role FDG-PET in unknown primary head and neck cancers. They reported that FDG-PET was able to detect primary tumors in 24.5% (range, 5% to 73% in various studies) cases where conventional methods were unsuccessful. The primary site was found at the base of tongue in 27 patients (24.3%), tonsils in 20 patients (18.0%) and below the clavicle in 27 patients (24.3%). The sensitivity, specificity, and accuracy of FDG-PET in the detection of primary tumors were 88.3%, 74.9%, and 78.8%, respectively. Interestingly, PET scans were able to identify an additional 16% regional nodal metastases and 11% distant metastases previously undiscovered. Several prospective studies have confirmed these findings[22,25]. Rudmik et al[25] recently reported results of 30 patients who underwent PET/CT. PET/CT was performed after conventional workup and prior to operative panendoscopy. The surgeons were blinded to the results. Patients had routine examination under anesthesia and directed biopsies, and the PET/CT results were then revealed to the surgeon intraoperatively. Additional biopsies were taken if the PET/CT was positive. The traditional work-up identified tumors in 25% of patients, whereas PET/CT-directed biopsies revealed the primary lesion in 55% of patients. The sensitivity, specificity, positive predictive value, and negative predictive value of PET/CT in detection of primary tumor were 92%, 63%, 79%, and 83%, respectively.
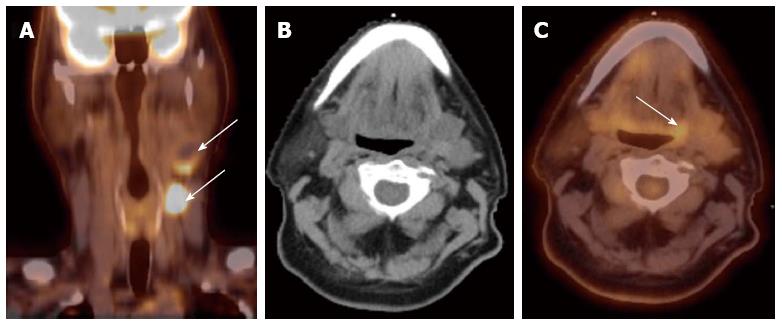
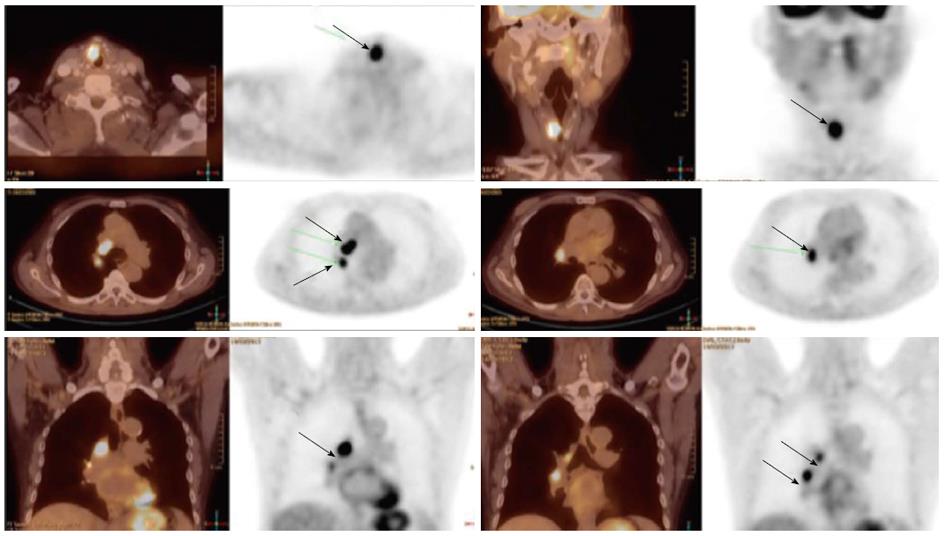
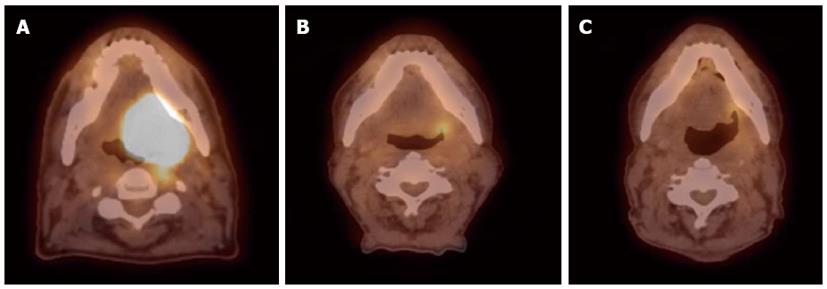
Detection of the site of primary cancer allows directed therapy to the tumor (surgery or radiation therapy) while sparing or minimizing the toxicity to uninvolved mucosal areas or tissues. The current paradigm for diagnosis and staging work-up for unknown primary cancers involves obtaining a PET/CT and then obtaining directed biopsies from the suspicious areas. Figure 1 illustrates a patient who presented with multiple left neck nodes. Conventional workup failed to identify the primary tumor but PET showed the primary tumor in left base of tongue.
