Published online Aug 28, 2010. doi: 10.4329/wjr.v2.i8.298
Revised: July 29, 2010
Accepted: August 5, 2010
Published online: August 28, 2010
Acute pancreatitis is characterized by acute chemical injury of the pancreatic parenchyma and peripancreatic tissue. The increased frequency of death in acute pancreatitis is directly correlated with the degree and progress of pancreatic necrosis. Moreover, the occurrence of some local complications in acute pancreatitis, such as pancreatic hemorrhage, peripancreatic abscess or large pseudocyst, and pseudoaneurysm, could influence the choice of treatment for these patients. Magnetic resonance imaging (MRI) can be used to help evaluate the presence and degree of pancreatic necrosis, and is crucial for identifying complications of acute pancreatitis and predicting prognosis. The purpose of this article is to describe MRI techniques for acute pancreatitis, to review the spectrum of pancreatic and peripancreatic patterns, as well as to survey various complications secondary to acute pancreatitis on MRI. The role of MRI in the initial evaluation and staging of acute pancreatitis is emphasized.
- Citation: Xiao B, Zhang XM. Magnetic resonance imaging for acute pancreatitis. World J Radiol 2010; 2(8): 298-308
- URL: https://www.wjgnet.com/1949-8470/full/v2/i8/298.htm
- DOI: https://dx.doi.org/10.4329/wjr.v2.i8.298
Acute pancreatitis is caused by acute chemical injury of the pancreas, and the leakage of activated pancreatic enzymes leads to autodigestion of the pancreatic parenchyma and peripancreatic tissues[1-3]. Alcoholism and choledocholithiasis are the most common etiological factors for this disease[2-6]. The clinical variety of mild acute pancreatitis (70%-80% of patients), also called edematous interstitial pancreatitis, exhibits a self-limiting disease with no or minimal organ dysfunction, without complications, and with a favorable prognosis[1]. Severe acute pancreatitis (20%-30%), also called hemorrhagic necrotizing pancreatitis, is characterized by conspicuous organ dysfunction, a high incidence of local complications and a dramatic increase in mortality rate (10%-23% in necrotizing pancreatitis)[4,7].
Increased levels of serum and/or urinary pancreatic amylase and lipase have been detected in most individuals with acute pancreatitis after the onset of symptoms. However, these pancreatic enzymes have no role in the assessment of disease severity[8]. Imaging for acute pancreatitis has a significant role in confirming the diagnosis of this disease, which helps to detect pancreatic necrosis and diagnose local complications[9]. Furthermore, imaging is useful in the early assessment of disease severity[5,10,11].
Ultrasonography (US), a convenient and inexpensive imaging technique, can help evaluate the presence of gallbladder and/or common duct stones in acute pancreatitis. However, visualization of the pancreas is often disturbed by overlying gastrointestinal gas, which is an important limitation for US applications in this disease[8].
Contrast-enhanced computerized tomography (CT) is often used to aid the diagnosis of pancreatic necrosis and help evaluate the presence and development of local complications in acute pancreatitis[12]. CT severity index (CTSI), a very good imaging grading system for disease severity, has shown excellent correlation with the incidence of death in patients with acute pancreatitis[4,8]. However, CT has the potential aggravation of pancreatic injury that results from the use of iodinated contrast media and an increased radiation burden that can result from follow-up scans[13].
As with the development of high-field-strength magnetic resonance imaging (MRI), it has been established that several techniques such as abdominal rapid gradient-echo breath-hold, magnetic resonance cholangiopancreatography (MRCP) and three-dimensional dynamic contrast-enhanced sequences are performed to depict satisfactorily the normal pancreas and pancreatic disorders[14,15].
Advantages for using MRI in patients with acute pancreatitis are as follows: (1) it is a diagnostic imaging method without radiation hazard; (2) it is particularly useful in patients who cannot receive iodinated contrast material owing to allergic reactions or other contraindications; (3) MRCP has the unique capability of providing noninvasive images of pancreatic parenchyma and pancreatic duct integrity, and it has the advantage of demonstrating possible communication of a pancreatic pseudocyst with pancreatic ducts[8,15]; (4) MRI has a potential advantage over CT in detecting bile duct lithiasis and pancreatic hemorrhage of pseudocysts or pseudoaneurysm, which can help plan surgery; (5) non-enhanced MRI seems to be more accurate and reliable for the early assessment of severity and prognosis of acute pancreatitis than is contrast-enhanced CT[12,16,17]; and (6) non-enhanced MRI is superior to CT for depiction and confirmation of mild forms of acute pancreatitis[18].
Nevertheless, there are several limitations for using MRI in this disease. (1) It requires patient cooperation and breath holding, otherwise, there can be motion artifacts that affect the visualization of the pancreas and its adjacent structures[7,8]. In our clinical practice, this requirement is difficult for patients with severe pancreatitis, who are too old or too weak to hold their breath for long enough; (2) On MRCP, pancreatic duct visibility can be decreased by the overlap of fluid-containing organs (e.g. stomach and duodenum); (3) MRI is time-consuming and relatively expensive with comparison to US or CT; and (4) MR contrast media (e.g. gadolinium) have a potential risk of developing nephrogenic systemic fibrosis in patients with severe acute pancreatitis associated with renal insufficiency after performing MR enhancement[19].
For a comprehensive assessment of acute pancreatitis, it is necessary to evaluate the pancreatic parenchyma, the peripancreatic tissues and vasculature[7]. MRI for acute pancreatitis requires the combined use of T1-weighted imaging (e.g. fast spin-echo imaging with multiple breath-hold acquisitions or single-breath-hold gradient echo imaging); T2-weighted imaging [e.g. fast recovery fast spin-echo or single-shot fast spin-echo (SSFSE) imaging]; and MRCP (e.g. a thick-slab, SSFSE T2-weighted sequence). The characteristics of these are as follows. (1) T1-weighted imaging with fat suppression improves the delineation of pancreatic borders and the pancreas itself, and it additionally has important value in evaluation of pancreatic hemorrhage and hemorrhagic complications of acute pancreatitis[7,8]; (2) T2-weighted imaging has significant advantage in demonstrating fluid-filled lesions in or around the pancreas and the pancreatic ducts. Fat-suppressed T2-weighted imaging is additionally helpful for evaluating the mild forms of acute pancreatitis[18,20]; and (3) MRCP has an excellent capability of allowing noninvasive evaluation of pancreatic ducts, side-branches and the whole extrahepatic biliary tract, and it provides few respiratory artifacts or susceptibility effects[7].
Furthermore, after the intravenous administration of contrast agent, the dynamic contrast-enhanced MRI (e.g. T1-weighted imaging performed with liver acquisition with volume acceleration is utilized, and magnetic resonance angiography (MRA) is performed to provide information for better visualization of the pancreatic vascular network.
In acute pancreatitis, the patterns of pancreas and peripancreatic tissue involvement can be depicted very well on MRI.
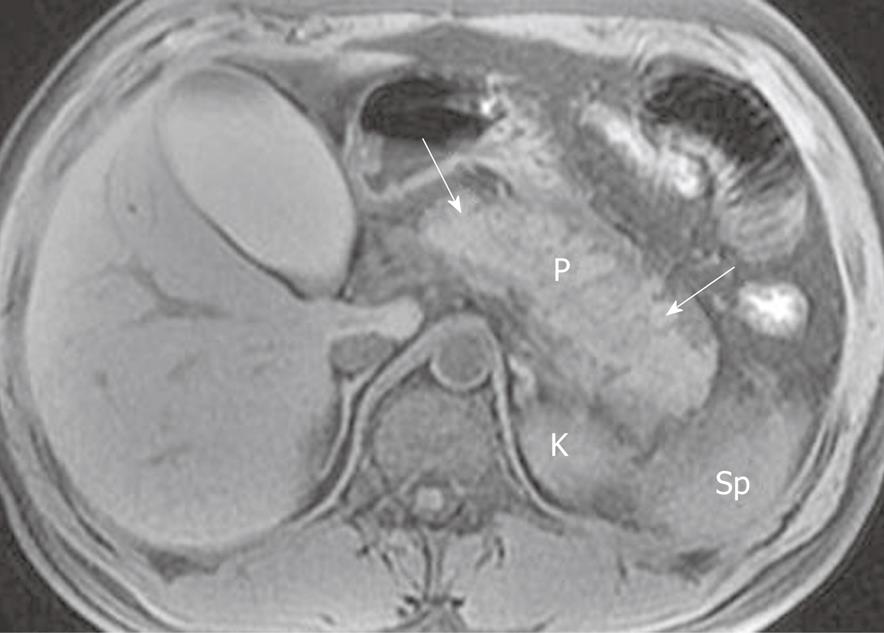
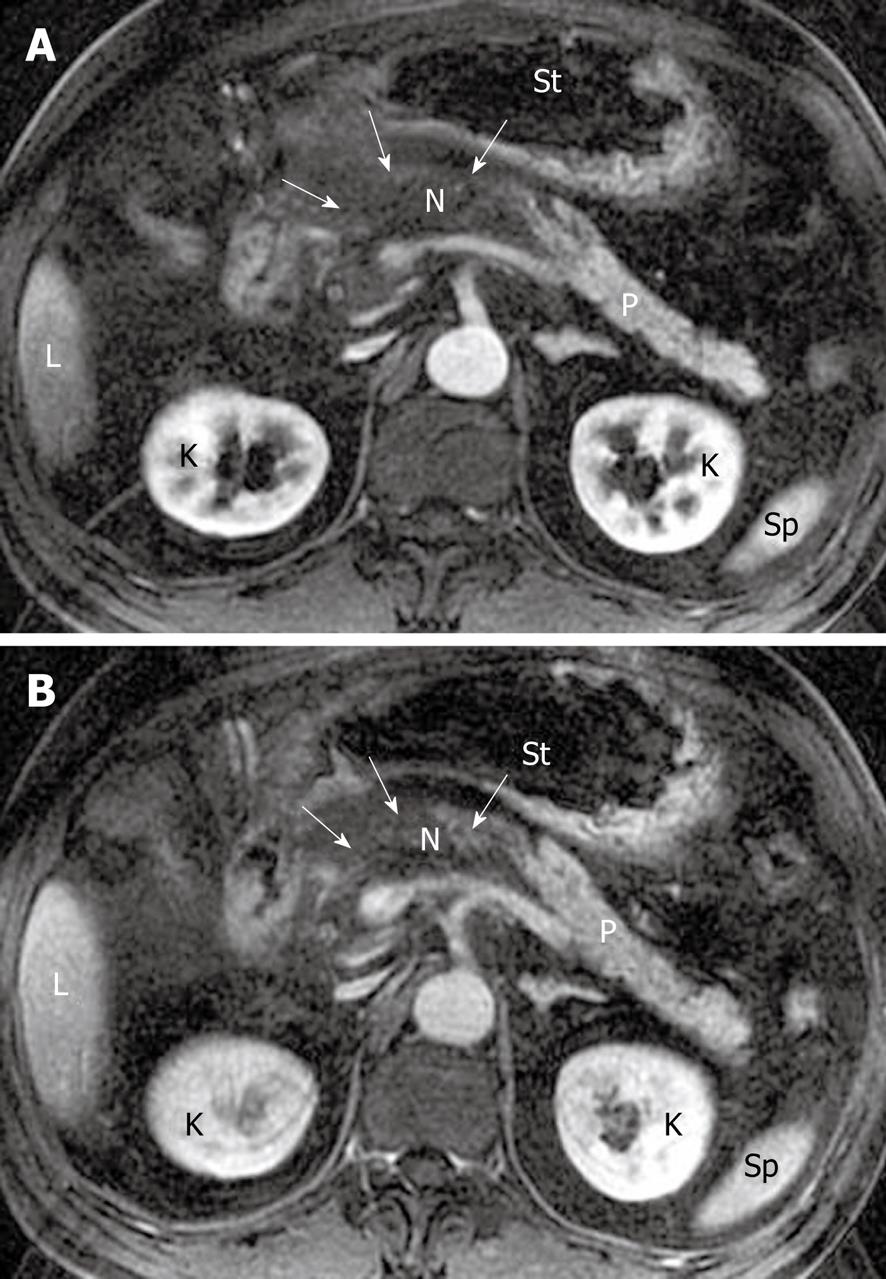
Morphology of the pancreas: MR T1-weighted with fat-suppression images are particularly useful for defining diffuse or a focal enlarged pancreatic gland. Lace-like contours of the pancreas can disappear and pancreatic boundaries are blurred (Figure 1). However, it should be emphasized that a few patients with edematous pancreatitis have no morphological changes of the pancreas[8,21]. In such circumstances, the objective assessment of this disease should be based on clinical manifestations and several laboratory markers.
