Published online Oct 28, 2010. doi: 10.4329/wjr.v2.i10.384
Revised: August 27, 2010
Accepted: September 3, 2010
Published online: October 28, 2010
Arterial spin labeling (ASL) is a magnetic resonance imaging technique for measuring tissue perfusion using a freely diffusible intrinsic tracer. As compared with other perfusion techniques, ASL offers several advantages and is now available for routine clinical practice in many institutions. Its noninvasive nature and ability to quantitatively measure tissue perfusion make ASL ideal for research and clinical studies. Recent technical advances have increased its sensitivity and also extended its potential applications. This review focuses on some basic knowledge of ASL perfusion, emerging techniques and clinical applications in neuroimaging.
- Citation: Petcharunpaisan S, Ramalho J, Castillo M. Arterial spin labeling in neuroimaging. World J Radiol 2010; 2(10): 384-398
- URL: https://www.wjgnet.com/1949-8470/full/v2/i10/384.htm
- DOI: https://dx.doi.org/10.4329/wjr.v2.i10.384
Perfusion refers to the delivery of oxygen and nutrients to tissue by means of blood flow. It is classically measured using a diffusible tracer that can exchange between the vascular compartment and tissue and is quantified in tissue-specific units of mL/g per minute. However, in clinical practice, the term “perfusion imaging” refers to a broad range of quantitative and qualitative measures of blood flow and blood hemodynamic properties, including blood volume, blood velocities and blood transit times. These measurements have been made with exogenously administrated tracers detected by a variety of imaging techniques, such as positron emission tomography (PET), single-photon emission computed tomography (SPECT), CT perfusion and dynamic susceptibility contrast (DSC) magnetic resonance imaging (MRI) imaging.
DSC MRI has been the primary method to assess cerebral perfusion in clinical settings. It relies on the measurement of the T2 or T2* signal decrease during the first passage of an exogenous endovascular susceptibility contrast agent through the cerebral vasculature[1]. It requires ultrafast imaging, such as echo planar imaging (EPI), principles of echo shifting with a train of observations (PRESTO) or spiral imaging. Gradient echo (GRE) or spin echo (SE) sequences can be used, but the signal change (∆T2*) measured with GRE is greater than that measured with SE (∆T2), allowing one to use a short echo time and less contrast agent. DSC is a qualitative method and relative changes in multiple hemodynamic parameters, including cerebral blood flow (CBF), cerebral blood volume (CBV), mean transit time (MTT) and time to peak (TTP) can be estimated from temporal characteristics of the first-pass time course[2]. The use of an exogenous tracer and the qualitative nature of this technique are the main disadvantages of DSC MRI perfusion.
Arterial spin labeling (ASL) is a non-ionizing and completely non-invasive MRI technique for measuring tissue perfusion (blood flow), which uses magnetically labeled arterial blood water protons as an endogenous tracer[3,4]. These benefits make ASL very suitable for perfusion studies in healthy individuals, patients with renal insufficiency and those who need repetitive follow-ups. It is also an impressive method for studying perfusion in pediatric populations in which the use of radioactive tracers or exogenous contrasts agents may be restricted[5]. Another advantage of ASL, as compared with conventional bolus techniques, is that ASL can be quantitative. Absolute perfusion quantification allows recognition of global hypo- or hyper-perfusion states and also permits comparison between multiple measurements in a longitudinal study[2,3,6,7].
ASL MRI perfusion was conceived more than 15 years ago[2,8,9]. The original ASL method for labeling arterial spins was proposed by Williams et al[9]. In 1992 these authors measured rat brain cerebral blood flow using water as a freely diffusible tracer. Two years later, Detre et al[2] extended the application of ASL to human brain studies at 1.5T MRI scans. Since then the technique has been mostly used in research, mainly because of the complex post-processing requirements and technical difficulties. Recent refinements in sequence robustness, decreased acquisition time, increased image resolution and lesser artifacts, as well as advances in post processing capabilities have made ASL available for routine clinical practice[6]. In order to interpret ASL perfusion studies, basic knowledge about technique, pitfalls and limitations is important. This review provides some basic principles of ASL, emerging techniques and clinical applications in neuroimaging.
