Revised: March 2, 2011
Accepted: March 9, 2011
Published online: March 28, 2011
AIM: To investigate intra-operator variability of semi-quantitative perfusion parameters using dynamic contrast-enhanced ultrasonography (DCE-US), following bolus injections of SonoVue®.
METHODS: The in vitro experiments were conducted using three in-house sets up based on pumping a fluid through a phantom placed in a water tank. In the in vivo experiments, B16F10 melanoma cells were xenografted to five nude mice. Both in vitro and in vivo, images were acquired following bolus injections of the ultrasound contrast agent SonoVue® (Bracco, Milan, Italy) and using a Toshiba Aplio® ultrasound scanner connected to a 2.9-5.8 MHz linear transducer (PZT, PLT 604AT probe) (Toshiba, Japan) allowing harmonic imaging (“Vascular Recognition Imaging”) involving linear raw data. A mathematical model based on the dye-dilution theory was developed by the Gustave Roussy Institute, Villejuif, France and used to evaluate seven perfusion parameters from time-intensity curves. Intra-operator variability analyses were based on determining perfusion parameter coefficients of variation (CV).
RESULTS: In vitro, different volumes of SonoVue® were tested with the three phantoms: intra-operator variability was found to range from 2.33% to 23.72%. In vivo, experiments were performed on tumor tissues and perfusion parameters exhibited values ranging from 1.48% to 29.97%. In addition, the area under the curve (AUC) and the area under the wash-out (AUWO) were two of the parameters of great interest since throughout in vitro and in vivo experiments their variability was lower than 15.79%.
CONCLUSION: AUC and AUWO appear to be the most reliable parameters for assessing tumor perfusion using DCE-US as they exhibited the lowest CV values.
- Citation: Gauthier M, Leguerney I, Thalmensi J, Chebil M, Parisot S, Peronneau P, Roche A, Lassau N. Estimation of intra-operator variability in perfusion parameter measurements using DCE-US. World J Radiol 2011; 3(3): 70-81
- URL: https://www.wjgnet.com/1949-8470/full/v3/i3/70.htm
- DOI: https://dx.doi.org/10.4329/wjr.v3.i3.70
Tumor angiogenesis is a process involving the proliferation of new blood vessels which penetrate tumors supplying them with nutrients and oxygen[1,2]. Nowadays, research is focused on the development of anti-angiogenic treatments whose aim is to destroy neoblood vessels which often occur initially without any morphological changes[3,4].
Until now, treatment evaluation has been based on Response Evaluation Criteria in Solid Tumors (RECIST)[5]. Even though RECIST criteria were recently revised, they still only concern morphological information[6]. It is commonly recognized that this criterion is no longer optimal for the early assessment of anti-angiogenic treatments which primarily target microvascularization. Functional imaging is currently establishing itself as the best modality for evaluating such therapies, by determining a series of semi-quantitative perfusion parameters related to tumor perfusion. These were defined as semi-quantitative as they only provided a relative evaluation of the physiological parameters such as blood flow or blood volume based on the dye dilution theory.
Nowadays, dynamic contrast-enhanced ultrasonography (DCE-US) is becoming increasingly widespread[7,8] as it allows functional imaging[9,10]. However, one of its major drawbacks is its intra-operator variability which could have a major impact on measurements, leading to erroneous interpretations. A small difference in perfusion could be interpreted as a functional change but might simply be due to operator variability.
Until now, information on intra-operator variability has been lacking. Consequently, the purpose of our study was to evaluate the intra-operator variability of semi-quantitative perfusion parameter measurements using DCE-US, both in vitro and in vivo, following bolus injections of SonoVue® (Bracco, Milan, Italy).
DCE-US studies were performed using bolus injections of SonoVue®, a second generation contrast agent, consisting of microbubbles of sulphur hexafluoride (SF6) stabilized by a shell of amphiphilic phospholipids[11,12]. The SF6 gas does not interact with any other molecules found in the body as it is a very inert gas. The size of the microbubbles, ranging from 1 to 10 μm[12], allows them to circulate through the whole blood volume. In addition, SonoVue® is a purely intravascular contrast agent which makes it ideal for the evaluation of perfusion[12]. Insonated at low acoustic power, SonoVue®, whose nonlinear harmonic response is high[13], provides continuous real-time ultrasonographic (US) imaging without destroying the microbubbles[14].
Before any US exam, the contrast agent was reconstituted by introducing 5 mL of 0.9% sodium chloride into the vial, containing a pyrogen-free lyophilized product, followed by manual shaking for at least 20 s. One experiment involved several injections of SonoVue®. As the microbubbles tended to accumulate at the upper surface because of buoyancy, the preparation was systematically manually checked before each injection in order to recover the required homogeneous solution. Each experiment lasted less than 2 h on account of the stability of SonoVue® over time which is 6 h after its reconstitution[11].
The time intensity method is essentially based on the dye dilution theory: after the injection of the indicators, contrast agent concentration is monitored as a function of time, generating a time intensity curve (TIC) from which a series of semi-quantitative perfusion parameters is extracted and analyzed[15,16]. To be valid, a series of assumptions must be verified[17]: (1) Flow has to be constant so that the amount of microbubbles injected has no effect on the flux; (2) Blood and contrast agent must be adequately mixed to obtain a homogeneous concentration; (3) Recirculation should not interfere with the first pass; and (4) The mixing of the contrast agent must exhibit linearity and a stable condition[18]. Linearity refers to the linear relationship between bubble concentration and signal intensity and was previously confirmed for low doses by Greis[12] as well as by Lampaskis et al[19] in the context of bolus injection.
In our study, conditions were assumed to be satisfied. The semi-quantitative perfusion parameters that we analyzed were therefore directly extracted from the TIC.
US protocol: SonoVue® bolus injections were performed through a 1-mL syringe (Terumo®, Belgium) and a “26Gx1/2” needle (Terumo®, Belgium). The injection site was marked so that the same site was used for all the in vitro studies. It was positioned at a distance of 30 cm from the input of the phantom.
According to the Guidelines for Evaluating and Expressing the Uncertainty of NIST (National Institute of Standards and Technology) Measurements Results, intra-operator variability studies must be performed under the following conditions: (1) The same measurement procedure; (2) The same observer; (3) The same measuring instrument, used under the same conditions; (4) The same location; and (5) Repetition over a short period of time.
Thus, all the experimental conditions as well as the operators injecting the SonoVue® and manipulating the ultrasound scanner were the same for all acquisitions.
To minimize errors which might have been due to possible SonoVue® residues in the injection materials, a new syringe and a new needle were used for each injection. In addition, the circuit was entirely emptied, rinsed and reset with degassed water between each acquisition. Thus, no contrast agent residues were present in the circuit so that the initial conditions were exactly the same for all the experiments.
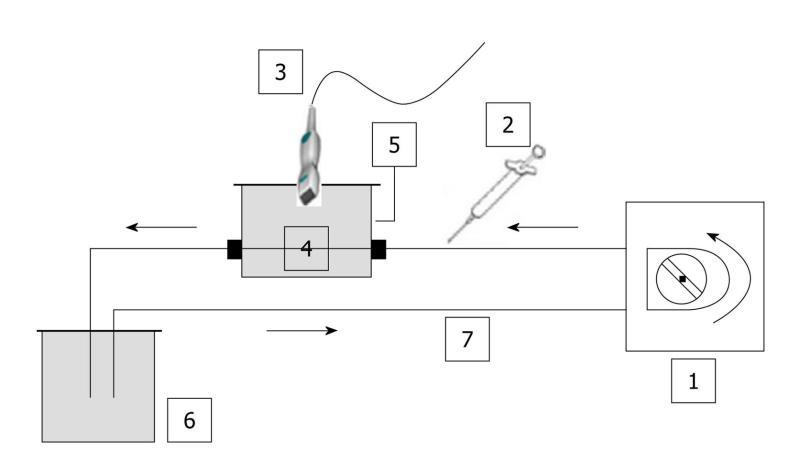
The three phantoms consisted of a closed-circuit flow model as no contact with ambient air was possible. Consequently, as the water was degassed and the circuit was closed, no significant amount of gas was trapped in the set-up (Figure 1).