Signal intensity of pancreatic parenchyma: Due to inflammation and edema of the pancreas in acute pancreatitis, the signal intensity of the pancreatic parenchyma might be hypointense relative to the liver on T1-weighted images, and hyperintense on T2-weighted images (Figure 2). However, signal changes between the pancreas and liver have been reported in a minority of patients with acute interstitial edematous pancreatitis[22].
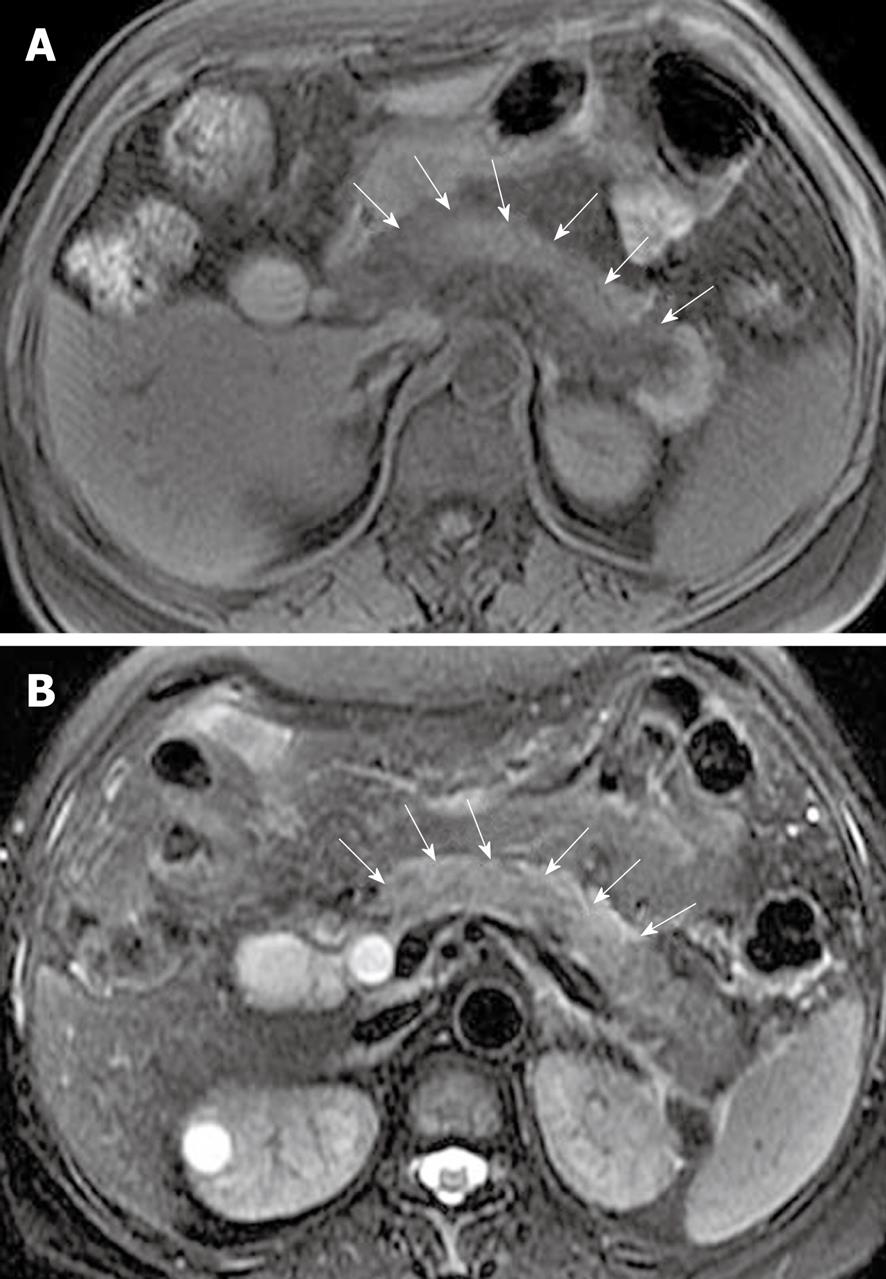
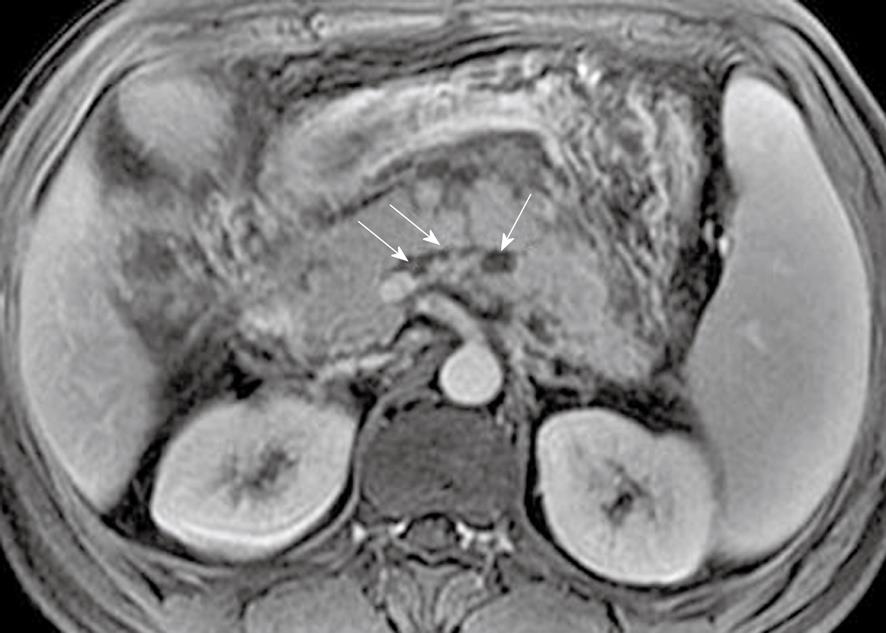
Pancreatic interlobular septa: Acute pancreatitis can result in pancreatic interlobular septal inflammation, edema, and fluid collections[7,23]. MR T2-weighted with fat-suppression images can accurately depict subtle interlobular septal abnormalities, such as threadlike hyperintense structures (Figure 3).
Pancreatic necrosis: Pancreatic necrosis refers to a pathological collection of devitalized tissue in the pancreas, and it can be focal or diffuse, or superficial or deep in the pancreatic gland[1,4,8]. Patients with pancreatic necrosis are routinely monitored in the intensive care unit because the increased mortality has been shown to correlate directly with the presence and degree of pancreatic necrosis[4]. Thus, the early detection of pancreatic necrosis is a prognostic indicator in these patients[5]. However, recognition of pancreatic necrosis by means of clinical examination is unreliable, therefore, the importance of diagnostic imaging for that purpose is clinically emphasized.
Accepted criteria for the diagnosis of pancreatic necrosis, similar to contrast-enhanced CT, have been defined as areas of diminished or non-enhanced pancreatic parenchyma depicted on dynamic contrast-enhanced MRI[11,13,21]. Furthermore, intravenous administration of contrast material is essential to enable differentiation of real pancreatic necrosis from transient pancreatic ischemia. The visualization of non-enhanced pancreatic parenchyma during the entire processes, including the arterial, venous and delayed phases, supports the diagnosis of the real pancreatic necrosis[8]. The diagnosis of pancreatic necrosis can be made in the course of 2-3 d after onset of acute pancreatitis[24].
