Published online Jun 28, 2012. doi: 10.4329/wjr.v4.i6.258
Revised: May 18, 2012
Accepted: May 25, 2012
Published online: June 28, 2012
AIM: To evaluate safety and utility of coronary computed tomography angiography (CCTA) compared to invasive coronary angiography (ICA) in new cardiomyopathy.
METHODS: Eighteen patients (mean age 56.5 years, 10 males) who presented for evaluation of new onset heart failure with evidence of systolic dysfunction (ejection fraction < 40%) on echocardiography and recent ICA were prospectively enrolled. Patients with known coronary artery disease, atrial fibrillation, creatinine > 1.5 g/dL, and contraindication to intravenous contrast administration were excluded. CCTA was performed using a dual source 64-slice scanner. Mean heart rate was 75 beats per minute. Stenosis was graded for each coronary segment as: none, mild (< 50%), moderate (50%-70%), severe (> 70%), or non-evaluable. Ischemic cardiomyopathy (ICM) was diagnosed if severe stenosis was present in the left main, proximal left anterior descending artery, or two or more major arteries.
RESULTS: Two patients were diagnosed with ICM by ICA. CCTA correctly identified 2 patients with ICM and 16 patients as non-ICM. CCTA successfully evaluated 240/246 coronary segments with an accuracy of 97.5%, sensitivity 70%, specificity 98.7%, positive predictive value of 70%, and negative predictive value of 98.7% for identifying severe stenosis on a per-segment level.
CONCLUSION: Dual source 64-slice multi-detector CCTA is a safe, accurate, and non-invasive technique for diagnosing ICM in patients presenting during the acute phase of newly diagnosed cardiomyopathy.
- Citation: Srichai MB, Fisch M, Hecht E, Slater J, Rachofsky E, Hays AG, Babb J, Jacobs JE. Dual source computed tomography coronary angiography in new onset cardiomyopathy. World J Radiol 2012; 4(6): 258-264
- URL: https://www.wjgnet.com/1949-8470/full/v4/i6/258.htm
- DOI: https://dx.doi.org/10.4329/wjr.v4.i6.258
The etiology of new onset heart failure may be non-ischemic or related to underlying coronary artery disease (CAD) (ischemic) cardiomyopathy Evaluation of the underlying etiology is important to direct therapeutic strategies and assess prognosis given that an ischemic etiology is a significant independent predictor of worse long-term outcomes[1,2]. Ischemic cardiomyopathy (ICM) is the underlying cause of heart failure in approximately two-thirds of patients[3], and is defined by the presence of any of the following: (1) history of myocardial infarction or revascularization procedure; (2) ≥ 75% stenosis of left main or proximal left anterior descending artery; or (3) ≥ 75% stenosis of two or more epicardial vessels[4]. Clinical assessment of etiology is unreliable, particularly since up to 40% of patients with a non-ischemic etiology may report a history of typical angina symptoms[4-7]. Thus, current guidelines recommend assessment for underlying obstructive CAD[8], either through non-invasive testing or invasive X-ray coronary angiography (ICA). However, traditional noninvasive testing with nuclear imaging and echocardiography have not been shown to consistently differentiate between ischemic and nonischemic etiologies[9-18], and patients are frequently referred for ICA.
Coronary computed tomography angiography (CCTA) has been demonstrated in several studies to have excellent diagnostic accuracy for evaluating the presence of significant CAD[19,20], particularly in unselected populations with no known CAD[21]. Patients with heart failure represent a unique clinical population for which prior CCTA study results are not necessarily applicable. Patients with heart failure often have elevated resting heart rates and do not tolerate large doses of β-blocker medications, commonly utilized in general CCTA populations to slow the heart rate for the purpose of improving overall image quality and increasing the number of interpretable coronary segments visualized with CCTA. In addition, left ventricular (LV) hemodynamics influence coronary artery opacification, and it has been shown that patients with cardiomyopathy tend to have impaired image quality due to reduced contrast opacification compared to patients with normal cardiac function[22]. As such, it is often not advisable to perform CCTA in the early and acute phase of heart failure diagnosis, and studies evaluating the CCTA in heart failure patients have performed CCTA during the more stable phase after prolonged β-blocker therapy[23,24].