Images were acquired using a Toshiba Aplio® ultrasound scanner (Toshiba, Japan) version 6, release 5, connected to a 2.9-5.8 MHz linear transducer (PZT, PLT 604AT probe). Harmonic imaging was performed using the “Vascular Recognition Imaging” (VRI) mode combining: (1) Fundamental B mode imaging, which allows simultaneous but independent grey scale visualization of morphological structures; (2) Doppler imaging, which provides vascular information; and (3) Harmonic imaging, based on the pulse inversion mode, which consists of summing echoes resulting from two waves which are inverted copies of each other. As microbubbles exhibit non-linear responses, the resulting sum of the echoes will be different from 0 implying a non-null signal[20].
Acquisitions lasting 50 s were obtained at a low mechanical index (MI = 0.1) and at a rate of 5 frames per second (fps).
Single straight pipe phantom: The first assembly consisted of a single straight silicone pipe phantom, immersed in a custom-made water tank which was connected to a peristaltic pump (SP vario/PD 5101, Heidolph®, Germany) providing a defined water flow rate of 42.4 mL/min. This phantom was used to mimic blood flow. Tubing was made of a 1 mm thick silicone pipe with a 2 mm internal diameter. A custom-made probe holder was used to keep the probe still throughout the experiments.
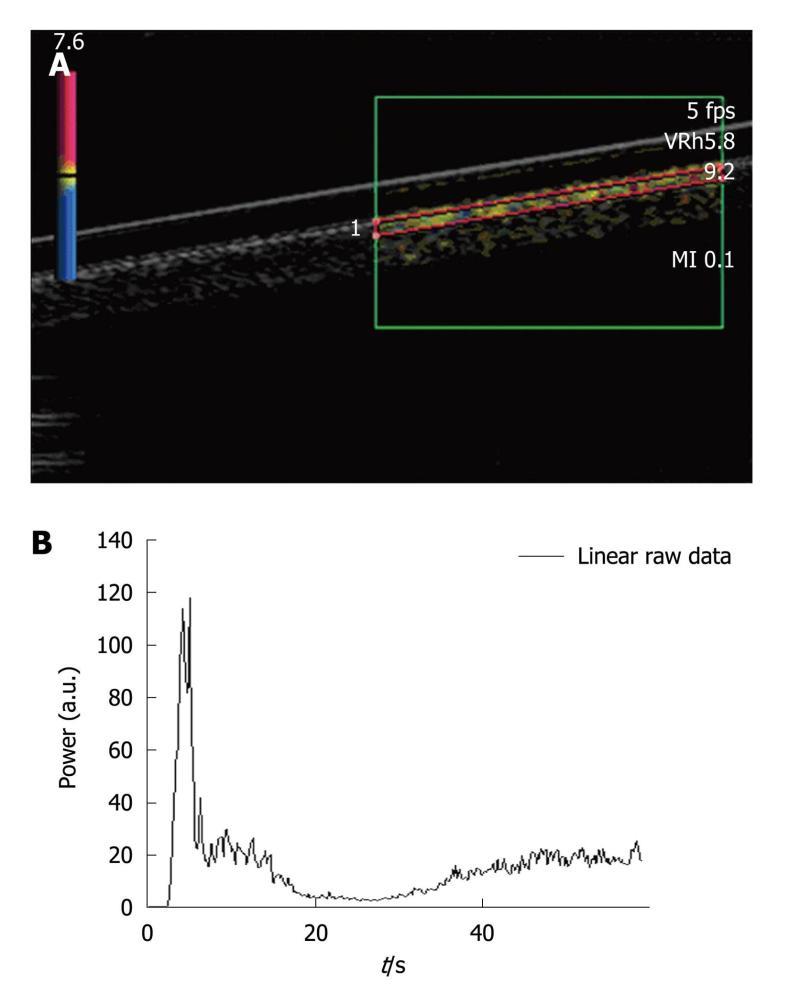
A region of interest (ROI) was set in the upper part of the pipe (Figure 2A). Each injection involved a new ROI and its associated TIC (Figure 2B). Three volumes of SonoVue® were tested. The first experiment involved five 0.02 mL bolus injections of contrast agent for a total amount of water set at 50 mL in the closed-circuit. This ratio respected that granted by marketing approval (“Autorisation de Mise sur le Marché”: AMM) (2.4 mL of SonoVue® for 5 L of blood). The second experiment was performed by injecting five times a 0.05 mL bolus of SonoVue®. This volume involved a ratio routinely used for clinical exams (4.8 mL of SonoVue® for 5 L of blood) and particularly in four studies performed at the Gustave Roussy Institute (IGR), involving 117 patients and 823 DCE-US exams[21,22] as well as a national project supported by the “Institut National du Cancer” (French National Cancer Institute)[23]. The last experiment involved five 0.08 mL bolus injections of SonoVue®. As the phantom became more complex, we focused on the IGR ratio previously validated in IGR studies.
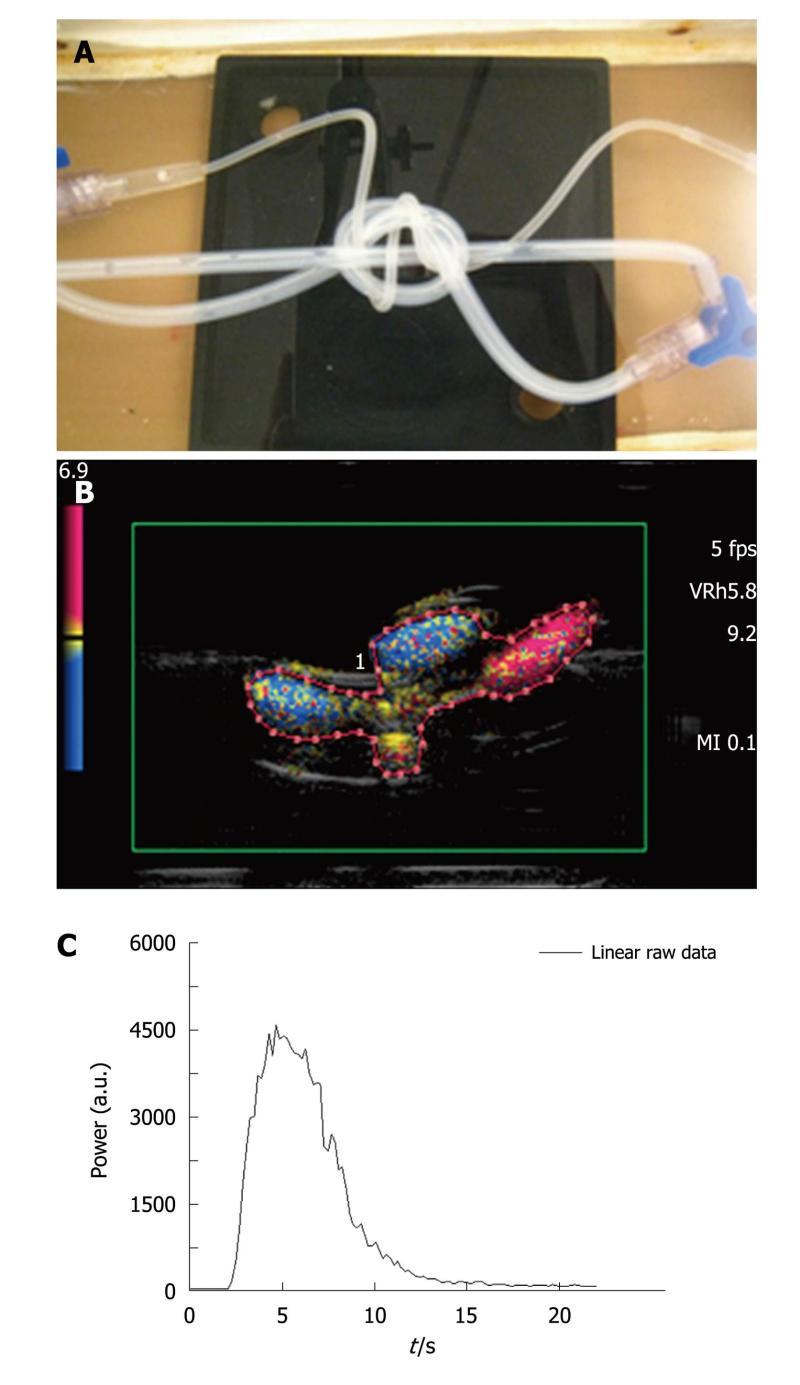
Three intertwined pipe phantom: A second phantom consisted of three intertwined silicone pipes immersed in a custom-made water tank. Two of the three tubes were in silicone with an internal diameter of 2 mm and a 1 mm thick wall as in the case of the previous phantom. The third one, originating from a catheter (Surflo® winged infusion set, Terumo®, Belgium), had a 1 mm internal diameter and a 0.5 mm thick wall. The input and the output of the phantom were composed of three-way taps (Discofix®, B. Braun, Melsungen, Germany) allowing linkage between the three pipes (Figure 3A). The phantom was connected to the same pump as previously described, providing a defined water flow rate set at 42.4 mL/min. Such an assembly was designed to mimic a complex structure akin to that of vessels in tumors.