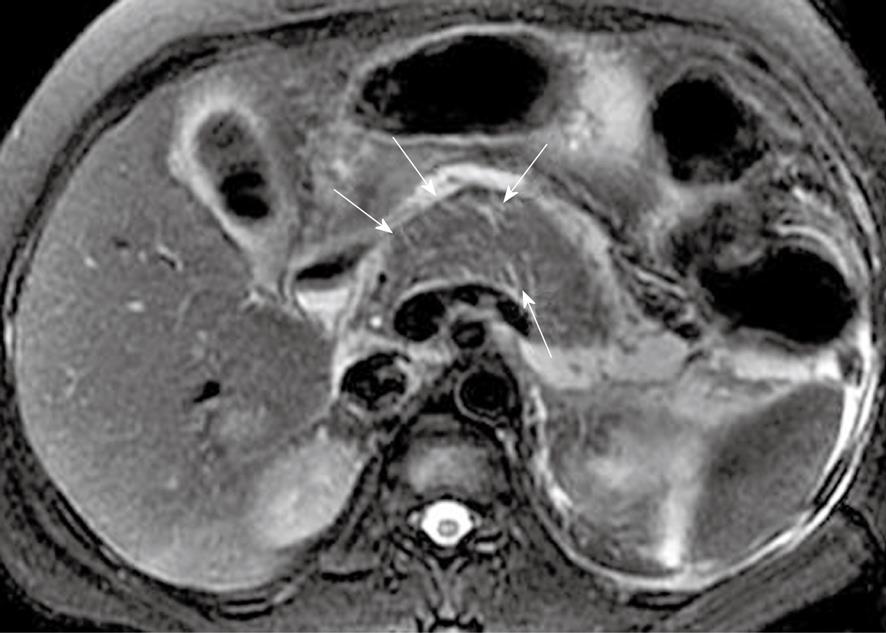
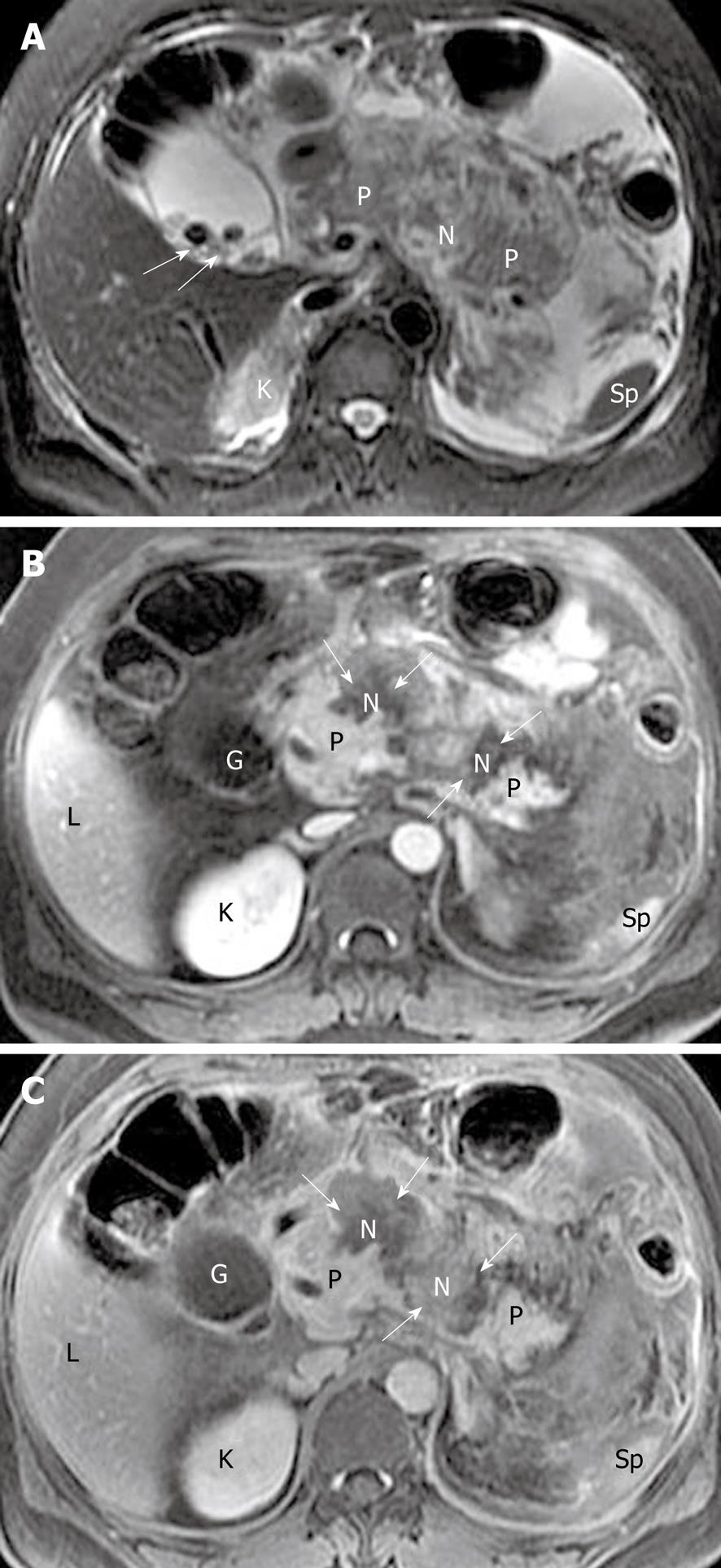
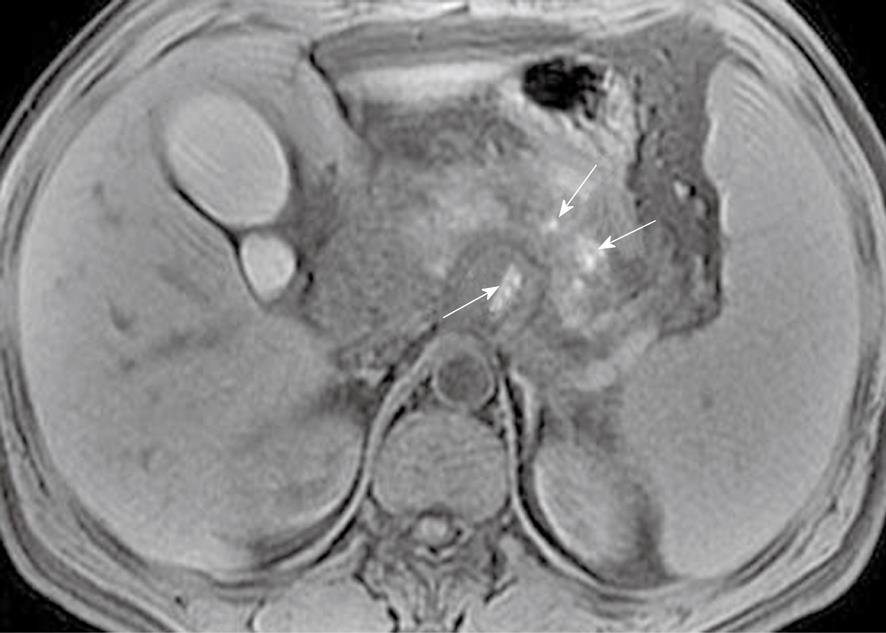
Focal pancreatic necrosis is characterized by spotted, patchy non-enhanced areas (like “pepper”) on contrast-enhanced MR images (Figure 4). The large, diffuse, non-enhanced zones of the pancreas (like “black pancreas”) on enhanced MR images reflect pancreatic diffuse necrosis (Figure 5). A special type of the diffuse necrosis is gland-liquefied necrosis. In this setting, the edge of the pancreas becomes discontinuous or the pancreatic head is not consistent with the body and/or tail, which exhibits “rupture of the pancreas” (Figure 6).
The extent of necrosis can be further quantified to less than 30% (mild), 30%-50% (moderate), and > 50% (severe) of the pancreatic gland, which is based on Balthazar’s criteria and grade points[8]. MR severity index (MRSI) derived from CTSI, which combines a consideration of both Balthazar grading scores and scores of the extent of pancreatic necrosis, can also be tested. It has been reported that MRSI is significantly correlated with CTSI, Ranson score, C-reactive protein levels, appearance of systemic complications, duration of hospitalization, and clinical outcome[12,25]. Moreover, there is an additional and inevitable problem that infection cannot be excluded in areas of gland necrosis (mentioned below).
Pancreatic hemorrhage: Pancreatic hemorrhage, also called hemorrhagic pancreatitis, is seen in 2%-5% of patients with acute pancreatitis and commonly occurs in the setting of severe forms of pancreatitis[8-10,23]. With conversion of hemoglobin to methemalbumin in the hemorrhagic zones, MRI shows the spotted or patchy (like “salt”) (Figure 7) or threadlike or girdle-shaped hyperintensity (Figure 8) on T1-weighted images with fat suppression. To the best of our knowledge, MRI is better than CT for detecting hemorrhagic pancreatitis. This is because the signal intensity changes of hemorrhage on MRI can be sustained for a long time, and have different MR features of hemorrhage at various times (e.g. serum methemalbumin with hyperintensity on T1-weighted images with fat suppression, and hemosiderin with hypointensity on T2-weighted images)[10,13,21]. Other complications of acute pancreatitis, such as pancreatic pseudocyst or pseudoaneurysm, also can show hemorrhage (mentioned below).
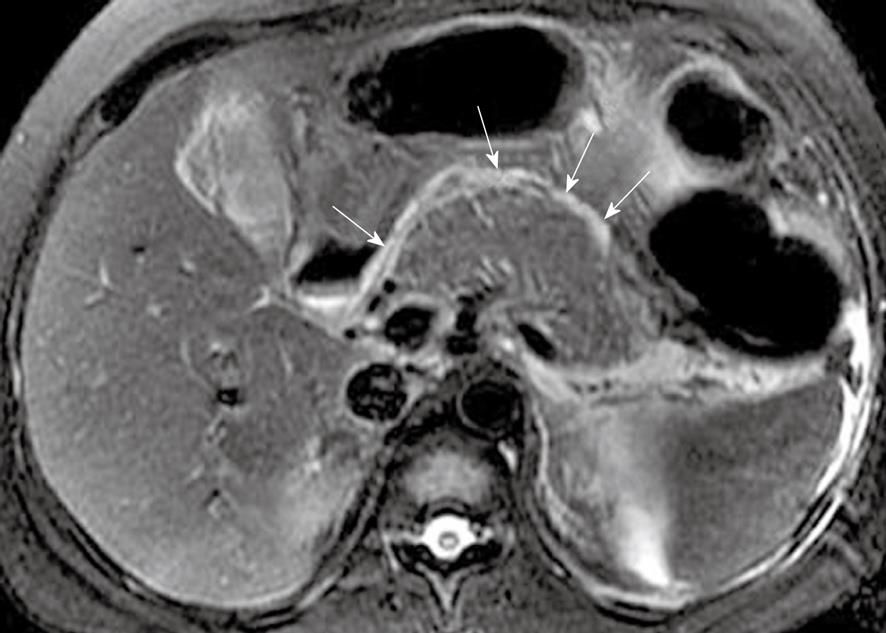
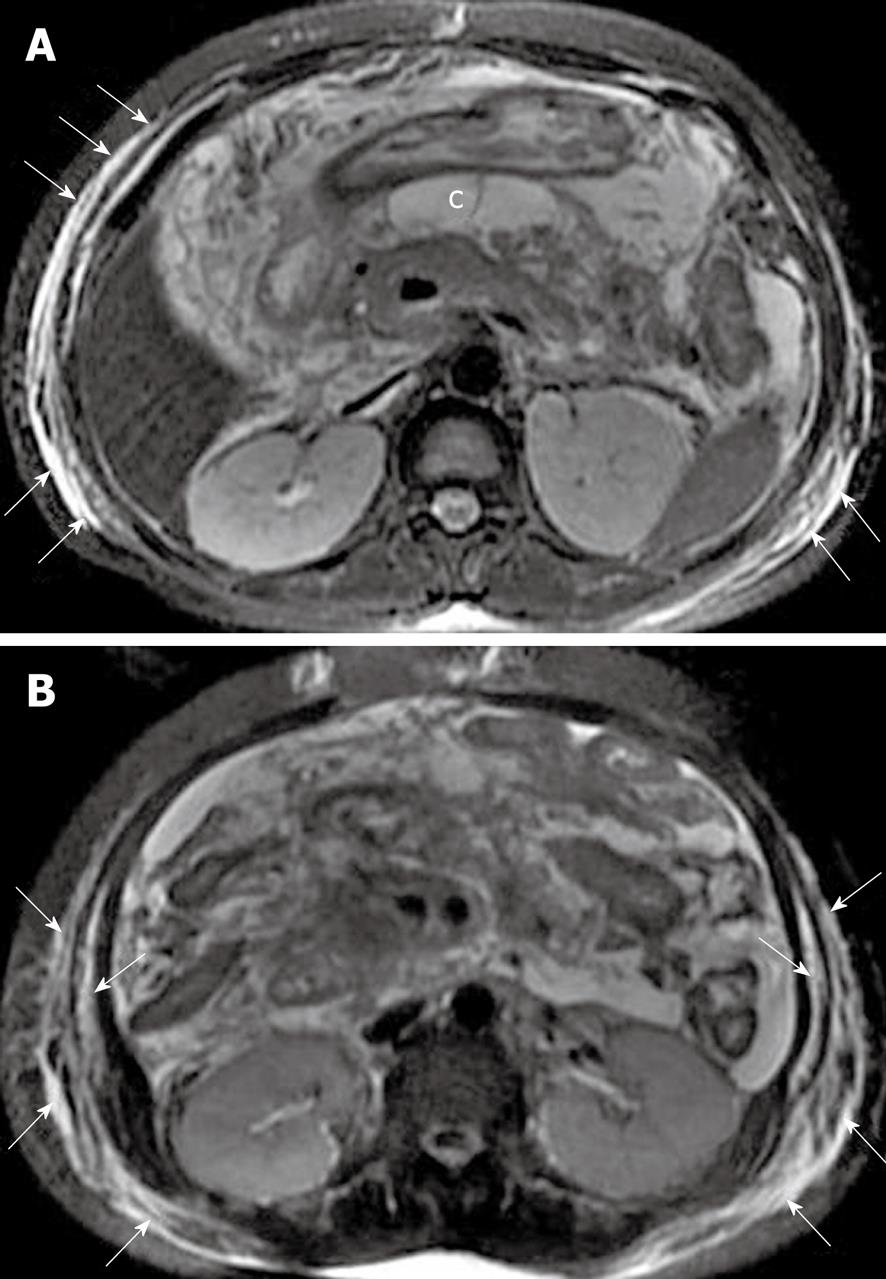
Pancreatic capsule: The pancreas is surrounded by the formation of a capsule, which is actually loose connective tissue[7,8]. In normal individuals, the pancreatic capsule is not seen on CT and MR images, whereas, in patients with acute pancreatitis, abnormalities of the covering of the pancreas, such as edematous thickening of the pancreatic capsule and subcapsular fluid collections, can be accurately depicted on MR T2-weighted with fat-suppression images[22] (Figure 9). In our clinical practice, these pancreatic capsule changes might be the most common findings in patients with edematous or interstitial pancreatitis, and MRI is more helpful than CT to depict them.
