INTRODUCTION
For non-surgical anticancer strategies such as conventional radiotherapy and chemotherapy, the main disadvantage is lacking specificity for cancer tissue, i.e. concomitant cytotoxic effects on normal tissues. In order to find more selective treatments, researchers have made efforts to exploit morphological, physiological and microenvironmental differences between normal and malignant tissues, including microvasculature, oxygenation and necrosis. One of the most prominent differences lies in the tumor neovasculature[1].
Tumor vasculature is a crucial component of pathophysiology in solid tumors, which affects growth, metastasis and therefore, response to therapy. Compared with the normal vasculature, tumor vessels are less mature in structure and leakier, where blood flow is spatially and temporally heterogeneous and often compromised. Furthermore, hyperpermeability of the vascular wall and lack of functional lymphatics within tumors elevate interstitial fluid pressure in solid tumors[2,3]. The molecular mechanisms of abnormal tumor vasculature may result from the imbalance between pro- and antiangiogenic regulating factors in tumor as well as host stromal cells[4]. Such vascular characteristics of solid tumors are sufficiently different from those of normal tissues and thus provide a unique target for tumor treatment[1].
Drugs developed for vascular targeting therapies can be divided into two different groups: antiangiogenic agents for inhibiting the formation of new vessels and vascular disrupting agents (VDAs) for destroying the existing vessels[5]. Hallmark characteristics with VDAs are selective reduction in tumor blood flow, induction of ischemic tumor necrosis, presence of viable neoplastic cells at the tumor periphery, and effect on delaying tumor growth[6]. According to their action mechanisms, VDAs can be further categorized into ligand-directed VDAs and small molecule VDAs. Small molecule VDAs include flavonoids such as 5,6-dimethylxanthenone-4-acetic acid (DMXAA/ASA404), and tubulin-destabilizing agents[7]. As a tubulin-destabilizing VDA, cis-1-(3,4,5,-trimethoxyphenyl)-2-(4’-methoxyphenyl)ethene-3’-0-phosphate or combretastatin A-4-phosphate (CA4P/Oxi2021) is most representative, and has been under phase III clinical trials.
Unlike other conventional chemotherapies, VDAs are cytostatic rather than cytotoxic to malignant cells. They starve and indirectly kill tumor cells by depleting their blood supply, and can only delay tumor growth but not eradicate the tumor. Given this novel action mechanism, imaging biomarkers have been elaborated to detect and quantify non-invasively VDA-induced morphological, functional and metabolic alterations. Relative to the conventional clinical endpoints such as mortality and morbidity, these imaging biomarkers work in a more prompt, predictable and precise way[8,9]. Hereby, the term biomarker is adopted more broadly than its traditional definition, i.e. a biomarker can be derived not only from biofluid samples with the techniques of biochemistry and molecular biology, but also from modern imaging metrics including magnetic resonance imaging (MRI), computed tomography (CT), positron emission tomography (PET) or single photon emission tomography (SPECT), ultrasound, and optical imaging[10]. In this article, we review the action mechanisms of tubulin-destabilizing VDAs and the preclinical and clinical results of two lead VDAs, CA4P and ZD6126 (N-acetylcolchinol-O-phosphate), with the emphasis on the role of MRI in the preclinical evaluation of VDA effects.
VDAs
Pathophysiological features of tumor vessels as targets of VDAs
Oxygen diffusion distance from capillaries is only 150-200 μm. Because of the unrestrained growth, tumor cells growing outwith this effective diffusion distance become hypoxic and eventually necrotic[11-13]. Therefore, a tumor has to develop its own vessels to maintain its growth, i.e. angiogenesis, when its diameter exceeds about 0.5 mm[14].
These newly developed tumor vessels are often immature: the endothelial cells are irregular-shaped with larger interendothelial conjunctions[15,16] and poor connections between the endothelial lining and irregular basement membrane[17,18]. Due to these characteristics, tumor vessels are hyperpermeable and interstitial fluid pressure is higher than in normal tissues. Such high pressure is also contributed by the inefficient drainage with dysfunctional tumor lymphatics, which can be caused by rapid proliferation of tumor cells in a confined space, which creates mechanical stress that compresses intratumor lymphatics[19,20]. Besides, malignant tumors are known to feature with lymphatic deficiency or retarded development of lymphatics[19,20]. Tumor vessels are tortuous, disorganized and non-hierarchical, with complex branching of heterogeneous length and diameters, leading to high resistance to perfusion[21]. Under such conditions, any slight fluctuation of blood perfusion may cause catastrophic events in tumor vessels, while it has little effect on normal tissue, because mature vessels are more robust against perfusion changes due to efficient regulating mechanisms[21].
Role of cytoskeleton in the regulation of endothelial barrier function
The endothelial barrier keeps the blood cells from exposure to surrounding tissues. Endothelial cells (ECs) line the inner surface of blood vessels and rely on their cytoskeleton to maintain the structural integrity of confluent monolayer and flat shape. Dysfunction in cellular shape can cause subsequent vascular hyperpermeability[22]. The cytoskeleton consists of three distinct components: microtubules, actin microfilaments and intermediate filaments[23], and the former two are associated via linking proteins, which, in turn, interact with these two cytoskeletal components for signaling[24]. As the scaffolding of the cell, the cytoskeleton plays a vital role in cell motility, division, shape maintenance, and signal transduction[24]. In tumor vessels, actin is ill-developed and thus the maintenance of cell shape depends more on microtubules[25,26].
The delicate dynamic balance between the centripetal tension and centrifugal force to ensure the cellular shape is finely modified by cytoskeleton and intercellular junctional complexes of membrane-binding proteins that provide intercellular adherence, which is regulated by several signaling pathways[22]. Reorganization of actin leads to the assembly of bundled stress fibers, and therefore, increased cellular contractility. The main constituent of intercellular junctions is vascular endothelial (VE) cadherin/β-catenin complex anchored to actin[22]. Disruption of the VE-cadherin/β-catenin pathway causes the loss of intercellular junctional organization, dysfunction of monolayer barrier, and eventual rounding up of ECs[27].
Mechanisms of VDA action
The mechanisms of action with VDAs still need to be fully elucidated. It has been speculated that CA4P binds to tubulin of microtubule at or close to the colchicine-binding site[28]. Unlike the antitumor effect with colchicine that is only achievable at a dose close to the maximum tolerated dose (MTD), the effect with VDAs is observed within a wide therapeutic window lower than the MTD. Their ability to selectively target the cytoskeleton and compromise the endothelial intercellular junctions is vital to their mechanisms of action[7]. CA4P has been most extensively studied. Therefore, we take CA4P as an example to discuss the potential molecular and cellular mechanisms of action, which are likely to be applicable to other tubulin-binding VDAs such as ZD6126.
On a long-term basis, CA4P inhibits the microtubule dynamics, interferes with the mitotic spindle function and leads to cell cycle arrest, which results in proliferation blockage and/or apoptosis[29]. Although such a direct cytotoxic or antiproliferative effect may contribute to the antivascular effects of CA4P, it would be too slow to account for the rapid vascular shutdown observed in vivo, which can occur within minutes after CA4P treatment in animal models[30]. Rather, immediate morphological and functional changes are more likely to be involved in such vascular collapse.