A new ROI containing both pipes and water spaces (Figure 3B), was drawn for each injection and the associated TIC was obtained (Figure 3C). The total amount of water in the circuit was set at 60 mL. Two series of acquisitions were obtained involving, respectively, five 0.03 mL (AMM ratio) and 0.06 mL (IGR ratio) bolus injections of contrast agent.
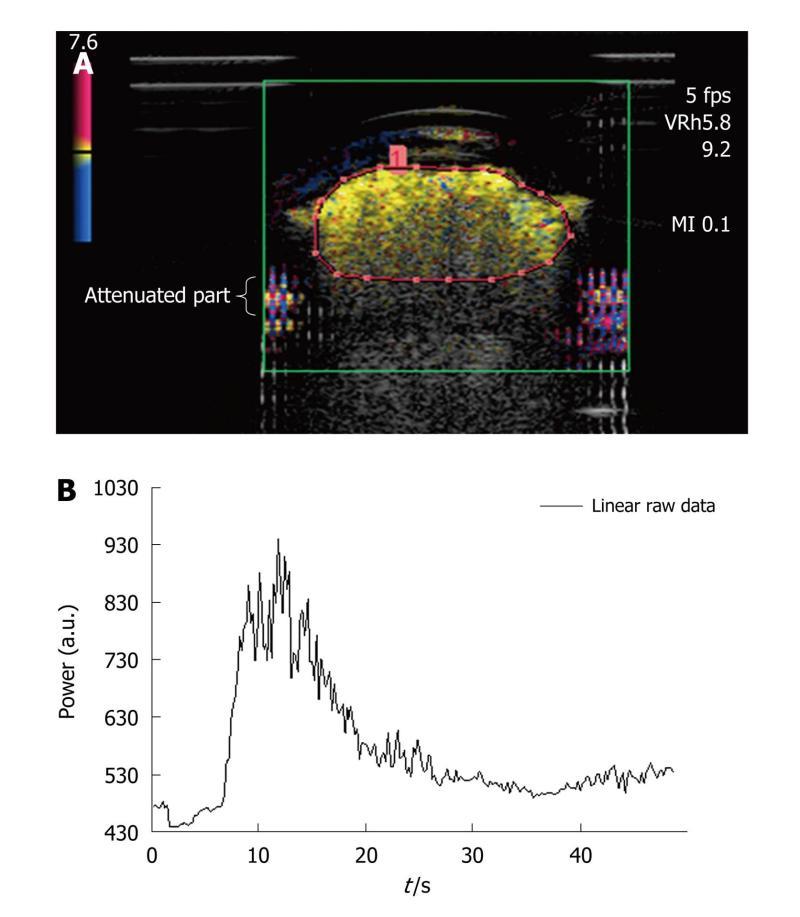
Dialyzer: The third assembly was composed of a dialyzer (FX PAED, Fresenius Medical Care, France). The dialysis cartridge, consisting of about 1550 capillaries with an internal diameter of 220 μm, was connected to the peristaltic pump providing a water flow rate of 42.4 mL/min and was immersed in water. To minimize attenuation due to the original plastic case of the dialyzer, a rectangular part of it was removed and replaced by a cellophane sheet to maintain the circuit closed[24]. The advantage of such a phantom was that the capillaries of the dialyzer were comparable in dimension to that of one type of vessel found in the microvascularization (arteriole diameter < 300 μm). In addition, as the size of SonoVue® microbubbles ranged from 1 to 10 μm[12], they were about a thousand-fold the dimensions of capillary pores (2.4 nm), thus ensuring the intravascular property of the ultrasound contrast agent.
As shown in Figure 4A, attenuation occurs in the lower part of the dialyzer: it is characterized by a strong decrease in amplitude and a loss of the contrast signal. An ROI was drawn for each of the repeated measurements and only contained the upper part of the dialyzer so that the acquisition was not prone to attenuation phenomena[25]. Based on the selected ROI, the associated time intensity curve was obtained for the analysis (Figure 4B). The total amount of water was 100 mL and a volume of 0.10 mL of SonoVue® was tested five times (IGR ratio).
Animals and tumor model: Experiments were conducted with nude female mice aged from 6 to 8 wk with the approval of the European Convention for the Protection of Vertebrate Animals used for experimental and other scientific purposes (Strasbourg, 18.III.1986; text amended according to the provisions of protocol ETS No. 170 as of its entry into force on 2nd December 2005).
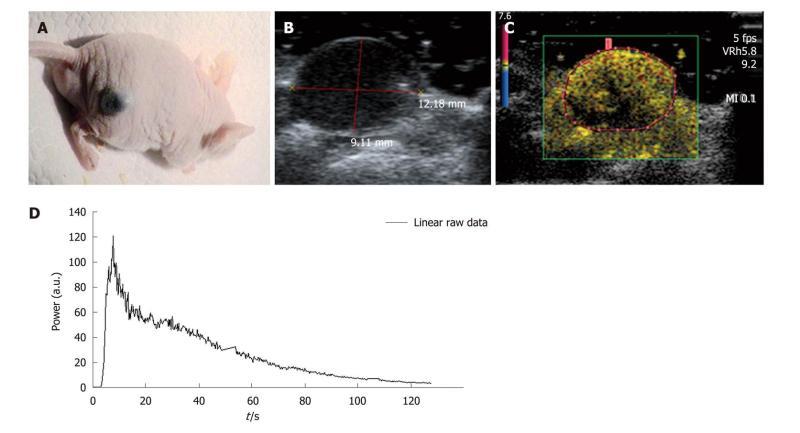
The selected tumor model was the B16F10 (CRL-6475, ATCC, American Type Culture Collection) melanoma cell line which is a murine skin cancer. The tumor cells were cultured in DMEM (Dulbecco minimum essential medium) (Gibco Life Technologies, France) combined with 10% fetal bovine serum, 1% penicillin/streptomycin and glutamate (Invitrogen Life Technologies, Inc., France) to avoid bacterial contamination of the solution. While growing, cells were maintained in an incubator at 37°C. Tumors were xenografted onto the right flank of five mice (Figure 5A) through a subcutaneous injection of 2 × 106 melanoma cells in 0.2 mL of Phosphate Buffered Saline (PBS). DCE-US exams were performed following three 0.02 mL, 0.05 mL or 0.1 mL retro-orbital bolus injections of SonoVue® according to the methodology used in our lab.