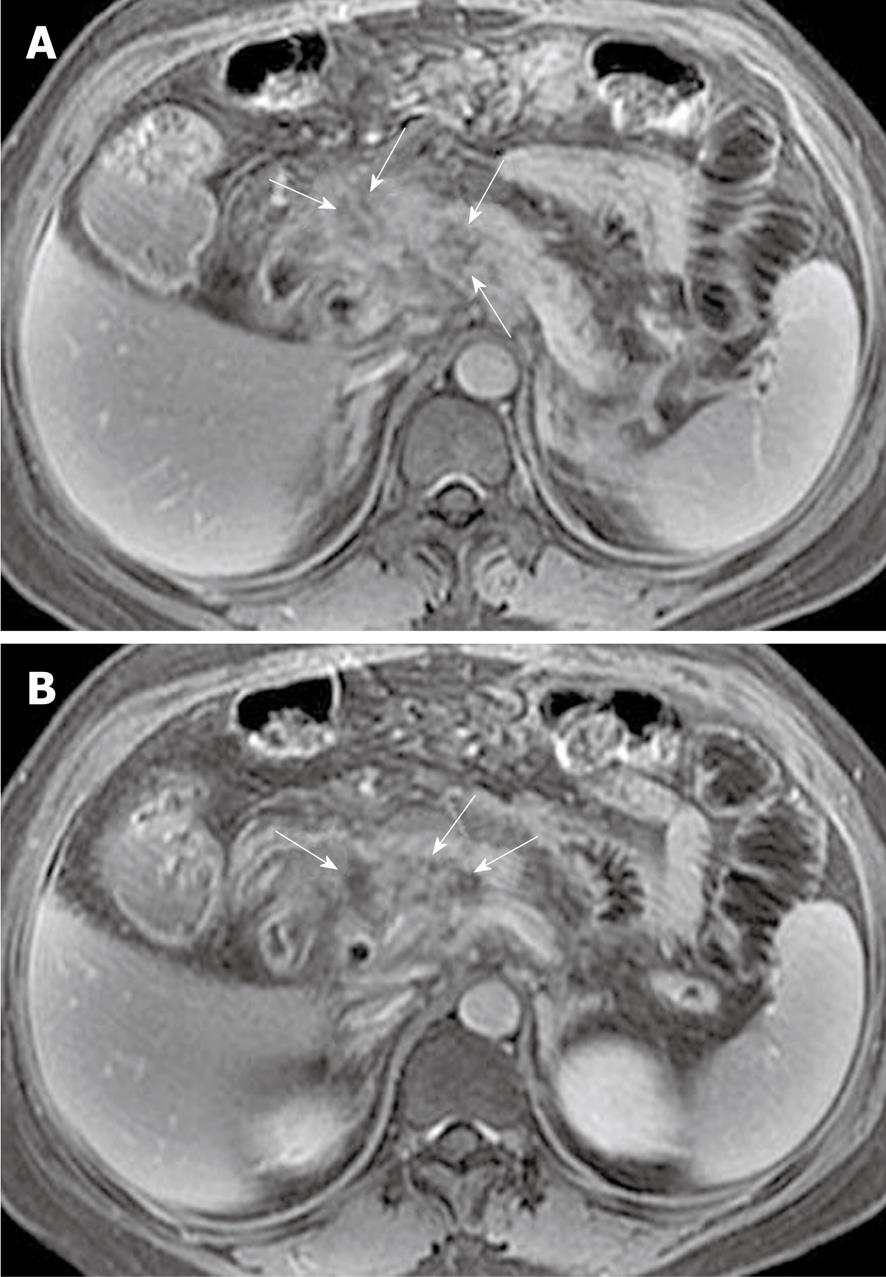
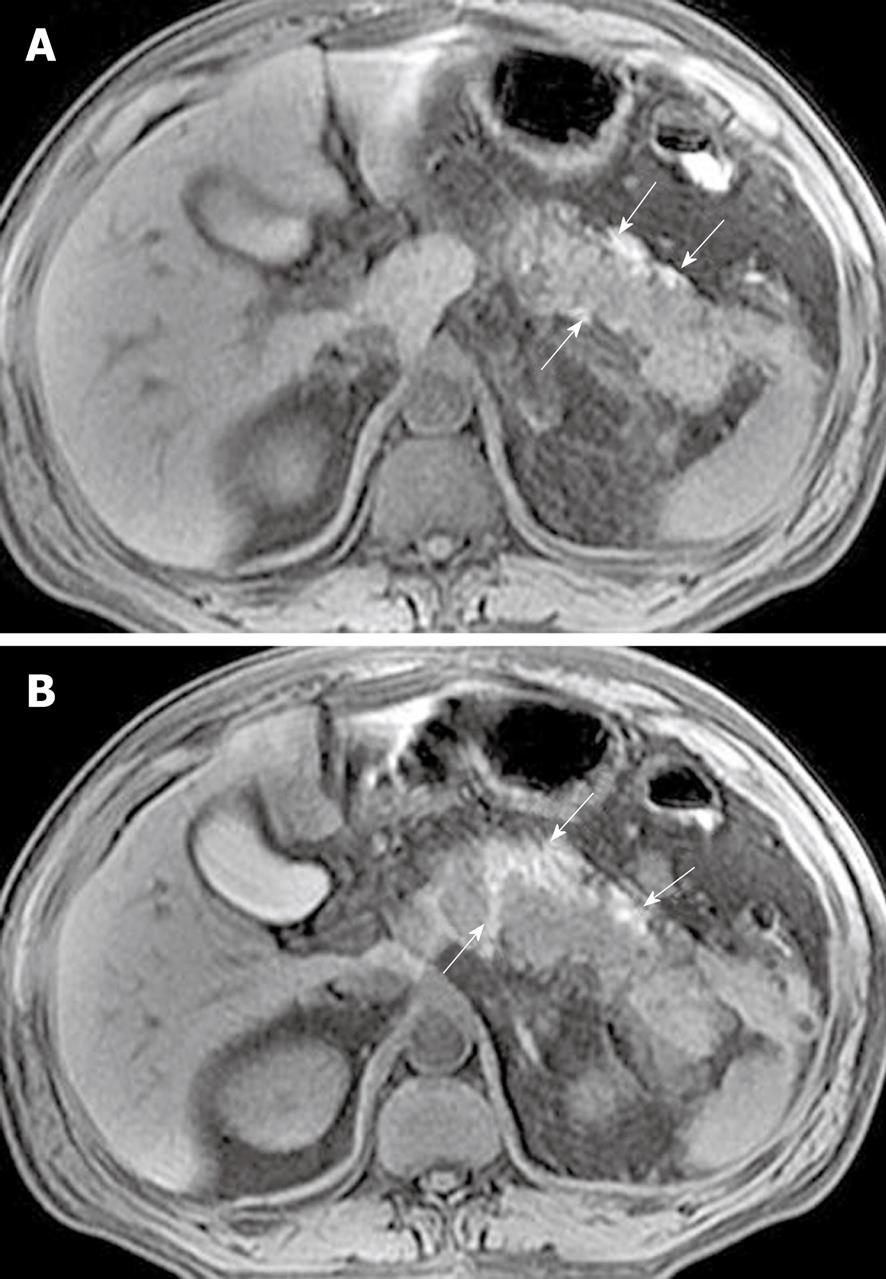
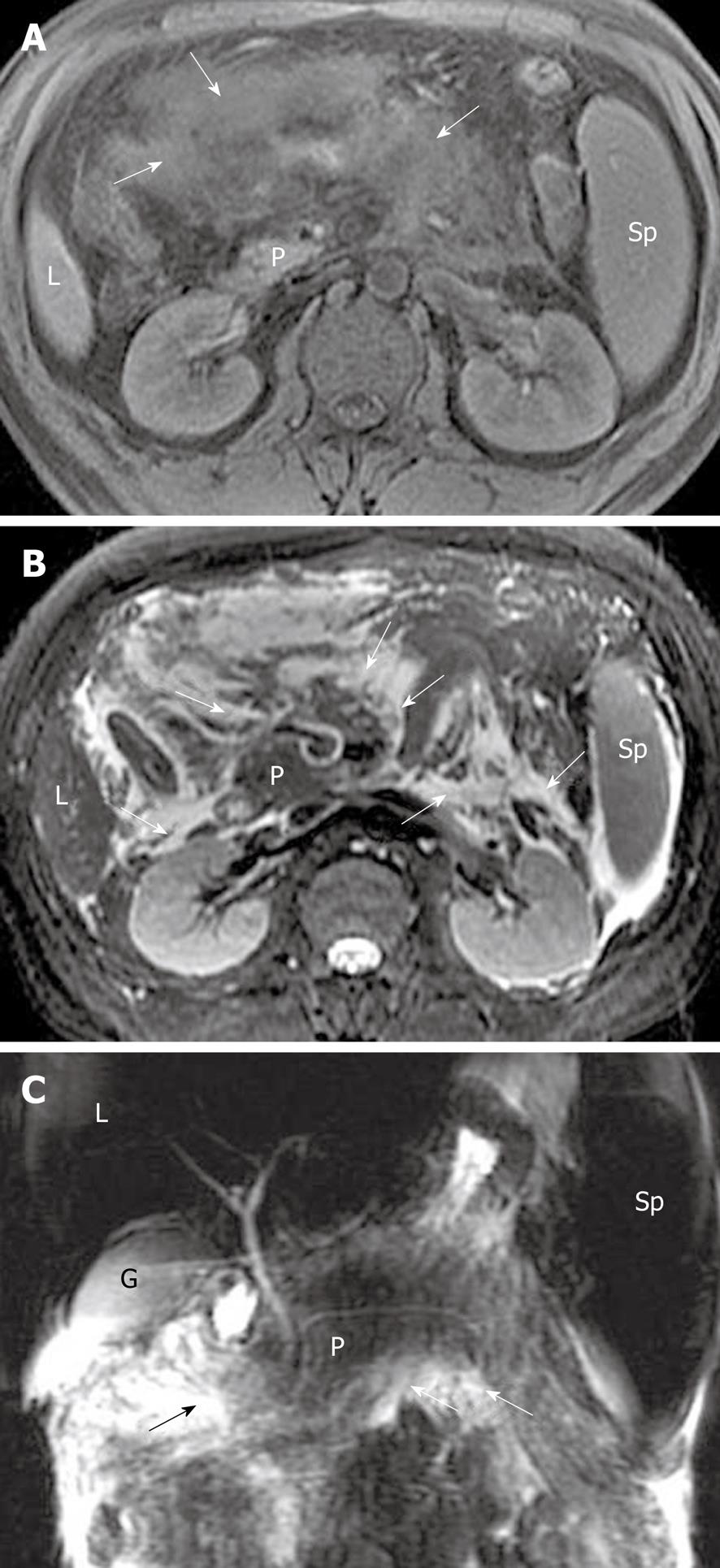
Peripancreatic and retroperitoneal fat changes: The pancreatic capsule almost forms no barrier to inflammatory extension of acute pancreatitis, because of the previously mentioned anatomical characteristics. Extravasation of activated pancreatic enzymes (such as pancreatic lipase) after an episode of acute pancreatitis can induce the development of peripancreatic fat edema and necrosis to variable degrees; a common phenomenon that occurs in patients with or without parenchymal necrosis[2,8,13]. However, it is difficult to differentiate pathological changes of peripancreatic fat edema from peripancreatic fat necrosis because they are combined with each other and have similar findings on MR images. Some patients with severe pancreatitis have shown extensive fat edema and necrosis located in omental and mesenteric zones and the extrapancreatic retroperitoneal fatty tissue regions (Figure 10). The inflammatory involvement of retroperitoneal fat edema and necrosis is often consistent with an anterior pararenal space of the left kidney, the right kidney, or both. Cross-sectional T2-weighted imaging and fat-suppressed T1-weighted imaging combined with MRCP might be helpful for comprehensively assessing the range of peripancreatic and retroperitoneal fat changes in acute pancreatitis, which also has an advantage over transverse CT examinations.