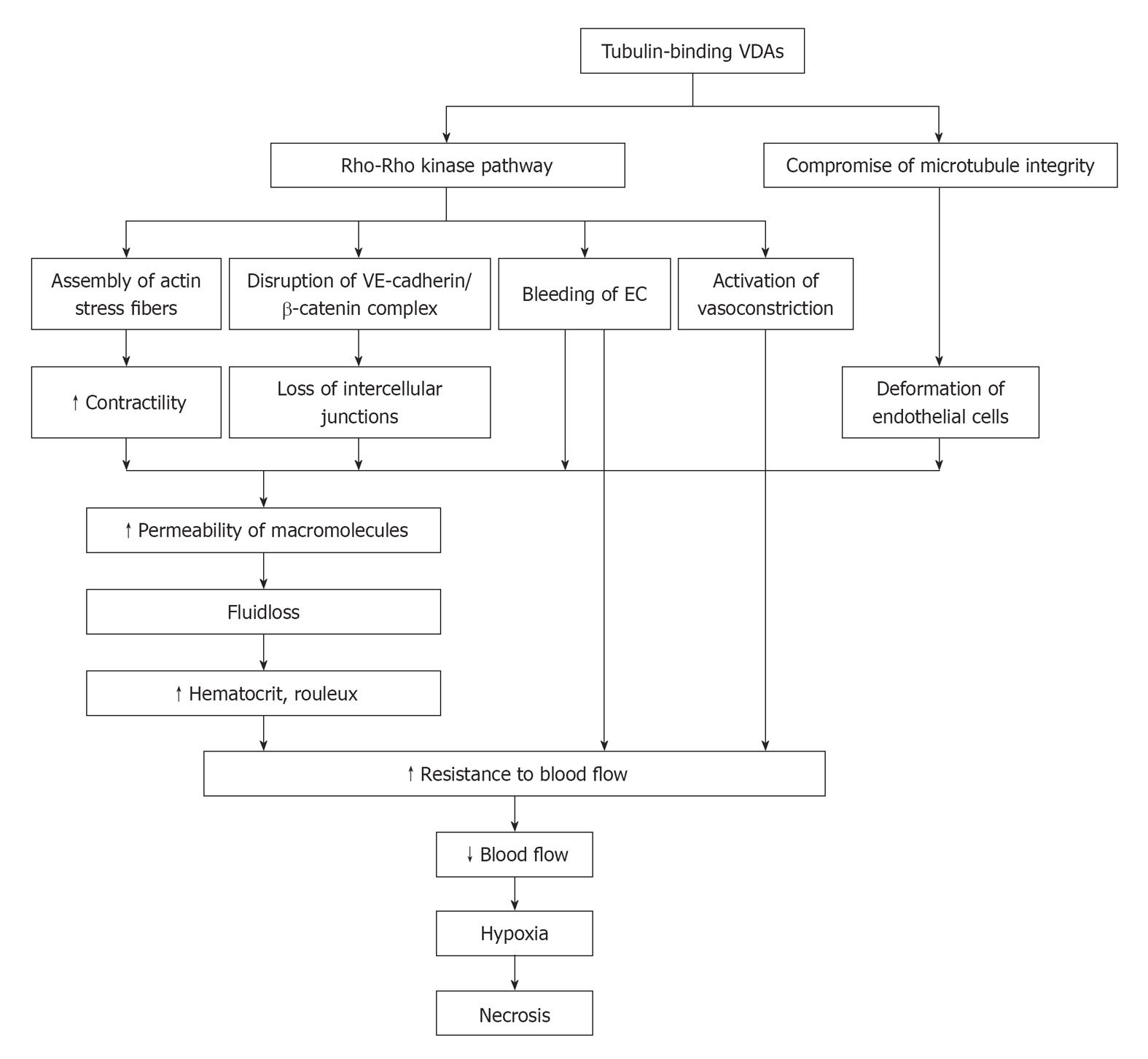
In vitro, it has been shown that Rho-GTPase plays an important role in the capillary-like collapse (Figure 1). Belonging to signaling G protein (GTPase), Rho proteins (Ras homologous proteins) are interconnected with microtubules[31]. The members of the Rho-GTPase family are essential in converting and amplifying external signals into cellular effects, including regulation of actin dynamics and cadherin/β-catenin pathway[32,33].
Figure 1 Schematic mechanisms of action with tubulin-binding vascular disrupting agents.
VE: Vascular endothelial; VDAs: Vascular disrupting agents; EC: Endothelial cell.
CA4P selectively binds to microtubules and depolymerizes tubulin, which results in the activation of Rho-GTPase and its associated Rho kinase[34-36] (Figure 1). Activation of the Rho/Rho-kinase pathway may cause downstream morphological and/or functional changes in ECs, which can lead to dysmorphism and hyperpermeability: (1) assembly of actin stress fibers and fortified contractility of ECs[24]; (2) disruption of the VE-cadherin/β-catenin complex to induce the loss of intercellular adhesion and the appearance of paracellular gaps[22]; (3) blebbing of ECs with regulation of stress-activated protein kinase p38 (SAPK-2/p38) to bring about increased monolayer permeability and resistance to blood flow[36,37]; and (4) vasoconstriction to give rise to increased geometric resistance to blood flow[38]. In addition, the direct binding of CA4P to tubulin compromises the integrity of cytoskeleton, and morphological changes of endothelial monolayer architecture further deteriorates[7,39] (Figure 1).
With the increased vascular permeability, the consequent leakage of plasma macromolecule into extravascular extracellular space (EES) results in fluid loss, increased hematocrit and formation of rouleaux[40]. As a result, the resistance to blood flow is increased. After EC damage, direct exposure of basement membrane to flowing blood initiates coagulation and hemorrhage[40]. Accordingly, the drop in blood flow induces hypoxia and deprivation of nutrients and subsequent necrosis of tumor (Figure 1).
In vivo, the increase in permeability may be the key event responsible for the VDA-induced vascular collapse[41]. Although the primary effects of CA4P have been confirmed in vivo, including morphological changes in ECs, such as blebbing[42] and increased permeability and vasoconstriction in arterioles[38], direct evidence of mechanisms via the activation of Rho/Rho-kinase pathway are still sparse. However, the CA4P-induced vascular shutdown effect is attenuated in combination with Rho or Rho kinase inhibitors[7,36,43], while amplified in combination with an anti-VE-cadherin agent[44], which may be considered indirect proof of the link between the cytoskeletal remodeling and permeability.
Dose of VDAs
Some VDAs are orally active, e.g. ABT-751[39] and CYT997[45], while intraperitoneal (ip) and intravenous (iv) administrations are most frequently applied in the treatment of tumors in rodent models. The ip injection is convenient for the handing of rodents, while it fails to mimic the clinical practice where iv injection is applied. Successful iv injection ensures an effective dose of VDA in the systemic circulation.
For single doses of CA4P, the MTD is estimated to be around 68 mg/m2 in patients[46], which gives the clinically relevant dose of about 10 mg/kg in rats[47,48]. In mice, the roughly estimated MTD is 1000-1500 mg/kg[49]. However, the lowest effective dose is 25 mg/kg, which is already higher than the MTD in humans. Therefore, the CA4P effect with higher doses in mice is difficult to translate into humans[50].