Settings: During the experiments, mice were placed on the left flank and maintained at a constant temperature through a warming pad (TEM, Bordeaux, France) connected to a Gaymar T/Pump® (Gaymar Industries, Inc., USA). The temperature was regulated with the adjustable thermostat of the pump and was set at 40°C (internal mouse temperature: 39°C). Each mouse was weighed and the tumor volume was determined before each DCE-US exam. The ROI was set to exclusively include the tumor (Figure 5B-C). An ROI was drawn for each of the repeated measurements and once it was selected, the ultrasound scanner directly allowed access to the time intensity curve associated with the selected ROI. The linear raw data to be modeled were converted through text files and extracted to obtain a graph using Excel® (Figure 5D).
A break of at least 15 min was observed between each injection in addition to 3 min of insonation at a high mechanical index (MI = 1.4), so that contrast agent could be eliminated. Mice were kept asleep no more than 2 h. This duration included the time required for the mice to obtain a stationary heart rate after the administration of anesthesia, acquisition time and the duration of the break between each injection. Three injections per mouse were considered for the data analysis. Mice underwent either chemical or gaseous anesthesia.
Chemical anesthesia: The amount of chemical anesthesia was determined based upon mouse body weight. Once weighed, the required amount of anesthesia was prepared and injected intraperitoneally using the 1-mL syringe and a “30Gx1/2” needle (Microlance™, Ireland). The solution consisted of ketamine (10 mg/mL, Ketalar®, Parapharm, France) and xylazine (2%, Rompun®, Bayer, France). To ensure that the mice remained asleep throughout the experiment, 150 μL/g per mouse were systematically injected.
Gaseous anesthesia: Mice were anaesthetized through a 2 L/min inhalation of O2 combined with 2.5% of isoflurane. It was possible to modify the flow rate during the experiment so that mice could be maintained asleep without being endangered throughout the experiment.
US protocol: Preliminary fundamental B-mode imaging using a 14 MHz PLT 1204AT (Toshiba, Japan) probe was performed to determine the tumor volume prior to the SonoVue® injection (Figure 5B). The largest longitudinal and transversal sections allowed us to determine the tumor volume by measuring the three perpendicular tumor diameters[26] according to the following formula: V = 1/2 × (depth × width × length).
Harmonic imaging was performed in the same way as for the in vitro experiments with a mechanical index set at 0.1 and a rate of 5 fps. Acquisitions lasting 3 min were recorded allowing visualization of both the wash-in and wash-out parts of the TICs.
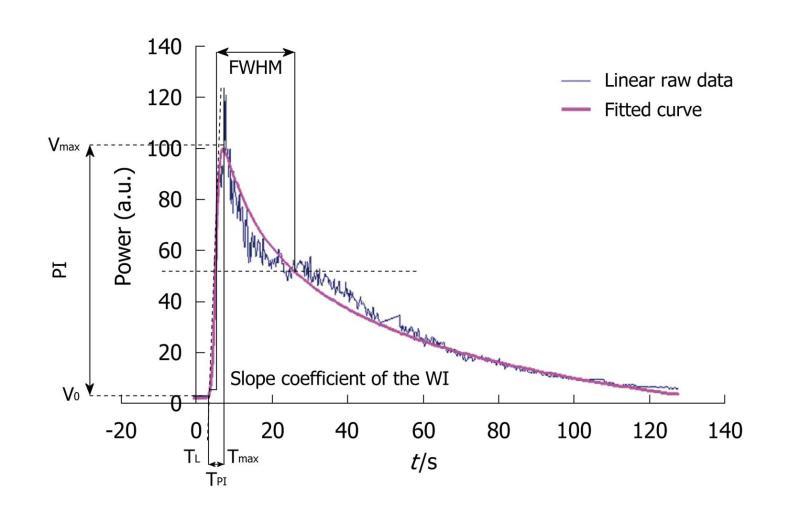
As already described in the literature, linear raw data (uncompressed linear data obtained before standard video-visualization) have the advantage of exhibiting a linear dynamic range which is the essential part of the evaluation of semi-quantitative perfusion parameters from TICs[27]. Acquisitions involved recording harmonic images after the SonoVue® injection, using dedicated software, CHI-Q®, on the Toshiba Aplio® ultrasound scanner. In a manually outlined ROI, the mean US signal intensity induced by contrast uptake was obtained and expressed in arbitrary units. This was linearly linked to the number and size of microbubbles in the ROI (Aplio® procedure)[28,29]. Then, the corresponding TIC to be modeled was converted through text files generated by the ultrasound scanner and extracted to obtain a graph using Excel®. Seven semi-quantitative perfusion parameters were extracted from this curve: the peak intensity (PI) was the difference between Vmax [maximal intensity value: V(Tmax)] and V0 [Initial intensity value: V(T0)] and represented the highest intensity value attained by the TIC for the defined ROI. The time to peak intensity (TPI), corresponding to Time to Peak Intensity, was the time required for the contrast agent to arrive in the tumor and reach the PI. It corresponded to the difference between the latency time (TL), defined as the time between the injection of the product and the beginning of contrast uptake, and Tmax. From a mathematical point of view, TL corresponded to the time at the intersection between y=V0 and the tangent at Tmax/2. The AUC, AUWI and AUWO corresponded to the area under the curve, the area under the wash-in (from T0 to Tmax) and the area under the wash-out (from Tmax to Tend corresponding to the end of the acquisition) of the TIC. The area calculations were based on the trapezoidal rule: the region under the graph of the fitted curve was approximated by trapezoids whose areas were calculated to provide approximations of the areas under the curve, the wash-in or the wash-out. An additional operation of subtracting the offset on the y-axis was performed so that the total area under the curve was independent of it. The limits of integration were defined as follows:
Where T0 is the initial time. The slope coefficient of the wash-in (WI) was literally defined as the slope of the tangent of the wash-in at half maximum. Finally, the full-width at half maximum (FWHM) was defined as the time interval during which the value of the intensity was higher than Vmax/2. This parameter is commonly considered as a first approximation of the mean transit time (MTT) (Figure 6)[8,26,30]. However, as the linear raw data provided by the ultrasound scanner were too noisy, extraction was performed after TIC fitting. This was based on the mathematical model developed at the IGR (Patent: WO/2008/053268 entitled “Method and system for quantification of tumoral vascularization”).
where I(t) describes the variation in the intensity of contrast uptake as a function of time; a0 is the intensity before the arrival of the contrast agent; a1 is linked to the maximum value of contrast uptake; a2 is linked to the rise time to the peak intensity; p is a coefficient related to the increase in intensity; q is a coefficient related to the decrease in intensity; A and B are arbitrary parameters.
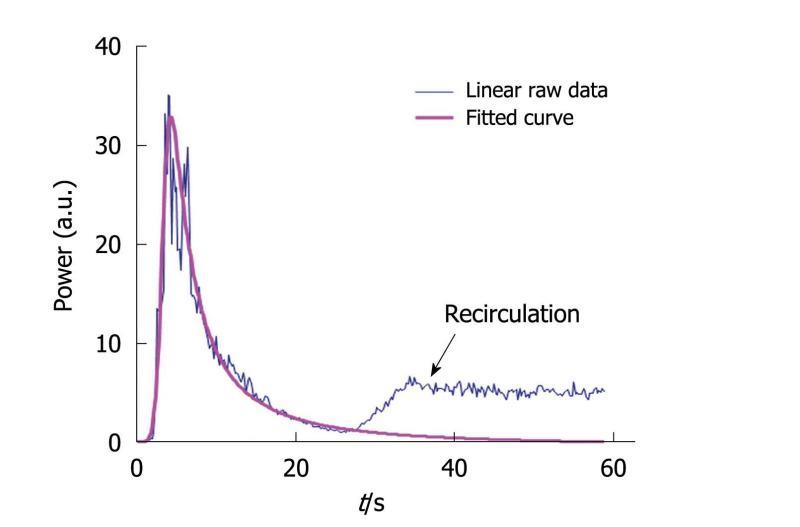
The fitted curve was obtained by adjusting all the coefficients of the equation (called the IGR equation) to obtain a sum of the least-squares differences between the linear raw data and the modeled values as close to 0 as possible. Equation parameters were derived through the mathematical model so that asymptotic values were properly defined. This fitting was performed using the Solver program in Excel®. Through this modeling step, it was possible to avoid recirculation so that the conditions required to apply the time intensity method were fulfilled. Indeed, the IGR equation was used to perfectly fit the wash-in part of the curve while the wash-out part was adjusted so that recirculation was not taken into account in the final fitted curve (Figure 7). This adjustment is often performed[31] to ensure that the conditions required for the time intensity method are adequately verified. Once the fitted curve was determined, the semi-quantitative perfusion parameters were derived according to their previously described definitions.
