Published online Nov 28, 2013. doi: 10.4329/wjr.v5.i11.430
Revised: October 18, 2013
Accepted: November 2, 2013
Published online: November 28, 2013
Processing time: 102 Days and 12 Hours
AIM: To report our preliminary experience with a new generation aspiration catheter in the treatment of symptomatic pulmonary embolism (PE).
METHODS: A retrospective database search for pulmonary artery embolectomy since introduction of the Pronto .035” and XL extraction catheter (Vascular Solutions, Minneapolis, MN) at our institution in 10/2009 was performed. Ten consecutive patients were identified in which the Pronto .035” or XL catheter was used between 01/2010 and 03/2013. All patients were referred for catheter based embolectomy due to contraindications to systemic lysis, or for being in such a critical clinical condition that immediate percutaneous treatment deemed warranted. The computed tomography (CT) right to left heart ratio as predictor for the severity of the PE was retrospectively evaluated on standard axial views. The difference between pre- and post-procedure pulmonary pressure measures was taken to assess the procedural effect.
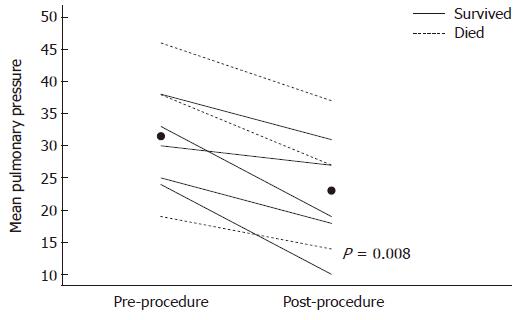
RESULTS: Extensive PE was confirmed angiographically in all patients. Measured right- to left ventricle (RV/LV) ratios were elevated beyond one in seven of the eight available CTs. Acute procedural success defined as clinical removal of visible thrombus and improvement in mean pulmonary artery pressure was seen in all recorded patients (n = 8), the mean pulmonary pressures declined from a median (range) of 35.5 (19-46) to 23 (10-37, P = 0.008) mmHg. Neither death nor other complications occurred intra- or immediately periprocedural, yet short term mortality within 30 d was found in 6 out of 9 patients, one patient was lost in follow up. The cause of death within 30 d in the 6 patients was identified as: Circulatory failure in direct connection with the PE (n = 2), stroke, sepsis, or succumbing to malignancy in a hospice setting (n = 2).
CONCLUSION: Success in thrombus removal with improved pulmonary hypertension and systemic hypotension suggests this aspiration technique to be effective. Aspiration catheters should be part of further trials.
Core tip: We present a new aspiration catheter for use in pulmonary embolism (PE) (Pronto .035” and XL extraction catheter, Vascular Solutions, Minneapolis, MN) in a case series of ten patients. The aspiration catheter allowed fast thrombus removal and lowered mean pulmonary artery pressure. No peri-procedural complications occurred, but high 30-d mortality remained. Catheter based aspiration embolectomy should be considered in acute symptomatic PE, since it is fast and does not require additional special equipment, thus signifying a widely applicable technique. Aspiration embolectomy should be included in further trials treating symptomatic PE.
- Citation: Heberlein WE, Meek ME, Saleh O, Meek JC, Lensing SY, Culp WC. New generation aspiration catheter: Feasibility in the treatment of pulmonary embolism. World J Radiol 2013; 5(11): 430-435
- URL: https://www.wjgnet.com/1949-8470/full/v5/i11/430.htm
- DOI: https://dx.doi.org/10.4329/wjr.v5.i11.430
The treatment of massive pulmonary embolism (PE) remains an ongoing challenge due to acute onset of right heart failure and cardiogenic shock limiting the time for therapeutic success. Systemic thrombolysis is often contraindicated and when given involves > 20% complications including 3% intracranial bleeding with a 30 d mortality of 25%-65% depending on severity and case selection[1,2]. In this setting intervention with percutaneous mechanical thrombectomy is appealing, aiming to rapidly reduce the clot burden, pulmonary artery pressure, right heart strain, and to reestablish circulation[3-6].