We evaluated the safety and diagnostic accuracy of CCTA compared to the gold standard of ICA for evaluating CAD in patients during the acute phase of newly diagnosed cardiomyopathy.
We prospectively enrolled 18 patients who presented for evaluation of new onset heart failure symptoms with evidence of systolic dysfunction [ejection fraction (EF) < 40%] on echocardiography and recent or planned ICA between August 2006 and April 2008. Patients with history of myocardial infarction, prior revascularization, or recent revascularization procedure were excluded from the cohort. Patient with contraindications to CCTA including history of severe reaction to iodine contrast, creatinine levels < 1.5 mg/dL, and significant cardiac arrhythmia were excluded. Patients with intervening clinical event between time of ICA and CCTA were also excluded. Our study was approved by the institutional review board at the medical center and was compliant with the Health Insurance Portability and Accountability Act. All participating patients gave written informed consent.
Clinical 2D and Doppler echocardiography was performed in all patients using standard echocardiographic views for quantitative and qualitative assessment of global and regional ventricular size and function, increased wall thickness and valvular abnormalities. Valvular abnormalities were considered significant if there was evidence of at least moderate stenosis or regurgitation in the aortic or mitral valve. Relevant information on overall EF, presence of regional vs global wall motion abnormalities, and presence of significant valvular abnormalities was extracted from the clinical report and verified by a cardiologist with Level 3 training in echocardiography.
CCTA was performed with a dual source 64-slice scanner (Siemens Definition, Erlangen, Germany). Prior to image acquisition, 18- or 20-gauge intravenous access was obtained and electrocardiographic (ECG) leads were placed. All patients received sublingual nitroglycerin prior to scan commencement. Intravenous metoprolol was administered to patients with resting tachycardia (heart rate > 100 bpm) or frequent premature contractions. A test bolus injection method was used to calculate scan delay time, and 60-116 mL of 320-370 mg I/mL of nonionic contrast [iodixanol (Visipaque 320, GE Healthcare), iopamidol (Isovue 370, Bracco Diagnostics) or iopromide (Ultravist 370, Bayer Healthcare Pharmaceuticals)] was intravenously administered at a rate of 5-7 mL/s (mean 6 mL/s) with 50 mL saline chaser. First pass CCTA acquisition was obtained from carina through the diaphragm using thin collimation in conjunction with retrospective ECG gating. Scan parameters for first pass dual source CT scan included gantry rotation 330 ms, tube voltage 120 kV for both tubes, average tube output 351 mAs (automatically adjusted for patient body habitus with CareDose 4D, range 287-426 mAs), retrospective gating with pitch automatically adjusted for patient heart rate (range 0.2-0.5), ECG dose modulation (using window of 30%-80% of the R-R interval), 32-mm × 2-mm × 0.6-mm collimation with z-flying focal spot technique and 82.5 ms temporal resolution. Images were reconstructed with 0.75 mm slice thickness and 0.5 mm increment at least motion cardiac phases as well as 10% phase intervals from 0%-90% of the RR wave for evaluation of coronary arteries and LV function.
