INTRODUCTION
Acute intestinal ischemia is an abdominal emergency occurring when blood flow to the bowel loops decreases because of mesenteric arterial hypoperfusion, impaired venous drainage or occlusion[1-3]. It is estimated that nearly 1% of patients presenting with acute abdomen have ischemic intestinal disease involving the small bowel or colon[3-5].
Bowel ischemia is considered a potentially transient and reversible event; however, it may lead to intestinal infarction that requires surgical or interventional management. For this reason, early diagnosis is important to improve survival rates[6]. In most cases of late or missed diagnosis, the mortality rate from intestinal infarction is very high, with a reported value ranging from 60% to 90%[6-8].
Multi-detector computed tomography (MDCT) is a fundamental imaging technique that must be promptly performed in all patients with acute abdomen and suspected bowel ischemia. In fact, MDCT allows for correct diagnosis, contributes to appropriate treatment planning and provides important prognostic information thanks to its ability to define the nature of the disease and the extent of the anatomical damage[9].
ETIOLOGY AND PATHOPHYSIOLOGY
The causes of intestinal ischemia can be occlusive or non occlusive. Occlusive causes are due to the embolic or thrombotic occlusion of arterial or venous vessels and account for about 80% of all cases of intestinal ischemia[10]. Between 36% to 50% of intestinal infarctions are caused by embolic obstruction of the superior mesenteric artery in patients with cardiac pathology, while in 50%-60% of cases intestinal ischemia is caused by arterial thrombosis[10,11]. Venous thrombosis accounts for about 10%-15% of all cases of intestinal ischemia[10-12]. The most frequent cause of venous infarction is secondary to bowel closed-loop obstruction. This event does not lead to vascular thrombosis but to the twisting of the loops on their vascular pedicle which produces severe venous stasis. Another cause of venous intestinal ischemia is bowel obstruction, which causes an overdistension of the bowel wall, preventing the outflow of the venous blood[13-18]. More rarely, intestinal infarction of the occlusive type can be due to generalized vasculitis or hypercoagulable states[10,16].
Non occlusive causes account for about 20%-30% of all intestinal ischemia[17]. In these forms, there is a significant reduction in blood flow within the arteries and veins. Hypovolemic shock, severe heart failure, abnormal blood concentration, episodes of neurogenic vasodilation and vasoconstriction secondary to drugs determine non occlusive bowel infarction in most cases[7].
In cases of arterial and non occlusive ischemia, the first bowel reaction is in the spasm of the involved loops. In a more advanced phase, the microvascular wall damage causes hemorrhagic foci on the thinned bowel wall and the microflora proliferation leads to the hypotonic bowel dilatation. If the bowel ischemia persists for long enough, the entire bowel wall becomes necrotic and intramural air spreads through the mesenteric veins and into the portal venous system. If the underlying pathological process is removed, blood plasma or red blood cells may migrate from the disrupted mucosa into the lumen[1,2,9].
In cases of venous ischemia, the initial phase is characterized by spastic reflex ileus and is followed by hypotonic reflex ileus masked by progressive intestinal intramural and mesenteric edema. Prolonged stasis reduces the arterial flow and the progression to intestinal infarction causes an extensive submucosal hemorrhage and edema. The consequent loss of bowel wall integrity and the intestinal bacteria proliferation cause intestinal necrosis and peritonitis[9,10].
DIAGNOSIS AND COMPUTED TOMOGRAPHY
In the 1950s, the diagnosis of bowel ischemia was generally performed only during surgical exploration or at autopsy, but since the 1970s, the constant progress in imaging technology has gradually changed the diagnostic possibilities. Traditional radiography detects advanced stage findings such as bowel overdistension with the presence of air-fluid levels, pneumatosis and gas in the portal venous system. Ultrasonography is generally difficult to perform in patients with mesenteric infarction due to the presence of abundant intestinal meteorism and the poor compliance of these patients. However, its use is justified in order to rule out other diseases that could cause acute abdominal symptoms[19,20]. The gold standard investigation for detecting intestinal ischemia is angiography with its diagnostic and therapeutic applications[21]. However, this investigation is invasive, has a high cost and is often not available[10,22,23].