For single doses of ZD6126, the MTD in patients is about 112 mg/m2, which gives the clinically relevant dose of about 10 mg/kg in rats[51]. In mice, the MTD is about 750 mg/kg[52].
The tumor response to various VDAs depends mainly on drug type, tumor model and dosing regimen in preclinical studies. Generally speaking, the higher dose of VDAs can induce more striking antivascular effect, while the results cannot be convincingly translated into clinical practice if the dose for animal models exceeds the MTD in patients. Therefore, the results with clinically relevant doses in tumor models may better predict the outcomes in patients.
In vivo effect
After VDA treatment, a rapid increase in tumor vascular permeability triggers the catastrophic cascade of vessel collapse in vivo. A decrease in blood flow occurs almost immediately, and reaches the maximum in the following several hours. The collapsed blood supply induces central necrosis of the tumor. However, tumor sparing still exists at the periphery, leading to relapse after single-dose treatment[53-56]. The efficacy of such therapy relies largely on how fast blood supply is recovered. This restoration is unavoidable, because the tumor cells at the periphery can obtain a direct supply of oxygen and nutrients from neighboring normal tissues and engulfed normal vessels during the fast growth of malignancies[7,56]. Thus, growth of the tumor is only delayed due to the compromised blood supply and it cannot be eradicated.
Histopathologically, VDA-induced necrosis is located in the center of the tumor with a characteristic viable rim of a few cell layers adjacent to the normal tissue surrounding the tumor mass, which persists irrespective of differences in potency and efficacy of VDAs. In addition, hemorrhage often occurs together with necrotic tumor cells several hours after treatment[57]. Besides, the infiltration by inflammatory leukocytes may also contribute to the vascular-disrupting effect[41,42].
After VDA treatment, tumors may become phenotypically more aggressive due to hypoxia. With the regulation of hypoxia inducible factor 1α (HIF-1α), expression of angiogenic gene is activated and the level of vascular endothelial growth factor (VEGF) is thus increased[55,58]. Therefore, antiangiogenic therapy may be complementary to VDA, providing dual targeting at both preexisting and new vessels.
ANIMAL TUMOR MODELS
In vivo cancer research in clinically relevant animal models bridges the in vitro studies of cell culture and biochemical assays with the more costly, time-consuming clinical practice. Considering the greater costs and stricter ethical regulations on human studies, a variety of rodent tumor models have been introduced particularly in combination with multiparametric imaging biomarkers to envisage the internal real-life events in experimental VDA research.
These animal models with various tumor cell lines can be classified according to several features. For examples, they can be categorized by locations such as subcutaneous[51,59], intramuscular[60-62] or visceral organ[57] tumors; by destination relative to source graft such as orthotopic[63-65] or ectopic tumors; by carcinogenesis such as primary[26,48,65,66] or secondary tumors; by graft origins such as allograft[63] or xenograft human[61,67,68] or animal[69] tumors; and by immune status of tumor recipient such as the tumors growing in immunocompetent or immunodeficient[64,68] animals.
A wide range of diverse VDA effects have been observed in various tumor models[70,71]. Tumor microenvironment and host-tumor interaction may account for such discrepancy in responsiveness. Besides tumor cells with gene mutations, host stromal cells are also greatly involved in the tumor initiation, progression, invasion, and metastasis. For instance, with the expression of VEGF, stromal fibroblasts play a role in the formation and maintenance of tumor vessels[2]. Accordingly, when transplanted into various host locations or organs, the same neoplastic graft may have different angiogenesis and vascular functions. Thus, response to the same treatment may differ depending on tumor location and host-tumor interaction, because the organ-specific regulation of the balance between pro- and anti-angiogenic factors is responsible for the different angiogenesis activities[2,4,62,72]. As a result, tumor models of orthotopic transplantation into visceral organs of host animals with intact immune functions are thought to be more relevant to the conditions of clinical patients in terms of better mimicking tumor microenvironment, therefore, the treatment outcomes are more translatable into patients[62,72].
For imaging studies of VDA effects in small rodents, image quality has been shown to be satisfactory, even for organs susceptible to motion artifacts with non-respiratory-gated acquisition at a clinical magnet[56]. However, imaging in mice is more challenging than in rats, because the body weight of a mouse is about one-tenth of a rat, which results in lower signal-noise ratio (SNR) and poorer spatial resolution. In addition, success rate is sometimes compromised for the repetitive cannulations for intravenous injection of VDAs or contrast agents in mice during the dynamic follow-up of treatment monitoring, leading to some missing data.
MEASURING TUMOR RESPONSE TO VDAs WITH IN VIVO IMAGING BIOMARKERS
VDAs have been shown to induce vascular shutdown in tumors within minutes, and how to evaluate accurately and promptly such effects remains a challenge to preclinical research and clinical practice. Ineffective treatment may not only hamper or delay the effective alternative therapies, but also cause unnecessary side effects and waste of resources. Considering the presence of possible non-responders to certain therapies, it is of immense importance to individualize the treatment regimens, in which early feedback after VDA treatment is deemed crucial.
For the assessment of anticancer effects, traditional clinical endpoints are difficult to quantify and may require lengthy and larger scales to complete[8,9]. Thus, it is impractical to perform such endpoints in the assessment of early effects with VDAs. Recently, multiparametric imaging biomarkers have been developed as “surrogate endpoints” to act as indispensable substitutes for such clinical endpoints. The quantitative structural, functional and metabolic information derived from these imaging biomarkers may enable more comprehensive assessments and predictions of clinical outcomes, and in this case, the possibility for timely therapeutic justification and adjustment in oncological patients under the VDA regimen.
Out of various imaging modalities, MRI has been most frequently applied for the evaluation of VDA effects due to its advantages such as excellent spatial and temporal resolution, imaging in arbitrary planes, no ionizing radiation and ability to provide morphological, functional and metabolic information (with MR spectroscopy) for serial post-treatment follow-up. In the following section, we focus on the role of MRI in the evaluation of VDAs and its validation with other robust and specific techniques.
Clinical and high-field-strength MRI scanners
For preclinical research and clinical trials of VDAs, some animal studies have been performed with clinical 1.5 T MRI scanners[56,59,73], and more studies on small-bore research scanners[51,53,62,64,68,69,74,75]. The clinical and animal scanners are different in terms of availability in research centers, accessibility during working hours, usability, difficulty in method development, and translatability. Most important, with some parametrical optimization of built-in sequences, clinical scanners yield more translational results from small rodents to clinical patients than do dedicated animal scanners.
Recently, 3.0 T clinical scanners have become widely available with a trend for introducing even higher field whole body scanners (7-11 T) throughout the industry, since the safety approval of 3.0 T scanners in patients in 2002[76]. For intracranial tumors, 3.0 T scanners have shown better SNR, spatial and temporal resolution, contrast-to-noise ratio, and spectral resolution than 1.5 T scanners with the same acquisition parameters[76-79]. However, the applications in other regions of the body, the added value of 3.0 T compared with 1.5 T scanners is still controversial, due to issues such as specific absorption rate and motion and susceptibility artifacts. The modification of acquisition parameters and development of new coils may lead to wider applications in body imaging with 3.0 T MRI[80,81].