Intra-operator variability was measured as the coefficient of variation (CV) which is the ratio of the SD of a specific parameter to its mean. CV = SD/mean.
For each set of experiments, the CV was evaluated and recorded for each of the seven semi-quantitative perfusion parameters. An overall range of variation was additionally provided in the “Results” part.
Three different phantoms were tested. Acquisitions were obtained after five injections of SonoVue®.
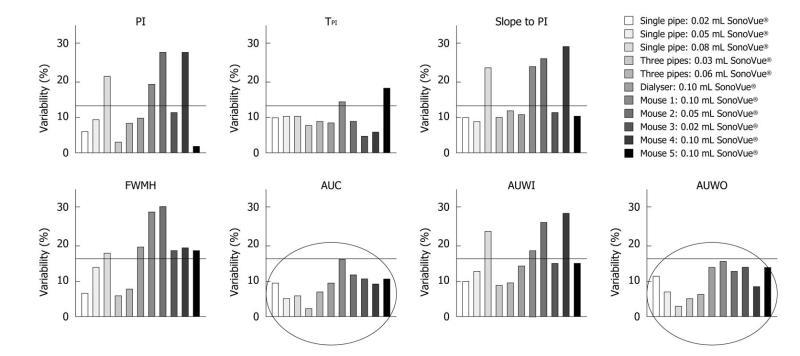
1st phantom - Single straight pipe phantom results: Three volumes of SonoVue® were tested: 0.02, 0.05 and 0.08 mL. For each volume, five fitted TICs were obtained following the five contrast agent injections. Data analysis was performed according to the protocol detailed in the “Materials and Methods” part: semi-quantitative perfusion parameters were extracted directly from the fitted TICs and their associated CV values were evaluated. The 0.02, 0.05 and 0.08 mL five injections respectively led to CV values ranging from 6.03% to 11.11%, from 5.23% to 13.24% and from 2.94% to 23.72%. Table 1 shows perfusion parameter CV values obtained following each set of experiments.
| PI | TPI | Slope of the WI | FWHM | AUC | AUWI | AUWO | |
| 0.02 mL/CV (%) | 6.03 | 9.78 | 9.57 | 6.43 | 9.40 | 9.97 | 11.11 |
| 0.05 mL/CV (%) | 9.27 | 10.14 | 8.28 | 13.24 | 5.23 | 12.76 | 6.84 |
| 0.08 mL/CV (%) | 20.87 | 10.10 | 23.52 | 17.43 | 5.87 | 23.72 | 2.94 |
2nd phantom - Three intertwined pipe phantom results: The five fitted TICs resulting from the series of 0.03 and 0.06 mL bolus injections of SonoVue® were analyzed using the IGR model. Intra-operator variability values were found to respectively range from 2.33% to 9.65% and from 6.12% to 11.62%. CV values associated with each perfusion parameter are shown in Table 2.
| PI | TPI | Slope of the WI | FWHM | AUC | AUWI | AUWO | |
| 0.03 mL/CV (%) | 3.16 | 7.69 | 9.65 | 5.56 | 2.33 | 8.55 | 5.32 |
| 0.06 mL/CV (%) | 7.85 | 8.60 | 11.62 | 7.53 | 6.73 | 9.52 | 6.12 |
For both these phantoms, maximum CV values were found in correspondence with the highest injected dose of SonoVue®. This observation might impact on the linear assumption. However, as previously mentioned in the “Time Intensity Method” part, to transfer in vitro results into clinical context, the concentration of interest is the IGR one which remains in the linear range.
3rd phantom - Dialyzer results: Data analysis was performed following five 0.10 mL bolus injections of SonoVue®. This series of experiments led to intra-operator variability values ranging from 8.11% to 19.11%. Table 3 provides more detailed results associated with each evaluated semi-quantitative perfusion parameter.
| PI | TPI | Slope of the WI | FWHM | AUC | AUWI | AUWO | |
| 0.10 mL/CV (%) | 9.66 | 8.11 | 10.27 | 19.11 | 9.31 | 13.89 | 13.36 |
Intra-operator variability studies were performed on five mice following three 0.02 mL, 0.05 mL or 0.1 mL bolus injections of SonoVue®. Experiments were performed over 5 d.
Chemical anesthesia: Four mice underwent chemical anesthesia. The body weight was found to be 23.5 g (min: 22.6 g; max: 25 g) throughout the experiments. As experiments lasted over 5 d and due to the rapid doubling time of melanoma cells[32-34], tumor volume ranged from 93.8 to 599.7 mm3 with a mean tumor value of 339.39 mm3. We calculated the semi-quantitative perfusion parameters directly from the TICs using the IGR mathematical model. CV values ranged from 1.48% to 29.97%. Table 4 shows variability values associated with each perfusion parameter for each mouse.
| Mouse | PI | TPI | Slope of the WI | FWHM | AUC | AUWI | AUWO | ||
| 0.02 mL/ 0.05 mL/ 0.1 mL/ CV (%) | Chemical anaesthesia | 1 | 18.99 | 14.04 | 23.85 | 28.87 | 15.79 | 18.43 | 15.46 |
| 2 | 27.60 | 8.33 | 25.75 | 18.90 | 9.06 | 28.39 | 8.12 | ||
| 3 | 11.39 | 4.41 | 1.48 | 18.44 | 10.76 | 14.73 | 13.58 | ||
| 4 | 27.88 | 5.68 | 28.82 | 29.97 | 11.21 | 26.22 | 12.42 | ||
| Gaseous anaesthesia | 5 | 1.90 | 17.63 | 10.09 | 24.96 | 12.32 | 12.43 | 12.79 |
Gaseous anesthesia: One mouse underwent gaseous anesthesia. It weighed 24.6 g. The tumor volume was 503.56 mm3. CV values were determined based on the fitted TICs and ranged from 1.90% to 24.96%. More detailed results are provided in Table 4.
In this study, intra-operator variability was assessed through the determination of the coefficients of variation of semi-quantitative perfusion parameters using three distinct phantoms as well as in vivo. Throughout the experiments, CV values were consistently lower than 30% (Figure 8). The area under the curve and the area under the wash-out exhibited CV values below 15.79% both in vitro and in vivo.
For each set of experiments, conditions such as the probe, the phantom, the whole circuit and the settings of the ultrasound scanner were maintained unchanged so that the assembly remained identical throughout the acquisitions.
The first source of variation could come from the ultrasound contrast agent itself. Indeed, before each injection, SonoVue® was reconstituted by manual agitation. Consequently, the solution may not have been identically homogeneous throughout the experiments. In addition, the precision of the syringe used to administer SonoVue® (within 0.01 mL) was low compared to the injected doses. Consequently, variations may have occurred even prior to any acquisition.
The second source of variation could come from the injection step. Manual injections were performed by the same operator and a mark was drawn on the injection site so that the same site was used throughout the experiment. A new syringe was used for each injection. However, even if the mark allowed us to maintain the same injection site, it did not provide information concerning the exact position of the syringe within the pipe. In addition, even though the injection was administered by the same operator, variations could have occurred as it was performed manually (rate or angle of the injection). Other errors may have affected the data analysis part. A region of interest had to be selected to compute the TICs. It had to be the same for all TICs. However, as a new ROI was manually drawn for each injection, results may have been tarnished because of mistakes. Additional sources of variation can occur in vivo. Indeed, even if mice were maintained asleep, their physiological parameters were not entirely monitorable and variations may have occurred during the experiments.
