Published online Dec 28, 2012. doi: 10.4329/wjr.v4.i12.462
Revised: October 12, 2012
Accepted: October 19, 2012
Published online: December 28, 2012
AIM: To evaluate the role of fluorine-18-labeled fluorodeoxyglucose positron emission tomography (18F-FDG PET) in various rheumatic diseases and its potential in the early assessment of treatment response in a limited number of patients.
METHODS: This study involved 28 newly diagnosed patients, of these 17 had rheumatoid arthritis (RA) and 11 had seronegative spondyloarthropathy (SSA). In the SSA group, 7 patients had ankylosing spondylitis, 3 had psoriatic arthritis, and one had non-specific SSA. Patients with RA were selected as per the American College of Rheumatology criteria. One hour after FDG injection, a whole body PET scan was performed from the skull vertex to below the knee joints using a GE Advance dedicated PET scanner. Separate scans were acquired for both upper and lower limbs. Post-treatment scans were performed in 9 patients in the RA group (at 6-9 wk from baseline) and in 1 patient with psoriatic arthropathy. The pattern of FDG uptake was analysed visually and quantified as maximum standardized uptake value (SUVmax) in a standard region of interest. Metabolic response on the scan was assessed qualitatively and quantitatively and was correlated with clinical assessment.
RESULTS: The qualitative FDG uptake was in agreement with the clinically involved joints, erythrocyte sedimentation rate, C-reactive protein values and the clinical assessment by the rheumatologist. All 17 patients in the RA group showed the highest FDG avidity in painful/swollen/tender joints. The uptake pattern was homogeneous, intense and poly-articular in distribution. Hypermetabolism in the regional nodes (axillary nodes in the case of upper limb joint involvement and inguinal nodes in lower limb joints) was a constant feature in patients with RA. Multiple other extra-articular lesions were also observed including thyroid glands (in associated thyroiditis) and in the subcutaneous nodules. Treatment response was better appreciated using SUVmax values than visual interpretation, when compared with clinical evaluation. Four patients showed a favourable response, while 3 had stable disease and 2 showed disease progression. The resolution of regional nodal uptake (axillary or inguinal nodes based on site of joint involvement) in RA following disease modifying anti-rheumatoid drugs was noteworthy, which could be regarded as an additional parameter for identifying responding patients. In the SSA group, uptake in the affected joint was heterogeneous, low grade and non-symmetrical. In particular, there was intense tendon and muscular uptake corresponding to symptomatic joints. The patients with psoriatic arthritis showed intense FDG uptake in the joints and soft tissue.
CONCLUSION: 18F-FDG PET accurately delineates the ongoing inflammatory activity in various rheumatic diseases (both at articular and extra-articular sites) and relates well to clinical symptoms. Different metabolic patterns on FDG-PET scanning in RA and SSA can have important implications for their diagnosis and management in the future with the support of larger studies. FDG-PET molecular imaging is also a sensitive tool in the early assessment of treatment response, especially when using quantitative information. With these benefits, FDG-PET could play a pivotal clinical role in the management of inflammatory joint disorders in the future.
- Citation: Vijayant V, Sarma M, Aurangabadkar H, Bichile L, Basu S. Potential of 18 F-FDG-PET as a valuable adjunct to clinical and response assessment in rheumatoid arthritis and seronegative spondyloarthropathies. World J Radiol 2012; 4(12): 462-468
- URL: https://www.wjgnet.com/1949-8470/full/v4/i12/462.htm
- DOI: https://dx.doi.org/10.4329/wjr.v4.i12.462
With more than two decades of fluorine-18-labeled fluorodeoxyglucose positron emission tomography (18F-FDG PET) use in the evaluation of oncological diseases, it is now perceived by the medical fraternity that inflammatory lesions concentrate 18F-FDG to a sufficient extent to provide diagnostic and functional information on inflammatory disease activity. This observation has led several investigators to use 18F-FDG PET imaging to assess various inflammatory joint disorders, particularly rheumatoid arthritis (RA). These highly morbid rheumatic disorders (RDs) affect approximately 1%-2% of the total world population[1]. Among these diseases, the incidence of RA increases with age up to about the seventh decade of life (a peak in the 5th decade)[2].