Since its introduction into clinical practice, computed tomography (CT) has been used more and more often for recognizing early signs of intestinal ischemia and infarction. While the first results reported in the literature did not to be appear very encouraging[24,25], the spread of spiral CT equipment has certainly increased the potential of this investigation[26].
MDCT, thanks to the increased spatial and temporal resolution, high-quality multi-planar reconstructions (MPR), maximum intensity projections (MIP) and three-dimensional rendering, is considered the gold standard for the diagnosis of intestinal ischemia, with reported sensitivity, specificity, positive and negative predictive values of 64%-93%, 92%-100%, 90%-100% and 94%-98%, respectively. It allows the visualization of the early signs of bowel ischemia and infarction and the etiological diagnosis of the disease, which is crucial for treatment planning in acute patients[9,10].
Recent experiences reported that dual-energy CT (DE-CT) provides a variety of post-processing options in the assessment of abdominal vascular diseases. In fact, DE-CT has also been reported to improve diagnostic accuracy and reduce radiation exposure and contrast material dose in the field of mesenteric vascular diseases. Besides, it seems to improve image quality in the study of abdominal and lower extremity arteries by virtual manipulation of keV-settings[27-30].
CT TECHNIQUE
The use of CT devices with volumetric image acquisition and high temporal resolution is crucial because of the critical clinical conditions and consequent poor compliance of patients suspected of having bowel infarction and referred for CT examination. The study protocol consists of unenhanced and contrast enhanced scans from the diaphragm dome to the pubic symphysis with the patient in the supine position.
After the intravenous injection of contrast medium (120-140 mL at a flow rate of 3-3.5 mL/s), scans are performed with a biphasic technique in the arterial (40 s mean delay) and venous (65 s mean delay) phases, with different technical parameters according to the CT device being used. Contrast medium injection may not be necessary if the unenhanced images clearly demonstrate the presence of the typical alterations of advanced stage bowel infarction. With single detector row spiral CT, the following parameters are used: slice thickness, 5 mm; reconstruction index, 2.5-5; pitch, 1.5; tube rotation time, 1 s. With multi-detector row CT, the parameters are: slice thickness, 0.5 mm; reconstruction index, 1-2.5 mm; pitch, 1.25-1.75; tube rotation time, 0.5-0.75 s. The administration of oral contrast medium to distend the bowel loops is not recommended because of the severe clinical condition and in order to keep the investigation time to a minimum. It is always recommended to visualize the obtained images with different window and level settings. In fact, a window set on the soft tissue values (width-W: 300-350; level-L: 40-50) can demonstrate alterations of the bowel wall, abdominal organs, mesentery and vascular structures; the window setting used to visualize the lung parenchyma (W: 450-1000; L: -100-0) will aid the recognition of extraluminal gas[10].
Unenhanced CT is reportedly required for the diagnosis of intestinal ischemia[31,32] in order to evaluate submucosal hemorrhage, hyperdense/calcified thrombi and atherosclerotic plaque and to obtain a baseline attenuation measurement of the bowel wall for the assessment of the enhancement. The arterial phase is performed for evaluating arterial stenoses, thrombi/emboli and occlusion[32,33], while the venous phase is for evaluating venous patency and abdominal organs which may be affected by ischemia.
On the other hand, a recent study which aims to reduce the radiation dose demonstrates that unenhanced CT is not necessary for the diagnosis of acute mesenteric ischemia because bowel enhancement can be assessed by using normal enhancing bowel as an internal reference and because of the low sensitivity of submucosal hemorrhage for the diagnosis. Major abdominal lesions can be readily diagnosed by using standard venous phase imaging, although important errors occur when relying only on the portal phase to assess the arterial system. The arterial phase is an integral component in the diagnosis of intestinal ischemia and should not be excluded to achieve dose reduction[32-34].
CT POST-PROCESSING
The widespread introduction of MDCT has revolutionized the field of computed tomography thanks to the high spatial and temporal resolution and the ability to create isotropic voxel data and, consequently, reliable MPR and three-dimensional (3D) reconstructions. Specialized 3D reconstruction techniques allow the visualization of the anatomical details which are difficult to evaluate by using axial images alone. Such details may require the use of oblique or curved reconstructions, or more complex methods such as MIP, minimum intensity projection (MinIP), surface-shaded volume rending (SS-VRT) and virtual endoscopy[35-37].