The next step concerned TIC fitting. To ensure that no variations could come from the solver itself, a series of ten fittings were performed on the same TIC. The parameters provided by the IGR mathematical model were exactly the same for all the curves which allowed us to conclude that only the first three steps mentioned above may have induced variations in the results.
First, in vitro, the fluid used was water at ambient temperature which did not exhibit the same ultrasound properties as blood[35-37] (Table 5). In addition, none of the phantoms contained tissue-mimicking material[38,39]: the first two were composed of silicone pipes leading to the same remark as with the fluid: the ultrasound properties of the silicone are different from those of vessels. The properties of the third phantom were more similar to those of the microvascularization due to its dimensions. However, the parallelism with the capillaries did not reflect the complex and irregular structure of tumor microvascularization. Consequently, the three increasingly complex phantoms we worked with were mainly used for intra-operator variability evaluations and their properties tend to mimic only some in vivo properties which might induce difficulties in transferring in vitro results to in vivo ones. Moreover, in vivo, variability measures were based on three injections. Having more injections to interpret might have provided us with a better statistical analysis: this last limitation was offset by the number of mice we worked on. Another limitation might concern the stability of the ultrasound contrast agent. Indeed, each experiment duration was less than 2 h on account of the stability of SonoVue® over time which is 6 h after its reconstitution as described by Schneider[11]. However, recent studies reported a significant incidence of spontaneous gas diffusion phenomena on temporal evolution of contrast microbubble size[40-42]. In the following study, gas diffusion phenomena occurring for 2 h from initial formation of contrast agent was neglected: this assumption might impact the results. Finally, as previously mentioned, the precision of the syringe was a source of variability and the absence of any measurements performed to accurately evaluate microbubble concentration at the injection time might be a limitation.
Several intra-operator variability studies using MRI, CT and PET have been described in the literature. These reported an overall intra-observer variability ranging from 3% to 30%[43-50]. The ranges of the semi-quantitative perfusion parameters we evaluated were consistent with those reported in the literature. Evelhoch et al[45] analyzed CV for the initial area under the gadolinium diethylenetriaminepentaacetate uptake vs time curve (IAUC) in 19 patients examined with two scans. The CV was found to be 18%. Wells et al[47] evaluated regional flow and the volume of tissue distribution of the contrast agent in tumor and normal tissue in 5 patients who underwent two PET-CT using inhaled C15O2 1 wk apart. They obtained CV values ranging from 9% to 14% (11% in the tumor) for the flow and from 3% to 13% (6% in the tumor) for the volume distribution. Myocardial perfusion values using CT imaging were determined by Groves et al[49] using two different approaches: the maximum-slope method and the peak method. CV values ranging from 12.6% to 23.7% for intra-observer agreement were observed. No published studies on intra-operator variability using DCE-US were found in the literature. Our results are concordant with these previous findings since the overall intra-operator variability of the semi-quantitative parameters observed both in vivo and in vitro ranged from 1.48% to 29.97%.
Anti-angiogenic treatments inhibit the formation of neoblood vessels in tumors obstructing their dissemination because of the lack of a blood supply. Such drugs exert activity primarily on the microvascularization: they may be effective even if no obvious change in tumor shape is observed after their administration. In order for functional imaging to be efficient in assessing such treatments, the variability of any semi-quantitative perfusion parameters should be lower than any reduction in perfusion. Thomas et al[51] found a decrease of 40% in AUC values, using DCE-MRI, at day 28 in 43 patients suffering from advanced cancers (24 colorectal, 1 breast, 2 mesothelioma, 6 neuroendocrine, 2 renal and 8 others) and who received single-agent PTK/ZK. Other studies demonstrated that reductions in perfusion following anti-angiogenesis treatments, using MRI, CT, PET or US, range from 30% to greater than 90%[26,52-55]. For example, Lavisse et al[26] studied the evolution of the peak intensity (PI), the TPI and the FWHM after the administration of AVE8062. They found that 6 h after the injection, the PI value represented 7% of the initial PI, TPI was three-fold higher than the initial TPI and FHWM was twice the initial FWHM.
In the light of these data, the CV values found in the current DCE-US study justified its use as an imaging method for assessing anti-angiogenic treatments.
In addition, in this study, the AUC and AUWO exhibited both in vitro and in vivo CV values below 15.79%. This is a major result considering the previous findings reported by Lassau et al[22,56,57] in different types of tumors such as GIST or RCC: among the seven semi-quantitative perfusion parameters evaluated, the two parameters which always correlated with the RECIST response and overall survival were the AUC and the AUWO. These two complementary observations highlight the reliability of such parameters in assessing anti-angiogenic treatments: AUC and AUWO are associated to a good correlation to RECIST response as well as to low intra-operator variability values. The TPI may also be considered a parameter of interest as it exhibited CV values in a range noticeably similar to AUC and AUWO: it ranged from 4.41% to 17.63%.
Dynamic T1-weighted MRI and CT are commonly used in assessing tumor angiogenesis as DCE-US suffers from some limitations. One of its major drawbacks is its operator dependence. In addition, CV values might depend on the organ as some can be more challenging to image such as the liver suffering from respiration artifacts. There is also a limited depth penetration making imaging of deepest regions difficult[50,58]. Finally, some anti-angiogenic treatments mainly involve change in tumor vasculature permeability without tumor flow. Consequently, as ultrasound contrast agents are purely intra-vascular, DCE-US can only provide tumor blood flow information: no information concerning tumor permeability can be determined[50].
On the other hand, further studies involving intra-operator variability might support DCE-US as the imaging method of choice for the early evaluation of anti-angiogenic drugs because it also has many additional specific advantages. Contrast agents used in DCE-MRI are extracellular ones and no linear relationship exists, whatever the dose between the measured signal and the concentration of the MRI contrast agents[50]. DCE-US involves working with purely intravascular contrast agents. In addition, within a certain range, the measured intensity exhibits a linear relationship with microbubble concentration making it possible to assess perfusion parameters. In spite of the advantage of a linear relationship between the change in CT intensity and the concentration of the contrast agent as well as a high spatial resolution, DCE-CT has a low sensitivity and high concentrations of contrast agent can be toxic[50]. PET and SPECT modalities are highly sensitive to very low tracer concentrations but exhibit a poor resolution[50] compared to that of DCE-US. In addition DCE-US has advantages linked to ultrasound imaging: it is non-invasive, easy to use, not expensive, rapid and widely available.
In this study, intra-operator variability measurements were based on semi-quantitative parameters, i.e. parameters extracted from the measured TIC within phantoms or tumors. Such parameters are often determined to estimate physiological parameters but do not take into account variations linked to physiological effects[31]. Blood flow and blood volume could be determined using methods that take into account patient hemodynamic conditions as well as the way the contrast agent is injected[31] which are elements included in the arterial input function. Consequently, further studies including the arterial input function will have to be performed to determine its influence on the DCE-US technique.
This work performed using in vitro and in vivo models demonstrated that among the seven semi-quantitative perfusion parameters, two (the AUC and the AUWO) linked to the blood volume and blood flow[9,10] exhibited an intra-operator variability value below 15.79% and could be the most reliable for early evaluation of anti-angiogenic treatments.
COMMENTS
Early functional imaging of anti-angiogenesis treatments in oncology is of major importance. Dynamic contrast-enhanced ultrasonography (DCE-US) is now commonly recognized as a functional imaging technique able to evaluate such therapies as it is a sensitive and highly available modality allowing early prediction of tumor response to treatments based on changes in vascularity before any morphological ones occur.
Microbubble contrast agents for DCE-US have developed during the past 10 years and are currently approved in Europe, Asia and Canada. Nowadays, ultrasound provides an ideal imaging modality for angiogenesis: it is widely available, not ionizing, low cost and provides real time imaging. However, until now, information on intra-operator variability in perfusion parameter measurements performed using DCE-US is lacking. In this study, the authors evaluated such variability and highlighted values similar to previous findings using other imaging modalities.