Figure 1 Computed tomography.
A: A patient presented with multiple left neck nodes (arrows); B: The conventional methods as well as the computed tomography (CT) imaging could not identify the primary tumor; C: A positron emission tomography/CT scan showed increased fluorodeoxyglucose uptake in the left base of tongue (arrow) and a directed biopsy of this area confirmed the primary site.
As noted above, PET scans have also helped in identification of previously unidentified involved cervical lymph nodes. Various CT and MRI based criteria have been developed to label lymph nodes are being involved by cancer or not[26]. However, this may still result in 20%-30% rate of false-positive and false-negative results. Various reports comparing FDG-PET with other imaging modalities including ultrasound, CT and MRI have consistently reported a much higher sensitivity and specificity for PET scans when compared with the gold-standard, surgical lymph node dissection[27,28]. This has specially been helpful in detecting lymph nodes which are at a distance from the primary or in the contralateral neck, especially when the lymph node has not reached size criteria by CT/MRI. PET is also very helpful in detecting involved lymph nodes in the lower neck where there are complex muscular and vascular structures (Figure 2). The average sensitivity and specificity for PET scan to detect involved nodes are reported to be 90% or higher[27].
Figure 2 Increased fluorodeoxyglucose positron-uptake in the low neck revealed a metastatic lymph node which would otherwise be difficult to detect because of the presence of muscular and vascular structures in this region of the neck.
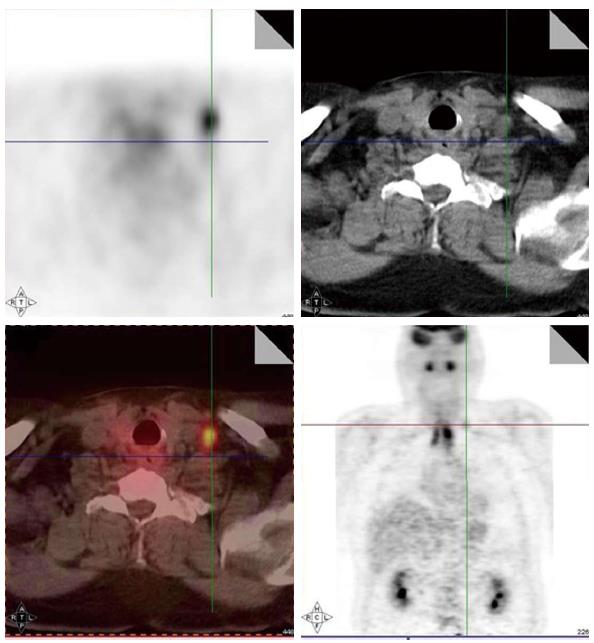
Local-regionally advanced head and neck cancers metastasize to mediastinal lymph nodes, lungs, bone and liver. PET scans are also helpful in ruling out presence of distant metastases as PET images in head and neck cancer are often obtained from skull base to hips and PET are more sensitive in small metastasis than CT. Various reports have documented the incidence of distant metastases as detected by PET scans ranging from 6% to 25% for stage III/IV head and neck cancers[29-34]. The sensitivity and specificity of PET scans for the detection of metastases are 77% and 94%, respectively. Hearle et al[35] noted distant metastases in 10% of 299 patients evaluated with 97% sensitivity and 96% specificity. These PET findings resulted in a change in the management plan in 8% to 15% of cases[30,36]. Figure 3 shows a head and neck cancer patient with small bone metastases detected by PET scan but these lesions were missed in the CT scan.
Figure 3 Small bone metastases detected in a patient with head and neck squamous cell carcinoma (arrow).
These lesions were not visible on the computed tomography scan.
