Published online Jul 28, 2011. doi: 10.4329/wjr.v3.i7.188
Revised: May 25, 2011
Accepted: May 9, 2011
Published online: July 28, 2011
AIM: To determine whether acoustic radiation force impulse (ARFI) elastography is a reliable method for predicting fibrosis severity in patients with chronic hepatitis C virus (HCV) hepatitis.
METHODS: We performed a multicenter study including 274 subjects with HCV chronic hepatitis in which we compared ARFI with liver biopsy (LB). In each patient we performed LB (evaluated according to the Metavir score) and ARFI measurements (using a Siemens Acuson S2000™ ultrasound system: 10 valid measurements were performed and median values were calculated and expressed in meters/second (m/s).
RESULTS: A direct, strong, correlation (Spearman r = 0.707) was found between ARFI measurements and fibrosis (P < 0.0001). For predicting the presence of fibrosis (F ≥ 1 Metavir), significant fibrosis (F ≥ 2), severe fibrosis (F ≥ 3) and cirrhosis (F = 4), the cut-off values of 1.19, 1.21, 1.58 and 1.82 m/s were determined, respectively, liver stiffness measurements had 73%, 84%, 84% and 91% Se respectively; 93%, 91%, 94%, 90% Sp, respectively; with AUROCs of 0.880, 0.893, 0.908 and 0.937, respectively.
CONCLUSION: ARFI measurement is a reliable method for predicting the severity of fibrosis in HCV patients
- Citation: Sporea I, Şirli R, Bota S, Fierbinţeanu-Braticevici C, Petrişor A, Badea R, Lupşor M, Popescu A, Dănilă M. Is ARFI elastography reliable for predicting fibrosis severity in chronic HCV hepatitis? World J Radiol 2011; 3(7): 188-193
- URL: https://www.wjgnet.com/1949-8470/full/v3/i7/188.htm
- DOI: https://dx.doi.org/10.4329/wjr.v3.i7.188
The evaluation of liver fibrosis is essential in chronic hepatitis since a decision about treatment is linked, in most cases, to the severity of the disease (especially in chronic hepatitis C), and because the prognosis of the disease is based on the severity of fibrosis.
Until a few years ago, the evaluation of fibrosis was made only by liver biopsy (LB), which was considered to be the “gold standard” for hepatological evaluation[1], but in latter years, non-invasive methods (recently developed) are beginning to replace this invasive procedure.
The disadvantages of LB are: it is an invasive procedure, there are rare, but possible, post-biopsy complications, it is a stressful medical procedure for many patients and sometimes, not enough histological material is obtained.
Non-invasive methods for the evaluation of liver fibrosis using ultrasound waves include: transient elastography (TE) (FibroScan)[2-4]; SonoElastography (real-time tissue elastography: RT-E)[5-9] and acoustic radiation force impulse (ARFI) elastography[10-13]. The advantages of ultrasound-based methods for liver stiffness (LS) evaluation are that they are noninvasive, well tolerated by patients; a rapid estimation is obtained regarding the disease severity (a few minutes); and sometimes the elastography module is integrated in the available ultrasound machine (RT-E Hitachi and ARFI Siemens). However, it is unknown whether these non-invasive ultrasound-based methods are accurate enough to replace, at least partially, LB.
The aim of our study was to determine whether ARFI elastography is a reliable method for predicting the severity of fibrosis in patients with chronic hepatitis C virus (HCV) hepatitis.
We performed a multicenter study in 3 university hospitals (Timisoara, Bucharest and Cluj-Napoca) that included 274 patients, 113 men and 161 women, mean age 50.1 ± 11.8 years with HCV chronic hepatitis (all with PCR HCV RNA +) in which ARFI and LB were compared.
In each patient LB and LS measurements by means of ARFI were performed, the LB in the previous 6 mo before entering the study, and none of the patients had received antiviral therapy.
All patients agreed to participate in this study, which was approved by the local Ethical Committee.