DCE-US is supported by the French National Cancer Institute which is currently studying the technique in several pathologies to establish the optimal perfusion parameters and timing for quantitative anticancer efficacy assessments: currently 400 patients with 1096 DCE-US demonstrated that the area under the curve quantified at 1 mo could be a robust parameter to predict response at 6 mo. The following study is the first one analyzing intra-operator variability in perfusion parameter measurements performed using DCE-US. Our study would suggest that DCE-US technique has comparable intra-operator variability than other functional imaging modalities that are currently used in routine.
By evaluating intra-operator variability in perfusion parameter measurements performed using DCE-US, this study may confirm the interest of dynamic contrast enhanced-ultrasonography as functional imaging in anti-angiogenic therapy evaluations.
DCE-US involves the use of microbubble contrast agents and specialized imaging techniques to evaluate blood flow and tissue perfusion. Intra-operator variability studies aim to determine the variation in measurements performed by a single operator and instrument on the same item and under the same conditions.
This is an interesting study and may draw the readers’ attention because the authors evaluated the feasibility and reproducibility of DCE-US through in vitro and in vivo. Moreover, I think that this study would guide the readers about the methods of basic research in the areas of radiology. Unfortunately, strength of reported findings is limited by some experimental constraints and manuscript presentation needs several improvements before publication.
Peer reviewers: Chan Kyo Kim, MD, Assistant Professor, Department of Radiology, Samsung Medical Center, Sungkyunkwan University School of Medicine, 50 Ilwon-dong, Kangnam-gu, Seoul 135-710, South Korea; Sergio Casciaro, PhD, Institute of Clinical Physiology - National Research Council,Campus Universitario Ecotekne, Via Monteroni, 73100 Lecce, Italy
S- Editor Cheng JX L- Editor O’Neill M E- Editor Zheng XM
| 1. | Lanza GM, Caruthers SD, Winter PM, Hughes MS, Schmieder AH, Hu G, Wickline SA. Angiogenesis imaging with vascular-constrained particles: the why and how. Eur J Nucl Med Mol Imaging. 2010;37 Suppl 1:S114-S126. |
| 2. | Eisenbrey JR, Forsberg F. Contrast-enhanced ultrasound for molecular imaging of angiogenesis. Eur J Nucl Med Mol Imaging. 2010;37 Suppl 1:S138-S146. |
| 3. | Kalva SP, Namasivayam S, Vasuedo Sahani D. Imaging Angiogenesis. Cancer Drug Discovery and Development: Antiangiogenic Agents in Cancer Therapy. Totowa, NJ: Humana Press 2008; 189-203. |
| 4. | Lassau N, Chami L, Péronneau P. Imagerie de contraste ultrasonore pour l’évaluation précoce des thérapeutiques ciblées. Echographie de contraste. Paris: Springer 2007; 81-86. |
| 5. | Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205-216. |
| 6. | Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S, Mooney M. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228-247. |
| 7. | Quaia E. Microbubble ultrasound contrast agents: an update. Eur Radiol. 2007;17:1995-2008. |
| 8. | Ignee A, Jedrejczyk M, Schuessler G, Jakubowski W, Dietrich CF. Quantitative contrast enhanced ultrasound of the liver for time intensity curves-Reliability and potential sources of errors. Eur J Radiol. 2010;73:153-158. |
| 9. | Claassen L, Seidel G, Algermissen C. Quantification of flow rates using harmonic grey-scale imaging and an ultrasound contrast agent: an in vitro and in vivo study. Ultrasound Med Biol. 2001;27:83-88. |
| 10. | Schwarz KQ, Bezante GP, Chen X, Mottley JG, Schlief R. Volumetric arterial flow quantification using echo contrast. An in vitro comparison of three ultrasonic intensity methods: radio frequency, video and Doppler. Ultrasound Med Biol. 1993;19:447-460. |
| 11. | Schneider M. Characteristics of SonoVuetrade mark. Echocardiography. 1999;16:743-746. |
| 12. | Greis C. Technology overview: SonoVue (Bracco, Milan). Eur Radiol. 2004;14 Suppl 8:P11-P15. |
| 13. | Quaia E, Calliada F, Bertolotto M, Rossi S, Garioni L, Rosa L, Pozzi-Mucelli R. Characterization of focal liver lesions with contrast-specific US modes and a sulfur hexafluoride-filled microbubble contrast agent: diagnostic performance and confidence. Radiology. 2004;232:420-430. |
| 14. | von Herbay A, Vogt C, Willers R, Häussinger D. Real-time imaging with the sonographic contrast agent SonoVue: differentiation between benign and malignant hepatic lesions. J Ultrasound Med. 2004;23:1557-1568. |
| 15. | Hamilton WF, Moore JW, Kinsman MJ, Spurling RG. Studies on the circulation. IV. Further analysis of the injection method, and of changes in hemodynamics under physiological and pathological conditions. Am J Physiol. 1932;99: 534-551. |
| 16. | Kinsman JM, Moore JW, Hamilton WF. Studies on the circulation. I. Injection method: physical and mathematical considerations. Am J Physiol. 1929;89:322-330. |
| 17. | Mischi M, Del Prete Z, Korsten HHM. Indicator dilution techniques in cardiovascular quantification. Biomechanical Systems Technology: Cardiovascular Systems. Singapore: World Scientific Publishing Company 2007; 89-156. |
| 18. | Li PC, Yang MJ. Transfer function analysis of ultrasonic time-intensity measurements. Ultrasound Med Biol. 2003;29:1493-1500. |
| 19. | Lampaskis M, Averkiou M. Investigation of the relationship of nonlinear backscattered ultrasound intensity with microbubble concentration at low MI. Ultrasound Med Biol. 2010;36:306-312. |
| 20. | Whittingham TA. Contrast-specific imaging techniques: technical perspective. Contrast media in ultrasonography: basic principles and clinical applications. Berlin, Heidelberg, New-York: Springer 2005; 43-70. |
| 21. | Lassau N, Koscielny S, Chebil M, Chami L, Bendjilali R, Roche . A, Escudier B, Le Cesne A, Soria J () Functional imaging using DCE-US: which parameters for the early evaluation of antiangiogenetic therapies? J Clin Oncol. 2009;27:3524. |
| 22. | Lassau N, Koscielny S, Albiges L, Chami L, Benatsou B, Chebil M, Roche A, Escudier BJ. Metastatic renal cell carcinoma treated with sunitinib: early evaluation of treatment response using dynamic contrast-enhanced ultrasonography. Clin Cancer Res. 2010;16:1216-1225. |
| 23. | Lassau N, Lacroix J, Aziza R, Vilgrain V, Taeb S, Koscielny S. French Multicentric Prospective Evaluation of Dynamic Contrast-enhanced Ultrasound (DCE-US) for the Evaluation of Antiangiogenic Treatments. Radiological Society of North America. 95th Scientific Assembly and Annual Meeting; 2009 No 29-Dec 4. . |
| 24. | Veltmann C, Lohmaier S, Schlosser T, Shai S, Ehlgen A, Pohl C, Becher H, Tiemann K. On the design of a capillary flow phantom for the evaluation of ultrasound contrast agents at very low flow velocities. Ultrasound Med Biol. 2002;28:625-634. |
| 25. | Meyer-Wiethe K, Cangür H, Seidel GU. Comparison of different mathematical models to analyze diminution kinetics of ultrasound contrast enhancement in a flow phantom. Ultrasound Med Biol. 2005;31:93-98. |