Several devices have been developed for this task, but each comes with its limitations. The AngioJet (Medrad Interventional/Possis, Minneapolis, MN) and Hydrolyzer (Cordis, Miami, FL) thrombectomy systems use negative pressure created by high-pressure saline injection to aspirate the thrombus[7]. The AngioJet device has been associated with bradyarrhythmias and deaths when used in the pulmonary arteries and carries a black box warning for that use[8,9]. Angioplasty balloons or a rotational pigtail catheter (Cook, Bloomington, IN) have been applied to mechanically fragment a large central thrombus into smaller pieces. However, the fragments can end up occluding previously perfused arteries, potentially increasing pulmonary artery pressures and right heart strain[10]. The principle of aspiration embolectomy has proven successful in small and moderate sized arteries like coronaries. The Greenfield suction embolectomy catheter (Boston Scientific, Natick, MA) was the only PE catheter device approved by the FDA. Bulky design with difficult steerability compromised widespread utilization[11]. Lang et al[12] reported successful treatment with a 14 F 90-cm-long Ultrathane non tapered catheter (Cook, Bloomington, IN), but this catheter was not steerable. Extensive manipulation for repeat access to specific pulmonary artery sites was required for each aspiration pass. This slowed the procedure greatly. Both are no longer available.
We report our preliminary experiences with a new generation of aspiration catheters, the 10 F Pronto .035″ and 14 F XL extraction catheter (Vascular Solutions, Minneapolis, MN) in the treatment of acute symptomatic pulmonary embolism. Compared to the preceding suction catheters, these catheters are quickly manipulated over a wire, combining protection from pulmonary artery wall perforation with a rapid return to the target position after a run of aspiration.
Institutional review board (IRB) approval was obtained. A retrospective database search for pulmonary artery embolectomy since introduction of the catheters in 10/2009 was performed. Ten consecutive patients were identified in which the Pronto .035” or XL catheter were used between 01/2010 and 03/2013.
Patient characteristics are demonstrated in Table 1. The median age of patients was 62.5 (range 33-74) years. All patients were referred for catheter based embolectomy due to contraindications to systemic lysis, or the circulatory status was judged so critical that thrombolytic therapy was deemed not likely to be effective in the available time[13]. The computed tomography (CT) right to left heart ratio as predictor for the severity of the PE was measured on standard axial views[14-16]. Tissue plasminogen activator (tPA) and systemic Heparin were given at the discretion of the operator in dosages as shown in Table 1. Recorded blood loss varied widely from the common 120-300 mL to the maximum volume of 800 mL in one patient. An intraprocedural blood transfusion was required in that patient. Most patients received an IVC filter as secondary prophylaxis after aspiration.