There is a paucity of tests which can be regarded as pathognomonic for RDs. Although a few serological tests have been shown to be specific for RDs, viz. rheumatoid factor (RF), anti-cyclic citrullinated protein antibodies for RA and human leukocyte antigen-B27 for spondyloarthropathies (SPA), many patients with an obvious clinical diagnosis have negative serum markers. Thus, RDs are also frequently classified grossly as “seropositive and seronegative rheumatoid diseases” referring to those with positive RF and negative RF, respectively. This also determines the treatment strategies for RDs. Radiographic examinations are routinely used for the assessment of joint erosions, and features of disease progression usually conclude that the “point of no return” has been reached in such disorders. However, this modality lacks the ability to detect early inflammation. Recently, magnetic resonance imaging (MRI), especially for finger lesions, has become important as it delineates synovial inflammation as contrast-enhanced lesions with excellent anatomical resolution. However, the tendency of RDs to involve large joints, especially in SPA (asymmetrically throughout the body), limits the application of MRI.
The usefulness of PET using FDG-PET to assess synovial inflammation, has been evaluated by a few investigators[3-7], especially in the setting of RA. Most of these studies produced promising results and were able to quantify inflammation in the joints. Despite these reports, translation into routine clinical practice requires further data including an examination of its potential to assess response to standard therapeutic regimens in RDs. To date, disease modifying anti-rheumatoid drugs (DMARDs) are the mainstay of treatment in RDs. With the advent of biological therapies (e.g., tumor necrosis factor-α), treatment strategies are changing from symptomatic relief to remission of the disease. This also demands a modality that is reliable for determining the resolution of inflammatory processes in the affected joints and possibly at extra-skeletal sites. The role of routine cross-sectional imaging modalities is substantially limited. Thus, in the present study, we endeavoured to evaluate early treatment response to DMARDs in a group of patients with RA, while assessing the whole body (WB) metabolic patterns on FDG-PET in patients with various RDs.
Twenty eight patients with different RDs underwent WB FDG-PET studies (Table 1), 17 of these patients had RA (seropositive) and 11 patients had seronegative spondyloarthropathy (SSA). Of the 11 patients with SSA, 7 had ankylosing spondylitis (AS), 3 had psoriatic arthritis (PsA) and 1 had non-specific SSA (nsSSA). The study protocol was approved by the Institutional Ethics Committee, and informed consent was obtained from each patient.
| No. | Characterstics | Ankylosing spondylitis | Psoariatic arthritis | Non specific SSA | Rheumatoid arthritis |
| 1 | Total number of patients | 7 | 3 | 1 | 17 |
| 2 | Male: female ratio | All male | 2:1 | Male | All female |
| 3 | Age range | 17-40 yr (mean = 28.6 yr) | 34-53 yr (mean = 42 yr) | 19 yr | 27-60 yr (mean = 40 yr) |
The selected RA patients fulfilled the American College of Rheumatology revised criteria[8-10] for the diagnosis of RA. The diagnosis of SSA was purely based on clinical assessment. Treatment response was focused only on RA patients, with one PsA patient undergoing response evaluation (RE). The detailed clinical and laboratory parameters undertaken by the rheumatologist are shown in Table 2.
| Inclusion criteria |
| Positive ACR criteria[8-10] (at least 4 out of 7) for designating RA |
| Clinical diagnosis of specific seronegative spondyloarthropathy1 |
| Positive or negative RA factor |
| Classical symptoms with raised ESR (> 30 mm/h) |
| Classical symptoms with raised C-reactive protein (> 20 mg/L) |
| HLA-B27 positive with supportive clinical diagnosis1 |
| Newly diagnosed and not received any form of treatment. |
| Exclusion criteria |
| Had been treated with any DMARD/steroids earlier. |
| Uncontrolled diabetics |
Imaging was performed after ensuring more than 6 h fasting and blood glucose levels below 150 mg/dL. Approximately 10 mCi of 18F-FDG was injected intravenously through a secure IV line. One hour after FDG injection, WB PET scanning was performed from the skull vertex to below the knee joints using a GE Advance dedicated PET scanner. Separate scans were acquired for upper limbs (elbow, wrist and hands) and leg (ankle and feet). The PET data were reconstructed using a Gaussian filter with an ordered-subset expectation maximization algorithm (2 iterations, 8 subsets). Standard axial, coronal, sagittal and maximum intensity projection images were generated. Post-treatment scans were performed in 9 patients in the RA group at 6-9 wk after initiating therapy, with protocols maintaining the same standards as an earlier study (i.e. blood glucose < 150, waiting duration of 60 min, and similar reconstruction algorithms).