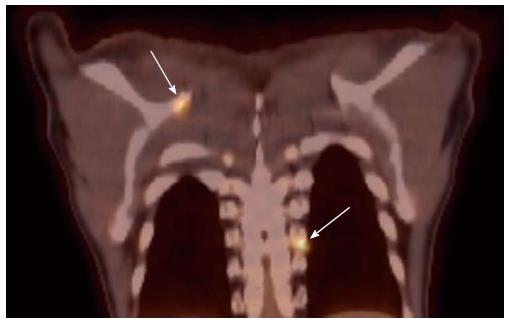
The use of tobacco products (chewing and smoking) and alcohol have been associated with the development of head and neck cancers. This risk extends to other sites of the aero-digestive tract including lung and esophageal cancers. Synchronous primary cancers have been noted in approximately 10% of head and neck cancer patients. Strobel et al[37] reported 69 synchronous primary cancers in 62 patients among 589 consecutive patients imaged with PET scans. Most (80%) of these were in the upper aero-digestive tract. A recent report from Japan noted a higher rate (18%) of second primary cancers among 230 head and neck cancer patients[38]. Evaluation of the diagnostic sensitivity showed that PET scans were most likely to detect second primaries in other head and neck sites and lungs while the sensitivity for finding gastric and esophageal cancers was much lower at 25% and 7.6%, respectively. Needless to say, the discovery of these second primary cancers resulted in a change in the management plan for these patients. Figure 4 shows a patient who presented with right oral tongue cancer and PET was obtained as part of the workup that revealed he also had a cancer in the soft palate as well in the upper esophagus. Figure 5 is an example of a patient with a synchronous laryngeal and lung squamous cell carcinomas.
Figure 4 A positron emission tomography scan was obtained in a patient with a diagnosis of right oral tongue cancer (anterior arrow in axial views).
The positron emission tomography/computed tomography revealed two additional primary cancers, one in the soft palate and the other in the upper esophagus.
Figure 5 A patient with a synchronous laryngeal and lung squamous cell carcinomas.
A 70-year-old male with diagnosis of squamous cell carcinoma of the glottic larynx T3N0M0 (A and B; arrow). He underwent a positron emission tomography/computed tomography scan which revealed additional lesions in the right lung which were biopsied endobronchial and shown to be a second primary lung cancer with mediastinal lymphadenopathy (C and D; arrows).
RADIATION THERAPY PLANNING
Radiation treatment plays an important role in the management of head and neck cancer. Radiotherapy is given as a definitive treatment when the tumor is not resectable or when organ preservation is preferable[5,39]. Radiotherapy is also given to patients who have high risk pathology features after surgery[40,41]. Chemotherapy is often administrated concurrently with radiotherapy in locally advanced disease.
In the past decade, intensity-modulated radiotherapy (IMRT) has become a standard radiation technique in head and neck cancer[42,43]. IMRT is a highly conformal radiation technique which allows delivery of different radiation dose to different adjacent structures, also called dose painting, thus enabling delivery of high dose to the tumor targets while sparing the normal tissues. The use of IMRT has led to a reduction in xerostomia and improvements in quality of life following treatment[44-46]. However, highly conformal treatments can lead to disease not being included in the high-dose radiation treatment volume, resulting in locoregional failures. On the other hand, over-drawing the target volumes can result in high-dose radiation being unnecessarily delivered to normal tissues that may lead to increased toxicities. Therefore, accurate delineation of the tumor volume and regions at risk are critical in order to achieve the best treatment outcomes.