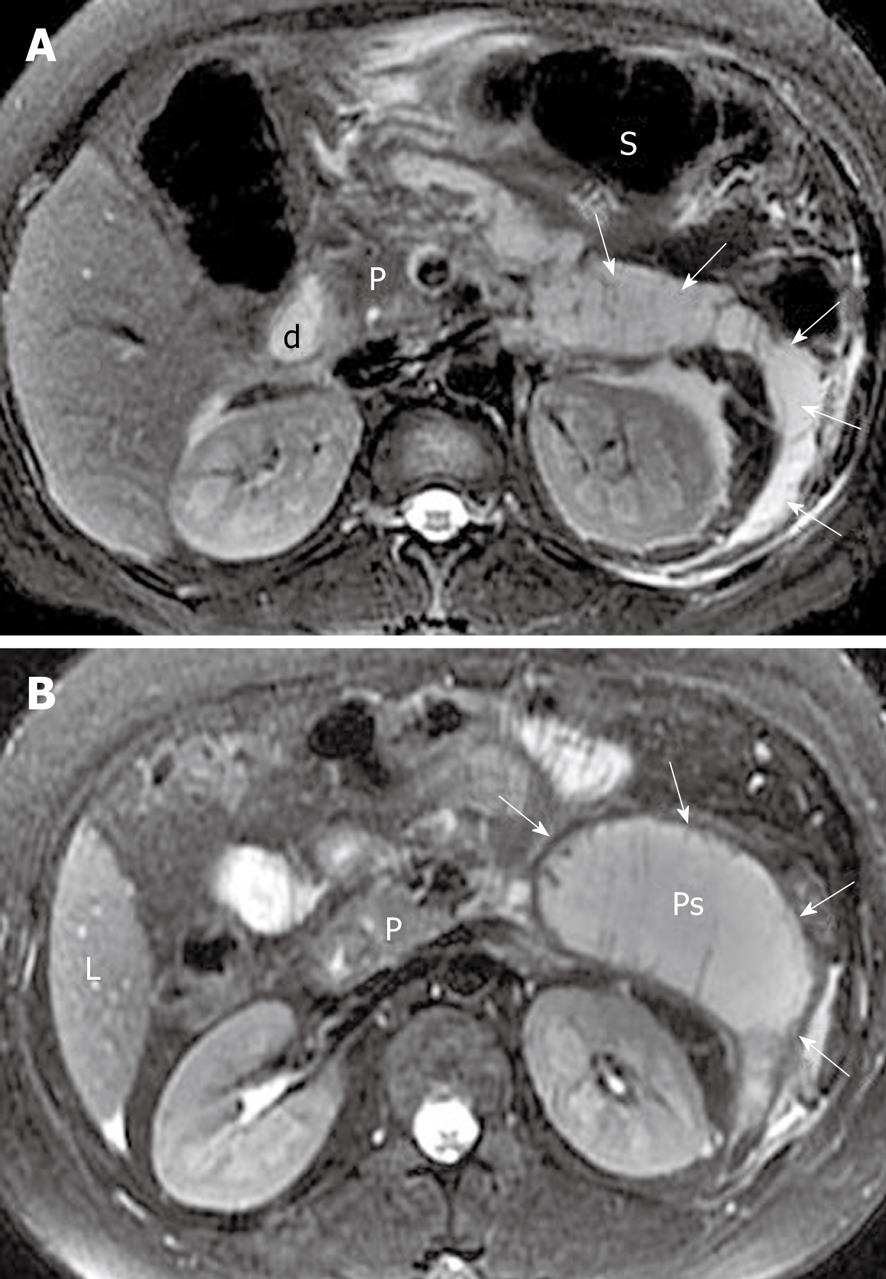
Peripancreatic and retroperitoneal fluid collections: As previously mentioned, extravasation of activated pancreatic enzymes results in extrapancreatic chemical injury and multiple areas of fat edema and tissue necrosis, which favor secondary peripancreatic and retroperitoneal fat tissue liquefaction and further develop fluid collections[8,21]. MRI, particularly fat-suppressed T2-weighted imaging, can accurately depict simple or complex fluid collections. The latter one includes: (1) areas of hemorrhage in fluid collections; or (2) pancreatic and/or fat tissue necrosis pieces or fragments in fluid collections (Figure 11). The sequelae of fluid collections might: (1) be absorbed completely; (2) progress to pseudocysts; or (3) be concomitant with infection to form pancreatic cellulitis or abscesses (mentioned below).
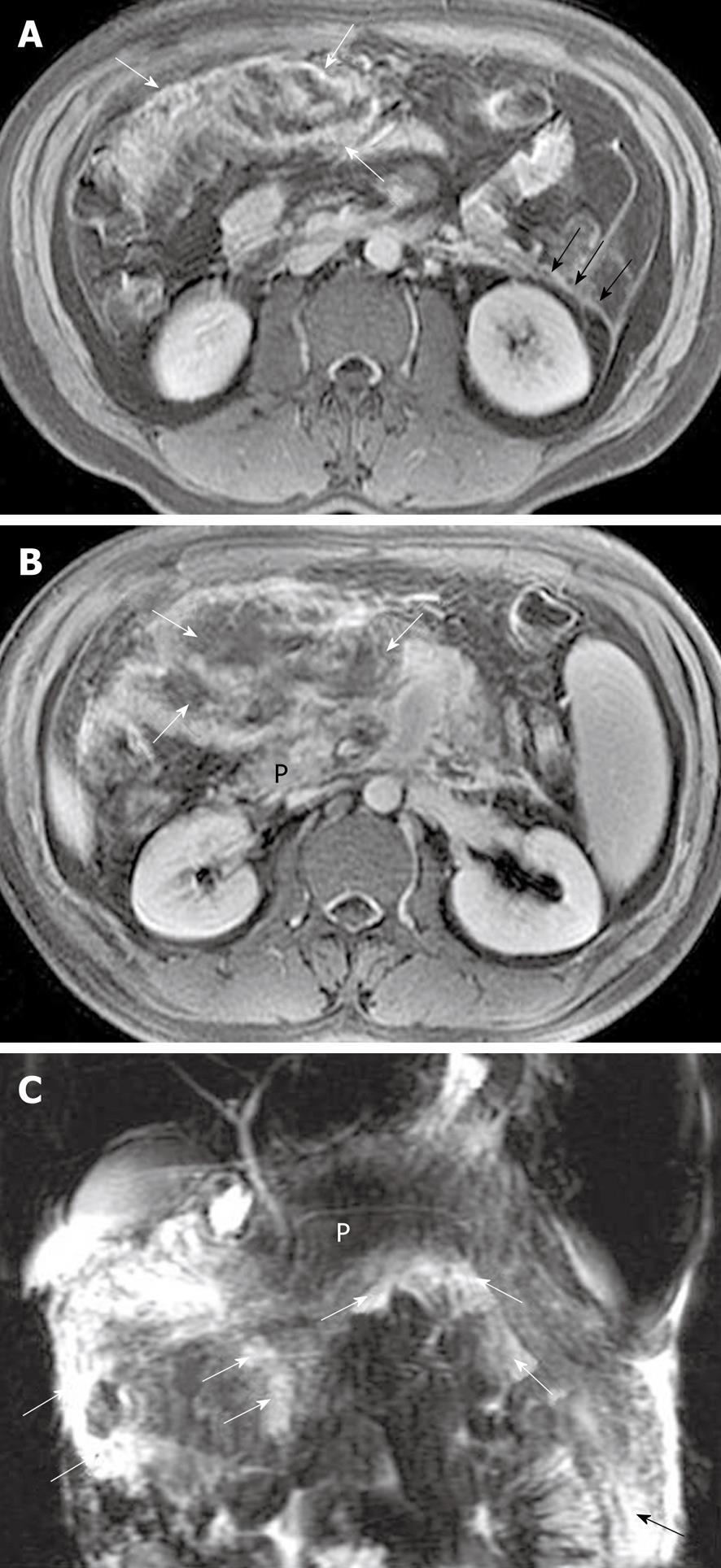
Peritoneal and fascial changes: Acute pancreatitis can exhibit peritoneal and fascial abnormalities. Both of them are secondary to extrapancreatic inflammatory extension and occur simultaneously in most patients with this disease[7,8]. Anterior fascia of the kidney is the most common one that is invaded by peripancreatic inflammatory extension, due to its proximity to the pancreas. The inflammatory extravasation also can involve one or several structures of the peritoneum such as omentum, mesentery, and colic mesentery[1,8]. With the development and progress of this disease, these peritoneal structures develop edema or swelling with/without fat necrosis or fluid collections. Contrast-enhanced MRI can show the irregular thickening and heterogeneous enhancement of the intestinal wall, and MRCP can manifest the range of edematous mesentery and fluid collections with hyperintensity (Figure 12).