Biomarkers from conventional MRI sequences
Conventional MRI biomarkers are derived from T2-weighted imaging (T2WI), T1-weighted imaging (T1WI) and contrast-enhanced T1WI (CE-T1WI). Despite the topographic information such as tumor location, shape and volume, the quantification of tumor signal intensity (SI) on T2WI can help to detect VDA-induced hemorrhage[64]. SI on T2WI can also help to differentiate the viable tissue from necrosis on a pixel-based image texture analysis[82]. The heterogeneous SI on T2WI after VDA treatment is associated with (hemorrhagic) necrosis and complicated by evolving stages of necrosis and/or deoxyhemoglobin. Accordingly, SI change in T2WI is not considered a consistent imaging biomarker of hemorrhagic necrosis[51,71].
To date, the most frequently used surrogate endpoint for therapeutic evaluation of tumor response is the change in tumor size[83]. Tumor size can be measured linearly with 1D or 2D longest axis, although it may often lead to the overestimation of tumor volume of irregular shape. Manual delineation of tumor in tumor-containing slices or computer-assisted 3D analysis is more accurate for the estimation of tumor volume. Tumor volume of 3D analysis is predictive of survival in patients with tumors[84,85]. However, the change in tumor size/volume always falls as a late event behind the earlier and complex changes in microstructure and function induced by the downstream molecular and cellular events after VDA treatment[42,57], because VDAs only slow down the tumor growth without tumor eradication or size reduction[86]. Therefore, tumor size/volume is not a suitable imaging biomarker for very early assessment of the outcomes with VDAs (Figure 2).
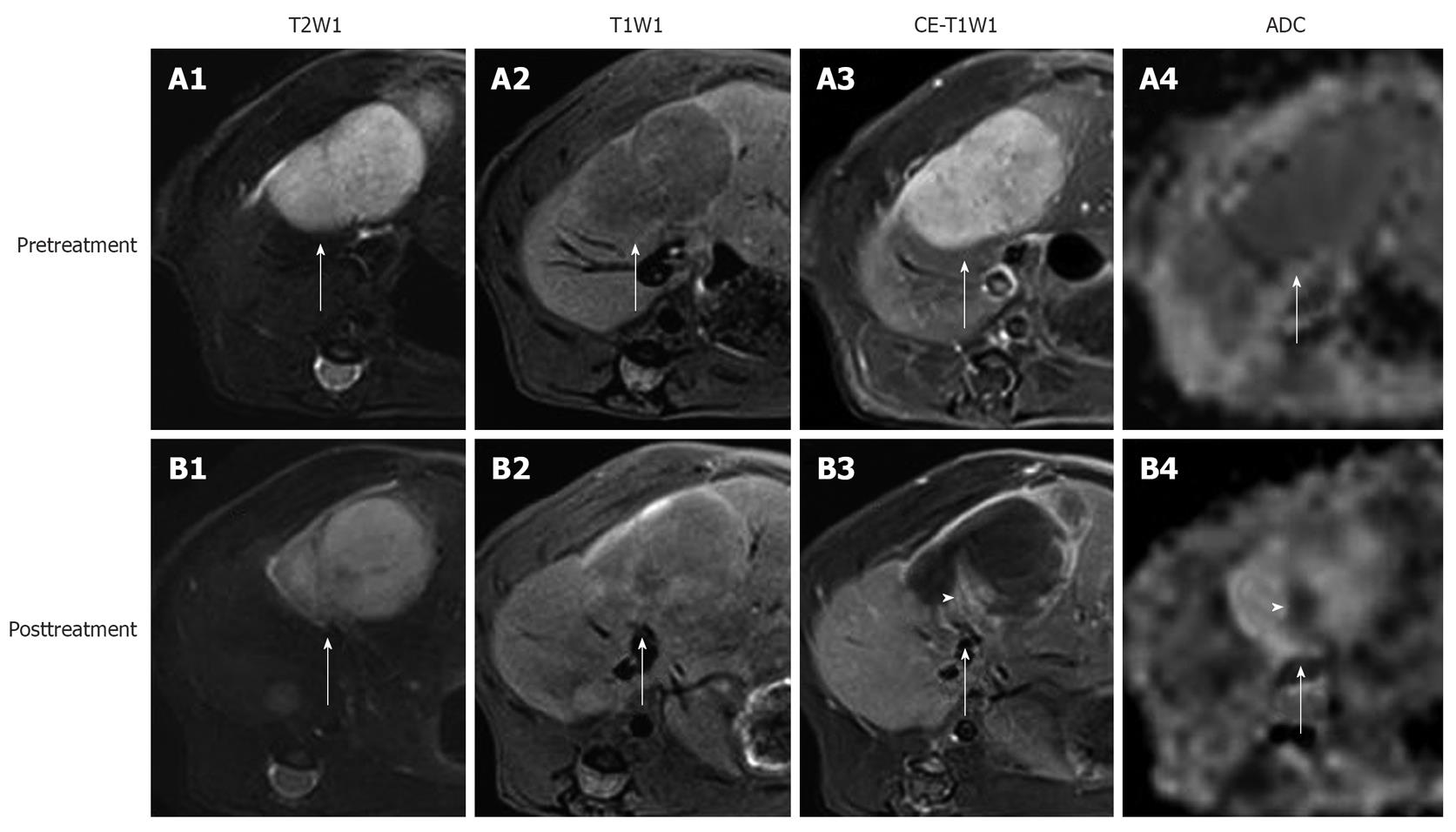
Figure 2 In vivo magnetic resonance imaging findings of an implanted tumor in rat liver.
Before treatment, the tumor (arrows) appeared hyperintense on T2WI (A1); hypointense on T1WI (A2); strongly enhanced on CE-T1WI (A3); and slightly hypointense on ADChigh (b = 500, 750, 1000 s/mm2) (A4). At 24 h after the intravenous treatment with CA4P at 10 mg/kg, obvious vascular shutdown was observed. The tumor (arrows) was still hyperintense on T2WI (B1) and hypointense on T1WI (B2). On CE-T1WI, the tumor (arrow) appeared hypointense in the center with an enhanced rim of viable neoplastic cells (B3). On ADChigh map (B4), the hyperintensity in the center corresponded to necrosis, and the isointense ring was concordant with the viable tumor rim (arrow) on CE-T1WI. Note the viable tumor nodule at the periphery, shown as hyperintensity (arrowhead) on CE-T1WI (B3), and hypointensity (arrowhead) on ADChigh (B4).
Enhancement ratio is defined as the enhancement degree of tumor post-treatment on CE-T1WI relative to that before treatment[56]. It largely reflects the proportional distribution of contrast agent in blood vessels and EES of viable tumor tissues, and can be used for roughly assessing tumor vascularity, but it lacks the specific physiological meaning (Figure 2).