CCTA images were reviewed on a 3D workstation equipped with multi-planar and maximum projection reformations (Multimodality Workplace, Siemens Healthcare) by two expert reviewers (MBS, cardiologist with 6 years experience and EH, radiologist with 4 years experience) blinded to all clinical information and results of the ICA study. Coronary arteries were evaluated using the 15 segment American Heart Association model of the coronary artery tree[25], and each segment was graded based on diameter luminal stenosis severity as follows: 0 = none, 1 = mild (< 50%), 2 = moderate (50%-70%), 3 = severe (> 70%), and 4 = non-evaluable. Significant stenoses were defined as those with greater than 50% luminal narrowing. Patients were defined as having an ICM by CCTA if there was grade 3 stenosis in the left main, proximal left anterior descending artery, or 2 or more major arteries[4]. Additionally, LV end-diastolic volume, end-systolic volume and EF were calculated using specialized post-processing software (Circulation, Siemens Healthcare). All coronary artery segments were evaluated regardless of size.
All patients underwent clinically indicated ICA for evaluation of cardiomyopathy. Conventional ICA was performed according to standard clinical protocols[25]. Multiple projections were acquired in standard views for evaluation of maximal coronary artery stenosis.
ICA images were reviewed by two expert reviewers (JS and ER, cardiologists with 20 and 4 years experience, respectively) blinded to all clinical information and results of the CCTA study. The coronary arteries were evaluated in the same manner as the CCTA images utilizing the 15 segment coronary segmentation model; coronary artery lesions was graded by visual estimation of percentage diameter stenosis. Patients were identified as having an ICM by ICA using the same criteria as that used for CCTA.
Continuous variables were reported as mean and standard deviation values and compared using the Mann-Whitney U test, whereas categorical variables were described as percentages and compared using Fisher’s exact test. The sensitivity, specificity, positive predictive value, and negative predictive values were calculated using standard formulas. Coronary segments that were not evaluable by the readers on CCTA secondary to small vessel size or coronary motion were removed from analysis. All reported P-values are 2-sided and results were declared statistically significant when P < 0.05. SPSS Version 18 statistical software (SPSS Inc., Chicago, IL) was used for all computations.
There were 18 patients [mean age 56 ± 13 years, 10 (56%) men] enrolled who met inclusion and exclusion criteria (Table 1). Most patients were enrolled within 1 mo (n = 10) or 2 mo (n = 16) of their diagnosis. Two patients were enrolled more than 2 mo following initial diagnosis and treatment due to timing of ICA. All patients were treated with β-blocker therapy. The majority of patients had already been receiving carvedilol (n = 17) at a relatively small dose (average dose 10.7 ± 7.7 mg twice a day). There were a total of 5 patients (28%) with significant stenosis in at least 1 coronary segment by ICA. Two patients (11.1%) met criteria and were diagnosed with ICM. There were no significant differences in the baseline characteristics between the group with ICM compared to non-ICM (Table 1).