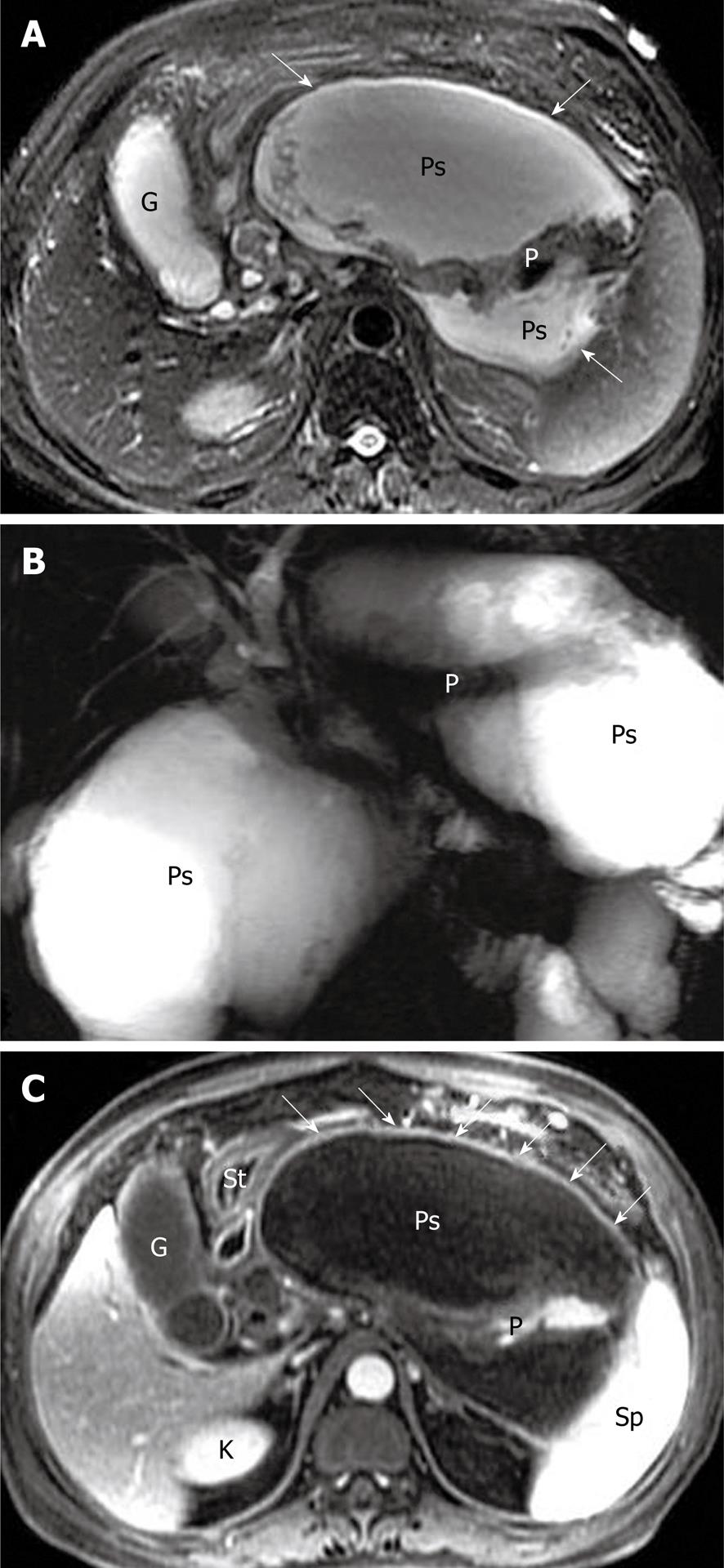
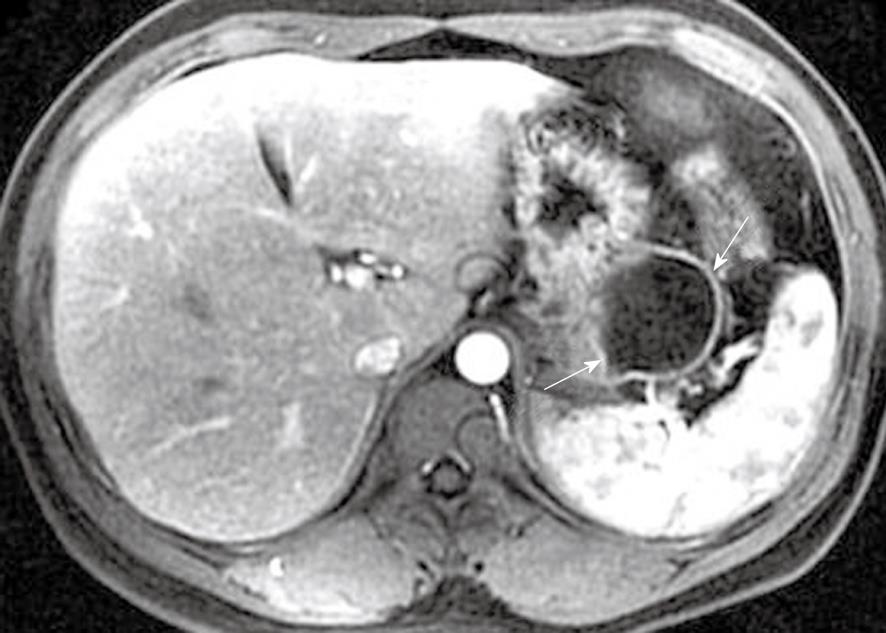
Pancreatic pseudocyst is a very common type of local complications of acute pancreatitis, and it develops in most patients who are already suffering from post-necrotic pancreatitis[7,8,26]. It is defined as a localized collection of pancreatic juices by a fibrous membrane without an epithelium, and occurs approximately 4-6 wk after a first episode of acute pancreatitis[27,28]. According to spatial locations, pancreatic pseudocysts are classified as intraparenchymal (within the pancreatic tissue) or extrapancreatic (surrounding the pancreas), or both. The intraparenchymal pseudocyst might be communicating with pancreatic ducts and associated with partial pancreatic ductal obstruction, whereas the extrapancreatic pseudocyst might be related to the development of peripancreatic fluid collections[7,27,28]. The other classification is based on whether the pseudocyst is accompanied by mucus, protein and hemorrhage. The simple pseudocyst is present as a round or oval fluid collection surrounded by a thin or thick wall and homogeneous water-like signal intensity (Figure 13), and the complex pseudocyst is present as a round or oval heterogeneous lesion dominated by hyperintensity on T1-weighted images with fat suppression (Figure 14). In our experience, when one or several pseudocysts are suspected of bleeding, MRI is more favorable than CT (previously mentioned in pancreatic hemorrhage).
In acute pancreatitis, secondary bacterial contamination can occur in the setting of: (1) pancreatic necrosis; (2) peripancreatic fat necrosis; (3) retroperitoneal fat necrosis; (4) peripancreatic fluid collections; or (5) pseudocysts. It has been noted that 40%-70% of patients with pancreatic necrosis have secondary necrosis or severe bacterial contamination[8,29]. Pancreatic liquefied necrosis with or without multiple areas of fat necrosis might progress to pancreatic cellulitis, whereas peripancreatic fluid collections or pseudocysts might become infected to develop abscesses. Once the infectious complications occur, whether pancreatic cellulitis or abscesses, they constitute a major mortality risk for patients with acute pancreatitis.
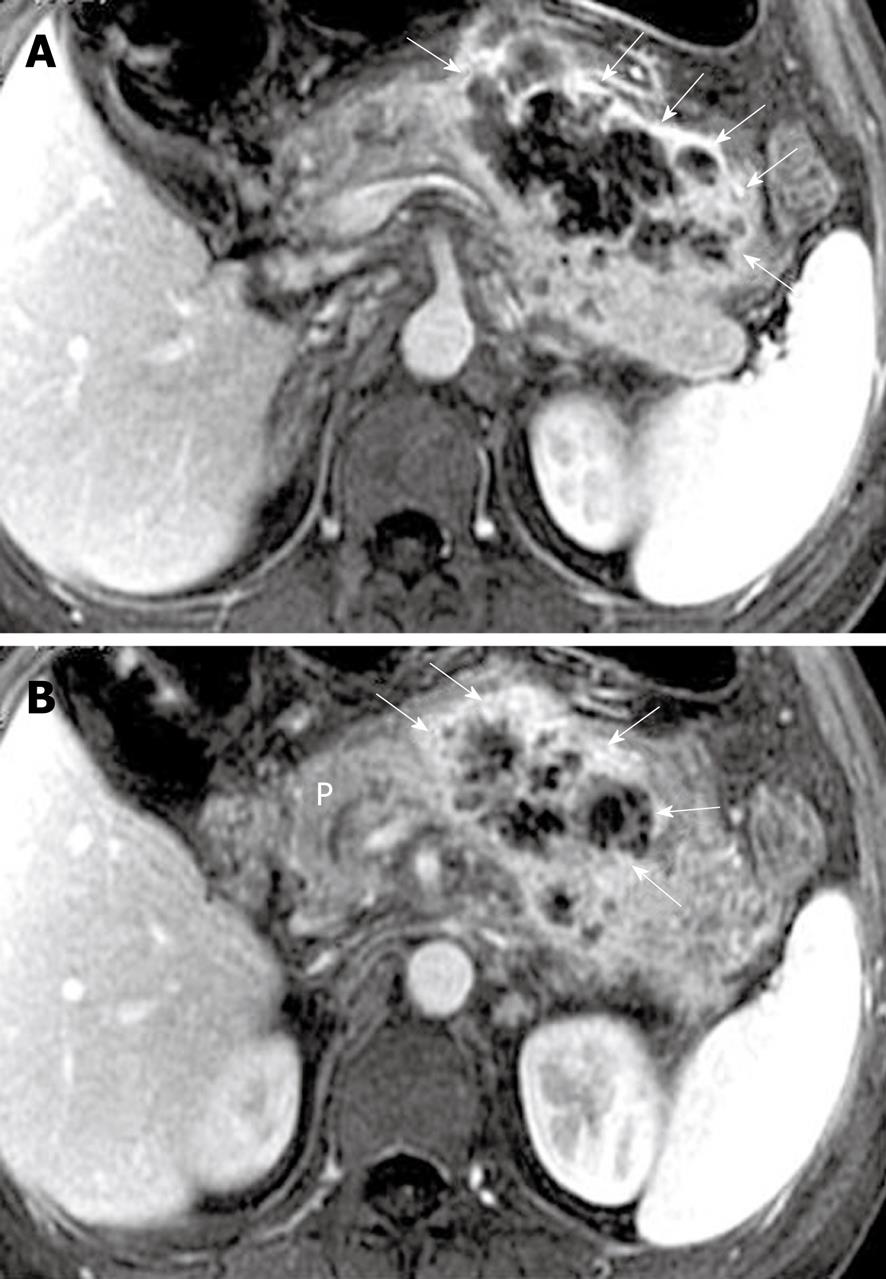
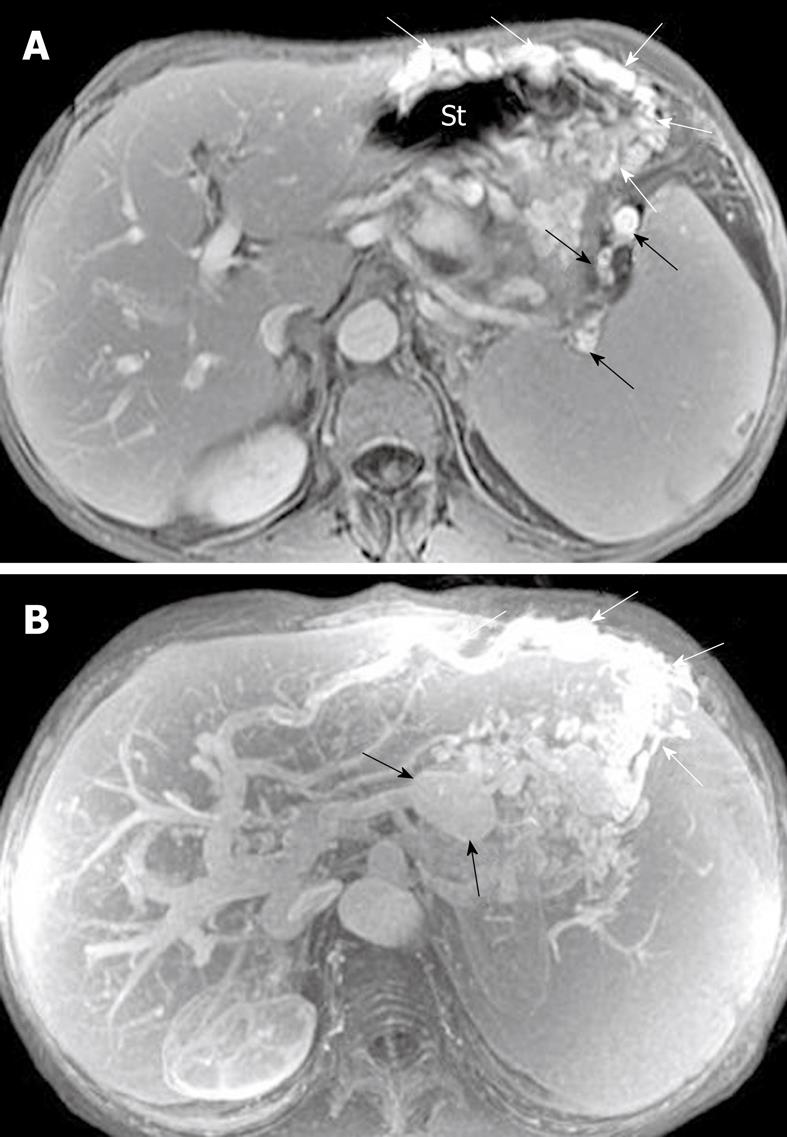
Pancreatic cellulitis, with an incidence that varies from 8.3% to 10.6%, is clinically difficult to differentiate from pancreatic abscess[30]. It is an inflammatory entity that is situated at the intraparenchymal region of the pancreas and is associated with peripancreatic zones, as a result of pathological pancreatic swelling and necrosis, inflammatory cell infiltration and peripancreatic fat tissue necrosis[29,30]. After administration of contrast material, an ill-defined multilocular mass (like “hornets’ nest”) exhibits ring-like and separated enhanced areas with fragments of post-necrotic gland and fat tissue, and non-enhanced tissue liquefaction (Figure 15).