3D reconstructions are obtained by means of dedicated computer software that can handle the volumetric data of CT. Even if the use of 3D reconstructions increases the total exam evaluation time, it has been demonstrated how using 3D reconstruction techniques for examining volumetric data improves the interpretation, recognition and description of specific clinical conditions[37-39].
In cases of intestinal ischemia, MPR and 3D reconstructions allow easy detection and understanding of CT findings. In fact, reconstructions such as curved MPR, MIP, vessel probe (VP), SS-VRT and tissue transition projections (TTP) are particularly useful for the assessment of the vascular and bowel signs of intestinal ischemia.
Curved MPR are a subcategory of MPR reconstructions; they display all voxels contained in a selectable curved surface as a single bi-dimensional image which allows following winding structures along their natural path of development in a single image. This technique is particularly useful for the study of the vascular system. The vessel is displayed as a straight line resulting in vascular defects, stenoses and dilatations being easily detected.
MIP is a data visualization method that enables the detection of highly dense structures, distinguishing them from the surrounding tissues in order to better understand the extension of some structures, such as vessels, nodules, calcifications, surgical clips and foreign bodies. MIP is used in CT angiography because it can follow the complete course of vessels even if they are tortuous, allowing the evaluation of eventual enlargements and defects[35].
VP is a program that allows vessels to be simultaneously examined in 3D, curved reformat and cross-sectional reformat views. It can study and measure arteries from between 0.5 and 18 mm in diameter and calculate the degree of stenosis. It can display images in a variety of formats, including automatic and simultaneous orthogonal cross-sections, orthogonal MPR, oblique and curved MPR, 3D and curved reformat views. This fast and simple software has also been used in the field of the preoperative T staging of esophageal and gastric cancer for examining the visceral wall[40,41].
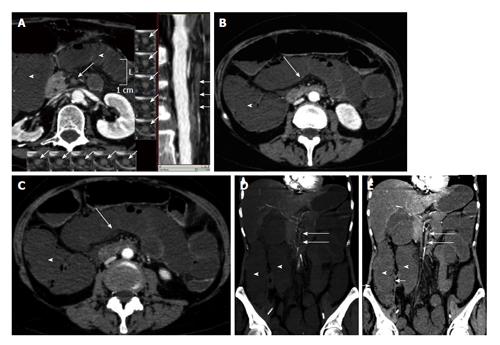
In cases of intestinal ischemia, curved MPR, MIP and VP are useful for evaluating the course of mesenteric vessels, searching for any filling defect (Figure 1A).
Figure 1 Arterial mesenteric ischemia.
A: Vessel probe in multiplanar (MPR) mode reconstructions showing bowel ischemia caused by the occlusion of the superior mesenteric artery (arrows). Bowel loop dilatation is associated (arrowheads); B and C: Computed tomography transverse scans show bowel wall dilatation (arrowheads) with loss of wall enhancement (arrows) in a case of arterial bowel ischemia diagnosed in the early stage; D: Coronal maximum intensity projection reconstruction; E: Coronal MPR reconstruction. Bowel ischemia caused by the occlusion of the superior mesenteric artery. Bowel loop dilatation (arrowheads) and parietal pneumatosis of the right colon (short arrows) are associated.
SS-VRT is a technique that creates a 3D visual illustration of CT volumetric data from any desired perspective. SS-VRT images provide a three-dimensional view that is significantly superior to other volume rendering techniques[35,37]. These techniques typically select voxels to be included in a surface rendering based on a selected range of Hounsfield values. By properly choosing the Hounsfield range, different types of tissues can be selected: parenchyma, bone, airways and vessels. By analyzing a combination of Hounsfield ranges, a volume of CT data can be segmented into several tissue types. The main diagnostic utility of SS-VRT techniques is its ability to reproduce structures of a specific density with great detail. With successive interactive steps of exclusion/inclusion of different tissue types and resizing/trimming of the regions of interest, surfaces that would otherwise be very difficult to visualize can be detected. In patients with intestinal ischemia due to vascular occlusion, SS-VRT images allow a 3D assessment of the course of mesenteric vessels.
