Revised: December 28, 2010
Accepted: January 4, 2011
Published online: February 28, 2011
AIM: To evaluate the effects of using CO2 as negative contrast agent in decreasing the overlapping on the pancreaticobiliary system from intestinal fluids.
METHODS: We evaluated the magnetic resonance cholangiopancreatography (MRCP) images in 117 patients divided into two groups (group 1, without taking gas producing crystals to produce CO2, n = 64; group 2, with CO2, n = 53) in a 1.5T unit using MRCP sequence. Anatomic locations of intestinal fluids distribution, overlapping with common bile duct (CBD) and pancreatic duct (PD), were evaluated.
RESULTS: In the group with CO2, the decrease in distribution of intestinal fluids was significant in the gastric antrum (P = 0.001) and duodenal bulb (P < 0.001), but not in the gastric fundus and body and in the second portion of the duodenum (P = 1.000, P = 0.171, and P = 0.584 respectively). In the group with CO2, the decrease in overlapping with CBD was significant (P < 0.001), but the decrease in overlapping with PD was not (P = 0.106).
CONCLUSION: MRCP with carbon dioxide as negative contrast agent would decrease intestinal fluids in the gastric antrum and duodenal bulb, thereby decreasing overlapping with the CBD.
- Citation: Chen CW, Liu YS, Chen CY, Tsai HM, Chen SC, Chuang MT. Use of carbon dioxide as negative contrast agent for magnetic resonance cholangiopancreatography. World J Radiol 2011; 3(2): 47-50
- URL: https://www.wjgnet.com/1949-8470/full/v3/i2/47.htm
- DOI: https://dx.doi.org/10.4329/wjr.v3.i2.47
Magnetic resonance cholangiopancreatography (MRCP) is a safe and noninvasive technique used to evaluate the pancreaticobiliary system[1-3]. With the use of thin-section single-shot fast-spin echo sequences and thick-section heavily T2-weighted sequences, MRCP can demonstrate the anatomy of the biliary tract, pancreatic duct (PD) and gallbladder, since the fluid within them serves as an intrinsic contrast medium[4]. However, high signal intensity from intestinal fluids would decrease the quality of MRCP images due to superimposition with the biliary tract[4-6]. Therefore, how to decrease the high signal intensity from intestinal fluids has always been problematic for radiologists.
Previous researchers have used oral negative contrast agents (including blueberry and pineapple juices) to suppress the high signal of the gastrointestinal tract[4,7-9]. Although blueberry juice has been shown to be a well-tolerated and effective oral contrast agent during MRCP, pure blueberry juice is not widely available. Pineapple juice is another negative contrast medium. However, the patient has to take the 400 mL of pineapple juice 15 to 30 min before MRCP is performed, thereby limiting its clinical usage. A potential risk of loss of signal intensity of the common bile duct (CBD) caused by reflux of oral negative contrast medium agent in patients with a history of endoscopic sphincterotomy has also been reported[10].
Based on the experience of upper gastrointestinal series, we understand that CO2 accumulates in the gastric antrum after gas-producing crystals are taken. This phenomenon will cause intestinal fluids to be retained in the gastric fundus and therefore decrease the overlapping with the CBD. There are also several advantages to using CO2 as the negative contrast medium: it is widely available, inexpensive and easily prepared. To our knowledge, this is the first study using CO2 as negative contrast agent for MRCP in the literature.
The purpose of our study was to evaluate the effects of using CO2 as a negative contrast medium in decreasing the overlapping of intestinal fluids on the pancreaticobiliary system.
From October 2007 to September 2008, a total of 117 consecutive patients (70 men, 47 women; age range, 20-82 years; mean age, 58 years), who met the inclusion criteria, were enrolled in our study. Patients were included if they were referred for evaluation of biliary tract problems. We excluded patients if they had had cholecystectomy or gastric surgery, or were unable to take gas-producing crystals. The Institutional Review Board of our hospital approved the study. Written informed consent was obtained from each patient after the purpose and protocol of the study had been fully explained.