The goal of ASL MRI perfusion is to produce a “flow labeled image or tag image” and a “control image” in which the static tissue signals are identical, but the magnetization of the inflowing blood is different[5]. In this technique, arterial blood water is magnetically tagged before it enters the tissue of interest. This is performed with a radiofrequency (RF) pulse that inverts or saturates the water protons in flowing blood supplying the imaged region[4,7,9]. By adding a delay between labeling and image acquisition, called inversion delay (TI) in pulsed ASL (PASL) or post-labeling delay (PLD) in continuous ASL (CASL)[10], labeled blood is allowed to reach the capillaries where it gives rise to perfusion signal[5,6]. The magnetic tracer decays with the longitudinal relaxation rate T1[2,11] and the relaxation time for water in blood or tissues is about 1- 2 s, thus only small amounts of arterial spin-labeled water accumulate in the brain[2,4]. ASL signal-to-noise ratio (SNR) is inherently low, because the signal from the labeled inflowing blood is only 0.5%-1.5% of the full tissue signal. This signal depends on many parameters, such as flow, T1 of blood and tissue, as well as the time it takes blood to travel from the site of labeling to imaging region[5]. Tag and control images are acquired in a temporally interleaved fashion[12]. Subtraction of labeled images from control images eliminates static tissue signal and the remaining signal is a relative measure of perfusion proportional to cerebral blood flow (CBF)[6]. Multiple labeled-control image pairs are acquired and averaged for the generation of CBF maps.
The temporal resolution is also inherently poor. The low SNR combined with the poor temporal resolution results in a low contrast-to-noise ratio (CNR). A repetition time (TR) of 2 s or more is typically used in ASL experiments. Therefore, one tag and control image pair is acquired every 4 s. A typical acquisition lasts between 5 and 10 min. Methods for improving temporal resolution include turbo-ASL[13] and single-shot ASL[14]. Turbo-ASL shortens the imaging time by using much shorter TR than those usually employed with little reduction in ASL signal. In single-shot ASL techniques, background suppression is used to suppress static tissue eliminating the need for a control image. These techniques can improve temporal resolution, but the quantification of the signal is more complex[15]. In most ASL applications EPI is used for ASL acquisition because of its high SNR and fast acquisition time. However, EPI can introduce distortions in regions of high magnetic field susceptibility, particularly at the base of the brain[4]. Recently, fast three-dimensional (3D) sequences have been introduced for ASL image acquisition to improve image quality, providing higher SNR and reducing image distortion.
Another approach for increasing SNR in ASL is the use of a phase array receiver coil, which can be optimized for parallel imaging to shorten the image acquisition time. Although there is usually reduction in SNR with parallel imaging, in ASL perfusion much of this SNR cost can be regained through shortened TE along with reduced distortion from susceptibility artifacts[4].
High magnetic field strength is also beneficial for ASL, not only does image SNR increase but T1 also lengthens, allowing more spin label to accumulate[4]. The pitfalls of high field strength are the susceptibility to field inhomogeneities and higher energy deposition[16].
Since ASL is a subtraction technique, it is sensitive to subject movement. Filters have been developed to detect and discard bad subtraction pairs related to large movements or transient hardware gradient malfunctions. However, the best way to ensure a proper subtraction of labeled from control scans is to use fast imaging techniques, such as spiral or EPI.
The major source of error in perfusion quantification is the arterial transit time, which is the time it takes blood to travel from the tagging region to the imaging slices. Originally, the first models used for absolute quantification of ASL, did not take into account this effect. However, Buxton et al[17] demonstrated that arterial transit time is one of the most important parameters needed for the quantification of both PASL and CASL techniques. An absolute quantitative perfusion map can be obtained using the General Kinetic Model, developed by Buxton et al[17]. The amount of delay between labeling and imaging acquisition should be chosen according to the subject’s condition. In healthy volunteers, a delay of 1 sec is suitable, while in patients with cerebrovascular diseases, longer delays are necessary[5].