ARFI elastography was performed in all patients using a Siemens Acuson S2000™ ultrasound system. The ultrasound probe automatically produces an acoustic “push” pulse that generates shear-waves which propagate into the liver. Their speed, measured in meters/second (m/s), is displayed on the screen. The propagation speed increases with fibrosis. The operator can select the depth at which liver elasticity is evaluated, by placing a “measuring box” (10 mm long and 5 mm wide) in the desired place
(Figure 1). In all our patients, Virtual Touch Tissue quantification was performed, using the Acuson system. The patients were examined in left lateral decubitus, with the right arm in maximum abduction. Scanning was performed between the ribs in the right liver lobe (e.g. segment 8) (in order to avoid cardiac motion), 1 cm under the capsule, with minimal scanning pressure applied by the operator, while the patients were asked to stop breathing for a moment, in order to minimize breathing motion.
We performed 10 measurements in each patient, and a median value was calculated, the result being measured in m/s. The time interval between LB and ARFI measurements was less or equal to 6 mo.
LB was performed in all patients using the echoguided TruCut technique, with a 1.8 mm (14 G) diameter automatic needle device - Biopty Gun (Bard GMBh), or echoassisted, using Menghini-type modified needles, 1.4 and 1.6 mm in diameter. Only LB fragments including at least 6 portal tracts were considered adequate for pathological interpretation and included in our study. The LBs were assessed according to the Metavir score, by a senior pathologist (one at each center). Fibrosis was staged on a 0-4 point scale: F0 - no fibrosis; F1 - portal fibrosis without septa; F2 - portal fibrosis and few septa extending into lobules; F3 - numerous septa extending to adjacent portal tracts or terminal hepatic venules and F4 - cirrhosis.
The data obtained from our patients were collected in a Microsoft Excel file, and statistical analysis was performed using the SPSS program. Predictors for the stage of fibrosis (ARFI measurements) were numeric variables, thus the mean and standard deviation were calculated.
The ANOVA test was used to compare the mean values obtained by ARFI for each degree of fibrosis. Associations between assay results and fibrosis stage according to the Metavir scoring system (range: 0-4, ordinal scale), were described using the Spearman rank correlation coefficient (r).
The diagnostic performances of ARFI elastography were assessed using receiver operating characteristics (ROC) curves. ROC curves were thus built for the detection of: significant fibrosis (F ≥ 2 Metavir), severe fibrosis (F ≥ 3 Metavir) and cirrhosis. Optimal cut-off values were chosen to maximize the sum of sensitivity (Se) and specificity (Sp). Se and Sp were calculated according to standard methods. Exact CI of 95% were calculated for each predictive test.
Our study included 274 patients. Of the 262 patients with valid ARFI measurements, 29 subjects (11.1%) did not have fibrosis (F0 Metavir on LB), 43 subjects (16.4%) were classified as F1, 74 subjects (28.2%) as F2, 51 patients (19.5%) as F3 and 65 patients (24.8%) had liver cirrhosis (F4 Metavir on LB). No serious complications occurred following LB, and only minor complications, such as post-biopsy pain were observed.
Of 274 patients, valid ARFI measurements were obtained in 262 subjects (95.6%). Measurements of LS ranged from 0.67 to 4.15 m/s.