Moreover, the application of a range of densitometric values corresponding to the transition zone between the bowel content (air or contrast medium) and the surrounding tissue makes the bowel wall transparent. These reconstructions, called tissue transition projections, allow the evaluation and extent of loop dilatation which are prognostic indicators in patients affected by intestinal ischemia[35,37].
CT FINDINGS
There is a significant correlation between the CT findings of bowel ischemia and the pathological damage. Unenhanced scans can show atherosclerotic calcification and the hyperdense aspect of the vascular structure involved in cases of recent thrombosis, parietal pneumatosis, air in the mesenteric vessels or portal branches, and pneumoperitoneum or retropneumoperitoneum. In all other cases, intravenous injection of contrast medium is essential in order to detect the causes and signs of the ischemia. In occlusive, embolic or thrombotic forms, CT allows visualization of the site of the vascular obstruction, appearing as a defective opacification of the vascular lumen. It is most easily recognized when the occlusion is at the level of the main trunks[10,26]. Most changes occurring during the initial phase of ischemia affect the cells and cannot therefore be visualized. The earliest recognizable alteration in the course of ischemia is vasodilation; in this phase, CT examination after contrast medium injection will show diffuse parietal hyperdensity at the level of the involved loops[3,10,26,42]. This hyperdensity is already evident in the arterial phase and persists during the venous phase; it can most easily be recognized by comparing the density of the normal and pathological segments of the bowel[3,10]. This sign occurs in about 51% of patients and indicates that the ischemia has not yet caused irreversible damage of the bowel and can be treated conservatively[10,43]. Without treatment, the mentioned alterations will evolve parallel with the anatomical damage. The hyperemic phase is followed by intense vasoconstriction that is aggravated by the compression of the intramural capillaries due to the overdistension of the bowel wall. The characteristic CT findings in this phase are the reduced or absent enhancement of the ischemic bowel after intravenous injection of the contrast medium. The persistence of the vasospasm causes an increase in capillary permeability with submucosal edema leading to the wall thickening and the interruption of the peristaltic activity, followed by bowel dilation[26] (Figure 1B and C). In venous infarction, venous stasis and hemorrhagic bulging of the mucosal and sub-mucosal layers lead to a higher degree of wall thickening than in the arterial type, associated with the typical target appearance of the loop (Figure 2)[26,44]. In 30%-70% of cases, vascular stasis causes edema and vascular bulging at the level of the mesentery which can be detected as an irregular hyperdensity of the mesenteric adipose tissue. These density changes indicate the onset of cellular necrosis that starts at the level of the mucosal epithelium and then extends to the other wall layers. In fact, the damage to the mucosal epithelium removes an important mechanical barrier between the bowel lumen and the bowel wall, causing the migration of intestinal gas, enzymes and anaerobic bacteria from the lumen into the wall thickness, as well as the passage of fluid from the wall into the bowel lumen. In this phase, CT shows the presence of air within the wall thickness, a sign known as parietal pneumatosis (Figure 1D and E). This is certainly one of the most important lesions in cases of suspected bowel ischemia; it has an incidence ranging between 22% and 72% in the literature and indicates advanced stage disease. The air within the wall may be arranged in a linear or curved shape; the bubbles are sometimes located in the central part of the wall and take on the ray-like appearance described as the “kiwi sign”. In order to determine whether any gas is present within the intestinal wall, other diseases or factors that could induce pneumatosis must first be excluded, such as lung disease, peptic ulcer, collagen disturbances and steroid treatments. However, the clinical data need to be considered for a differential diagnosis[10,26,44,45]. Reactive endoabdominal fluid collections may be observed in this phase, generally located close to the ischemic loops. With the progression of the anatomical damage, air may migrate from the bowel wall into the branches of the portomesenteric veins. If air can be seen at the level of the main branch of the portal vein or the intrahepatic portal branches, this is a sure sign of a very advanced phase of mesenteric infarction (present in 9%-36% of cases), although it does not necessarily indicate transmural bowel necrosis. The only pathognomonic sign of transmural necrosis is bowel perforation, which is seen as pneumoperitoneum or retropneumoperitoneum (described in 6%-20% of cases) and diffuse ascites (20%-22% of cases)[10,26,44].