The pattern of FDG uptake was analysed in the small joints of hands and feet, wrists, elbows, shoulders and sacroiliac (SI) joints, knees, hips and ankle joints as well as the atlanto-axial joint (total of 21 joints). The small joints of the hands and feet (interphalangeal, metacarpo/tarso-phalangeal and carpal/tarsal joints) were collectively considered as single units for the ease of quantitation. A dedicated work station was used to draw circular regions of interest covering each joint and to calculate the maximum standardized uptake value (SUVmax) of each joint. FDG uptake in extra-articular sites such as lymph nodes, tendons, soft tissue nodules was also noted and SUVmax was measured. FDG uptake in affected joints was also evaluated qualitatively and categorised as follows; no uptake (same as background); mild uptake if more than background, but less than liver; moderate uptake (same as in liver); high uptake if higher than in liver, but less than brain, and intense uptake if equal to or more than brain. The method was based on the scoring system by Kubota et al[7] with modifications.
The number of painful/swollen joints, the white blood cell count, erythrocyte sedimentation rate (ESR) and C-reactive protein (CRP) level were evaluated within 5 d of the PET examination during pre- and post-treatment scans. The patients who were evaluated for response received standard regimens of DMARDs either as monotherapy or a combination of two drugs with or without steroids.
This study included 28 newly diagnosed patients with either RA or SSA. Of these patients, 17 had RA (all RF positive) and 11 had SSA (7-AS, 3-PsA, 1-nsSSA) (Figures 1-4). The clinical and serological details of the patients are shown in Tables 1 and 3. Female predominance was noted in the RA group, while males predominated in the SSA group.
| Type of RD | No. of patients | ESR range (mm/h) | CRP levels (mg/L) |
| RA | 17 | < 10 to 640 | < 5 to 46.14 |
| AS | 7 | 30 to 92 | < 5 to 20 |
| PsA | 3 | 27 to 30 | 20 to 29 |
| nsSSA | 1 | 31 | 23.1 |
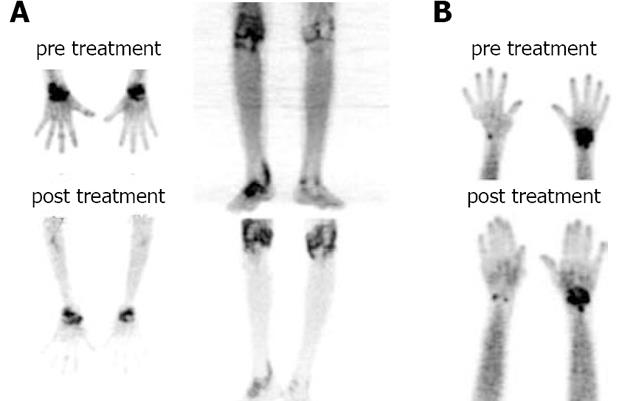
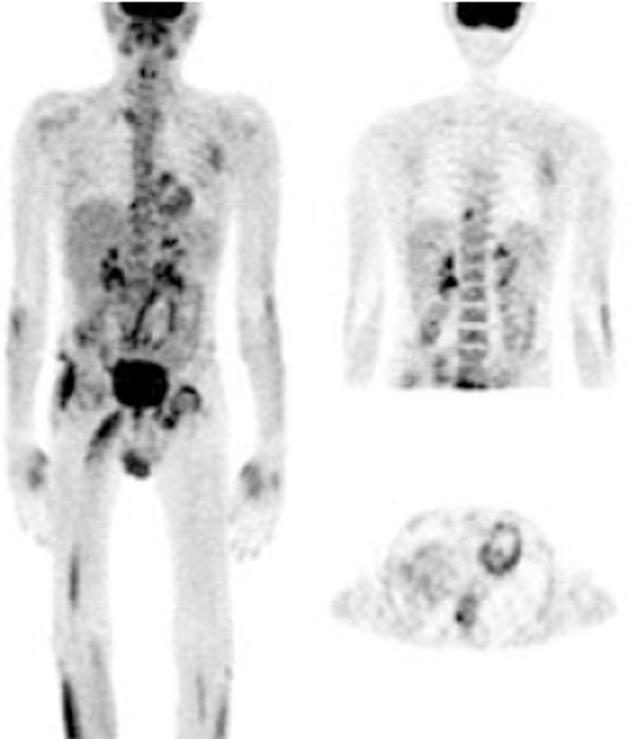
FDG-PET imaging features in the joints of RA patients: Seventeen patients with newly diagnosed RA were studied in this group of which 9 were evaluated for RE following therapy. Eight patients were lost to follow up. FDG uptake was in agreement with clinical presentation and symptoms; as all the painful and/or swollen and/or tender joints showed considerable FDG avidity (in the high to intense category). Metabolically, the wrist joint was the most common and predominantly affected (13/17) followed by the ankle joints. Although RA is known to involve multiple joints, 4 patients showed intense uptake in only one joint, predominantly (3) in the wrists. Only one patient showed uptake in the small joints of the feet.