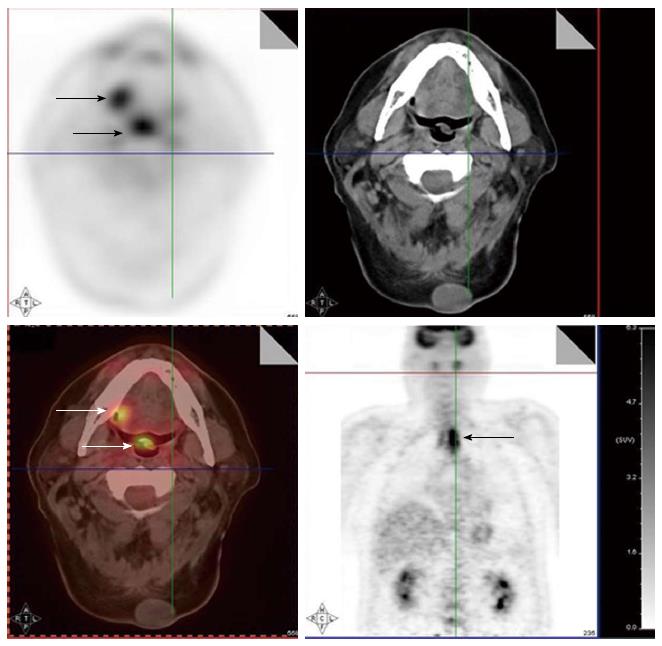
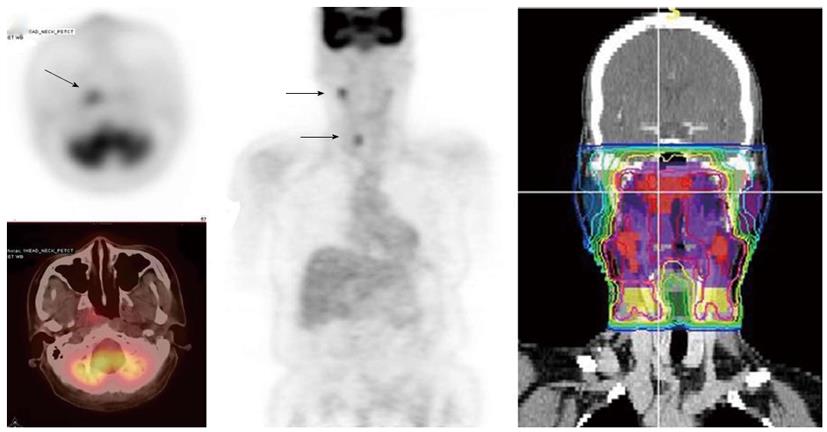
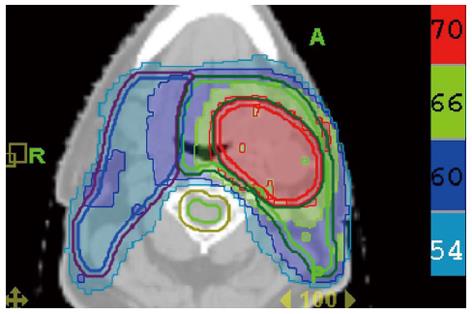
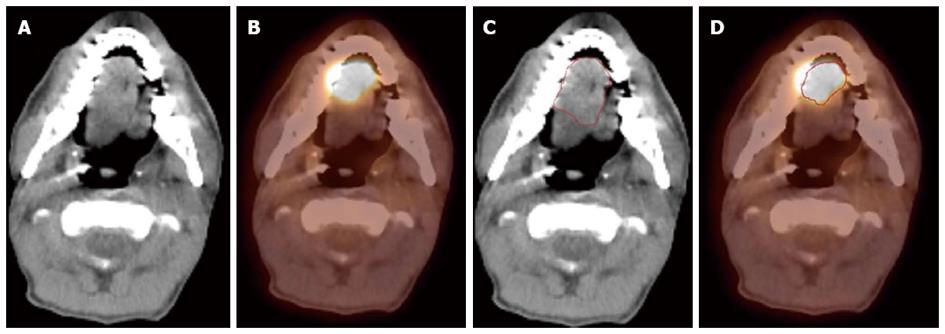
Since FDG PET scan has a high sensitivity in detecting tumor, it plays an important role in radiation treatment planning especially in IMRT planning. FDG PET scan is routinely obtained and co-registered with treatment planning CT images for treatment planning. Currently there are following several practical applications of PET in radiation treatment planning: (1) Detecting small lymph nodes that are not size criteria in CT and including these nodes in high dose target. In general, lymph nodes less than 1.0 cm in CT or MRI are usually called benign. However, PET scan has higher resolution and can detect malignant node as small as 0.5 to 0.6 cm. As mentioned above, PET has a high sensitivity for malignant lymph node, reported being up to 90%. Therefore, PET avid nodes are included in the high dose radiation targets especially when biopsy confirmed to be malignant. Figure 6 shows a patient with nasopharyngeal cancer, initially staged as T1N0 after conventional workup. However, FDG PET revealed hypermetabolic foci in the primary tumor in the nasopharynx and in bilateral level II lymph nodes which were small and were not called as lymphadenophy in the CT and MRI (Figure 6B). Fine needle biopsies of the right level II lymph node was obtained and confirmed to contain metastatic disease. Therefore, these lymph nodes were included in the high dose area in the IMRT plan (Figure 6C); (2) Detecting the primary tumor in patients who present with “unknown primary” and including the primary tumor in the high dose target. As mentioned above, in head and neck cancer with unknown primary, FDG PET can detect primary tumor in 25% of patients where conventional work up were unsuccessful. Most of these primary tumors are in the oropharynx, such as tonsil and the base of the tongue. When the primary tumor is detected, the patient is treated with radiation field tailored to the primary tumor, thus avoiding radiating the whole pharyngeal axis which is the standard radiation technique in patients with unknown primary. Figure 7 illustrates the IMRT plan for the patient with the primary tumor detected in PET. The left base of tongue tumor detected by PET was included in the high dose field while the larynx and nasopharynx were spared; (3) Accurate delineation of the edge of the primary tumor. Accurate delineation of the edge of tumor to generate gross tumor volume (GTV) is the first step in target delineation for IMRT planning. It is often difficult to separate the tumor from surrounding soft tissue and muscle in CT imaging which is the primary imaging modality in radiation treatment planning, especially for tumor in the oral tongue and oropharynx. Figure 8 shows a patient with oral tongue cancer, comparing CT (Figure 8A) vs PET (Figure 8B). The border of the tumor in the CT was not very clear, difficult to separate from the tongue muscle. Yet, the PET showed sharp contrast between the tumor and sounding tissue. The GTV based on CT (Figure 8C) is much larger than that based on PET (Figure 8D). Several studies have published comparing GTV generated by CT vs those when PET