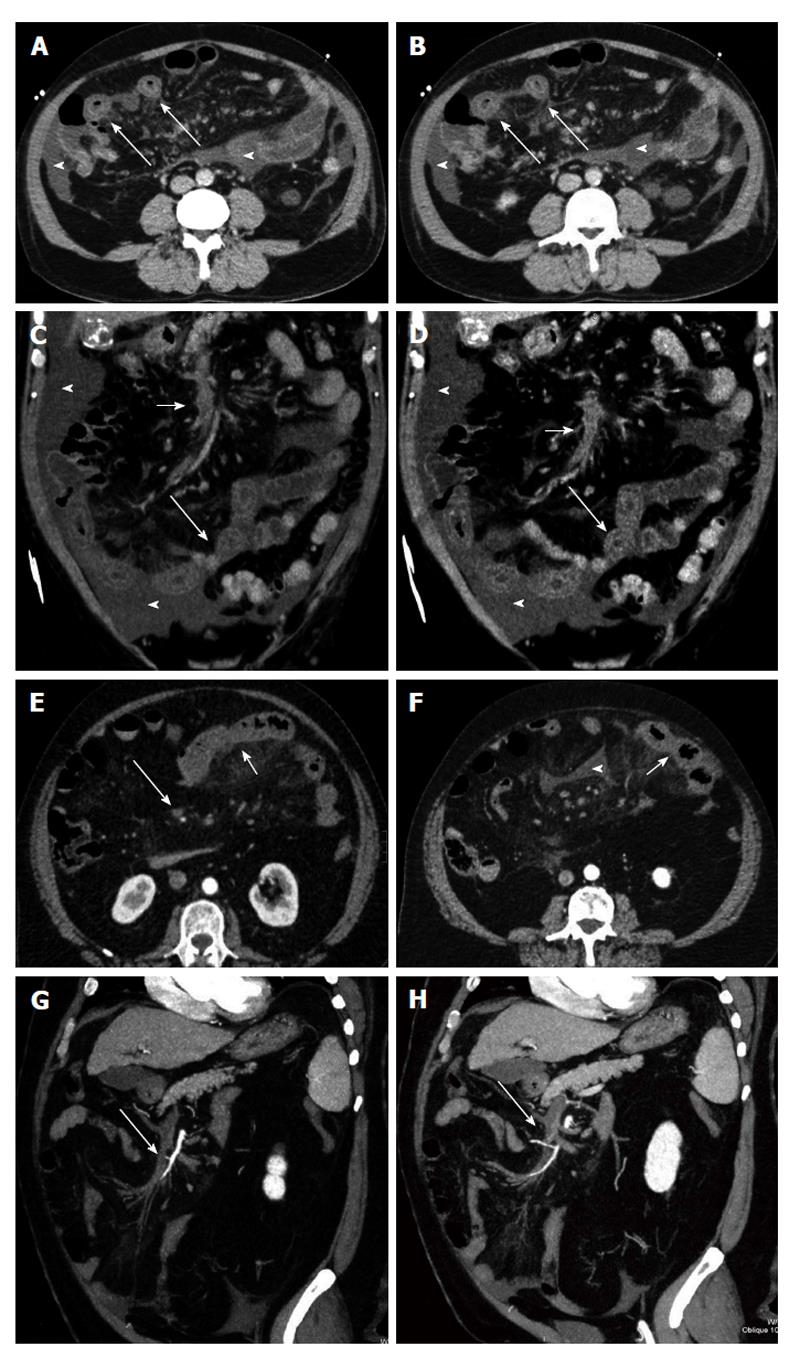
Figure 2 Venous mesenteric ischemia.