The necrosis ratio as an imaging biomarker for the evaluation of anticancer therapy has been endorsed as well as tumor size by the European Association for the Study of the Liver and the American Association for the Study of Liver Diseases[87,88]. The necrosis ratio can be measured on CE-T1WI, exploiting the perfusion deficit caused by the vascular shutdown in the non-viable tumor tissue (Figure 2). However, in this way, the necrosis ratio with non-specific contrast agent is underestimated due to inward diffusion of the contrast agent from the viable rim to the necrotic center of the tumor, when correlated with the necrosis ratio measured by histopathology[56,89]. Another method is to delineate the necrotic part on dynamic contrast-enhanced MRI (DCE-MRI) in order to minimize the diffusion of contrast agent[90]. Nevertheless, DCE-MRI has a relatively poor spatial resolution despite its high temporal resolution, i.e. the viable and necrotic tumor is sometimes difficult to discern on DCE-MRI. It needs to be explored which way to determine necrosis ratio can correlate better with the histopathological results. As an alternative to histopathology, the necrosis ratio from MRI may provide an imaging tool for assessing necrosis for the serial follow-up of patients after reliable necrosis develops. The ultimately reliable determination of necrosis may only be realized with the use of necrosis-avid contrast agents, which are not clinically available[91,92].
Conventional MRI biomarkers are easier to acquire and analyze, while they only reveal incomplete pathophysiological processes, and are often too late compared with very rapid shutdown after VDA treatment. Thus, it is imperative to develop more prompt, accurate, quantifiable, and specific imaging biomarkers for characterizing those early molecular and cellular changes, which can be clinically applicable to depicting early functional and metabolic changes, offering the insight into VDA mechanisms of action, dictating the course of therapy, and predicting treatment outcomes. Fortunately, the recent rapid advances in MRI and other modalities have made such requirements feasible for developing functional imaging biomarkers.
Diffusion-weighted imaging
First applied in neuroimaging, diffusion-weighted imaging (DWI) has rapidly evolved into a non-invasive oncological tool in the body, including the brain[93,94]. As a quantitative functional biomarker for detection and characterization of tumor, DWI is easy-to-perform and contrast-agent-free, and its innate imaging contrast is not significantly affected by exogenous contrast agents[95,96]. Therefore, DWI can be applied in patients with renal dysfunction, where contrast agents are contradicted, for repetitive monitoring after VDA treatment[97,98].
Basic principles: At a microscopic level, all water molecules undergo thermally driven random movement in three dimensions, so-called Brownian motion. Diffusion is a measure for the effective moving distance of water molecules within a given time[99,100]. In biological tissue, the mobility of water molecules are unavoidably hampered by their interaction with cell membranes, intracellular organelles and macromolecules, so that their apparent diffusion coefficient (ADC) within tissues in physiological or pathological conditions is determined by tissue cellularity, tissue components, and tortuosity of EES[101,102]. On the other hand, ADC is also affected by microscopic flow due to microcirculation within a voxel and water exchange between intracellular and extracellular compartments[8,103]. In general, ADC reflects the information of cellular density and membrane integrity, as well as different weighting of perfusion components, depending on the various diffusion gradients applied in the acquisition[103].
DWI can be obtained by applying two symmetrical diffusion-sensitizing gradients on the either side of a 180° refocusing pulse to a T2-weighted sequence. In a DWI sequence, moving water molecules undergo a phase shift after the first diffusion gradient and their phase shift cannot be canceled out as for static molecules after the second gradient, which causes the signal loss of moving water molecules on DWI. The imaging contrast between mobility-restricted and normal water molecules is thus created on SI[99]. For example, tumor tissues normally have higher cellular density, and after VDA treatment, edema with restricted mobility of water and necrosis with elevated diffusion in EES can be differentiated from normal tissues on DWI[86,104]. A diffusion gradient is characterized by the amplitude, duration and direction of diffusion-sensitizing factor (b value with the units s/mm2), and the weighting of diffusion on SI depends on b value[99]. For quantification of ADC, gradients are applied in three directions (X, Y and Z axes). However, in tissues in which mobility of water molecules is restricted by some structural barriers such as fiber bundles in the brain, diffusion anisotropy is quantified in more than six directions on diffusion tensor imaging (DTI)[105].
ADC-Quantification of DWI: Frequently expressed in a unit of 10-3 mm2/s, ADC is more robust against the influence of magnetic field strength and T2 shine-through effect, which facilitates intra- and inter-subject comparisons[106]. ADC can be quantified with the following least-squares algorithm[99]: ADC = ln(S0/Si)/bi (1), where Si is the SI measured on the ith b value image and bi is the corresponding b value. S0 is a variable that estimates the intrinsic SI (for b = 0 s/mm2). In tumors, such quantification requires at least two values in one direction, while more than three values are used to reduce noise[107]. ADC value can be generated with mono-exponential fit between SI and b value for each voxel, and displayed as a parametric map for all voxels[94].
It is important to bear in mind that intravoxel incoherent motion (IVIM) may dominate ADC values in biological tissues when lower b values are used. This means that, for a given voxel, not only the diffusion of water molecules contributes to its ADC, but also the microcirculation of blood in capillary within the voxel[103]. In tumors, rapid blood flow leads to non-linearity of ADC fitting within lower range of b values, i.e. small increase in b value causes bigger attenuation of SI[108,109].
For the calculation of ADC, the usual method is to obtain an overall ADC fitting of mono-exponential decay through a range b values from 0 to about 1000 s/mm2, or more specifically, flow-sensitive ADClow with lower b values (< 100-200 s/mm2) and diffusion-sensitive ADChigh with higher b values (> 500 s/mm2) can be quantified. The difference between ADClow and ADChigh can be defined as ADCperfusion to assess the perfusion fraction roughly[59,110] (Figure 3). Taking advantage of simplified calculation, mono-exponential analysis neglects the non-linearity of signal decay. To characterize the decay curve more adequately, bi- or multi-exponential models, as well as their alternative method, stretched model, are also explored in order to derive the perfusion fraction (f) and true diffusion coefficient (D). Despite the wider range of b values, longer acquisition time and requirement for higher SNR, the advantage of these more complicated models over mono-exponential methods still needs to be fully elucidated[8,108,111]. For any analytic method, the noise should be reduced whenever possible to ensure accurate fitting of ADC.