| All patients (n = 18) | Ischemic (n = 2) | Non-ischemic (n = 16) | P-value | |
| Male sex | 10 (56) | 2 (100) | 8 (50) | 0.32 |
| Age (yr) | 56 ± 13 | 67.5 ± 14.8 | 55.2 ± 12.3 | 0.86 |
| Hypertension | 16 (89) | 2 (100) | 14 (88) | 1.00 |
| Diabetes mellitus | 6 (33) | 2 (100) | 4 (25) | 0.10 |
| Hyperlipidemia | 11 (61) | 2 (100) | 9 (56) | 0.50 |
| Tobacco use | 3 (17) | 0 (0) | 3 (19) | 1.00 |
| Body mass index | 27.9 ± 4.5 | 30.3 ± 5.4 | 28.3 ± 4.6 | 0.26 |
| Creatinine clearance | 83.5 ± 29.4 | 60.4 ± 6.6 | 86.3 ± 29.9 | 0.052 |
| Baseline medications | ||||
| Lasix | 13 (72) | 2 (100) | 11 (69) | 1.00 |
| Beta blocker | 18 (100) | 2 (100) | 16 (100) | NS |
| ACE inhibitor/angiotensin receptor blocker | 17 (94) | 2 (100) | 15 (94) | 1.00 |
| Aldactone | 4 (22) | 0 (0) | 4 (25) | 1.00 |
| Digoxin | 3 (17) | 1 (50) | 2 (13) | 0.31 |
| Statin use | 11 (61) | 2 (100) | 9 (56) | 0.50 |
| Aspirin | 12 (67) | 2 (100) | 10 (63) | 0.53 |
| Echocardiography | ||||
| LV EF | 22.8 ± 8.6 | 17.5 ± 3.5 | 23.4 ± 8.9 | 0.33 |
| Regional wall motion abnormalities | 12 (67) | 2 (100) | 10 (63) | 0.53 |
| Valvular abnormalities | 6 (33) | 0 (0) | 6 (38) | 0.53 |
For the two cases of ICM identified by ICA, echocardiography demonstrated regional wall motion abnormalities, normal wall thickness and no evidence for significant valvular heart disease, suggestive of an ICM. Using the presence of regional wall motion abnormalities and no significant valvular heart disease as diagnostic criterion for ICM, echocardiography demonstrated an accuracy of 61%, sensitivity 100%, specificity 56%, positive predictive value 22%, and negative predictive value of 100%. In the 5 cases with significant stenosis in at least 1 coronary segment, echocardiography demonstrated an accuracy of 56%, sensitivity 60%, specificity 54%, positive predictive value 33%, and negative predictive valve of 78% for diagnosing significant CAD.
All patients received sublingual nitroglycerin 0.4 mg, and 3 patients received intravenous β-blocker (5 mg maximum) for frequent premature contractions as part of their CCTA examination. No patient received β-blocker specifically to lower their heart rate during the examination. Mean heart rate during CCTA examination was 75 ± 16 bpm (range 52-101 bpm) and mean intravenous contrast amount administered was 91 ± 17 mL (Table 2). All patients were in sinus rhythm, although 4 had 1 or more premature contractions noted during image acquisition. There were no complications or adverse events noted as a result of the CCTA examination. ICA was performed via femoral arterial approach for all patients. Mean intravenous contrast amount administered during ICA was 64 ± 26 mL. There were 2 complications noted after ICA, femoral hematoma and femoral artery dissection, although neither complication required further intervention. The average time between ICA and CCTA was 31 d (range 6-74 d).
| All patients (n = 18) | Ischemic(n = 2) | Non-ischemic (n = 16) | P-value | |
| Sublingual nitroglycerin | 18 (100) | 2 (100) | 16 (100) | NS |
| Intravenous β-blocker | 3 (17) | 0 (0) | 3 (19) | 1.00 |
| Contrast amount | 91 ± 17 | 81 ± 15 | 93 ± 17 | 0.33 |
| Contrast rate | 6 ± 0.5 | 5.5 ± 0.7 | 6.1 ± 0.4 | 0.33 |
| Average heart rate | 75 ± 16 | 68 ± 11 | 76 ± 16 | 0.64 |