| Patients | Age (yr)/Sex | Pertinent risk factor | Axial CT RV/LV ratio | Systemic pressure pre therapy (Sys/Dia/HR) (mmHg) | Systemic pressure post therapy (Sys/Dia/HR) (mmHg) | Pulmonary pressure pre therapy (Sys/Dia/Mean) (mmHg) | Pulmonary pressure post therapy (Sys/Dia/Mean) (mmHg) | Concomitant Therapeutics Tpa(mg)/ Heparin Bolus (U) | Death ≤ 30d |
| Patient 1 | 67 F | Malignancy, Surgery | 1.6 | 90/66/145 | 81/65/135 | 53/37/46 | 48/31/37 | 6/6000 | Y |
| Patient 2 | 74 M | MVA, Surgery | 1.7 | 120/79/133 | 148/102/122 | 53/23/33 | 32/13/19 | 12/0 | N |
| Patient 3 | 33 F | Neurosurgery | 1.6 | 100/76/116 | 94/51/124 | 54/27/38 | 11/27/1934 | 12 | Y |
| Patient 4 | 47 M | MVA, Surgery | 1.3 | 133/57/143 | 115/54/131 | 7/19/1943 | 4/14/1931 | 10 | Y |
| Patient 5 | 47 F | Malignancy, PEs, COPD | 0.8 | 88/65/45 | 87/52/57 | NA/NA/24 | NA/NA/10 | 0 | N |
| Patient 6 | 57 M | Malignancy | NA | 126/102/112 | 102/79/97 | 50/35/40 | NA | 27 | Y |
| Patient 7 | 64 M | Malignancy, Surgery, COPD | 1.3 | 103/76/115 | 119/84/90 | 60/20/40 | NA | 0 | NA |
| Patient 8 | 62 M | Diabetes, Hypertension | 1.4 | 111/74/80 | 104/80/70 | 58/27/38 | 44/18/31 | 16/5000 | N |
| Patient 9 | 73 M | Malignancy | 1.5 | 116/72/115 | 116/69/131 | NA/NA/30 | NA/NA/27 | 6 | Y |
| Patient 10 | 63 M | Malignancy | NA | 165/82/108 | 66/57/62 | 39/15/25 | 25/14/18 | 4.5 | Y |
The nonparametric Wilcoxon signed rank test was used to examine the paired difference between pre- and post-procedure pulmonary pressure measures. Given the small sample size, which limited the power for detecting differences, survival was not statistically compared. Two-sided P-values less than 0.05 were considered statistically significant.
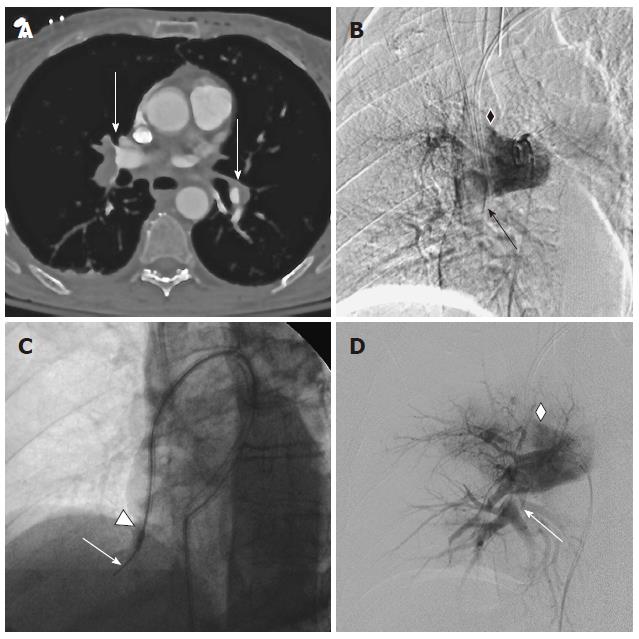
Use of the Pronto .035” or XL catheter is FDA approved in peripheral arteries or veins and is off-label in the pulmonary arteries. Embolectomy is achieved through a side-port proximal to the tip of the catheter (Figure 1). A pigtail tip configuration in 8 and 14 F is provided to improve large embolus aspiration in the large diameter of the main pulmonary arteries, while the straight and angled tip configuration enables advancement in segmental and branch arteries. The side-port has radio-opaque marker to allow controlled positioning in clot. Aspiration is achieved via a lockable 60 mL syringe connected to a sideport. Suction control is maintained and modified during use with a roller clamp near the syringe. In 8 out of 10 cases the 10 F Pronto .035’’ catheter was used, since the 8 and 14 F XL catheter only became available in 01/2012. In the remaining 2 cases the 14 F straight and pigtail catheters were used.