was incorporated, and noted a trend for decreasing GTVs when PET was used[47-52]. Some studies also showed that the interobserver variability deceased when PET was used for target delineation[47]; and (4) In postoperative radiation, detecting recurrent tumor even before radiation and including the recurrent tumor in high dose target. Patients with high risk pathology features are treated with adjuvant chemoradiation for better local regional control and survival. Postoperative radiotherapy is often given 4 to 6 wk after surgery when the surgical wound is fully healed. However, some patients may have local regional recurrences even before radiation. Because of the anatomical distortion and fibrotic changes after surgery, and flap reconstruction, these recurrences are difficult to be detected by physical examination and CT imaging. FDG PET is ideal imaging modality at this setting. Shintani et al[53] reported 91 consecutive patients referred to postoperative adjuvant radiation after complete surgical resection. These patients had FDG PET obtained at a median time of 28 d after surgery. They reported 27 patients with suspicious PET findings. Further biopsies led to changes in adjuvant treatment in 14 patients (15.4%), including increasing the radiation therapy dose in 6 patients, and extending the radiation therapy treatment volume and increasing the dose in 1 patient. Liao et al[54] also reported 29 patients who had a PET scan obtained before postoperative radiation. They found 7 patients with positive PET studies, 3 with distant metastases and 4 with local regional recurrences. For those who had local regional disease detected by the PET, the radiation volumes and radiation dose have to be changed, with higher dose delivered to the recurrent tumor. Thus, for patients with high risk features, especially for those who have a prolonged interval from surgery to radiation, a post-surgery and pre-radiation FDG PET will be valuable in treatment decision and radiation treatment planning.
Figure 6 A patient with nasopharyngeal cancer.
A: Initial stage was T1N0 when the patient was referred to our institution after conventional workup (arrow in axial image); B: Fluorodeoxyglucose positron emission tomography revealed hypermetabolic foci in the primary tumor in the nasopharynx and in bilateral level II lymph nodes which were small and were not called as lymphadenophy in her computed tomography and magnetic resonance imaging. Fine needle biopsies of these lymph nodes were obtained. The right level II node (arrows) was confirmed to contain metastatic disease, while the left level II lymph node was not diagnostic; C: Intensity modulated radiotherapy plan for this patient. The right level II node was treated to a high dose of radiation. The lower neck was treated with an anterior-posterior field (From Woods C, Sohn J, Machtay M, Yao M. Radiation treatment planning for head and neck cancer with PET. PET Clinics 2012; 7: 396; with permission).
Figure 7 Treatment plan for the patient described in Figure 1.
The base of tongue was found to be the primary cancer site and this area was included in the high dose intensity-modulated radiotherapy plan while sparing uninvolved mucosal areas.
Figure 8 A patient with oral tongue cancer.