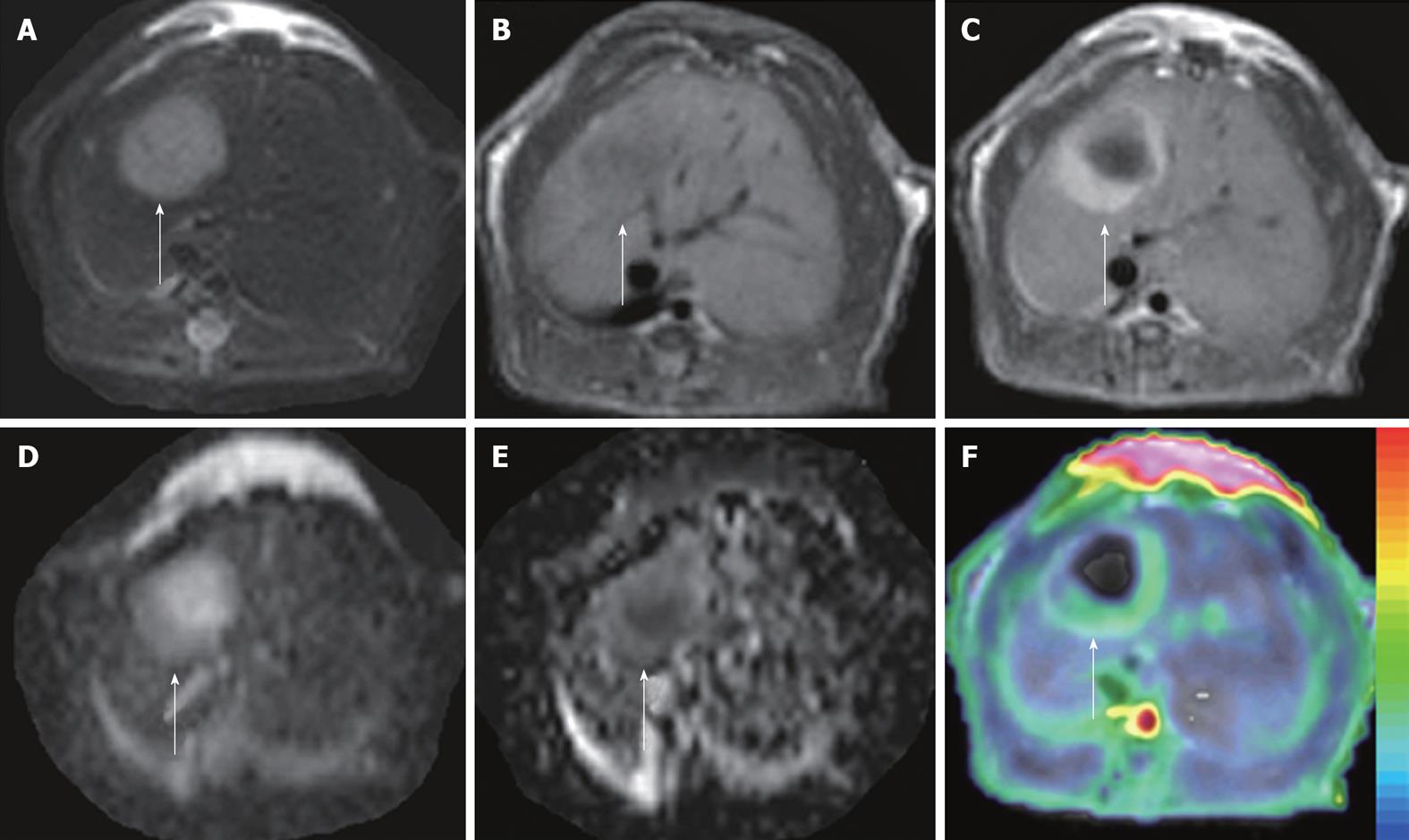
Figure 3 ADCperfusion and initial area under the gadolinium curve in an implanted tumor in rat liver.
At 48 h after iv treatment with CA4P at 10 mg/kg, obvious tumor recurrence with partial recovery of blood supply was demonstrated. The tumor (arrows) appeared hyperintense on T2WI (A) and hypointense on T1WI (B); On CE-T1WI, the tumor relapsed at the periphery, shown as ring enhancement of viable tumor cells (C); ADC10b (derived from 10 b values from 0 to 1000 s/mm2) revealed the hyperintense necrotic center and isointense viable tumor rim (D); On ADCperfusion (ADClow-ADChigh) maps, the relative hyperintensity at the periphery suggested the partial recovery of perfusion, compared to the hypointensity in the necrotic center with perfusion deficit (E); ADCperfusion matched well with CE-T1WI-overlyaed initial area under the gadolinium curve (IAUGC) map (F).
Visual interpretation: DW images can be evaluated on source DWI or quantitative ADC maps. DWI with b = 50 s/mm2 is often called black blood imaging, due to its nullification of blood signals to render vessels black. Black blood DWI has a better detection rate for small tumor lesions than T2WI has[112], and has been recommended as an alternative to T2WI with conspicuity of small lesion on 3.0 T scanners[113]. The combination of DWI with T2WI and CE-T1WI has been suggested to improve the diagnostic accuracy of small tumor lesions[94]. However, due to T2 shine-through, hyperintensity on high b value (800-1000 s/mm2) images does not always indicate increased cellularity, e.g. fluid shows hyperintense on both DWI and ADC maps. For this reason, ADC maps are preferable to DWI, and DWI should be always interpreted concurrently with the ADC map and all other available morphological images to prevent misinterpretation[104].
For the display of DWI or ADC maps, the inverted gray-scale (PET-like) for the suppression of background signal is used for whole-body DWI of high contrast from high b values, to detect multiple metastatic lesions[114]. With the co-registration between DWI or ADC maps with a color scale and structural T1WI or T2WI, fusion imaging can be obtained to integrate functional and anatomic information[8,94].
Quantitative interpretation: For the quantification of ADC, the most frequently used method is to draw a freehand region of interest (ROI) or volume of interest (VOI) over the whole tumor, and mean or median values of all pixels/voxels within the ROI/VOI are obtained. Such manual delineation is easy but fails to characterize tumor heterogeneity. Histogram-based analysis can reflect the frequency of pixels with different diffusion, and the pixels can be divided into subgroups according to their ADC values. Therefore, it may better reflect how many pixels undergo change in ADC after treatment[8,115]. It has been demonstrated that ADC histogram analysis may be a superior and quicker biomarker of tumor response to bevacizumab than tumor volume[116]. With the spatially varying change in ADC after treatment, an ideal approach is to analyze the pixels present both before and after treatment with spatial tags to detect any change in ADC pixel by pixel. By using a threshold diffusion map, the pixels can be categorized into decrease, increase or no change after treatment. The segmented tumor can be overlaid on structural images to demonstrate clearly the heterogeneous response of a tumor to treatment depending on different locations within the tumor[117]. However, the pixel-wise registration is more susceptible to motion, and its applications in the body is more difficult than in the brain[8].
Evolution of ADC changes: In tumors, the mobility of water molecules is restricted due to cellular membranes or interaction with structural proteins. The high tumor cellularity results in lower diffusivity and thus lower measured ADC in most tumors. However, the restriction on diffusion is multifactorial and also influenced by the unique intracellular water diffusion and microscopic tissue/tumor organizational characteristics. Consequently, the ADC of untreated tumors can occasionally be higher than that of native tissue[8,110], and it is vital to monitor intrasubject dynamic changes in ADC pre- and post-treatment.
Although the mechanism has not been fully explored, there is a temporary decrease in tumor ADC after VDA treatment. The probable reason may lie in abrupt decline in blood flow, subsequent cytotoxic edema due to the acute hypoxia, and resultant increased tortuosity of water molecules in EES[7,104]. Some preclinical studies have shown a transient decrease in ADC at 1 h after CA4P treatment[107,57]. The duration of decreased ADC was different in these two studies with the same tumor cell line but in different transplantation locations: ADC rebounded to pretreatment values in the intrahepatic tumor at 6 h[57]; however, ADC decreased gradually from 1 h until 6 h in the subcutaneous tumor[107]. The difference in doses of CA4P and tumor locations may have accounted for the phase discrepancy in ADC drop. On the other hand, the reduction in blood flow also contributed to the decrease in ADC[8,110], which was confirmed by the fact that ADClow decreased more significantly than ADChigh[56,107].