A and B: Transverse computed tomography (CT) scans; C and D: Coronal MPR reconstructions. Bowel ischemia caused by the occlusion of the superior mesenteric vein (short arrows). The target sign with concentric bowel wall thickening is well evident (long arrows). Ascites is associated (arrowheads); E and F: Transverse CT scans; G and H: Coronal curved multiplanar reconstructions. Bowel ischemia caused by the occlusion of the superior mesenteric vein (long arrows). The target sign with concentric bowel wall thickening is well evident (short arrows). Ascites is associated (arrowheads).
CT PROGNOSTIC CORRELATIONS
The prognostic value of CT findings of intestinal ischemia has been already reported in the literature and can be explained by the correlation between the progression of intestinal ischemic damage and the corresponding alterations detected on imaging.
Usually, outcome is closely correlated with the kind of vascular obstruction, with a reported mortality rate of 89% in the arterial and 11% in the venous forms[9].
Parietal hyperdensity, the absence of wall enhancement and bowel wall thickening all indicate a good outcome, whereas loop dilatation, parietal and portomesenteric pneumatosis and pneumoperitoneum/pneumoretroperitoneum are all indicators of unfavorable outcome[1-3,9,10,46].
Bowel wall hyperdensity reflects vasodilation, which is the first consequence of hypoxic damage. The absence of wall enhancement corresponds to the ensuing vasoconstriction; bowel-loop thickening and dilatation are related to the increased capillary permeability, pneumatosis and the presence of air within the mesenteric-portal system reflect the necrosis of the intestinal mucosa, whereas pneumoperitoneum/pneumoretroperitoneum corresponds to a transmural extension of the necrosis[3,9,10,47]. Therefore, wall hyperdensity, the absence of enhancement and wall thickening are an early stage of the disease, in contrast to loop dilation, parietal and portomesenteric pneumatosis and pneumoperitoneum/pneumoretroperitoneum which reflect an advanced stage of disease and are characterized by high mortality rates. Furthermore, hyperdensity and bowel wall thickening significantly correlate to venous forms, whereas loop dilatation, parietal pneumatosis, the presence of gas within the mesenteric venules and portal branches, as well as pneumoperitoneum/pneumoretroperitoneum are typical of arterial forms of ischemia. On the other hand, the absence of wall enhancement and the presence of ascites are not specific CT findings with respect to the nature of ischemia as their incidence is almost the same in both arterial and venous obstructions.
These findings may have a temporal justification; in fact, it has been demonstrated that in arterial obstructions, the pathophysiological events leading to wall necrosis occur in rapid succession, whereas a longer time interval is required for the initial vascular damage of venous occlusions to develop into the anatomical damage. Thus, the possibility of detecting wall abnormalities typical of the early phase of the disease is greater in venous infarctions than in the arterial forms. Wall thickening and the target appearance of the intestinal loops indicate a good prognosis, more frequently associated with venous infarction owing to a larger intramural hemorrhagic component and superinfection. Portomesenteric pneumatosis is considered to be a negative sign with an associated mortality rate of 75%-90%[10,48]; it indicates advanced bowel necrosis and correlates with unfavorable outcome, especially when associated with other ischemic wall abnormalities.
DIFFERENTIAL DIAGNOSIS
CT signs of bowel ischemia have different levels of specificity. For this reason, differential diagnosis with other abdominal disease is crucial.
Thickening of the bowel wall is the most common but least specific CT finding. Most bowel tumors present as focal thickenings, while benign conditions present as segmental and diffuse bowel thickenings extending for 6-40 cm or greater than 40 cm, respectively. Segmental or diffuse bowel wall involvement usually does not exceed 1 cm in thickness and may have a stratified white or grey attenuation pattern in contrast-enhanced scans[48,49].
The stratified pattern of attenuation, including the “target sign”, indicates bowel inflammation or ischemia. In cases of bowel ischemia, this finding should be evaluated in the clinical context and in association with other imaging findings, such as occlusion of the mesenteric vessels, intestinal pneumatosis, air in the mesenteric or portal veins, bowel dilatation and ascites. Bowel wall thickening with a stratified pattern may also be seen in the active phase of Crohn’s disease (CD). However, CD, which predominantly affects the ileum and right colon, is characterized by CT signs such as discontinuous involvement of the bowel wall, prominent vasa recta, fistulas and abscesses, and proliferation of the fat tissue along the mesenteric border of the bowel[44-46].