Extra-articular FDG uptake pattern in RA: Different areas of extra-articular soft tissue FDG uptake were found in 10 patients. The most common sites were axillary lymph nodes (9 patients), epitrochlear lymph node (1 patient), cervical lymph nodes (2 patients), thyroid gland (3 patients) and subcutaneous (possibly rheumatoid) nodules (2 patients). Only 2 patients with RA showed mild FDG uptake in the tendons associated with inflamed joints. One patient, who was anaemic, also showed increased FDG avidity in the bone marrow.
SUVmax values in the inflamed joints ranged from 3.1 to 11.5. During the RE scan, clinical evaluation in 9 patients showed symptomatic improvement in 4, mixed relief in 1, persistent symptoms but no progression in 2 and progressive disease in 2 patients. The SUVmax values on RE scans ranged from 2.2 to 9.3. Good correlation was observed between SUVmax values and clinical response to therapy. The detailed data of response assessments are shown in Table 4. However, on visual analysis of response, the uptake pattern in most of the joints remained in the high to intense category, although patients who had improved showed reduced joint FDG avidity, as measured by SUV estimation. Significant intra-observer and inter-observer variation was noted when comparing pre- and post-treatment scans in the visual assessment. Thus, SUVmax values were considered appropriate for the assessment of treatment response.
| Sr. No. | No. of joints involved | Clinical assessment | % change in SUVmax |
| 1 | 10 | Mixed symptoms | Knee = 64% ↓ |
| Ankle = no change | |||
| Wrist = 51.7% ↑ | |||
| Elbow = 47% ↓ | |||
| 2 | 4 | Progressive disease | Knee = 44% ↑ |
| Ankle = 33% ↑ | |||
| 3 | 2 | Improvement | Wrists = 62% ↓ |
| 4 | 6 | Improvement | Ankle = 23% ↓ |
| Wrist = 45.2% ↓ | |||
| 5 | 4 | Improvement | Wrist = 73 % ↓ |
| SJH* = 34% ↓ | |||
| 6 | 6 | Improvement | Ankle = 43.3% ↓ |
| Wrists = 23 % ↓ | |||
| SJF* = 24.5% ↓ | |||
| 7 | No response | No change | |
| 8 | No response | No change | |
| 9 | 2 | Progressive disease | Wrists = 48% ↑ |
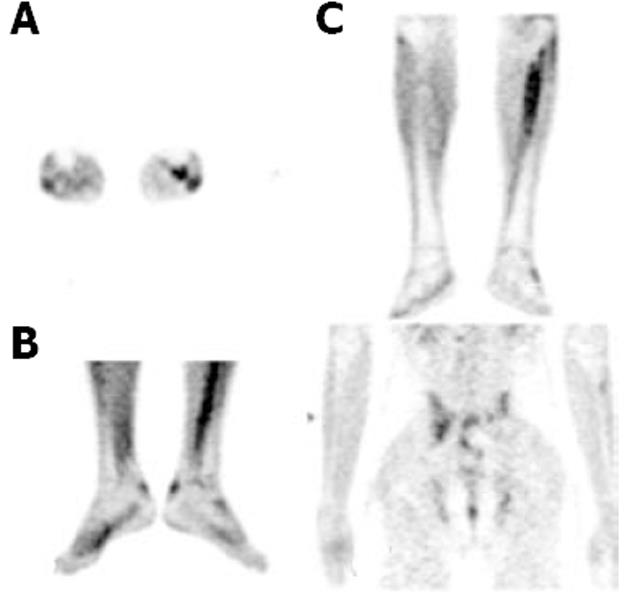
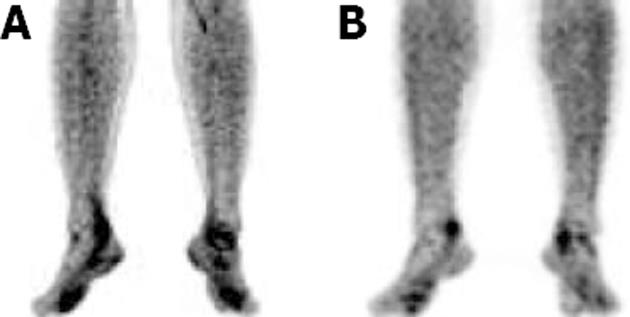
The FDG uptake pattern observed in SSA patients was different from that observed in RA patients. A total of 11 WB scans were acquired which predominantly included patients with AS (n = 7). FDG uptake in the affected joints was heterogeneous and of varying intensity (from mild to high). Interestingly, FDG uptake in patients with AS was not observed and uptake in the SI joints or the spine typically ranged from 2.66 to 3.703 (mean SUVmax 3.1), which were the predominant sites of pain in these patients. Enhanced FDG uptake was more asymmetrical than that observed in RA patients. FDG hypermetabolism was noted in the bilateral SI joints, and asymmetrical uptake was observed in the sternoclavicular, shoulders, hip and facet joints. There was significant evidence of tendon and muscular uptake corresponding to the symptomatic joints. A few patients also showed abnormal FDG uptake in the interosseous membranes of the legs (Figure 4A). This favours enthesitis as the proposed pathogenesis of SPA[11,12]. The most common tendons with abnormal FDG uptake were the calcaneal tendons (Figure 4B).