After the transient drop, ADC rebounds due to the collapsed cell membranes and decreased cellularity, and thus increased mobility of water molecules in EES throughout the progressing necrosis formation, during which the ADC value may reach the pretreatment baseline, and thus shows no significant difference from the baseline at some time points[74]; so-called pseudonormalization of ADC[118]. The onset and duration of pseudonormalization vary depending largely on tumor model and treatment strategy. If MRI falls within the window of pseudonormalization, there can be no significant change in ADC value, which, however, does not necessarily mean that ADC has not dynamically changed.
As necrosis develops, tumor cell volume is reduced with increased EES. The displacement of water molecules is less hampered, which increases ADC. The increase in ADC after VDA treatment has been shown in preclinical and clinical studies[53,57,68,119,120]. The peripheral sparing of tumor after VDA treatment has lower ADC, and can be distinguished from central necrosis of high ADC[8,110] (Figures 2 and 3). After single doses of VDA, the residual tumor unavoidably gives rise to recurrence, which in turn, leads to decreased overall ADC[59].
Derived from the different b values applied in DWI, ADChigh mainly reflects the true diffusion and is more accurate for the characterization of VDA-induced necrosis; ADClow, on the other hand, indicates the different weightings by several factors such as diffusion, microcirculation and structural barriers, which deteriorate its measurement reproducibility for individual or intergroup comparisons[119]; and ADCperfusion is most correlated with blood supply and can thus be used to approximate tumor blood perfusion as an alternative when venous access is limited[56,59] (Figure 3).
DCE-MRI
DCE-MRI enables quantitative characterization of microcirculation in terms of blood flow, blood volume and/or capillary permeability, as well as pathophysiological insight into the mechanism of VDA action in tumors. Therefore DCE-MRI has been applied as a promising imaging biomarker for the assessment of VDA effects[121,122].
Basic principles: DCE-MRI involves serial acquisition of sequential images before, during and after injection of a contrast agent to cover the volume of the tumor. By tracking the pharmacokinetics of injected contrast agent, DCE-MRI is capable of the non-invasive quantification of microvascular structure and function. In VDA studies, two kinds of contrast agents are often used: low molecular weight agents (< 1000 Da, e.g. gadolinium diethylenetriaminepentaacetic acid or Gd-DTPA) that rapidly traverse from capillary into the EES, but not into tumor cells; and large molecular agents (> 20 kDa) with low capillary permeability for prolonged intravascular retention, so-called blood pool agents[123]. DCE-MRI sequences can be designed to be T1-weighted or T2*-weighted, which exploit different physiological properties to derive different kinetic variables. T1-weighted DCE-MRI is sensitive to the presence of contrast agent in the EES and reflects microvascular blood flow, permeability and extracellular leakage space, whereas T2*-weighted DCE-MRI, or more specifically, dynamic susceptibility contrast (DSC) MRI, is sensitive to the vascular phase of contrast agent delivery and reflects blood flow and volume[124].
Upon bolus injection, the contrast agent enters arterioles and passes through the capillary network, known as the first pass of the contrast agent. Its paramagnetic properties render a decrease in both the T1 and T2* (or T2) relaxation times of water molecules. On T2*-weighted DEC-MRI, the transient drop of SI of nearby tissue is due to the presence of contrast agent within the capillary compartment. Therefore, such a sequence performs better in brain with intact blood brain barrier (BBB) or when combined with blood pool contrast agents, since the tracer largely remains intravascular[125]. Measurement of the T2* effect during the rapid decrease and subsequent recovery in SI necessitates rapid sampling acquisition to ensure high temporal resolution. T2*-weighted DEC-MRI is mostly applied in brain tumors due to the presence of the BBB[124,126]. In extracranial tumors, the contrast agent readily extravasates from the intravascular space into the EES at a rate determined by several physiological factors including tissue blood flow, permeability of the capillaries and surface area. On T1-weighted DCE-MRI, contrast agent in EES shortens the T1 relaxation time of nearby water hydrogen nuclei and causes increased SI. Therefore, T1-weighted DEC-MRI is widely applied in the extracranial tumors[127].
Quantification of DCE-MRI: For the quantification of DCE-MRI, we need to convert SI into the concentration of contrast agent at each time point during the acquisition. This is accomplished by measuring the T1 map on T1-weighted DCE-MRI, while it is more complicated in T2*-weighted DCE-MRI. It is usually necessary to derive arterial input function (AIF) by measuring the SI in arteries near the locations of tumor, and AIF is useful for the compensation of the influence of injection speed of contrast agent and cardiac output[128].
T2*-weighted DCE-MRI: The quantification of T2*-weighted DCE-MRI can be semi-quantitative or quantitative. The former method does not employ complicated kinetic modeling or AIF, and derived summary parameters from contrast agent concentration time curve (or SI time curve) include area under the peak (AUP), and time to peak (TTP). Such analysis is straightforward, while it does not provide pathophysiological information of perfusion related to vascular shutdown[129], and may also be complicated with the leakage of contrast agent into the EES, which is likely in tumors with high permeability[130].
For quantitative analysis of T2*-weighted DCE-MRI, the most robust biomarker is relative blood volume (rBV) from the first-pass technique, calculated as the integral area under the concentration-time curve, with the interpretation of AIF and kinetic models[131]. Relative blood flow can also be quantified, and mean transit time (MTT) is obtained according to the central volume theorem BF = BV/MTT. However, extracranial tumors are usually hyperpermeable, and the compartmentalization of contrast agent is usually lost. Thus quantification of these parameters are less reliable due to the leakage of contrast agent into the EES and subsequent T1 effect on T2*-weighted sequence. The possible solutions include the correction with gamma-variate function by using more complicated kinetic models, preloaded dose of contrast agent to eliminate the effect of its leakage into the EES or its recirculation, and dual or multiecho imaging sequences[130,132-135].
T1-weighted DCE-MRI: T1-weighed DCE-MRI exploits the distribution of contrast agent in the EES, which increases the T1 relaxation rate (1/T1) of nearby hydrogen nuclei. The concentration of gadolinium ions is known to be directly proportional to the change in 1/T1, and the latter is related to changes in SI on T1WI. With a low gadolinium dose, we can assume that there is a linear relation between the amount of contrast agent in the tissue and the resultant difference in relaxation time[136]. Semi-quantitative and quantitative analyses of T1-weighted DCE-MRI are possible.
For semi-quantitative analysis, the model-free method utilizes the enhancement curve in terms of curve shape, time from injection to arrival of contrast agent, gradient of upslope or wash out phase and maximal intensity[124]. The most frequently used parameter is initial area under the gadolinium curve (IAUGC) (Figure 3), as well as maximal initial slope of curve, TTP, and the slope of washout[56,127]. The simplicity of this method with computer routine enables its easy accessibility to many investigators, and it has been shown to be successful to monitor the responses to VDA[59,137]. However, these semi-quantitative measures fail to show any direct correlation with underlying physiological measures of tumor perfusion, permeability or leakage space, and only provide a mixed complex that hampers the interpatient or interscanner comparison[122,138].