Another vascular artifact that also interferes with the accuracy of CBF measurements comes from the remaining labeled blood in the large vessels. This signal may give artificially high perfusion values in CBF quantification. By applying bipolar “crusher” gradients before imaging the parenchyma, it is possible to suppress the moving signal that is within the vasculature. However, because ASL has inherently low SNR, removing any signal from the image, even if it is in the vessel, can lower the apparent quality of the perfusion image. According to Buxton et al[17], the signal from large arteries can be sufficiently destroyed during the time course of an echo-planar (EPI) acquisition without the need for additional bipolar gradients.
In the last few years, significant improvements in imaging acquisition techniques have been made. It is now possible to acquire the entire brain volume within a single-shot RF excitation using ultra-fast 3D sequences, such as a combined gradient and spin echo (GRASE) sequence[18]. Background suppression with a series of appropriately time inversion pulses can increase the ASL effect from approximately 1% of brain signal up to 100% of measured signal, dramatically increasing the sensitivity for detecting dynamic changes in CBF[19].
Currently there are four types of ASL techniques that differ, mainly according to the magnetic labeling process. CASL was the very first implementation of ASL[9]. Pseudo-continuous ASL (PCASL), PASL and velocity-selective ASL (VS-ASL) were developed to address limitations and technical challenges encountered in the first ASL methods[6,12].
Tagging based on location and velocity: CASL uses long and continuous RF pulses (2-4 s) in combination with a slice-selective gradient to induce a flow-driven adiabatic inversion of the arterial magnetization in a narrow plane of spins, usually just below the imaging plane[5,6,12,20]. The spins within a physiologic range of velocities traveling perpendicular to the tagging region can be inverted by adjusting the amplitudes of the gradients and the RF pulse through a phenomenon known as flow-driven adiabatic inversion[9]. The continuously inverted spins provide a theoretically higher SNR than that obtained with other ASL techniques, such as PASL[21].
However, CASL has several drawbacks. The long inversion pulses induce magnetization transfer (MT) effects[5] and cause large amount of RF energy deposition in the subject, resulting in a higher specific absorption rate (SAR)[12]. In the first implementation, the MT effects were compensated by applying a distal labeling during the control experiment. This produced identical saturation effects, but was only valid for single slice acquisition, because the labeling RF pulse was applied concurrently with a gradient pulse and therefore MT effects were dependent on slice position. For multislice acquisition, Alsop et al[8] proposed the use of two closely spaced inversion planes, called double adiabatic inversion. Theoretically, the magnetization gets inverted while traversing the first plane and returns to its original state during the passage through the second plane. Double inversion is achieved by applying a sinusoidal modulation of the RF waveform. Another method to control MT effects, called “simultaneously proximal and distal RF irradiation” (SPDI) was proposed by Talagala et al[22]. In this technique the RF pulse is distributed on both sides of the acquisition volume. The main limitation of these approaches is the double RF deposition, resulting in higher SAR. The use of separate coils for labeling the arterial blood is another way to avoid MT effects and reduce RF deposition, but the need for additional hardware restricts this solution to a small number of research centers[23,24].
Tagging efficiency of CASL may be affected by variations in flow velocity[21], which makes the average inversion efficiency of CASL (80%-95%) lower than PASL (95%). However, the closer inversion to the imaging plane minimizes the loss of perfusion signal caused by T1 relaxation and somehow compensates for the lower inversion efficiency[6]. As discussed previously for ASL quantification, CASL is sensitive to the transit time, but because of the steady state behavior of this type of sequence, the effect is smaller than in PASL[5]. Another limitation for clinical use of the CASL technique is the need for additional hardware to transmit continuous RF pulses, which are not typically available on commercial scanners[6].