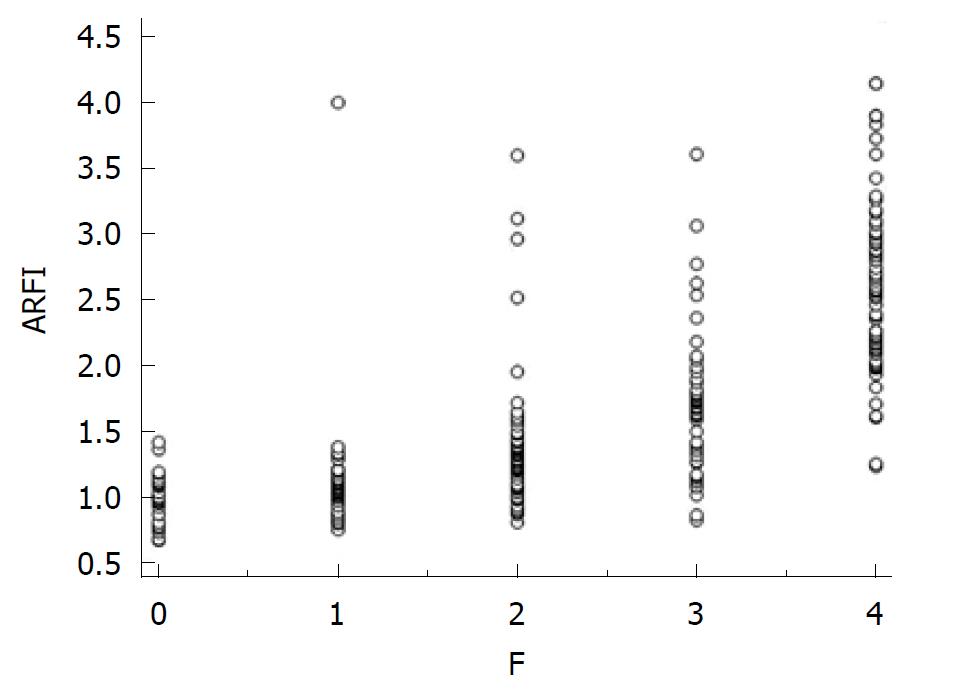
A direct, strong, correlation (Spearman r = 0.727) was found between ARFI measurements and fibrosis (P < 0.0001) (Figure 1).
The mean values of LS assessed by ARFI, according to the severity of fibrosis, were: F0 - 0.97 ± 0.19 m/s; F1 - 1.07 ± 0.13 m/s; F2 - 1.33 ± 0.48 m/s; F3 - 1.71 ± 0.52 m/s; F4 - 2.60 ± 0.67 m/s.
The mean LS values for contiguous stages of fibrosis were statistically significantly different: F0 vs F1: 0.97 ± 0.19 m/s vs 1.07 ± 0.13 m/s (P = 0.01); F1 vs F2: 1.07 ± 0.13 m/s vs 1.33 ± 0.48 m/s (P < 0.001); F2 vs F3: 1.33 ± 0.48 m/s vs 1.71 ± 0.52 m/s (P < 0.001); F3 vs F4 : 1.71 ± 0.52 m/s vs 2.60 ± 0.67 m/s (P < 0.001).
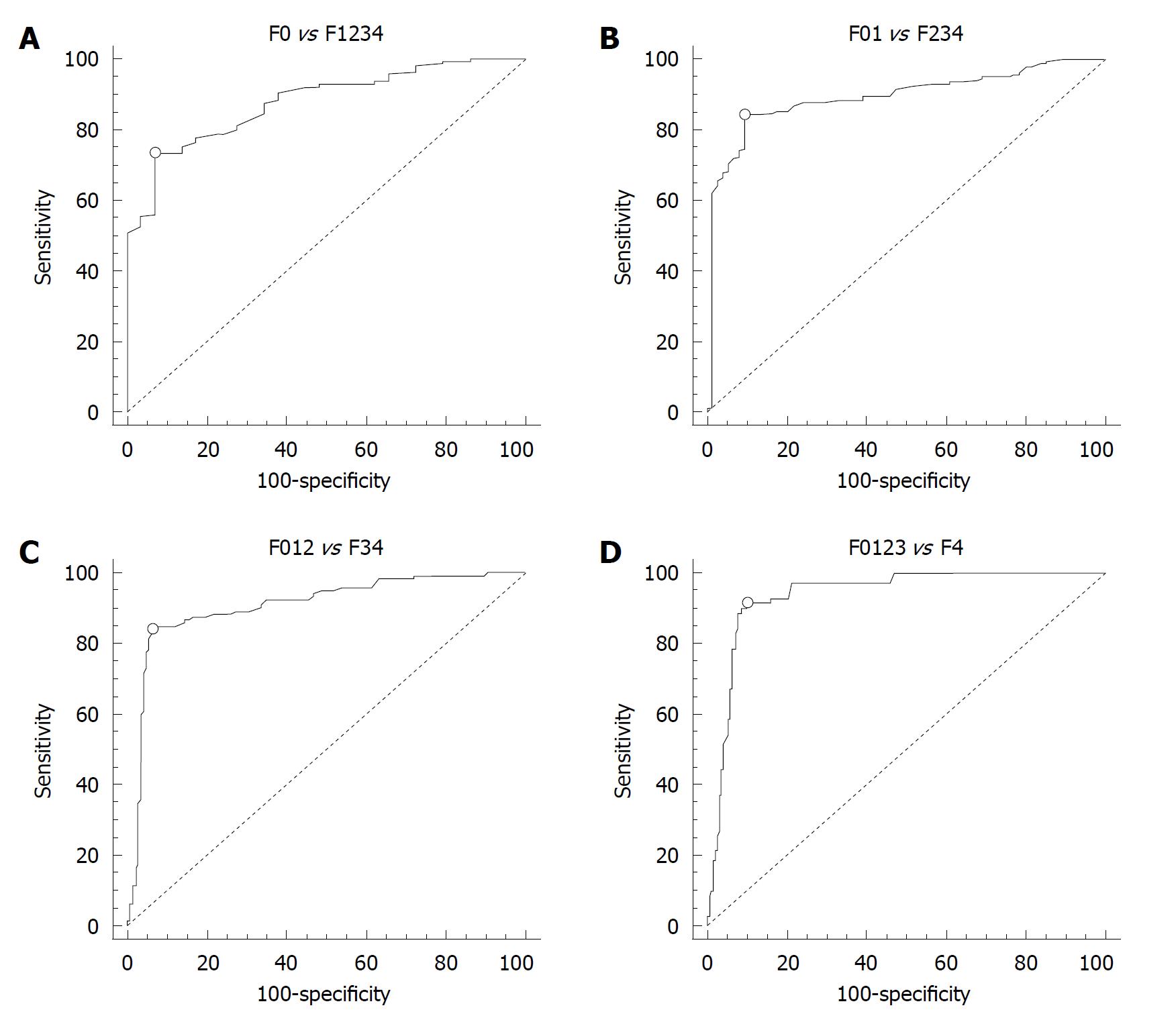
The presence of fibrosis (F ≥ 1 Metavir) was predicted by a cut-off value of 1.19 m/s, LS measurements had 73% Se, 93% Sp, 99% PPV and 30% NPV, with AUROC = 0.880 (Figure 2A, Table 1).
| Fibrosis | Cut-off (m/s) | AUROC | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) |
| ≥ F1 | 1.19 | 0.880 | 73 | 93 | 99 | 30 |
| ≥ F2 | 1.21 | 0.893 | 84 | 91 | 96 | 69 |
| ≥ F3 | 1.58 | 0.908 | 84 | 94 | 92 | 88 |
| F4 | 1.82 | 0.937 | 91 | 90 | 77 | 97 |
The presence of significant fibrosis (F ≥ 2 Metavir) was predicted by a cut-off value of 1.21 m/s, LS measurements had 84% Se, 91% Sp, 96% PPV and 69% NPV, with AUROC = 0.893 (Figure 2B, Table 1).
The presence of severe fibrosis (F ≥ 3 Metavir) was predicted by a cut-off value of 1.58 m/s, LS measurements had 84% Se, 94% Sp, 92% PPV and 88% NPV, with AUROC = 0.908 (Figure 2C, Table 1).
The presence of cirrhosis (F = 4 Metavir) was predicted by a cut-off value of 1.82 m/s, LS measurements had 91% Se, 90% Sp, 77% PPV and 97% NPV, with AUROC = 0.937 (Figure 2D, Table 1).
Virtual Touch™ tissue imaging application implements ARFI technology for the evaluation of deep tissues, not accessible to superficial compression elastography techniques. Using image-based localization and proprietary implementation of ARFI technology, shear wave speed may be quantified, in a precise anatomical region, focused on a region of interest, with a predefined size, provided by the system. Measurement value and depth are also reported and the elasticity results were obtained in m/s.