| Calcium score | 138 ± 330 | 789 ± 839 | 57 ± 114 | 0.03 |
| LV EDV | 198 ± 87 | 163 ± 47 | 203 ± 91 | 0.72 |
| LV EF | 37 ± 19 | 17 ± 4 | 40 ± 19 | 0.09 |
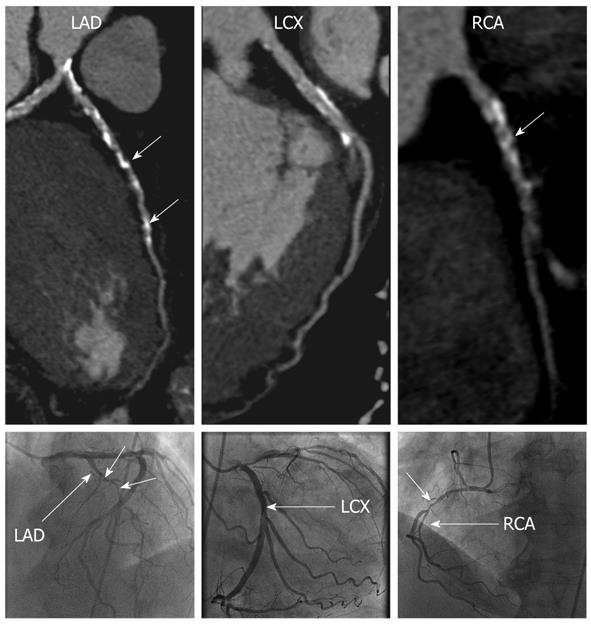
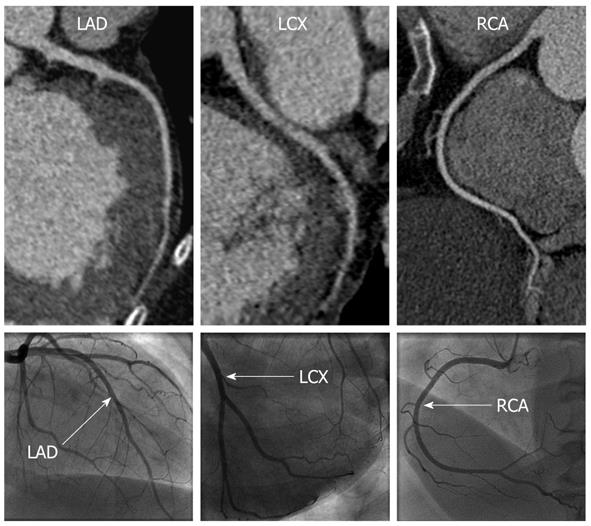
CCTA correctly identified the two patients with ICM, and the remaining patients as having non-ICM (Figures 1 and 2). Significant differences were noted in the mean total calcium score between the ischemic vs non-ICM groups (789 vs 57, P < 0.05), however, LV volumes and EF were not significantly different (Table 2).
Of the 246 total coronary segments available for analysis, 6 (2.4%) segments were unevaluable by CCTA due to small size (mean size 1.4 mm, n = 5) and motion artifact (n = 1). Of the 240 remaining segments, CCTA correctly detected significant stenosis in 10 of 16 (62.5%) segments identified on ICA as having significant stenosis. In the 6 segments with significant stenosis missed by CCTA, 5 were in branch vessels or distal segments measuring 1.7 mm or less in diameter, including the posterior descending artery (n = 2), obtuse marginal branch (n = 2) and diagonal branch (n = 1). Overall, CCTA had an accuracy of 96.7%, sensitivity 62.5%, specificity 99.1%, positive predictive value 83.3%, and negative predictive value of 97.4% for identifying significant stenosis (> 50%) on a per-segment basis. When a > 70% diameter stenosis criterion was used, CCTA had an accuracy of 97.5%, sensitivity 70%, specificity 98.7%, positive predictive value of 70%, and negative predictive value of 98.7%.
This study demonstrates that 64-slice dual source CCTA can provide a safe, accurate, and non-invasive technique for diagnosing ICM in patients presenting for evaluation of new cardiomyopathy during the acute phase of management and treatment, and with better diagnostic performance characteristics compared with resting 2D echocardiography. Although 3 of the patients received intravenous β-blocker prior to image acquisition, there were no adverse events or complications as a result of medication or contrast administration. In this small study, CCTA accurately identified the 2 patients with ICM and 16 patients with non-ICM. Although the mean total calcium score was significantly different between the two groups (789 vs 57), our study population was too small to evaluate the overall diagnostic value of a calcium score cut-off value for distinguishing between the two groups. However, other studies have demonstrated modest sensitivity (80%-85%) when using calcium score cut point values of 45-310[26,27]. Diagnostic accuracy, sensitivity and negative predictive values were high for CCTA detection of significant and severe coronary stenoses on a per-segment level. Compared to prior studies of CCTA for detection of CAD, our diagnostic sensitivity was relatively lower, primarily related to missed lesions in branch vessels or distal coronary segments. As all coronary artery segments were evaluated on CCTA regardless of size, our lower sensitivity may have been related to inclusion of small vessels that would have been excluded from prior studies due to small size < 1.5 mm and/or impaired image quality due to reduced contrast opacification of these smaller segments as a result of the patient’s underlying cardiomyopathy.