The patients with psoriatic arthritis showed high to intense FDG uptake, predominantly in the distal phalangeal joints. The SUVmax values depicting tendon or soft tissue uptake were obvious on visual assessment.
In this study, a varying pattern of articular and extra-articular activity was observed in various rheumatic diseases detected by WB PET scans. FDG PET delineated a varying pattern of inflammatory activity in the affected joints and extra-articular sites that could also be quantified using SUV values. The FDG uptake patterns were in agreement with the clinically inflamed/tender/swollen joints, the acute phase reactants (ESR, CRP) and the clinical assessment and diagnosis by the rheumatologist. The WB scan provided an assessment of disease extent by showing the variable intensity of metabolic activity in the inflamed synovial joints in RA and the enthesitis in SSA which correlated with the patient’s symptoms. The successful assessment of small as well as large joints in the present study supported the results of previous studies which specifically assessed either small[5] or large[7] joints. The efficacy of WB FDG-PET in RDs in this study was similar to that in these previous studies and suggests that WB FDG uptake represents disease activity in joints affected by RDs.
In RA patients, the uptake pattern was diffuse and homogeneous, and intense metabolic activity corresponding to the joint space was poly-articular in distribution. This in turn, correlated with the pathology of cytokine-induced hyperactive and hyperplastic inflamed synovial membrane (“pannus”) laden with macrophages. Macrophages are known to use glucose as a major metabolic substrate[13]. In contrast, the FDG uptake was mild to moderate in the affected joints of SSA patients. This also signifies the major difference between RA and SSA, where the latter lacks the “pannus”. However in SSA, increased metabolic activity was noted in fibrous tissue such as tendons, osseous membranes and muscle (fascia) corresponding to the affected joints. The primary pathology of SPA is “enthesitis” with chronic inflammation. Cytokines and other inflammatory markers are also important in the inflammatory process which ultimately leads to fibrosis and ossification at sites of enthesitis. Early lesions including subchondral granulation tissue erode the joint and is gradually replaced by fibrocartilage and then ossification[11,12]. This pathogenesis is proposed to lack the presence of a large population of inflammatory cells at the affected joints, which could be the reason for our observation of low grade metabolic activity in affected joints in this group of patients. The inflamed fibrous tissue in the peri-articular region showed high metabolic activity.
We found a different metabolic pattern in patients with RA and SSA (especially AS) which correlated with their pathogenesis. Such uptake patterns can have implications for differentiating RA and SSA from degenerative diseases (e.g., osteoarthritis) involving major joints such as knee, hip and SI regions in challenging situations. This will be particularly useful in elderly patients where it is difficult to differentiate between SSA and osteoarthritis. SSA also lacks the specific inflammatory markers associated with RA, therefore, FDG-PET may be useful in such cases. Further studies are required to assess the potential efficacy of FDG PET in this area.