The edge of the tumor was not very clear in the computed tomography (CT) image (A), but more obvious in the PET image (B). Gross tumor volume was outlined based on CT scan (C) vs with fluorodeoxyglucose positron emission tomography/CT (D). The volume included is larger with CT alone.
Following are some active investigations in further exploration how to use PET in radiation treatment in head and neck cancer: (1) Subvolume delineation and dose escalation. Tumors are not homogeneous. Since FDG uptake is correlated with tumor aggressiveness, the region of the tumor with higher FDG uptake may harbor more aggressive cancer cells and may require higher radiation dose to eradicate. Indeed, FDG-avid regions in the tumor have been shown to be correlated with hypoxia that is associated with tumor radioresistance[55,56]. With the dose painting capability of IMRT, a higher radiation dose can be delivered to these tumor subvolumes to achieve potentially better tumor control. Schwartz et al[57] studied theoretical IMRT models using PET derived volumes in 20 patients with head and neck cancer. They found that a mean dose of 74.9 Gy (range, 71.53-80.98 Gy) could be delivered to the PET-avid volume without overdosing the adjacent critical structures. Madani et al[58] conducted a Phase I study of dose escalation to FDG-avid subvolmes. They reported it was feasible to deliver a radiation boost of 30 Gy in 10 fractions to the PET tumor volume before standard IMRT treatment, and they are planning a randomized phase II trial comparing this treatment regimen with standard IMRT; and (2) Adaptive radiation therapy. During the course of radiation treatment, the patient can have significant physical/anatomical changes due to tumor response. A second CT simulation and replanning are required in order to ensure the tumor is being dosed appropriately. Changes also occur in the FDG uptake of the tumor during the course of radiotherapy and some investigators have explored adaptive radiotherapy and planning techniques to alter the plan based on the changes in PET imaging[59,60].
ASSESSMENT OF TREATMENT EFFICACY, FURTHER MANAGEMENT AND FOLLOW-UP
FDG PET/CT scans have been proven to be a useful technology in assessing treatment response in patients treated with definitive radiation and chemotherapy and for detecting residual and recurrent disease. PET/CT scans are usually performed 2 to 3 mo after treatment completion. The optimal timing of obtaining the scan has been debated in literature and based on many reports it has been determined that the 12-wk time point after completion of therapy may be the most appropriate[31,61,62]. Scans obtained at earlier time-points (< 8 wk) have a high rate of false-positive FDG uptake in the radiation treatment field due to inflammatory changes. Scans done too late (> 16 wk) may allow residual loco-regional disease to grow and metastasize. If increased FDG uptake is noted at the primary site, patients should undergo a biopsy followed by a surgical resection for residual disease. Figure 9 shows a serial PET/CT scans in a patient treated for oropharyngeal squamous cell carcinoma.
Figure 9 Sixty-six year old male was diagnosed with squamous cell carcinoma of the left base of tongue T4aN1M0.
He received external beam radiation therapy (70 Gy in 35 fractions) with concurrent cisplatin 100 mg/m2 (3 cycles). Positron emission tomography/computed tomography scans were done pre-treatment (A) and at 3-mo (B) and 8 mo (C) post-radiation therapy. Follow-up images show good response to treatment with sustained response at least 8 mo from treatment.
The role of FDG PET/CT in decision making for neck dissection after chemoradiation has also been extensively investigated. Yao et al[63] reported a 100% negative predictive value (NPV) and 43% positive predictive value (PPV) for PET scans done 12 wk after radiation in 53 patients who were noted to have a complete response at the primary site. A prospective study in 112 consecutive patients reported by Porceddu et al[64] also noted the utility of the 12-wk post radiochemotherapy PET scan in decision making for neck dissection. Patients who had equivocal PET results underwent another scan 4 to 6 wk later. Patients who had CT abnormalities but were PET-negative were observed and no subsequent neck node failures were noted in these patients. Nine patients continued to have PET-positive disease in the neck of which 8 underwent surgery. Residual disease was noted pathologically in 6 of these 8. Another prospective study from MD Anderson Cancer Center reported on 98 patients[65]. They stratified patients into low-risk and high-risk groups based on tumor stage, nodal stage, overall stage, tumor site, smoking history and HPV status. The most significant benefit of FDG PET/CT over CT scans was noted in detecting residual disease among high-risk patients. The NPV of PET/CT was 75% as compared to 37.5% for CT alone.