The catheter system was introduced through an appropriately sized 10 or 14 F sheath in the right common femoral vein. Access to the main pulmonary arteries was gained according to operator preferences, (six different attendings), either with a Montefiore pulmonary artery or pigtail catheter (Cook, Bloomington, IN) and pressure measurements were obtained. After angiography and positioning of a stable exchange length working wire in the target artery, the embolectomy catheter was introduced. XL 14 F pigtail catheters were used with rotation under suction to sweep the entire diameter of the large central arteries. The angulated 10 F Pronto .035” catheter and the straight 14 F XL catheter were wedged over the wire into clot bearing segmental arteries. Clot was suctioned from branches and of the main right or left central pulmonary artery during the withdrawal movement. If necessary, additional angiographic catheters were used to select challenging branch arteries. The number of passes and endpoint of the procedure was at the discretion of the operator, with angiographic flow improvement combined with improved pulmonary or systemic pressures as the usual criteria to halt the embolectomy (Figure 2).
All patients in our series were confirmed to have extensive PE on angiography. As risk factor for PE prior surgery was identified in 5 cases, malignancy as risk factor for PE and adverse outcome[15] was found in 6 patients. Measured right- to left- ventricle (RV/LV) ratios were elevated beyond one in seven of the eight available CTs (Table 1).
Acute procedural success defined as clinical removal of visible thrombus and improvement in mean pulmonary artery pressure (average of systolic and diastolic pressures) was seen in all recorded patients (n = 8, Figure 3); the mean pulmonary pressure declined from a median (range) of 35.5 (19-46) to 23 (10-37, P = 0.008) mmHg. No deaths occurred intra- or immediately periprocedural, also no minor complication such as access hematoma or arrhythmia was observed.
A short term mortality within 30 d was found in 6 out of 9 patients, one patient was lost in follow up. The cause of death within 30 d in the 6 patients was identified as: Circulatory failure in direct connection with the PE (n = 2), stroke, sepsis, or succumbing to malignancy in a hospice setting (n = 2).
Consistent success in thrombus removal with improvement in pulmonary hypertension and systemic hypotension suggests this aspiration technique is both successful and of physiologic importance in acute symptomatic PE.
Kucher et al[17] defined the requirements for an ideal percutaneous PE thrombectomy catheter as being (1) highly maneuverable, (2) effective in thrombus removal, and (3) safe without damaging cardiac or pulmonary structures. The presented catheter system seems to fulfill all of these requirements, and absence of complex and costly technology should favor widespread use with a real impact on this insidious and often fatal disease. Having two configurations available allowed to remove the most urgent central thrombus burden in the right or left main pulmonary artery first, whereas the angled tip and straight configuration was then used to reestablish flow in obstructed inter- and segmental arteries. The amount of discarded blood in our case series varied widely as well as the concomitant use of thrombolytics. Cell saving techniques are routine in operative cases, and may well be appropriate here. Requirements for blood transfusions need to be part of future investigations as well as establishing standardized concomitant drug regiments and firm therapeutic endpoints.
It is evident that a small retrospective series like ours has considerable limitations pertaining to generalization of our findings. It is also possible that a variety of treatment strategies may have their roles, since different types of thrombi and emboli may be encountered[18] and one treatment method may not fit all[19].
Although mortality has been reported to range from about 20%-65% in those presenting with shock[20], the high 66% mortality within 30 d (Table 1) could bring into question the efficacy of the aspiration system. Taking the RV/LV ratio of one as indicator for severity of the PE, seven of our measured eight patients presented with largely elevated ratios from 1.3-1.7. Even though the RV/LV ratio is a controversial prognostic indicator[21-24], it seems to outperform other radiologic surrogate measurements. Similarly, six patients had underlying malignancy as risk factor for adverse short term outcome[15]. The high mortality appears therefore rather to be a reflection of the critical condition of the patients than to refute the efficacy of percutaneous embolectomy. No death could be linked to a procedural complication. Considering that conservative management with systemic tPA results in numerous complications and death rates up to 65%[1,25,26], we propose an earlier role for catheter based embolectomy than in a moribund state.