The patients were randomly assigned to two groups: those who took and those who did not take gas-producing crystals, i.e. with CO2 and without CO2. Patients in the first group received 6 g of gas-producing crystals with 10 mL water orally, shortly before the MRCP examination. If there was insufficient air distension of the stomach, 3 additional grams of gas-producing crystals were given. For those who did not take gas-producing crystals, the MRCP examination was performed directly without any oral contrast.
All patients underwent MRCP with a 1.5-T MR scanner (Achieva; Philips Medical Systems, The Netherlands) using a four-element quadrature phased-array surface coil. The MRCP was performed as per the following parameters: single shot MRCP radial sequence: repetition time (ms)/echo time (ms), 8000/800; flip angle, 90°; field of view, 350 mm; matrix, 320 × 255; echo spacing, 4.2 ms; 6 radial sections of 40 mm thickness obtained at 12 degrees of rotation.
The MRCP images were reviewed by two gastrointestinal radiologists (HMT and MTC with 10 and 6 years of MRCP interpretation experience, respectively), who were blinded to the patients’ group allocation, and who had to reach a consensus. First, the reviewers were asked to identify the presence of intestinal fluids in different anatomic locations of the stomach (fundus, body and antrum) and duodenum (bulb and 2nd portion). Then, they were asked to identify the anatomic structures of intestinal fluids overlapping the CBD. Third, the reviewers were asked to identify the anatomic structures of intestinal fluids which overlapped the PD.
Differences between the two groups of data were assessed with χ2 test. The analyses were performed by using SPSS software (SPSS for Windows, version 11.0, 2001; SPSS, Chicago, Ill). A P value of less than 0.05 was considered to indicate a statistically significant difference for all analyses.
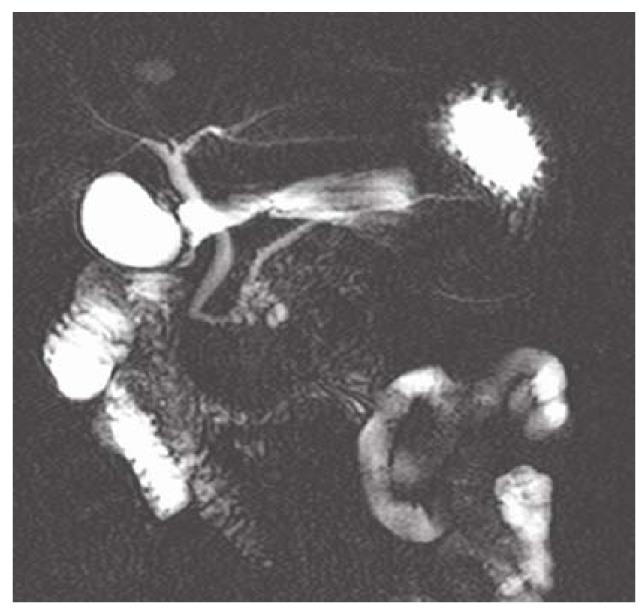
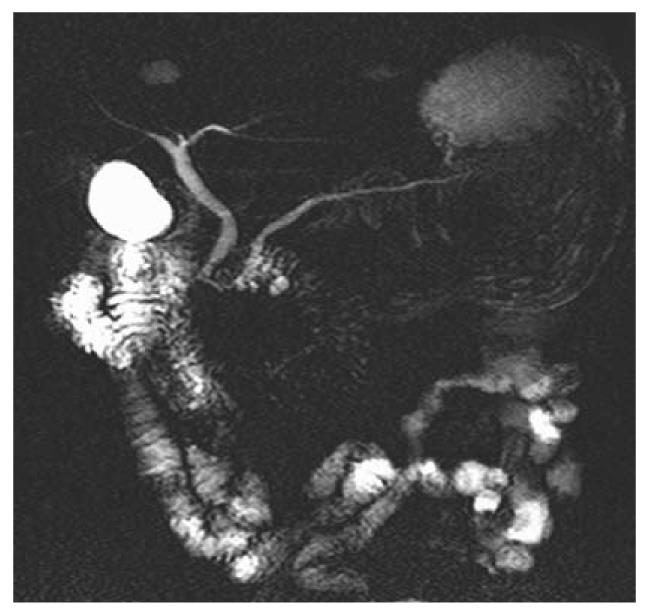
Among the 117 subjects, 53 persons took gas-producing crystals orally as negative contrast agent while 64 persons did not. Figure 1 shows hyperintense intestinal fluids in the gastric antrum and duodenal bulb overlapping the CBD. Figure 2 shows lack of overlap due to decreased hyperintense intestinal fluids in the gastric antrum and duodenal bulb. Table 1 summarizes the results of intestinal fluid distribution in different anatomic locations of the stomach and the duodenum in the two groups, i.e. with and without CO2 as negative contrast agent. In the group with CO2, the MRCP images showed significant improvement in decreased distribution of intestinal fluid in gastric antrum (P = 0.001) and