Pancreatic abscess, with an incidence that varies from 1% to 9% and a high mortality, occurs in the course of 2-5 wk after the onset of acute pancreatitis[31]. Unfortunately, in our clinical practice, it is not easy to differentiate pancreatic abscesses from pseudocysts due to the similar MR findings. Thus, confirmed diagnosis often depends on fine-needle aspiration biopsy of a lesion[28,31]. The bubble or gas attenuation within a lesion could indicate pancreatic abscess on non-enhanced CT images, however, in our experience, bubble or gas in an abscess cannot be easily identified on MR images. Therefore, we emphasize that the pancreatic abscess should be suspected to occur when patients have suffered from acute pancreatitis and develop fever, tachycardia, abdominal distention or an encapsulated liquid collection with an enhancing wall on MRI (Figure 16).
Vascular involvement is a common but ignorable local complication of acute pancreatitis[2,7,15,21]. Intraparenchymal and peripancreatic extravasation of activated digestive enzymes is responsible for damage to the pancreatic vascular network, including feeding arteries and draining veins. A spectrum of vascular abnormalities includes: (1) vasculitis; (2) artery bleeding or pseudoaneurysm; (3) phlebothrombosis or vein occlusion; (4) pancreatic regional portal hypertension; and (5) combination of these. MRA can be performed to supplement the information for visualization of vascular complications, besides common axial contrast-enhanced MRI.
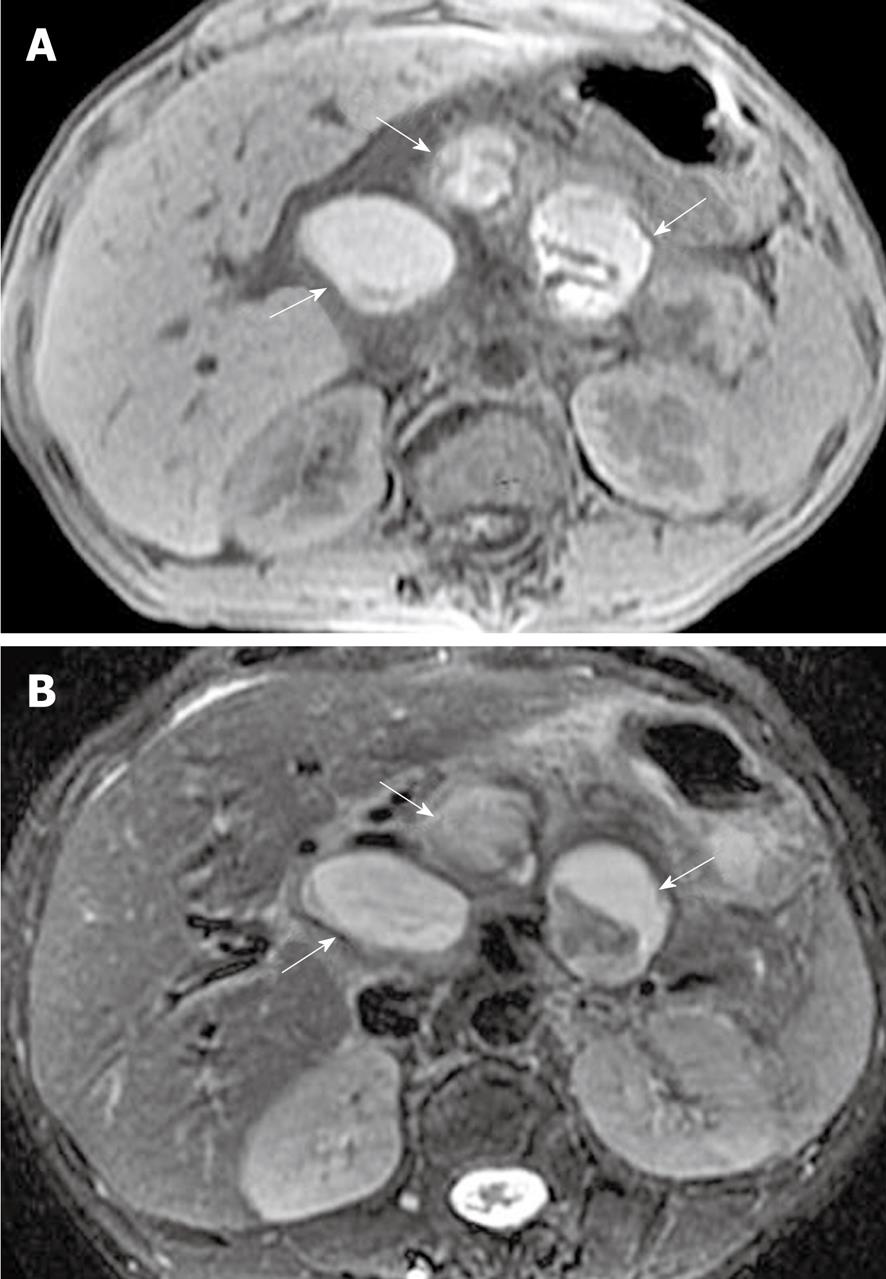
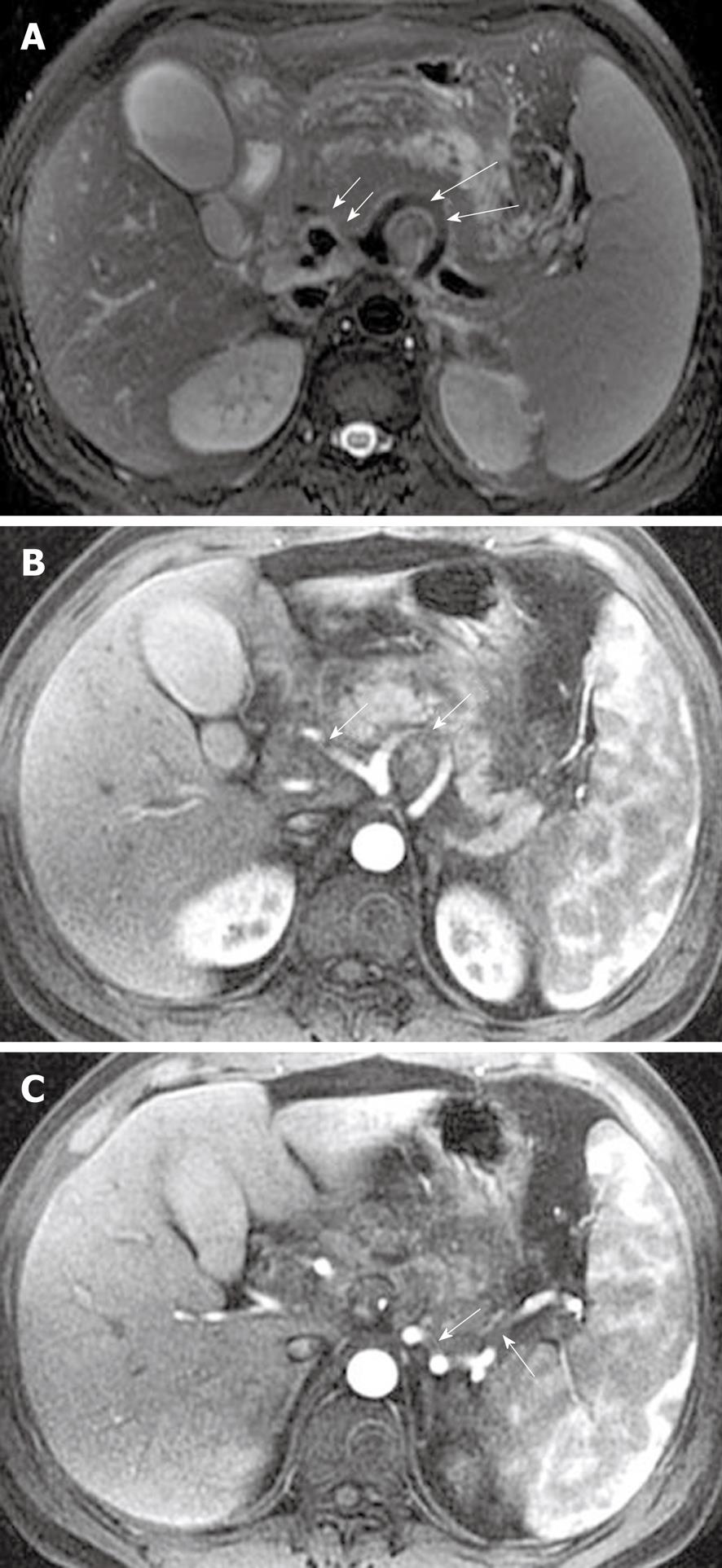
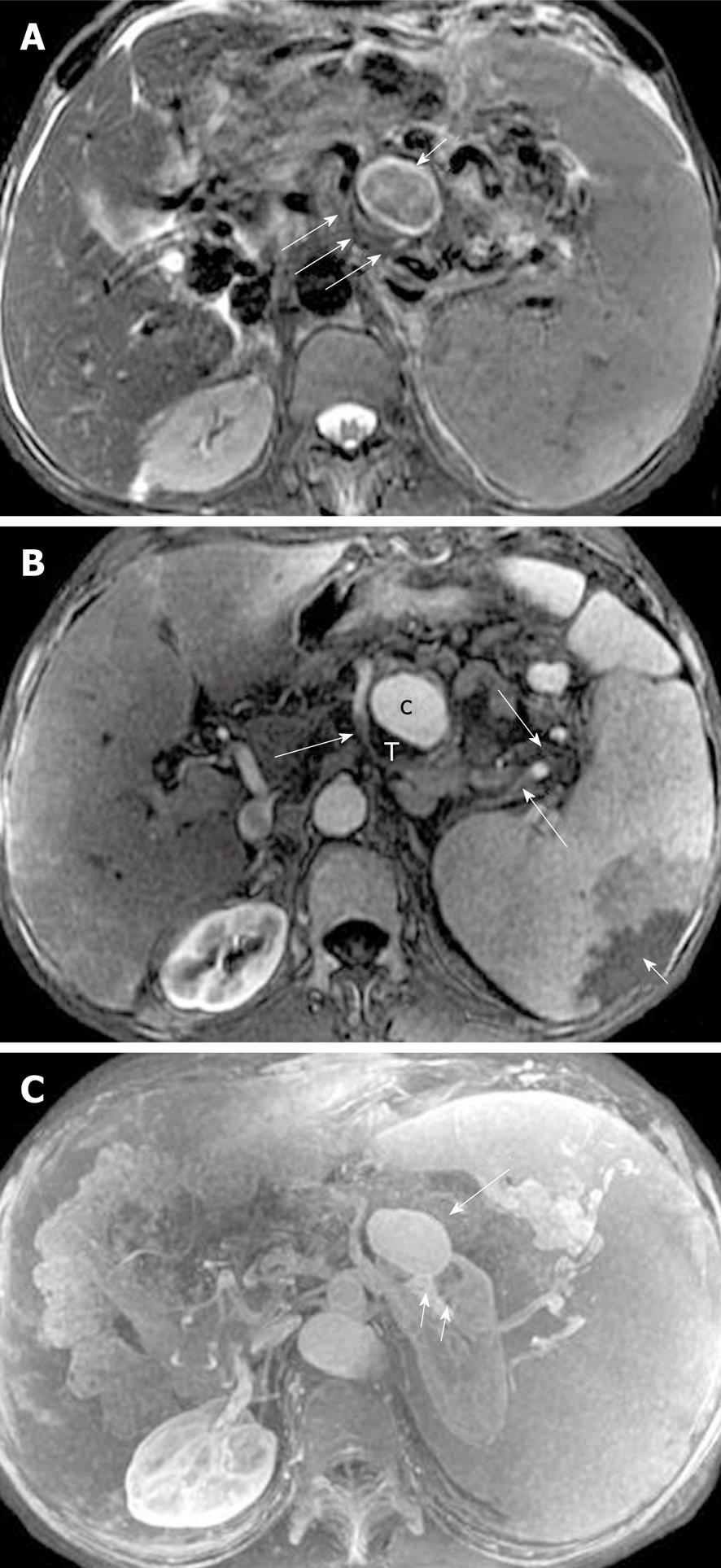
The splenic, gastroduodenal and pancreaticoduodenal arteries are more frequently involved than other peripancreatic arteries[23,32]. The arterial invasion exhibits the loss of the vascular flow voiding effect (“black blood”) on T2-weighted images, and poor or no enhancement on MR images during the contrast-enhanced arterial phase. The obscure and rough edges of the involved arterial wall are indicative of vasculitis (Figure 17). Vasculitis associated with incomplete arterial occlusion might favor secondary abdominal organ infarction, for example, of the spleen (mentioned below).