In contrast to prior studies in cardiomyopathy patients, the majority of patients in our cohort underwent CCTA during a more acute phase (< 2 mo) of diagnosis and treatment. This was reflected by the relatively higher average heart rate noted in our study population (mean 75 ± 16 bpm, range 52-101 bpm) compared to prior studies where the heart rate was notably lower, often < 65 bpm during the time of image acquisition[23,24,28]. As demonstrated by prior studies, pharmacologic reduction of heart rate to < 70 bpm improves image quality and diagnostic accuracy[23,24,28]. However, in the more acute phase of cardiomyopathy, elevated heart rate serves as a compensatory mechanism for the low stroke volume. Thus, aggressive pharmacologic measures to reduce the heart rate may be detrimental to a patient’s hemodynamic status and are often ineffective. The results of our study further demonstrate the utility of CCTA in the evaluation of newly diagnosed cardiomyopathy patients during a more acute phase of diagnosis and treatment despite these potential challenges to image quality.
The main limitations of this study are the small sample size and single center experience. Additional CCTA studies of cardiomyopathy patients are needed to further demonstrate feasibility and reliability in all stages of heart failure diagnosis.
In conclusion, our preliminary experience shows that 64-slice, dual source CCTA is a safe, accurate, and useful tool for the diagnosis of ICM in patients during the acute phase of evaluation for new onset cardiomyopathy.
Patients with heart failure represent a unique clinical population with elevated heart rates and poor left ventricular (LV) hemodynamics for which prior coronary computed tomography angiography (CCTA) study results are not necessarily applicable. It is often not advisable to perform CCTA in the early and acute phase of heart failure diagnosis. However, early and accurate diagnosis of significant coronary artery disease (CAD) is important to direct management in these patients. We demonstrate that dual source 64-slice multi-detector CCTA is a safe, accurate, and non-invasive technique for diagnosing ischemic cardiomyopathy (ICM) in patients presenting during the acute phase of newly diagnosed cardiomyopathy.
New developments in CCTA have focused on techniques to improve image quality and reduce radiation dose for a wide variety of patients undergoing examination. In particular, dual-source CT systems offer significant improvements in temporal resolution, allowing for diagnostic image quality without the need for β-blocker use in most patients.
Patients with heart failure often have elevated resting heart rates and poor LV hemodynamics which tends to impair image quality, but do not tolerate large doses of β-blockers. As such, it is often not advisable to perform CCTA in the early and acute phase of heart failure diagnosis, and studies evaluating the CCTA in heart failure patients have performed CCTA during the more stable phase after prolonged β-blocker therapy. However, early and accurate diagnosis of significant CAD is important to direct management in these patients. The use of dual source CCTA may provide a safe and reliable method for non-invasive imaging of the coronary arteries in patients with acute heart failure.
Dual source CCTA may provide a safe and reliable method for distinguishing between ischemic and non-ICM during the acute phase of presentation, and therefore direct further downstream management strategies.
The manuscript is interesting and of timely manner.