| 26. | Lavisse S, Lejeune P, Rouffiac V, Elie N, Bribes E, Demers B, Vrignaud P, Bissery MC, Brulé A, Koscielny S. Early quantitative evaluation of a tumor vasculature disruptive agent AVE8062 using dynamic contrast-enhanced ultrasonography. Invest Radiol. 2008;43:100-111. |
| 27. | Verbeek XA, Willigers JM, Prinzen FW, Peschar M, Ledoux LA, Hoeks AP. High-resolution functional imaging with ultrasound contrast agents based on RF processing in an in vivo kidney experiment. Ultrasound Med Biol. 2001;27:223-233. |
| 28. | Correas JM, Burns PN, Lai X, Qi X. Infusion versus bolus of an ultrasound contrast agent: in vivo dose-response measurements of BR1. Invest Radiol. 2000;35:72-79. |
| 29. | Gorce JM, Arditi M, Schneider M. Influence of bubble size distribution on the echogenicity of ultrasound contrast agents: a study of SonoVue. Invest Radiol. 2000;35:661-671. |
| 30. | Vicenzini E, Delfini R, Magri F, Puccinelli F, Altieri M, Santoro A, Giannoni MF, Bozzao L, Di Piero V, Lenzi GL. Semiquantitative human cerebral perfusion assessment with ultrasound in brain space-occupying lesions: preliminary data. J Ultrasound Med. 2008;27:685-692. |
| 31. | de Marco G, Dassonvalle P, M . C. H-F, Onen F, Idy-Peretti I. Cerebral perfusion: dynamic suceptibility contrast MR imaging. Part 2: vascular models and data extraction. Med Nucl. 2004;28:35-48. |
| 32. | Kwee JK, Mitidieri E, Affonso OR. Lowered superoxide dismutase in highly metastatic B16 melanoma cells. Cancer Lett. 1991;57:199-202. |
| 33. | Ohira T, Ohe Y, Heike Y, Podack ER, Olsen KJ, Nishio K, Nishio M, Miyahara Y, Funayama Y, Ogasawara H. In vitro and in vivo growth of B16F10 melanoma cells transfected with interleukin-4 cDNA and gene therapy with the transfectant. J Cancer Res Clin Oncol. 1994;120:631-635. |
| 34. | Yerlikaya A, Erin N. Differential sensitivity of breast cancer and melanoma cells to proteasome inhibitor Velcade. Int J Mol Med. 2008;22:817-823. |
| 35. | Gupta LC, Sahu UC. Diagnostic ultrasound. New Delhi: Jaypee Brothers Publishers 2007; . |
| 36. | Hedrick W, Hykes D, Starchman D. Ultrasound physics and instrumentation. 3rd ed. Saint-Louis, MO: Mosby 1995; . |
| 37. | Goldstein A, Powis RL. Medical ultrasonic diagnostics. Ultrasonic instruments and devices: reference for modern instrumentation, techniques, and technology. San Diego, CA: Academic Press 1999; 46-193. |
| 38. | Casciaro S, Demitri C, Conversano F, Casciaro E, Distante A. Experimental investigation and theoretical modelling of the nonlinear acoustical behaviour of a liver tissue and comparison with a tissue mimicking hydrogel. J Mater Sci Mater Med. 2008;19:899-906. |
| 39. | Casciaro S, Conversano F, Musio S, Casciaro E, Demitri C, Sannino A. Full experimental modelling of a liver tissue mimicking phantom for medical ultrasound studies employing different hydrogels. J Mater Sci Mater Med. 2009;20:983-989. |
| 40. | Sarkar K, Katiyar A, Jain P. Growth and dissolution of an encapsulated contrast microbubble: effects of encapsulation permeability. Ultrasound Med Biol. 2009;35:1385-1396. |
| 41. | Kwan JJ, Borden MA. Microbubble dissolution in a multigas environment. Langmuir. 2010;26:6542-6548. |
| 42. | Casciaro S, Errico RP, Conversano F, Demitri C, Distante A. Experimental investigations of nonlinearities and destruction mechanisms of an experimental phospholipid-based ultrasound contrast agent. Invest Radiol. 2007;42:95-104. |
| 43. | Ng CS, Raunig DL, Jackson EF, Ashton EA, Kelcz F, Kim KB, Kurzrock R, McShane TM. Reproducibility of perfusion parameters in dynamic contrast-enhanced MRI of lung and liver tumors: effect on estimates of patient sample size in clinical trials and on individual patient responses. AJR Am J Roentgenol. 2010;194:W134-W140. |
| 44. | Marcus CD, Ladam-Marcus V, Cucu C, Bouché O, Lucas L, Hoeffel C. Imaging techniques to evaluate the response to treatment in oncology: current standards and perspectives. Crit Rev Oncol Hematol. 2009;72:217-238. |
| 45. | Evelhoch JL, LoRusso PM, He Z, DelProposto Z, Polin L, Corbett TH, Langmuir P, Wheeler C, Stone A, Leadbetter J. Magnetic resonance imaging measurements of the response of murine and human tumors to the vascular-targeting agent ZD6126. Clin Cancer Res. 2004;10:3650-3657. |
| 46. | Morgan B, Utting JF, Higginson A, Thomas AL, Steward WP, Horsfield MA. A simple, reproducible method for monitoring the treatment of tumours using dynamic contrast-enhanced MR imaging. Br J Cancer. 2006;94:1420-1427. |
| 47. | Wells P, Jones T, Price P. Assessment of inter- and intrapatient variability in C15O2 positron emission tomography measurements of blood flow in patients with intra-abdominal cancers. Clin Cancer Res. 2003;9:6350-6356. |
| 48. | Cenic A, Nabavi DG, Craen RA, Gelb AW, Lee TY. Dynamic CT measurement of cerebral blood flow: a validation study. AJNR Am J Neuroradiol. 1999;20:63-73. |
| 49. | Groves AM, Goh V, Rajasekharan S, Kayani I, Endozo R, Dickson JC, Menezes LJ, Shastry M, Habib SB, Ell PJ. CT coronary angiography: quantitative assessment of myocardial perfusion using test bolus data-initial experience. Eur Radiol. 2008;18:2155-2163. |
| 50. | Miller JC, Pien HH, Sahani D, Sorensen AG, Thrall JH. Imaging angiogenesis: applications and potential for drug development. J Natl Cancer Inst. 2005;97:172-187. |
| 51. | Thomas AL, Morgan B, Horsfield MA, Higginson A, Kay A, Lee L, Masson E, Puccio-Pick M, Laurent D, Steward WP. Phase I study of the safety, tolerability, pharmacokinetics, and pharmacodynamics of PTK787/ZK 222584 administered twice daily in patients with advanced cancer. J Clin Oncol. 2005;23:4162-4171. |
| 52. | Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10:145-147. |
| 53. | Meijerink MR, van Cruijsen H, Hoekman K, Kater M, van Schaik C, van Waesberghe JH, Giaccone G, Manoliu RA. The use of perfusion CT for the evaluation of therapy combining AZD2171 with gefitinib in cancer patients. Eur Radiol. 2007;17:1700-1713. |
| 54. | Lassau N, Lamuraglia M, Vanel D, Le Cesne A, Chami L, Jaziri S, Terrier P, Roche A, Leclere J, Bonvalot S. Doppler US with perfusion software and contrast medium injection in the early evaluation of isolated limb perfusion of limb sarcomas: prospective study of 49 cases. Ann Oncol. 2005;16:1054-1060. |
| 55. | Miles KA, Griffiths MR. Perfusion CT: a worthwhile enhancement? Br J Radiol. 2003;76:220-231. |
| 56. | Lassau N, Chebil M, Koscielny S, Chami L, Bendjilali R, Roche A. Dynamic Contrast-enhanced Ultrasonography (DCE-US) with Quantification for the Early Evaluation of HCC Treated by Bevacizumab: Which Parameters? Radiological Society of North America. 95th Scientific Assembly and Annual Meeting; 2009 Nov 29-Dec 4. . |
| 57. | Lassau N, Chebil M, Chami L, Bidault S, Girard E, Roche A. Dynamic contrast-enhanced ultrasonography (DCE-US): a new tool for the early evaluation of antiangiogenic treatment. Target Oncol. 2010;5:53-58. |
| 58. | Quaia E, D'Onofrio M, Palumbo A, Rossi S, Bruni S, Cova M. Comparison of contrast-enhanced ultrasonography versus baseline ultrasound and contrast-enhanced computed tomography in metastatic disease of the liver: diagnostic performance and confidence. Eur Radiol. 2006;16:1599-1609. |