duodenal bulb (P < 0.001). Nevertheless, there were no significant differences in the distribution of intestinal fluid in gastric fundus (P = 1.000), gastric body (P = 0.171) and 2nd portion of duodenum (P = 0.584) between the two groups. Table 2 shows how the overlap of the intestinal fluid on the CBD was significantly decreased in the group with CO2 vs. that without CO2 (P < 0.001). On the other hand, there were no significant differences in the degree of overlap on the PD between the two groups (P = 0.106) (Table 2).
| Anatomic locations with intestinal fluids distribution | Patients | P-value | |
| Without CO2 (n = 64) | With CO2 (n = 53) | ||
| Gastric fundus | 62 (97) | 52 (98) | 1.000 |
| Gastric body | 25 (39) | 14 (26) | 0.171 |
| Gastric antrum | 24 (38) | 6 (11) | 0.001 |
| Duodenal bulb | 53 (83) | 22 (42) | < 0.001 |
| Duodenal 2nd portion | 57 (89) | 45 (85) | 0.584 |
| Overlapping structures | Patients | P-value | |
| Without CO2 (n = 64) | With CO2 (n = 53) | ||
| Common bile duct | 52 (81) | 21 (40) | < 0.001 |
| Pancreatic duct | 12 (19) | 4 (8) | 0.106 |
MRCP is a safe and noninvasive technique used to evaluate the pancreaticobiliary system[1-3,11]. It allows obtaining images of the pancreaticobiliary similar to the endoscopic retrograde pancreatography (ERCP) without the morbidity associated with the complications resulting from diagnostic ERCP[12-14]. Although MRCP has been shown to be accurate in the diagnosis of choledocholithiasis, malignant biliary obstruction and other congenital biliary tract anomalies, awareness of several potential pitfalls is crucial to avoid inappropriate interpretation[6]. High signal intensity from the intestinal fluids superimposed on the biliary tract is one of the pitfalls that interfere with the interpretation (Figure 1).
Previous researchers have used oral negative contrast agents (including blueberry and pineapple juices) to suppress the high signal of the gastrointestinal tract[4,7-9]. However, these negative contrast agents are not appropriate for routine use. Blueberry juice, although well-tolerated and effective, as oral contrast agent for MRCP, is not widely available. Pineapple juice, another negative contrast medium, is inconvenient to administer: patients have to drink 400 mL of pineapple juice 15 to 30 min before MRCP, thereby limiting its clinical usage. A potential risk of loss of signal intensity of the CBD caused by reflux of oral negative contrast medium agent in patients with a history of endoscopic sphincterotomy has also been reported[10].
All these disadvantages led us to look for a more suitable contrast agent for MRCP that might be widely available and timesaving.