Studies comparing LB to the non-invasive methods of evaluation in chronic liver disease have been performed in order to assess if these non-invasive methods are accurate enough to replace LB in the future.
Considering that fibrosis is heterogeneously distributed in the liver, LB has been criticized in the past, because it evaluates only a small part of the total volume of the liver, due to the small volume of tissue sample taken. It has been proven that liver fragments obtained in the same session, using laparoscopic biopsy, from the left and right liver lobe, can have different stages of fibrosis in almost half of cases assessed[14]. In addition, it was demonstrated that the smaller the liver sample, the higher the chance of sub-evaluating the severity of liver disease[15,16]. Using a mathematical model, Bedossa’s team[17] estimated that the chance of misdiagnosis in a fragment 2.5 cm in length, can reach 25% and that the optimal size of a LB sample is 4 cm (difficult enough to obtain in daily practice).
Also we must not forget that LB is an invasive procedure, which generates anxiety in the patient and is not totally risk free. Published data state that serious complications following diagnostic LB may occur in 1%-5% of cases[18,19], and that the death rate following diagnostic LB can reach 1-3/10 000 of biopsied cases[20].
In a study performed by Friedrich-Rust[10], in which ARFI was compared to LB and blood markers in 86 patients with chronic hepatitis (B or C), the Spearman correlation coefficients between the histological fibrosis stage and ARFI, TE, FibroTest and APRI scores indicated significant correlations: 0.71, 0.73, 0.66 and 0.45, respectively (P < 0.001). In this heterogeneous batch of individuals, the Spearman correlation coefficient between ARFI and LB was quite encouraging: 0.71. Since other elastographic methods, such as TE (FibroScan), showed great variability in chronic hepatitis B, we decided to perform this study only in patients with chronic hepatitis C. In an attempt to avoid LB sampling error, we included in our study only cases in which the tissue sample obtained by LB included at least 6 portal tracts.
In the study published by Lupşor and co-workers[13], 112 consecutive patients with chronic hepatitis C were evaluated by histology (Metavir score), ARFI and TE. In this study, ARFI was correlated with liver fibrosis (r = 0.717, P < 0.0001) and necroinflammatory activity (r = 0.328, P < 0.01), but not with steatosis (r = 0.122, P = 0.321). In this study, there was a significant increase in ARFI values in parallel with the increase in fibrosis stage: 1.079 ± 0.150 m/s (F0-F1), 1.504 ± 0.895 m/s (F2), 1.520 ± 0.575 m/s (F3), 2.552 ± 0.782 m/s (F4) (P < 0.0001), but there was a certain degree of overlap between the consecutive stages F1-F2 (P = 0.072) and F2-F3 (P = 0.965). In this study, the cut-off values (m/s) predictive of each fibrosis stage were: 1.19 (F ≥ 1), 1.34 (F ≥ 2), 1.61 (F ≥ 3) and 2.00 (F4). With regard to the comparison between ARFI and TE, this study found that the AUROCs were: 0.709 vs 0.902, P = 0.006 (F ≥ 1), 0.851 vs 0.941, P = 0.022 (F ≥ 2), 0.869 vs 0.926, P = 0.153 (F ≥ 3) and 0.911 vs 0.945, P = 0.331 (F4).
Our study was performed in a large cohort of patients with chronic viral hepatitis C, and ARFI was well correlated with liver fibrosis (r = 0.726, P < 0.0001) and the ARFI values increased in parallel with the stage of fibrosis. The cut-off values (m/s) predictive for each fibrosis stage were: 1.19 (F ≥ 1), 1.21 (F ≥ 2), 1.58 (F ≥ 3) and 1.82 (F = 4). According to our results, similar to TE, ARFI is not accurate enough to differentiate between F ≥ 1 vs F ≥ 2 Metavir. On the other hand, in daily practice, one of the most important points in chronic hepatitis assessment is discrimination between moderate and severe fibrosis (or cirrhosis), and the use of ARFI was able to differentiate between these two stages of fibrosis. One limitation of our study is that only a small proportion of our patients were F0 and F1 (11.1% and 16.4%, respectively), as compared to those with F2 - 28.2%, F3 - 19.5% and F4 - 24.8%.