| 1. | Franciosa JA, Wilen M, Ziesche S, Cohn JN. Survival in men with severe chronic left ventricular failure due to either coronary heart disease or idiopathic dilated cardiomyopathy. Am J Cardiol. 1983;51:831-836. [PubMed] |
| 2. | Fuster V, Gersh BJ, Giuliani ER, Tajik AJ, Brandenburg RO, Frye RL. The natural history of idiopathic dilated cardiomyopathy. Am J Cardiol. 1981;47:525-531. [PubMed] |
| 3. | Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97:282-289. [PubMed] |
| 4. | Felker GM, Shaw LK, O'Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research. J Am Coll Cardiol. 2002;39:210-218. [PubMed] |
| 5. | McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441-1446. [PubMed] |
| 6. | Raftery EB, Banks DC, Oram S. Occlusive disease of the coronary arteries presenting as primary congestive cardiomyopathy. Lancet. 1969;2:1146-1150. [PubMed] |
| 7. | Hunt SA, Baker DW, Chin MH, Cinquegrani MP, Feldman AM, Francis GS, Ganiats TG, Goldstein S, Gregoratos G, Jessup ML. ACC/AHA Guidelines for the Evaluation and Management of Chronic Heart Failure in the Adult: Executive Summary A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Revise the 1995 Guidelines for the Evaluation and Management of Heart Failure): Developed in Collaboration With the International Society for Heart and Lung Transplantation; Endorsed by the Heart Failure Society of America. Circulation. 2001;104:2996-3007. [PubMed] |
| 8. | Hunt SA. ACC/AHA 2005 guideline update for the diagnosis and management of chronic heart failure in the adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure). J Am Coll Cardiol. 2005;46:e1-e82. [PubMed] |
| 9. | Greenberg JM, Murphy JH, Okada RD, Pohost GM, Strauss HW, Boucher CA. Value and limitations of radionuclide angiography in determining the cause of reduced left ventricular ejection fraction: comparison of idiopathic dilated cardiomyopathy and coronary artery disease. Am J Cardiol. 1985;55:541-544. [PubMed] |
| 10. | Diaz RA, Nihoyannopoulos P, Athanassopoulos G, Oakley CM. Usefulness of echocardiography to differentiate dilated cardiomyopathy from coronary-induced congestive heart failure. Am J Cardiol. 1991;68:1224-1227. [PubMed] |
| 11. | Mody FV, Brunken RC, Stevenson LW, Nienaber CA, Phelps ME, Schelbert HR. Differentiating cardiomyopathy of coronary artery disease from nonischemic dilated cardiomyopathy utilizing positron emission tomography. J Am Coll Cardiol. 1991;17:373-383. [PubMed] |
| 12. | Iskandrian AS, Hakki AH, Kane S. Resting thallium-201 myocardial perfusion patterns in patients with severe left ventricular dysfunction: differences between patients with primary cardiomyopathy, chronic coronary artery disease, or acute myocardial infarction. Am Heart J. 1986;111:760-767. [PubMed] |
| 13. | Dunn RF, Uren RF, Sadick N, Bautovich G, McLaughlin A, Hiroe M, Kelly DT. Comparison of thallium-201 scanning in idiopathic dilated cardiomyopathy and severe coronary artery disease. Circulation. 1982;66:804-810. [PubMed] |
| 14. | Eisenberg JD, Sobel BE, Geltman EM. Differentiation of ischemic from nonischemic cardiomyopathy with positron emission tomography. Am J Cardiol. 1987;59:1410-1414. [PubMed] |
| 15. | Danias PG, Ahlberg AW, Clark BA, Messineo F, Levine MG, McGill CC, Mann A, Clive J, Dougherty JE, Waters DD. Combined assessment of myocardial perfusion and left ventricular function with exercise technetium-99m sestamibi gated single-photon emission computed tomography can differentiate between ischemic and nonischemic dilated cardiomyopathy. Am J Cardiol. 1998;82:1253-1258. [PubMed] |