In this study, we used carbon dioxide (CO2) as negative contrast medium produced by administering gas-producing crystals orally. There are several advantages using CO2 as a negative contrast medium: it is widely available, inexpensive and easily prepared. After taking oral gas-producing crystals, the CO2 will accumulate in the gastric antrum and duodenal bulb, because these areas are located upward during the supine position. The gastric antrum and duodenal bulb are also, anatomically, the nearest areas to the CBD and the PD. Therefore, the negative contrast medium effects caused by CO2 in these areas will not influence the high signal intensity at the level of the CBD and the PD during the MRCP, thereby improving the MRCP diagnostic value.
Our results (Table 1) showed significant improvement in the distribution of intestinal fluid in gastric antrum (P = 0.001) and duodenal bulb (P < 0.001). This subsequently decreased the degree of overlap of the intestinal fluids on the CBD (P < 0.001) (Table 2 and Figure 2), hence increasing the diagnostic value. However, our results failed to show a significant difference in the degree of overlap of the intestinal fluids on the PD between the two groups of patients, i.e. those taking vs. those non taking gas-producing crystals (P = 0.106). The overlap on the PD arises from the high signal intensity of the 3rd and 4th portions of the duodenum. However, the high signal source cannot be eliminated after production of CO2 by gas-producing crystals. A possible maneuver to decrease the overlap is the right hemi-decubitus position after taking oral gas-producing crystals. This position would decrease the high signal intensity of the 3rd and 4th portions of the duodenum, thereby decrease the overlap with the PD.
Our study has limitations. Firstly, although gas-producing crystals can produce carbon dioxide in the stomach, there were still 22 (41.5%) subjects in our study whose MRCP images were characterized by an insufficient amount of air. The possible reason was the fact of having taken just one pack of gas-producing crystals. This would have been improved simply by taking another pack. However, some patients might feel uncomfortable about the smell of the gas-producing crystals and hesitate to take another pack. Secondly, some patients took the gas-producing crystals too slowly, which may negatively influence the desired effects. This problem may be overcome by assuming the crystals faster. However, the physician should be aware of possible complications, such as choking. For those patients with nasogastric tube placement, the oral gas-producing crystal method could be replaced by manually injected air from the nasogastric tube. Thirdly, some patients had hiccups after they took the crystals. This would undoubtedly influence the results of the MRCP. Lastly, the CO2 might enter the papilla of Vater in patients with incompetent papilla. This would subsequently lead to pneumobilia which may mimic choledocholithiasis[6].
In conclusion, MRCP with carbon dioxide as negative contrast medium may decrease intestinal fluids in gastric antrum and duodenum, thereby decreasing their overlapping with the CBD.
MR cholangiopancreatography (MRCP) is a useful and noninvasive method to evaluate the normal anatomy and various pathologies of the pancreaticobiliary system. However, bile within the common bile duct (CBD) serving as an intrinsic bright contrast medium could be superimposed by hyperintensity from intestinal fluids on MRCP.
Several oral negative contrast agents (such as blueberry and pineapple juices) have been used to decrease the hyperintensity from intestinal fluids.
Carbon dioxide, a widely available, inexpensive and easily prepared contrast agent, used as a negative contrast agent during MRCP would accumulate in the gastric antrum, displace the intestinal fluids into the gastric fundus and therefore decrease the overlap with the CBD. This would greatly improve the diagnostic value of MRCP.
Administration of carbon dioxide as a negative contrast agent for MRCP is a practical method to decrease the overlap of intestinal fluids on the CBD.
MRCP is a noninvasive method by using thin-section single-shot fast-spin-echo sequence to enhance the bile juice and help clinician understand the normal anatomy and various pathologies of the pancreaticobiliary system. Cholodocholithiasis within the pancreaticobiliary system would appear as hypointense filling defects on MRCP.