The role of FDG-PET scans for long term follow-up surveillance for detection of loco-regional and distant metastatic recurrence has also been extensively investigated. Gupta et al[66] conducted a meta-analysis of 51 studies involving 2,335 patients. They reported a NPV of approximately 95% for both primary and neck disease for response assessment and surveillance. A recent report analyzed the role of long-term surveillance PET/CT scans in 214 patients with negative scans after completion of therapy[67]. Nine percent of these patients recurred on follow-up. This suggested a NPV for surveillance PET/CT of 91%. Based on these data the authors recommended that radiologic surveillance can be stopped early in those patients who are noted to have two consecutive negative PET/CT scans within 6 mo of each other.
PROGNOSIS
Many attempts have been made to establish PET/CT scan derived parameters as prognostic indicators. These studies have looked at pre-treatment, post-treatment and during treatment scans to obtain SUV and metabolic tumor volume (MTV) to serve as prognostic indices. Most of these studies have been retrospective. However, some prospective trials have also been reported. A recent review article summarizes these studies[68].
Allal et al[69] conducted a prospective study in 120 patients with HNSCC to evaluate the role of pre-treatment SUV in predicting for local control and disease-free survival. Seventy-three patients underwent radiation therapy with or without concurrent chemotherapy and 47 had surgery with or without adjuvant radiation therapy. At a median follow-up of 48 mo, 46 patients had recurrent and/or distant metastatic disease. In these patients the SUV was noted to be 5.8 vs 3.6 for those with disease controlled (P = 0.002). On the other hand, Vernon et al[70] reviewed 42 patients receiving PET/CT guided definitive radiotherapy and found that neither SUV of the primary tumor nor SUV of the lymph node was predictive of tumor recurrence.
In another prospective study, FDG PET was obtained 4 wk prior to chemoradiotherapy and 8 wk after completion of treatment in 98 patients[71]. Primary tumor and nodal SUVmax was calculated for both time points. The only prognostic factor for disease-specific survival was found to be the post-treatment primary tumor SUVmax. The mean SUVmax for those who failed was 7.2, compared to 4.2 for those who did not fail (P < 0.01). Pre-treatment SUVmax of primary tumor and lymph node were not found to be significantly associated with treatment outcomes. The conclusions from these studies are not consistent, partly due to the inherent problems with SUV measurement as it represents only a single point within the tumor but not represent the entire tumor. Additionally, there is heterogeneity in the patient population, heterogeneity in treatment modalities, small patient samples and the use of different endpoints.
In recent years, PET-based tumor volumes, i.e., metabolic tumor volume (MTV), are being explored. La et al[72] from Stanford University evaluated the prognostic value of MTV in 85 patients. A threshold of 50% maximal intensity was used to define the metabolic tumor volumes. They found that MTV had a significant relationship with disease-free survival (P < 0.001) and with overall survival (P < 0.001) on univariate analysis. An increase in MTV of 17.4 mL was significantly associated with an increased hazard of first event (recurrence or death). SUVmax did not show a significant relationship with either of these endpoints. Another report from the same group of investigators validated these findings in an additional 83 patients[73]. Recently the results of a sub-group of patients enrolled on the RTOG 0522 trial were presented[74]. Seventy-four patients underwent baseline and 8-wk post treatment PET scans. Baseline SUVmax or SUVpeak of the primary or nodal disease were not predictive for outcomes. However, patients having a primary tumor MTV above the cohort median had a significantly worse loco-regional control and progression free survival.
Some groups have also evaluated the utility of PET scans done during therapy. PET scans were done after neoadjuvant chemotherapy in 16 patients. These patients then underwent surgical resection and histopathologic responses were correlated with SUVmax. Comparisons between pathologic responders and non-responders revealed that there was significant difference in post-chemotherapy SUVmax and percent decrease in SUVmax[75].
The applicability of PET scans during the course of radiation therapy is harder to evaluate because of difficulty in image interpretation with inflammatory changes due to radiation and the cost of additional imaging. Such a study has, however, been done and reported on by Hentschel et al[76]. This prospective study evaluated the role of serial PET scans during early phase of radiation therapy and the ability of these scans to predict treatment outcomes. Patients were divided into two groups and all of them underwent 4 PET scans. In one group, PET scans were obtained before treatment, at 10 Gy, 30 Gy and 50 Gy. In the other group, PET scans were obtained before treatment, at 20 Gy, 40 Gy, and 60 Gy. Patients who had a rapid early response with > 50% decline in SUVmax at 10 Gy or 20 Gy as compared to the pre-treatment SUVmax had a significantly higher overall survival, loco-regional control and disease-free survival at 2 years. The 2-year overall survival was 88% for those who had more than 50% decline in SUVmax compared to 38% for those with less than 50% decline (P = 0.02). The 2-year disease-free survival was 75% and 31%, respectively for those with > 50% decline as compared to < 50% decline in SUVmax. And the 2-year local-regional control rate was 88% vs 40% for those with > 50% decline vs to those < 50% decline in SUVmax (P = 0.06).