A recently published study from Japan[21] also showed that ARFI has an excellent predictive value for the non-invasive assessment of fibrosis, with an AUROC of 0.94 for F2-F4 and F3-F4 and 0.96 for F4. In another Romanian study from 2009[22] similar very good results were obtained: AUROC of 0.90 for F ≥ 2 and 0.99 for F ≥ 3 and F4. In a German study[23], the AUROCs for F ≥ 2, F ≥ 3 and F4 were 85%, 92% and 87%, respectively, while in a French study[24], on an intention-to-diagnose basis, FibroScan and ARFI had similar predictive values for the diagnosis of significant fibrosis (F ≥ 2), with AUROCs: 0.791 ± 0.049 vs 0.793 ± 0.046 (P = 0.98).
These latter studies together with the present study, demonstrate that there is a strong correlation between histological fibrosis and LS measurements using ARFI. Therefore, the use of ARFI measurements could be an advantage, being a “real-time” evaluation of LS. It can also be used in patients in which valid measurements of LS by TE could not be obtained (since the location of ARFI measurements can be chosen under direct US guidance), and also in patients with ascites. Also, ARFI is a rapid method of liver fibrosis assessment, totally free of adverse events, comfortable for the patient and for the examiner (with a mean duration of approximately 5 min). Another advantage is the fact that LS can be evaluated with a device integrated in an ultrasound machine (already existing in some ultrasound laboratories). Thus, immediately after an ultrasound evaluation of the liver, ARFI measurements can be obtained, in order that information regarding the severity of liver fibrosis is available on the spot.
Some previous studies[13,25,26] showed that TE and ARFI are equal in the evaluation of moderate and severe fibrosis. In those cases, ARFI evaluation is the preferred method, considering that the rate of valid measurements is superior to TE, that it can be used in patients with ascites, and that there is no need to buy equipment (FibroScan).
In this quite large cohort of patients, we found that ARFI measurement is a reliable method for predicting the severity of fibrosis in HCV patients. However, the cut-off values for the different stages of fibrosis obtained must be validated. Further studies, including patients with other types of hepatopathies (chronic hepatitis B, ASH, NASH) are needed.
Non-invasive methods for fibrosis assessment in chronic hepatitis, such as transient elastography, are being accepted more and more, tending to replace invasive methods, especially in hepatitis C virus (HCV) chronic hepatitis. In recent years, a new elastographic method was developed for fibrosis assessment in chronic hepatitis - acoustic radiation force impulse (ARFI) elastography.
Single center studies including small numbers of patients have been published regarding the accuracy of ARFI elastography for fibrosis assessment in HCV hepatitis. This study included more than 250 patients from three university centers, thus increasing the relevance of the data.
The aim of this study was to determine whether ARFI elastography is a reliable method for predicting fibrosis severity in patients with chronic HCV hepatitis. In this quite large cohort of patients, the authors found that ARFI measurement is a reliable method for predicting the severity of fibrosis in HCV patients.
ARFI elastography involves targeting an anatomic region to determine its elastic properties using a region of-interest cursor, while performing real-time B-mode imaging. Tissue from the region of interest is mechanically excited to generate localized tissue displacements. These displacements result in shear-wave propagation away from the region of excitation which are tracked using US correlation-based methods. The shear-wave propagation velocity is proportional to the square root of tissue elasticity.
In this manuscript, the authors described the impact of ARFI elastography for predicting the fibrosis stage in Hep C patients. This topic is interesting.