| 16. | Medina R, Panidis IP, Morganroth J, Kotler MN, Mintz GS. The value of echocardiographic regional wall motion abnormalities in detecting coronary artery disease in patients with or without a dilated left ventricle. Am Heart J. 1985;109:799-803. [PubMed] |
| 17. | Sawada SG, Ryan T, Segar D, Atherton L, Fineberg N, Davis C, Feigenbaum H. Distinguishing ischemic cardiomyopathy from nonischemic dilated cardiomyopathy with coronary echocardiography. J Am Coll Cardiol. 1992;19:1223-1228. [PubMed] |
| 18. | Duncan AM, Francis DP, Gibson DG, Henein MY. Differentiation of ischemic from nonischemic cardiomyopathy during dobutamine stress by left ventricular long-axis function: additional effect of left bundle-branch block. Circulation. 2003;108:1214-1220. [PubMed] |
| 19. | Meijboom WB, Meijs MF, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CA, Nieman K, van Werkhoven JM, Pundziute G, Weustink AC. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52:2135-2144. [PubMed] |
| 20. | Mowatt G, Cook JA, Hillis GS, Walker S, Fraser C, Jia X, Waugh N. 64-Slice computed tomography angiography in the diagnosis and assessment of coronary artery disease: systematic review and meta-analysis. Heart. 2008;94:1386-1393. [PubMed] |
| 21. | Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, Scherer M, Bellinger R, Martin A, Benton R. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52:1724-1732. [PubMed] |
| 22. | Manghat NE, Morgan-Hughes GJ, Shaw SR, Marshall AJ, Roobottom CA. Impaired left ventricular function has a detrimental effect on image quality in multi-detector row CT coronary angiography. Clin Radiol. 2008;63:415-423. [PubMed] |
| 23. | Andreini D, Pontone G, Pepi M, Ballerini G, Bartorelli AL, Magini A, Quaglia C, Nobili E, Agostoni P. Diagnostic accuracy of multidetector computed tomography coronary angiography in patients with dilated cardiomyopathy. J Am Coll Cardiol. 2007;49:2044-2050. [PubMed] |
| 24. | Manghat NE, Morgan-Hughes GJ, Shaw SR, Broadley AJ, Gogola L, Marshall AJ, Roobottom CA. Multi-detector row CT coronary angiography in patients with cardiomyopathy -- initial single-centre experience. Clin Radiol. 2007;62:632-638. [PubMed] |
| 25. | Austen WG, Edwards JE, Frye RL, Gensini GG, Gott VL, Griffith LS, McGoon DC, Murphy ML, Roe BB. A reporting system on patients evaluated for coronary artery disease. Report of the Ad Hoc Committee for Grading of Coronary Artery Disease, Council on Cardiovascular Surgery, American Heart Association. Circulation. 1975;51:5-40. [PubMed] |
| 26. | Leber AW, Knez A, Mukherjee R, White C, Huber A, Becker A, Becker CR, Reiser M, Haberl R, Steinbeck G. Usefulness of calcium scoring using electron beam computed tomography and noninvasive coronary angiography in patients with suspected coronary artery disease. Am J Cardiol. 2001;88:219-223. [PubMed] |
| 27. | Budoff MJ, Jacob B, Rasouli ML, Yu D, Chang RS, Shavelle DM. Comparison of electron beam computed tomography and technetium stress testing in differentiating cause of dilated versus ischemic cardiomyopathy. J Comput Assist Tomogr. 2005;29:699-703. [PubMed] |
| 28. | Andreini D, Pontone G, Bartorelli AL, Agostoni P, Mushtaq S, Bertella E, Trabattoni D, Cattadori G, Cortinovis S, Annoni A. Sixty-four-slice multidetector computed tomography: an accurate imaging modality for the evaluation of coronary arteries in dilated cardiomyopathy of unknown etiology. Circ Cardiovasc Imaging. 2009;2:199-205. [PubMed] |
Peer reviewers: Zhonghua Sun, PhD, Discipline of Medical Imaging, Department of Imaging and Applied Physics, Curtin University of Technology, GPO Box U 1987, Perth, Western Australia 6845, Australia; Markus Weininger, Dr., Department of Radiology, University Hospital of Wuerzurg, Auf der Laeng 24, Wuerzburg 97076, Germany
S- Editor Cheng JX L- Editor A E- Editor Zheng XM