To generate best-possible evidence in the treatment of massive pulmonary embolism we suggest that percutaneous aspiration catheters be part of further trials and be included in nationwide pulmonary embolism treatment registries.
We gratefully acknowledge and thank Ms. Donna Ashlock, B.S., for her support in editing the images.
Massive pulmonary embolism remains a therapeutic challenge with high morbidity and mortality. Systemic thrombolysis is considered the standard of care, but may be unsatisfactory as sole treatment in cases with right heart failure and cardiogenic shock, with lysis being a time-consuming process bearing also considerable risks of severe hemorrhage. Analogous to therapies in other vascular beds, fast-onset and safe percutaneous treatment options have been proposed in cases of symptomatic pulmonary embolism.
Several percutaneous devices have been described, reaching from mechanical fragment disruption with catheters and balloons to rotational devices, all with their own short-comings. Currently, catheter-based thrombolysis is mostly deployed, in which thrombolytic drug amounts are greatly reduced by direct infusion into the pulmonary embolus. However, the process is still time consuming and has the potential for drug-induced hemorrhage. Further alternatives are therefore warranted.
The presented case study describes first experiences with a large-diameter thrombectomy catheter. The catheter can be directed and is used over a wire, therefore reducing the risks of vessel wall perforation. With a large volume syringe as suction device, the new catheter does not require a complex set-up, which facilitates widespread use.
The case study shows feasibility with no catheter related complication within 48 h of the intervention.
This is a nice report of the use of a new catheter in the treatment of symptomatic pulmonary embolism. Although the author reported that “no death could be linked to a procedural complication”, a high rate (6 out of 9 patients) of short term mortality within 30 d was really a big issue and should be considered seriously. The main result is that in all (8 of 10) patients where such data are available, mean pulmonary pressure dropped.
| 1. | Kasper W, Konstantinides S, Geibel A, Olschewski M, Heinrich F, Grosser KD, Rauber K, Iversen S, Redecker M, Kienast J. Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. J Am Coll Cardiol. 1997;30:1165-1171. |
| 2. | Aymard T, Kadner A, Widmer A, Basciani R, Tevaearai H, Weber A, Schmidli J, Carrel T. Massive pulmonary embolism: surgical embolectomy versus thrombolytic therapy--should surgical indications be revisited? Eur J Cardiothorac Surg. 2013;43:90-94; discussion 94. |
| 3. | Smulders YM. Pathophysiology and treatment of haemodynamic instability in acute pulmonary embolism: the pivotal role of pulmonary vasoconstriction. Cardiovasc Res. 2000;48:23-33. |
| 4. | Banovac F, Buckley DC, Kuo WT, Lough DM, Martin LG, Millward SF, Clark TW, Kundu S, Rajan DK, Sacks D. Reporting standards for endovascular treatment of pulmonary embolism. J Vasc Interv Radiol. 2010;21:44-53. |
| 5. | Wood KE. Major pulmonary embolism: review of a pathophysiologic approach to the golden hour of hemodynamically significant pulmonary embolism. Chest. 2002;121:877-905. |
| 6. | Kuo WT, van den Bosch MA, Hofmann LV, Louie JD, Kothary N, Sze DY. Catheter-directed embolectomy, fragmentation, and thrombolysis for the treatment of massive pulmonary embolism after failure of systemic thrombolysis. Chest. 2008;134:250-254. |