In the RE in a small group of RA patients and a patient with PsA, our study showed a variation in SUVmax values in inflamed joints which correlated with clinical assessment. The visual assessment of FDG uptake in inflamed joints was not found to be useful as major intra- and inter-observer variability was observed. This finding is in contrast to the response assessment performed by Elzinga et al[14], who studied WB FDG-PET in patients with RA using a visual evaluation score, and concluded that FDG uptake was significantly correlated with clinical evaluations of disease activity in patients with RA before and after treatment with infliximab. Our findings are in agreement with those of Kubota et al[7] and Goerres et al[6] who also recommend the use of a FDG uptake scoring system as a useful tool in the assessment of joint inflammation. The difference between the results of Elzinga et al[14] and the other studies could be due to the use of different treatment modalities (biological vs cytoreductive) and duration of the RE scan. However, based on our findings, we recommend the use SUVmax values as quantitative parameters for the assessment of treatment response in RDs. The additional evaluation using visual scoring methods may also be beneficial. The finding of regional lymph node hypermetabolism in RA patients (based on the involved joints) is in agreement with previous reports[15,16]. In patients with RA, we also observed resolution of regional nodal uptake (axillary or inguinal nodes based on the site of joint involvement) following DMARD therapy, which can be regarded as an additional parameter for identifying responding patients. The clinical implications of this observation require to be explored further. The limitations of this study include (1) small sample size and (2) low percentage of patients who took part in the post-treatment study. Therefore, further well-powered prospective studies would help to determine more definitive conclusions. In addition, the use of SUV values requires careful attention to the PET imaging protocol, patient preparation, measurement of blood glucose levels, waiting period, scan duration, reconstruction techniques and methods of SUV calculation.
Thus, we conclude that FDG-PET demonstrates varying patterns of metabolic activity in articular and extra-articular sites in various inflammatory joint disorders. Different metabolic patterns on FDG-PET studies in RA and SSA patients may play a diagnostic role in the future with the support of larger studies. FDG PET can also serve as an objective tool in the early assessment of treatment response in affected patients, especially with the use of quantitative information. With these benefits, FDG-PET could play a significant clinical role in the management of a wide spectrum of inflammatory joint disorders.
The potential of metabolic imaging with fluorodeoxyglucose positron emission tomography (FDG-PET) is being increasingly explored in several inflammatory disorders. The advantage of evaluating the whole body in a single examination is a major advantage of this modality which is preferable in systemic inflammatory disorders.
It is postulated that under the influence of cytokines and growth factors, activated inflammatory cells demonstrate enhanced FDG due to increased expression of glucose transporters. Recent reports have highlighted the increasing importance of FDG-PET in assessing various infective and inflammatory disorders such as osteomyelitis, pyrexia of unknown origin, painful joint prosthesis, vasculitis and sarcoidosis, and thus have demonstrated the potential of this technique in becoming the radionuclide imaging procedure of choice in many of these inflammatory conditions. However, there is a paucity of data on its usefulness in inflammatory joint disorders, the majority of which are systemic in nature. In the present study, the authors explored the usefulness of FDG-PET-based molecular imaging in depicting inflammatory activity at various skeletal and extra-skeletal sites.
Understanding the various metabolic patterns depicted by FDG-PET both at the articular and extra-articular sites can have important implications for their diagnosis and evolving therapeutic strategies in patients with inflammatory joint disorders. FDG-PET molecular imaging using quantitative methods may also prove to be an important diagnostic tool in the early assessment of treatment response. If similar findings are obtained in larger studies in the near future, FDG-PET could play a pivotal role in management of inflammatory joint disorders.
This is a retrospective assessment of rheumatic disease by FDG-PET. FDP-PET findings of RA and seronegative spondyloarthropathies are well illustrated, but clinical correlation is only limited to the relationship between standardized uptake value and disease activity. It is a good study.