| 1. | Gebo KA, Herlong HF, Torbenson MS, Jenckes MW, Chander G, Ghanem KG, El-Kamary SS, Sulkowski M, Bass EB. Role of liver biopsy in management of chronic hepatitis C: a systematic review. Hepatology. 2002;36:S161-S172. [PubMed] |
| 2. | Rockey DC. Noninvasive assessment of liver fibrosis and portal hypertension with transient elastography. Gastroenterology. 2008;134:8-14. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 107] [Cited by in RCA: 105] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 3. | Talwalkar JA, Kurtz DM, Schoenleber SJ, West CP, Montori VM. Ultrasound-based transient elastography for the detection of hepatic fibrosis: systematic review and meta-analysis. Clin Gastroenterol Hepatol. 2007;5:1214-1220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 357] [Cited by in RCA: 369] [Article Influence: 19.4] [Reference Citation Analysis (1)] |
| 4. | Sporea I, Sirli R, Deleanu A, Tudora A, Bota S, Cornianu M. Liver stiffnes evaluated through transient elastography in patients chronicaly infected with HBV. J Hepatol. 2009;50 Suppl 1:S143. |
| 5. | Friedrich-Rust M, Ong MF, Herrmann E, Dries V, Samaras P, Zeuzem S, Sarrazin C. Real-time elastography for noninvasive assessment of liver fibrosis in chronic viral hepatitis. AJR Am J Roentgenol. 2007;188:758-764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 229] [Cited by in RCA: 237] [Article Influence: 12.5] [Reference Citation Analysis (0)] |
| 6. | Tatsumi C, Kudo M, Ueshima K, Kitai S, Takahashi S, Inoue T, Minami Y, Chung H, Maekawa K, Fujimoto K. Noninvasive evaluation of hepatic fibrosis using serum fibrotic markers, transient elastography (FibroScan) and real-time tissue elastography. Intervirology. 2008;51 Suppl 1:27-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 72] [Cited by in RCA: 76] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 7. | Havre RF, Elde E, Gilja OH, Odegaard S, Eide GE, Matre K, Nesje LB. Freehand real-time elastography: impact of scanning parameters on image quality and in vitro intra- and interobserver validations. Ultrasound Med Biol. 2008;34:1638-1650. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 90] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 8. | Fujimoto K, Kato M, Wada S, Tonomura A, Oshita M, Mitake T. Non-invasive evaluation of hepatic fibrosis in patients with chronic hepatitis C using elastography. Medix. 2007;Suppl:24-27. |
| 9. | Popescu A, Sporea I, Focsa M, Sandra V, Ruta V, Deleanu A, Danila M, Sirli R. Assessment of Liver Fibrosis by Real Time SonoElastography (Hitachi) as Compared to Liver Biopsy and Transient Elastography. Ultrasound Med Biol. 2009;35 Suppl 8:S152. |
| 10. | Friedrich-Rust M, Wunder K, Kriener S, Sotoudeh F, Richter S, Bojunga J, Herrmann E, Poynard T, Dietrich CF, Vermehren J. Liver fibrosis in viral hepatitis: noninvasive assessment with acoustic radiation force impulse imaging versus transient elastography. Radiology. 2009;252:595-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 476] [Cited by in RCA: 468] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 11. | Sporea I, Sirli R, Deleanu AE, Popescu A, Focsa M, Danila M, Tudora A, Cornianu M. Transient Elastography (FibroScan®) as compared to Real Time-Elastography (Siemens) in patients with chronic hepatopathies. Gastroenterology. 2009;136:A-327. |
| 12. | Sporea I, Sirli R, Popescu A, Deleanu A, Focsa M. How Relevant is Real-Time Elastography (Siemens), for the Evaluation of Liver Stiffness? Ultrasound Med Biol. 2009;35 Suppl:S53. |
| 13. | Lupsor M, Badea R, Stefanescu H, Sparchez Z, Branda H, Serban A, Maniu A. Performance of a new elastographic method (ARFI technology) compared to unidimensional transient elastography in the noninvasive assessment of chronic hepatitis C. Preliminary results. J Gastrointestin Liver Dis. 2009;18:303-310. [PubMed] |