The manuscript addressed a novel procedure to improve the visualization of CBD or pancreatic duct during MRCP.
| 1. | Soto JA, Barish MA, Yucel EK, Siegenberg D, Ferrucci JT, Chuttani R. Magnetic resonance cholangiography: comparison with endoscopic retrograde cholangiopancreatography. Gastroenterology. 1996;110:589-597. |
| 2. | Fulcher AS, Turner MA, Capps GW, Zfass AM, Baker KM. Half-Fourier RARE MR cholangiopancreatography: experience in 300 subjects. Radiology. 1998;207:21-32. |
| 3. | Chan YL, Chan AC, Lam WW, Lee DW, Chung SS, Sung JJ, Cheung HS, Li AK, Metreweli C. Choledocholithiasis: comparison of MR cholangiography and endoscopic retrograde cholangiography. Radiology. 1996;200:85-89. |
| 4. | Riordan RD, Khonsari M, Jeffries J, Maskell GF, Cook PG. Pineapple juice as a negative oral contrast agent in magnetic resonance cholangiopancreatography: a preliminary evaluation. Br J Radiol. 2004;77:991-999. |
| 5. | Reinhold C, Bret PM. Current status of MR cholangiopancreatography. AJR Am J Roentgenol. 1996;166:1285-1295. |
| 6. | Irie H, Honda H, Kuroiwa T, Yoshimitsu K, Aibe H, Shinozaki K, Masuda K. Pitfalls in MR cholangiopancreatographic interpretation. Radiographics. 2001;21:23-37. |
| 7. | Hiraishi K, Narabayashi I, Fujita O, Yamamoto K, Sagami A, Hisada Y, Saika Y, Adachi I, Hasegawa H. Blueberry juice: preliminary evaluation as an oral contrast agent in gastrointestinal MR imaging. Radiology. 1995;194:119-123. |
| 8. | Papanikolaou N, Karantanas A, Maris T, Gourtsoyiannis N. MR cholangiopancreatography before and after oral blueberry juice administration. J Comput Assist Tomogr. 2000;24:229-234. |
| 9. | Hirohashi S, Hirohashi R, Uchida H, Kitano S, Ono W, Ohishi H, Nakanishi S. MR cholangiopancreatography and MR urography: improved enhancement with a negative oral contrast agent. Radiology. 1997;203:281-285. |
| 10. | Sugita R, Nomiya T. Disappearance of the common bile duct signal caused by oral negative contrast agent on MR cholangiopancreatography. J Comput Assist Tomogr. 2002;26:448-450. |
| 11. | Hekimoglu K, Ustundag Y, Dusak A, Erdem Z, Karademir B, Aydemir S, Gundogdu S. MRCP vs. ERCP in the evaluation of biliary pathologies: review of current literature. J Dig Dis. 2008;9:162-169. |
| 12. | Ong TZ, Khor JL, Selamat DS, Yeoh KG, Ho KY. Complications of endoscopic retrograde cholangiography in the post-MRCP era: a tertiary center experience. World J Gastroenterol. 2005;11:5209-5212. |
| 13. | Koito K, Namieno T, Ichimura T, Yama N, Hareyama M, Morita K, Nishi M. Mucin-producing pancreatic tumors: comparison of MR cholangiopancreatography with endoscopic retrograde cholangiopancreatography. Radiology. 1998;208:231-237. |
| 14. | Calvo MM, Bujanda L, Calderón A, Heras I, Cabriada JL, Bernal A, Orive V, Astigarraga E. Comparison between magnetic resonance cholangiopancreatography and ERCP for evaluation of the pancreatic duct. Am J Gastroenterol. 2002;97:347-353. |
Peer reviewer: Ahmed Mahmoud El-Tawil, MSc, MRCS, PhD, Department of Surgery, University Hospital of Birmingham, East Corridor, Ground Floor, Birmingham, B15 2TH, United Kingdom
S- Editor Cheng JX L- Editor Negro F E- Editor Zheng XM