| 7. | Kucher N, Goldhaber SZ. Mechanical catheter intervention in massive pulmonary embolism: proof of concept. Chest. 2008;134:2-4. |
| 8. | Dwarka D, Schwartz SA, Smyth SH, O’Brien MJ. Bradyarrhythmias during use of the AngioJet system. J Vasc Interv Radiol. 2006;17:1693-1695. |
| 9. | Jeyabalan G, Saba S, Baril DT, Makaroun MS, Chaer RA. Bradyarrhythmias during rheolytic pharmacomechanical thrombectomy for deep vein thrombosis. J Endovasc Ther. 2010;17:416-422. |
| 10. | Nakazawa K, Tajima H, Murata S, Kumita SI, Yamamoto T, Tanaka K. Catheter fragmentation of acute massive pulmonary thromboembolism: distal embolisation and pulmonary arterial pressure elevation. Br J Radiol. 2008;81:848-854. |
| 11. | Meyer G, Koning R, Sors H. Transvenous catheter embolectomy. Semin Vasc Med. 2001;1:247-252. |
| 12. | Lang EV, Barnhart WH, Walton DL, Raab SS. Percutaneous pulmonary thrombectomy. J Vasc Interv Radiol. 1997;8:427-432. |
| 13. | Hirsh J, Guyatt G, Albers GW, Harrington R, Schünemann HJ. Executive summary: American College of Chest Physicians Evidence-Based Clinical Practice Guidelines (8th Edition). Chest. 2008;133:71S-109S. |
| 14. | Ghaye B, Ghuysen A, Willems V, Lambermont B, Gerard P, D’Orio V, Gevenois PA, Dondelinger RF. Severe pulmonary embolism: pulmonary artery clot load scores and cardiovascular parameters as predictors of mortality. Radiology. 2006;239:884-891. |
| 15. | Furlan A, Aghayev A, Chang CC, Patil A, Jeon KN, Park B, Fetzer DT, Saul M, Roberts MS, Bae KT. Short-term mortality in acute pulmonary embolism: clot burden and signs of right heart dysfunction at CT pulmonary angiography. Radiology. 2012;265:283-293. |
| 16. | Lu MT, Demehri S, Cai T, Parast L, Hunsaker AR, Goldhaber SZ, Rybicki FJ. Axial and reformatted four-chamber right ventricle-to-left ventricle diameter ratios on pulmonary CT angiography as predictors of death after acute pulmonary embolism. AJR Am J Roentgenol. 2012;198:1353-1360. |
| 17. | Kucher N, Goldhaber SZ. Management of massive pulmonary embolism. Circulation. 2005;112:e28-e32. |
| 18. | Krueger K, Deissler P, Coburger S, Fries JW, Lackner K. How thrombus model impacts the in vitro study of interventional thrombectomy procedures. Invest Radiol. 2004;39:641-648. |
| 19. | Kennedy RJ, Kenney HH, Dunfee BL. Thrombus resolution and hemodynamic recovery using ultrasound-accelerated thrombolysis in acute pulmonary embolism. J Vasc Interv Radiol. 2013;24:841-848. |
| 20. | Goldhaber SZ, Visani L, De Rosa M. Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER). Lancet. 1999;353:1386-1389. |
| 21. | Araoz PA, Gotway MB, Harrington JR, Harmsen WS, Mandrekar JN. Pulmonary embolism: prognostic CT findings. Radiology. 2007;242:889-897. |
| 22. | Henzler T, Roeger S, Meyer M, Schoepf UJ, Nance JW, Haghi D, Kaminski WE, Neumaier M, Schoenberg SO, Fink C. Pulmonary embolism: CT signs and cardiac biomarkers for predicting right ventricular dysfunction. Eur Respir J. 2012;39:919-926. |
| 23. | Attinà D, Valentino M, Galiè N, Modolon C, Buia F, de Luca F, Bacchi-Reggiani ML, Zompatori M. Application of a new pulmonary artery obstruction score in the prognostic evaluation of acute pulmonary embolism: comparison with clinical and haemodynamic parameters. Radiol Med. 2011;116:230-245. |
| 24. | Moroni AL, Bosson JL, Hohn N, Carpentier F, Pernod G, Ferretti GR. Non-severe pulmonary embolism: prognostic CT findings. Eur J Radiol. 2011;79:452-458. |
P- Reviewers: Chen F, Sijens PE S- Editor: Qi Y L- Editor: A E- Editor: Liu XM