Quantitative analysis of T1-weighed DCE-MRI involves a pharmacokinetic model to characterize the underlying physiological process of the contrast agent in tissues, including its administration, first pass, transendothelial process, distribution in EES, and wash out[124,139]. On the basis of some simplifying assumptions, biological tissues can be regarded as several compartments, e.g. two-compartment model with blood plasma and EES, within which contrast agent is instantaneously mixed and uniformly distributed[127]. The Tofts model is one of the frequently used pharmacokinetic models to fit concentration-time serial data in order to derive physiological parameters[140,141]. The robust parameters include Ktrans (volume transfer constant of the contrast agent, unit/min), Kep (rate constant of wash out of contrast agent refluxing from EES into blood, unit/min) and Ve (the extravascular extracellular volume fraction, unit %).
Although quantification of Ktrans is often overestimated due to the innate assumptions in all kinetic models[142], and dedicated software has to be involved in the analysis, quantitative analysis of T1-weighted DCE-MRI highlights the underlying mechanism of VDA action in terms of the permeability change and subsequent perfusion collapse after VDAs, and it facilitates the direct comparison of these physiological parameters for intra- and inter-subject studies (Figure 4). Thus, the imaging biomarkers from DCE-MRI are most correlative to the VDA effects.
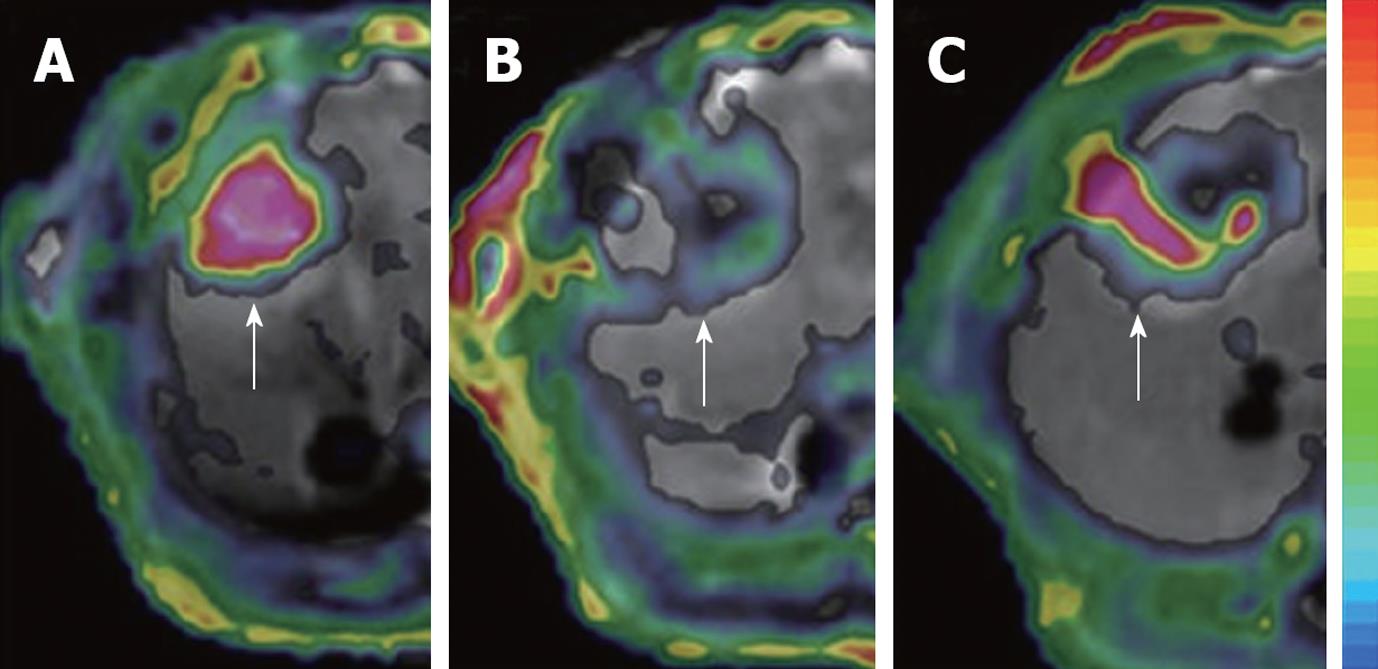
Figure 4 Dynamic changes in Ktrans.
The tumor (arrows) in rat liver showed an abundant blood supply with high Ktrans before treatment (A); At 6 h after CA4P treatment, vascular shutdown was indicated with low Ktrans in the center, surrounded by tumor residue at the periphery, with moderate Ktrans (B); At 48 h after treatment, the tumor relapsed upon the residue at the periphery with rebounding Ktrans (C).
Interpretation of DCE-MRI: In general, successful VDA treatment causes the immediate vascular shutdown of tumors, shown as a rapid drop in semi-quantitative and quantitative DCE-MRI parameters within minutes or hours, and neoplastic recurrence is reflected as recovery in such measures to baseline level, which depends on the dose of VDAs and tumor models[7,26,39,53,54,73,143] (Figure 4).
Ktrans reflects a composite of both blood flow and vascular permeability-area product, and therefore, its interpretation depends on the rate-limiting step between perfusion in vessels and diffusion into the EES. In untreated tumors, the vascular permeability-area product is often high, and the tissue is described as flow-limited, so that Ktrans approximates blood flow[140]; after the treatment with VDAs, the permeability transiently increases and then the blood flow drops abruptly, which decreases Ktrans. However, in this mixed situation, the blood flow and permeability cannot be decoupled and it is difficult to identify the dominating factor between the perfusion and permeability-area product[56,124,140] (Figure 4).
For example, in a rat subcutaneous tumor model, tumor perfusion decreased by 57% with ABT-751 treatment after 1 h, but recovered to near pretreatment levels within 6 h[39]. In a rat liver tumor model with ZD6126 treatment, Ktrans dropped to its lowest at 24 h and partially recovered at 48 h[56], while for the same tumor cell line but in subcutaneous model with CA4P, Ktrans decreased to its lowest level at 6 h and recovered at 9 d[59]. Values of DCE-MRI parameters are derived from an ROI covering the whole tumor in most studies, which however, ignores the tumor heterogeneity due to the persistence of the viable rim after VDA treatment. Therefore, inclusion of non-enhancing pixels in the center artificially underestimates the mean and/or median parameter values[144]. Some authors have defined the tumor center and periphery and have analyzed the DCE-MRI parameters respectively, and have successfully shown the different responses in necrotic center and viable rim, which have helped to elucidate tumor pathophysiology and drug action of VDAs[46]. However, the definition of core and rim is debatable[145] and manual delineation of tumor center and periphery suffers from relatively poor spatial resolution on DCE-MRI, even with cross reference to other structural images of higher spatial resolution such as that derived from CE-T1WI.
Alternatively, pixel-based analysis of DCE-MRI quantifies the value of each pixel within a tumor, and distribution histogram and mean and/or median values can be derived, which is especially helpful in the dynamic follow-up of VDA treatment[146,147]. Nonetheless, this pixel-based method suffers more from motion artifacts in extracranial tumors, than whole-tumor-based analysis, and the technique remains challenging for physiological motion, such as cardiac and respiratory movements[148].