| 1. | Irestein GS. Etiology and pathogenesis of rheumatoid arthritis. Kelly’s Textbook of Rheumatology. 6th ed. Philadelphia: WB Saunders 2001; 921-966. |
| 2. | Silman AJ, Hockberg MC. Epidemiology of the Rheumatic Diseases. Oxford: Oxford University Press 1993; . |
| 3. | Palmer WE, Rosenthal DI, Schoenberg OI, Fischman AJ, Simon LS, Rubin RH, Polisson RP. Quantification of inflammation in the wrist with gadolinium-enhanced MR imaging and PET with 2-[F-18]-fluoro-2-deoxy-D-glucose. Radiology. 1995;196:647-655. [PubMed] |
| 4. | Beckers C, Ribbens C, André B, Marcelis S, Kaye O, Mathy L, Kaiser MJ, Hustinx R, Foidart J, Malaise MG. Assessment of disease activity in rheumatoid arthritis with (18)F-FDG PET. J Nucl Med. 2004;45:956-964. [PubMed] |
| 5. | Beckers C, Jeukens X, Ribbens C, André B, Marcelis S, Leclercq P, Kaiser MJ, Foidart J, Hustinx R, Malaise MG. (18)F-FDG PET imaging of rheumatoid knee synovitis correlates with dynamic magnetic resonance and sonographic assessments as well as with the serum level of metalloproteinase-3. Eur J Nucl Med Mol Imaging. 2006;33:275-280. [PubMed] |
| 6. | Goerres GW, Forster A, Uebelhart D, Seifert B, Treyer V, Michel B, von Schulthess GK, Kaim AH. F-18 FDG whole-body PET for the assessment of disease activity in patients with rheumatoid arthritis. Clin Nucl Med. 2006;31:386-390. [PubMed] |
| 7. | Kubota K, Ito K, Morooka M, Mitsumoto T, Kurihara K, Yamashita H, Takahashi Y, Mimori A. Whole-body FDG-PET/CT on rheumatoid arthritis of large joints. Ann Nucl Med. 2009;23:783-791. [PubMed] |
| 8. | Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, Healey LA, Kaplan SR, Liang MH, Luthra HS. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315-324. [PubMed] |
| 9. | Fransen J, Stucki G, van Riel PLCM. Rheumatoid arthritis measures: Disease Activity Score (DAS), Disease Activity Score-28 (DAS28), Rapid Assessment of Disease Activity in Rheumatology (RADAR), and Rheumatoid Arthritis Disease Activity Index (RADAI). Arthritis Care Res. 2007;49:S214–S224. [RCA] [DOI] [Full Text] [Cited by in Crossref: 123] [Cited by in RCA: 124] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 10. | Fransen J, van Riel PL. The Disease Activity Score and the EULAR response criteria. Clin Exp Rheumatol. 2005;23:S93-S99. [PubMed] |
| 11. | McGonagle D, Gibbon W, Emery P. Classification of inflammatory arthritis by enthesitis. Lancet. 1998;352:1137-1140. [PubMed] |
| 12. | Muñoz-Villanueva MC, Muñoz-Gomariz E, Escudero-Contreras A, Pèrez-Guijo V, Collantes-Estévez E. Biological and clinical markers of disease activity in ankylosing spondylitis. J Rheumatol. 2003;30:2729-2732. [PubMed] |
| 13. | Matsui T, Nakata N, Nagai S, Nakatani A, Takahashi M, Momose T, Ohtomo K, Koyasu S. Inflammatory cytokines and hypoxia contribute to 18F-FDG uptake by cells involved in pannus formation in rheumatoid arthritis. J Nucl Med. 2009;50:920-926. [PubMed] |
| 14. | Elzinga EH, van der Laken CJ, Comans EF, Boellaard R, Hoekstra OS, Dijkmans BA, Lammertsma AA, Voskuyl AE. 18F-FDG PET as a tool to predict the clinical outcome of infliximab treatment of rheumatoid arthritis: an explorative study. J Nucl Med. 2011;52:77-80. [PubMed] |
| 15. | Seldin DW, Habib I, Soudry G. Axillary lymph node visualization on F-18 FDG PET body scans in patients with rheumatoid arthritis. Clin Nucl Med. 2007;32:524-526. [PubMed] |
| 16. | Sarma M, Vijayant V, Basu S. (18)F-FDG-PET assessment of early treatment response of articular and extra-articular foci in newly diagnosed rheumatoid arthritis. Hell J Nucl Med. 2012;15:70-71. [PubMed] |
Peer reviewers: Wenbao Wang, MD, Orthopaedic Department, Columbia University Medical Center, 106 Fort Washington Avenue, Apt 3H, New York, NY 10032, United States; Shigeru Ehara, MD, Professor, Chairman, Iwate Medical University School of Medicine, Morioka 020-8505, Japan
S- Editor Cheng JX L- Editor Webster JR E- Editor Xiong L