There is currently no consensus on what time points to use for PET scan based prognostication and what parameters are the most useful. There may be value in combining traditional prognostic factors with PET based parameters. Yao et al[77] showed that T-stage, N-stage and pretreatment SUV of the lymph node were significantly associated with distant metastasis. However, the combination of these factors can better predict distant metastasis-free survival. The 3-year distant metastasis-free survival was 98.1% for no factors, 88.6% for one factor, 68.3% for two factors, and 41.7% for three factors. Similarly, Moeller et al[65] incorporated HPV status in their mortality risk assessment in addition to post-treatment tumor SUVmax. They noted FDG PET/CT was predictive for outcomes in HPV-negative and non-oropharyngeal primaries.
FUTURE DIRECTIONS
FDG PET/CT scan has been proven to be a useful technology on many fronts in head and neck cancer and has significant impact on the management as discussed above. Currently, there are many exciting new areas of research and development that are being actively investigated and will continue to expand the role of this imaging modality in the future. These efforts can be broadly categorized as follows: (1) Protocol development and standardization: a: Head and neck cancer staging - There may be a possibility of combining anatomical information, which forms the basis of the current AJCC system, with functional information obtained from PET scans; b: Standardized uptake value (SUV) - Even though SUV is a widely used and reported parameter it suffers from some drawbacks that make it difficult to compare values between different institutions[19,78,79]. Further standardization in the way SUV is determined would allow inter-institutional collaboration and co-operative group clinical trials. Additional objectively measurable parameters which allow cross-platform and cross-hardware comparisons also need to be developed; c: PET/CT simulators - PET/CT scans are often co-registered with CT scans obtained for radiation therapy planning. PET/CT simulators are also available at some institutions which facilitate this. Consensus guidelines need to be developed for tumor and target volume delineation when using PET information for radiation treatment planning; d: PET as a prognostic indicator - The use of PET/CT scans as prognostic indicators in head and neck cancers and for follow-up and surveillance requires further research and investigation; and e: Economic analyses - Cost-benefit analyses suggest that PET/CT scans are cost-effective for diagnosis, staging and therapeutic decision making in head and neck cancers[80,81]. Common availability and decreasing costs of this technology will allow greater use and acceptance; (2) Newer imaging technology - Magnetic resonance imaging (MRI) offers superior imaging for soft-tissue delineation but offers little functional information unless MR spectroscopy is performed. MR spectroscopy can be performed in a limited volume. Recently MR/PET hybrid technologies have been developed for used in the clinic. This offers the advantages of high-quality soft tissue imaging from MR with whole-body and functional imaging from the PET component[82-84]. A recent review article highlights the potential for this new hybrid technology, the technical challenges and its use in clinical situations[85]; and (3) Newer radiopharmaceuticals - Many new radioisotopes and radiotracers are being developed to image further functional characteristics of tumors including hypoxia, tumor proliferation, amino acid metabolism and presence of EGFR on tumor cells. An excellent review on this topic has been provided by Wang et al[86]. As outlined in this review, a large number of efforts are being focused on hypoxia imaging. Hypoxia poses a major radiobiological disadvantage and confers radioresistance to the tumor. Hypoxic cells are not killed in response to radiation therapy and may be responsible for treatment failure, either locally or as distant metastasis. Some of the newer radiopharmaceuticals being used to image the hypoxic portion of the tumor include [18F]fluoromisonidazole (FMISO), copper-diacetyl-bis (N4-methylthiosamicarbazone) (Cu-ATSM) and [18F]fluoroazomycin arabinoside (FAZA). Once identified using PET scans, these hypoxic areas of the tumor can be preferentially targeted to receive a higher dose of radiation using IMRT technique.
The applications of FDG PET/CT scanning mentioned in this article highlight the extensive work done by groups across the world to the study the usefulness and application of this technology in various scenarios. Future work will continue to highlight the importance of this imaging modality in head and neck cancers.