| 14. | Regev A, Berho M, Jeffers LJ, Milikowski C, Molina EG, Pyrsopoulos NT, Feng ZZ, Reddy KR, Schiff ER. Sampling error and intraobserver variation in liver biopsy in patients with chronic HCV infection. Am J Gastroenterol. 2002;97:2614-2618. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1504] [Cited by in RCA: 1580] [Article Influence: 65.8] [Reference Citation Analysis (0)] |
| 15. | Abdi W, Millan JC, Mezey E. Sampling variability on percutaneous liver biopsy. Arch Intern Med. 1979;139:667-669. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 35] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 16. | Colloredo G, Guido M, Sonzogni A, Leandro G. Impact of liver biopsy size on histological evaluation of chronic viral hepatitis: the smaller the sample, the milder the disease. J Hepatol. 2003;39:239-244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 623] [Cited by in RCA: 601] [Article Influence: 26.1] [Reference Citation Analysis (1)] |
| 17. | Bedossa P, Dargère D, Paradis V. Sampling variability of liver fibrosis in chronic hepatitis C. Hepatology. 2003;38:1449-1457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1193] [Cited by in RCA: 1408] [Article Influence: 61.2] [Reference Citation Analysis (0)] |
| 18. | Thampanitchawong P, Piratvisuth T. Liver biopsy: complications and risk factors. World J Gastroenterol. 1999;5:301-304. [PubMed] |
| 19. | Piccinino F, Sagnelli E, Pasquale G, Giusti G. Complications following percutaneous liver biopsy. A multicentre retrospective study on 68,276 biopsies. J Hepatol. 1986;2:165-173. [PubMed] |
| 20. | Friedman LS. Controversies in liver biopsy: who, where, when, how, why? Curr Gastroenterol Rep. 2004;6:30-36. [PubMed] |
| 21. | Takahashi H, Ono N, Eguchi Y, Eguchi T, Kitajima Y, Kawaguchi Y, Nakashita S, Ozaki I, Mizuta T, Toda S. Evaluation of acoustic radiation force impulse elastography for fibrosis staging of chronic liver disease: a pilot study. Liver Int. 2010;30:538-545. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 172] [Article Influence: 10.8] [Reference Citation Analysis (0)] |
| 22. | Fierbinteanu-Braticevici C, Andronescu D, Usvat R, Cretoiu D, Baicus C, Marinoschi G. Acoustic radiation force imaging sonoelastography for noninvasive staging of liver fibrosis. World J Gastroenterol. 2009;15:5525-5532. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 133] [Cited by in RCA: 143] [Article Influence: 8.4] [Reference Citation Analysis (2)] |
| 23. | Goertz RS, Zopf Y, Jugl V, Heide R, Janson C, Strobel D, Bernatik T, Haendl T. Measurement of liver elasticity with acoustic radiation force impulse (ARFI) technology: an alternative noninvasive method for staging liver fibrosis in viral hepatitis. Ultraschall Med. 2010;31:151-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 24. | Boursier J, Isselin G, Fouchard-Hubert I, Oberti F, Dib N, Lebigot J, Bertrais S, Gallois Y, Calès P, Aubé C. Acoustic radiation force impulse: a new ultrasonographic technology for the widespread noninvasive diagnosis of liver fibrosis. Eur J Gastroenterol Hepatol. 2010;22:1074-1084. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 105] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 25. | Sporea I, Sirli RL, Deleanu A, Popescu A, Focsa M, Danila M, Tudora A. Acoustic radiation force impulse elastography as compared to transient elastography and liver biopsy in patients with chronic hepatopathies. Ultraschall Med. 2011;32 Suppl 1:S46-S52. [PubMed] |
| 26. | Sporea I, Sirli R, Popescu A, Danilă M. Acoustic Radiation Force Impulse (ARFI)--a new modality for the evaluation of liver fibrosis. Med Ultrason. 2010;12:26-31. [PubMed] |
Peer reviewers: Dr. Charikleia Triantopoulou, Konstantopouleion Hospital, Agias Olgas street, Athens, 14233 N. Ionia, 3-5, Greece; Yasunori Minami, MD, PhD, Assistant Professor, Division of Gastroenterology and Hepatology, Department of Internal Medicine, Kinki University School of Medicine, 377-2 Ohno-higashi, Osaka-sayama, Osaka, 589-8511, Japan
S- Editor Cheng JX L- Editor Webster JR E- Editor Zheng XM