The white pattern of attenuation is caused by intense enhancement of the bowel wall and may occur in both ischemic and inflammatory bowel disease (Figure 3). Hyperenhancement of the ischemic bowel may be due to the early vasodilation at the level of the involved loops, the mesenteric venous occlusion or to the reperfusion after occlusive or non-occlusive ischemia. Associated imaging findings of bowel ischemia have to be considered for the correct diagnosis.
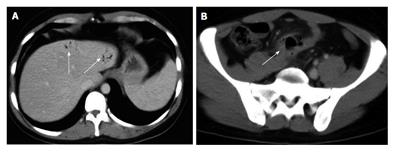
Figure 3 Crohn’s disease.
A: Computed tomography (CT) coronal reconstruction shows terminal ileal loop thickening with white attenuation pattern (arrow) and perivisceral vasa recta (arrowhead); B: Transverse CT scan shows thickening of the terminal ileal loop (arrow) associated with perivisceral endoperitoneal fluid (arrowhead).
The grey pattern of attenuation indicates decreased enhancement of the bowel wall. Its acute onset reflects a decreased blood supply and is pathognomonic of intestinal ischemia. The hypoattenuating bowel wall is caused by intense vasoconstriction and by wall edema in cases of mesenteric venous occlusion and bowel obstruction[49-51]. The delayed onset of grey pattern reflects transmural fibrosis and occurs in patients with chronic CD or chronic radiation enteritis.
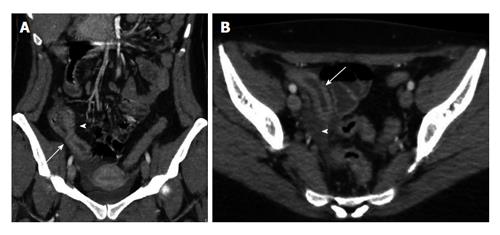
The combination of parietal pneumatosis and portomesenteric venous gas is a highly specific finding because it is associated with the presence of bowel ischemia in approximately 70% of cases. On the other hand, the sole finding of portomesenteric vein gas is associated with several causes besides mesenteric ischemia. In fact, it can be caused by some conditions such as wall alterations, bowel distension and abdominal sepsis. Wall alterations include several gastrointestinal ulcerative diseases, such as gastric ulcer, perforated gastric carcinoma and inflammatory bowel disease. Portomesenteric vein gas secondary to bowel distension can be due to iatrogenic visceral dilatation, paralytic or mechanical ileus, blunt trauma and barotrauma. Besides, some infectious abdominal processes, including diverticulitis, abdominal abscess and appendicitis, have been associated with portomesenteric vein gas (Figure 4)[49-51].
Figure 4 Intrahepatic portal air from diverticulitis.
A: Computed tomography transverse scan shows the presence of intrahepatic portal air (arrows); B: Pelvic sigmoid diverticulitis complicated by peridiverticular abscess (arrow).
Parietal pneumatosis has to be distinguished from pneumatosis cystoides intestinalis, an uncommon disease characterized by the presence of multiple gas-filled cysts in the submucosa and subserosa of the intestinal wall. The parietal cysts cause bowel obstruction in 16.3% of cases and the diagnosis of the disease is based on endoscopy. Plain radiography of the abdomen and CT easily detect the typical grape-like gas clusters into the bowel wall, allowing a differential diagnosis of intestinal pneumatosis with its typical linear shape[51,52].
CONCLUSION
Bowel infarction is an uncommon but often underestimated cause of non traumatic acute abdomen and early diagnosis is crucial in order to avoid irreversible damage to the bowel wall. MDCT is a fundamental imaging technique that must be promptly performed in all patients with acute abdomen and suspected bowel ischemia. Thanks to the dedicated reconstruction program, its diagnostic potential is much improved compared to the past and currently is superior to that of any other noninvasive technique. In the field of bowel ischemia, MDCT allows for correct diagnosis, is useful for appropriate treatment planning and provides important prognostic information as it is able to define the nature of the disease and the extent of the anatomical damage.