Pseudoaneurysm, a relatively uncommon and delayed complication of acute pancreatitis, can be a life-threatening emergency if rupture occurs[23]. The cavity of pseudoaneurysm communicates with the involved artery and can show marked enhancement on MR images after the administration of contrast material, whereas the non-enhanced zones reflect the mural thrombosis in the pseudoaneurysm (like the Chinese “Yin-Yang” sign)[33] (Figure 18).
The splenic vein is the most common of the veins that are involved, by inflammatory extension due to its proximity to the pancreas[23,32]. The involved vein might be complicated by local thrombosis and occlusion. MRI shows the loss of the vascular flow voiding effect on T2-weighted images. After administration of contrast agent, an intravenous asymmetrical filling defect can be seen on venous phase images (Figure 19).
Additionally, venous thrombosis and occlusion, particularly of the splenic vein, can result in pancreatogenic regional portal hypertension[34,35]. It shows the establishment of multiple, conspicuous collateral circulation, which usually involves gastric short veins around the fundus of the stomach, gastroepiploic veins close to the greater curvature of stomach, and splenic veins adjacent to the spleen hilum. Contrast-enhanced MRI shows numerous and circuitous blood vessels with abnormal enhancement, and splenomegaly with or without splenic infarction (Figure 20).
Pancreatic inflammatory extravasation in some patients with severe pancreatitis can result in extrapancreatic multiple injury, and even present as subcutaneous and intermuscular soft tissue involvement such as fat edema, swelling, necrosis, and small fluid collections (Figure 21).
The comprehensive assessment of acute pancreatitis is based on clinical, laboratory and imaging evaluation. MRI is an excellent noninvasive modality of choice to help stage the severity of inflammatory processes, and detect the presence and extent of pancreatic necrosis. The presence and development of complications of acute pancreatitis such as hemorrhage, fluid collections, pseudocysts, abscesses, pseudoaneurysm, and venous thrombosis are well-demonstrated by MRI.
| 1. | Scaglione M, Casciani E, Pinto A, Andreoli C, De Vargas M, Gualdi GF. Imaging assessment of acute pancreatitis: a review. Semin Ultrasound CT MR. 2008;29:322-340. |
| 2. | Balthazar EJ, Freeny PC, vanSonnenberg E. Imaging and intervention in acute pancreatitis. Radiology. 1994;193:297-306. |
| 3. | Lenhart DK, Balthazar EJ. MDCT of acute mild (nonnecrotizing) pancreatitis: abdominal complications and fate of fluid collections. AJR Am J Roentgenol. 2008;190:643-649. |
| 4. | Larvin M. Management of infected pancreatic necrosis. Curr Gastroenterol Rep. 2008;10:107-114. |
| 5. | Sandrasegaran K, Tann M, Jennings SG, Maglinte DD, Peter SD, Sherman S, Howard TJ. Disconnection of the pancreatic duct: an important but overlooked complication of severe acute pancreatitis. Radiographics. 2007;27:1389-1400. |
| 6. | Kwak SW, Kim S, Lee JW, Lee NK, Kim CW, Yi MS, Kim GH, Kang DH. Evaluation of unusual causes of pancreatitis: role of cross-sectional imaging. Eur J Radiol. 2009;71:296-312. |
| 7. | Matos C, Cappeliez O, Winant C, Coppens E, Devière J, Metens T. MR imaging of the pancreas: a pictorial tour. Radiographics. 2002;22:e2. |
| 8. | Balthazar EJ. Acute pancreatitis: assessment of severity with clinical and CT evaluation. Radiology. 2002;223:603-613. |
| 9. | Saokar A, Rabinowitz CB, Sahani DV. Cross-sectional imaging in acute pancreatitis. Radiol Clin North Am. 2007;45:447-460, viii. |
| 10. | Balci NC, Bieneman BK, Bilgin M, Akduman IE, Fattahi R, Burton FR. Magnetic resonance imaging in pancreatitis. Top Magn Reson Imaging. 2009;20:25-30. |
| 11. | Bollen TL, van Santvoort HC, Besselink MG, van Es WH, Gooszen HG, van Leeuwen MS. Update on acute pancreatitis: ultrasound, computed tomography, and magnetic resonance imaging features. Semin Ultrasound CT MR. 2007;28:371-383. |
| 12. | Stimac D, Miletić D, Radić M, Krznarić I, Mazur-Grbac M, Perković D, Milić S, Golubović V. The role of nonenhanced magnetic resonance imaging in the early assessment of acute pancreatitis. Am J Gastroenterol. 2007;102:997-1004. |
| 13. | Robinson PJ, Sheridan MB. Pancreatitis: computed tomography and magnetic resonance imaging. Eur Radiol. 2000;10:401-408. |
| 14. | Tkacz JN, Anderson SA, Soto J. MR imaging in gastrointestinal emergencies. Radiographics. 2009;29:1767-1780. |
| 15. | Kim DH, Pickhardt PJ. Radiologic assessment of acute and chronic pancreatitis. Surg Clin North Am. 2007;87:1341-1358, viii. |
| 16. | Kim YK, Kim CS, Han YM. Role of fat-suppressed t1-weighted magnetic resonance imaging in predicting severity and prognosis of acute pancreatitis: an intraindividual comparison with multidetector computed tomography. J Comput Assist Tomogr. 2009;33:651-656. |
| 17. | Viremouneix L, Monneuse O, Gautier G, Gruner L, Giorgi R, Allaouchiche B, Pilleul F. Prospective evaluation of nonenhanced MR imaging in acute pancreatitis. J Magn Reson Imaging. 2007;26:331-338. |
| 18. | Amano Y, Oishi T, Takahashi M, Kumazaki T. Nonenhanced magnetic resonance imaging of mild acute pancreatitis. Abdom Imaging. 2001;26:59-63. |
| 19. | Jain R, Levine M. Correction to the relative risk calculation for gadolinium-enhanced MR imaging and nephrogenic systemic fibrosis. Radiology. 2010;255:307-308; author reply 308. |
| 20. | Kim YK, Ko SW, Kim CS, Hwang SB. Effectiveness of MR imaging for diagnosing the mild forms of acute pancreatitis: comparison with MDCT. J Magn Reson Imaging. 2006;24:1342-1349. |
| 21. | Arvanitakis M, Delhaye M, De Maertelaere V, Bali M, Winant C, Coppens E, Jeanmart J, Zalcman M, Van Gansbeke D, Devière J. Computed tomography and magnetic resonance imaging in the assessment of acute pancreatitis. Gastroenterology. 2004;126:715-723. |
| 22. | Zhang XM, Feng ZS, Zhao QH, Xiao CM, Mitchell DG, Shu J, Zeng NL, Xu XX, Lei JY, Tian XB. Acute interstitial edematous pancreatitis: Findings on non-enhanced MR imaging. World J Gastroenterol. 2006;12:5859-5865. |
| 23. | Miller FH, Keppke AL, Dalal K, Ly JN, Kamler VA, Sica GT. MRI of pancreatitis and its complications: part 1, acute pancreatitis. AJR Am J Roentgenol. 2004;183:1637-1644. |
| 24. | Hirota M, Kimura Y, Ishiko T, Beppu T, Yamashita Y, Ogawa M. Visualization of the heterogeneous internal structure of so-called "pancreatic necrosis" by magnetic resonance imaging in acute necrotizing pancreatitis. Pancreas. 2002;25:63-67. |
| 25. | Arvanitakis M, Koustiani G, Gantzarou A, Grollios G, Tsitouridis I, Haritandi-Kouridou A, Dimitriadis A, Arvanitakis C. Staging of severity and prognosis of acute pancreatitis by computed tomography and magnetic resonance imaging-a comparative study. Dig Liver Dis. 2007;39:473-482. |
| 26. | Degen L, Wiesner W, Beglinger C. Cystic and solid lesions of the pancreas. Best Pract Res Clin Gastroenterol. 2008;22:91-103. |
| 27. | Kim YH, Saini S, Sahani D, Hahn PF, Mueller PR, Auh YH. Imaging diagnosis of cystic pancreatic lesions: pseudocyst versus nonpseudocyst. Radiographics. 2005;25:671-685. |
| 28. | Andrén-Sandberg A, Dervenis C. Pancreatic pseudocysts in the 21st century. Part I: classification, pathophysiology, anatomic considerations and treatment. JOP. 2004;5:8-24. |
| 29. | Maroun Marun C, Uscanga L, Lara F, Passareli L, Quiroz-Ferrari F, Robles-Díaz G, Campuzano-Fernández M. [Pancreatic phlegmon: a potentially fatal form of acute pancreatitis]. Rev Invest Clin. 1992;44:507-512. |
| 30. | Fan ST, Choi TK, Chan FL, Lai EC, Wong J. Pancreatic phlegmon: what is it? Am J Surg. 1989;157:544-547. |
| 31. | Hill MC, Dach JL, Barkin J, Isikoff MB, Morse B. The role of percutaneous aspiration in the diagnosis of pancreatic abscess. AJR Am J Roentgenol. 1983;141:1035-1038. |
| 32. | Mortelé KJ, Mergo PJ, Taylor HM, Wiesner W, Cantisani V, Ernst MD, Kalantari BN, Ros PR. Peripancreatic vascular abnormalities complicating acute pancreatitis: contrast-enhanced helical CT findings. Eur J Radiol. 2004;52:67-72. |
| 34. | Wang CX, Li R, Cai XJ, Peng Z, Liang FQ. [Diagnosis and treatment of pancreatogenic portal hypertension: analysis of 26 cases]. Zhonghua Yixue Zazhi. 2008;88:395-397. |
| 35. | Liu QD, Zhou NX, Zhang WZ, Wang MQ. Diagnosis and management of regional portal hypertension. Chin J Dig Dis. 2005;6:87-92. |
Peer reviewer: Charikleia Triantopoulou, MD, PhD, Head of Radiology Department, Konstantopouleio general Hospital, 3-5, Agias Olgas street, 14233 N. Ionia, Athens, Greece
S- Editor Cheng JX L- Editor Kerr C E- Editor Zheng XM