Tagging based on location: In 1994, Edelman et al[25] proposed the first PASL scheme. Instead of labeling blood as it flows through a plane, as used in CASL, PASL uses short RF pulses (5 to 20 ms to saturate or invert a thick slab (10-15 cm) of blood volume, known as a tagging region, proximal to the imaging region. The adiabatic pulses are generally used to obtain thick inversion slabs with sharp edges[26]. For the PASL sequences, MT-effects have to be considered as well, although these are much smaller compared with CASL. In the first PASL version with an “echo-planar MR imaging and signal targeting radio frequency” (EPISTAR) sequence, inversion was performed distal to the image slice during the control experiment to induce identical MT effects in both cases. Shortly afterwards, an alternative to this asymmetric method of labeling was proposed by Kwong et al[27] and independently published by Kim[28], who named it “flow alternating inversion recovery” (FAIR). In this approach, the label is applied using a non-selective inversion pulse, while the control employs a concomitant slice selective gradient pulse. The symmetric nature of this sequence automatically compensates for MT effects. Various kinds of tagging methods have been developed that differ in the location of the labeling plane and the choice of magnetically labeled state of blood for control and labeled images[4,6]. For most PASL techniques, the tagging efficiency is greater than 95%[29].
Because of the imperfect edges of the tag pulses in PASL, it is necessary to provide a spatial gap between the distal edge of the tagging slab and imaging plane. This results in a transit delay during which the tagged blood traverses this gap[26,29]. This transit delay is local and of unknown quantity and can be a large fraction of the TI. Errors associated with transit delay can cause problems in quantification of CBF by a PASL technique[29]. Sequences like QUIPSS II (quantitative imaging of perfusion using a single subtraction) and Q2TIPS (QUIPSS II with thin-slice TI1 periodic saturation) were developed to render ASL more transit time insensitive[5].
Several modular features for pulse sequence modification have been developed in order to improve tagging slice profiles [frequency-offset-corrected inversion (FOCI), variable-rate-selective excitation (VERSE)] and SNR (In-Plane Presat or background suppression techniques)[29]. For a detailed explanation of these sequences and modules, please refer to recent ASL reviews[20,26,29].
The ease of implementation and reduced practical problems as compared with CASL have made PASL a popular choice for clinical perfusion imaging[5,20].
PCASL has been introduced as an intermediate means to take advantage of CASL’s high SNR and PASL’s higher tagging efficiency[21]. First developed by Garcia and colleagues[6], this technique employs a train of discrete RF pulses together with a gradient wave applied between two consecutive RF pulses to mimic CASL’s flow-driven adiabatic inversion method for spin labeling[21]. PCASL provides less RF power deposition and MT effects compared to CASL without need of special hardware. Depending on the implementation, PCASL is susceptible to B0 in homogeneity and eddy currents[6].
Tagging based on location and velocity: In general, most ASL techniques are based on the proximal labeling of the arterial magnetization followed by acquisition of images in the tissue of interest after a delay time[20]. As described earlier, the main problem of such techniques is the finite arterial transit time between labeling and imaging[20] and variations of transit times are one of the largest potential sources of errors in quantifications of perfusion using ASL in human brain[13,30]. In VS-ASL, arterial spins are labeled everywhere (including in the volume of interest) based purely on flow velocity, therefore eliminating the effect of the transit delay time necessary for the labeled blood to reach a region of interest[13,20,29]. A velocity-selective tag pulse saturates or inverts blood above a cutoff velocity (VC), and data are acquired only from spins with velocity below VC[29,30]. In this manner, only spins that decelerated through the cutoff velocity during the inflow time TI will be observed and the signal will be proportional to TI CBF[29]. Theoretically, VS-ASL is inherently insensitive to transit delays and thus should be the method of choice for pathologies, such as stroke, where collateral or slow flow may otherwise result in long transit delays and grossly incorrect CBF measurements[6,13,29].
Territorial ASL (TASL) [selective ASL, regional perfusion imaging, vessel-encoded ASL (VE-ASL)] is a modified ASL technique that allows labeling and visualization of perfusion in territories of individual arteries[31-33]. Mapping vascular territories with ASL is based on labeling only blood flowing through an artery or arteries of interest, while leaving the others unlabeled[31]. TASL has been implemented by three different approaches: (1) using separate labeling/imaging coils positioned over the arteries of interest; (2) using a single head coil with selective inversion of spatially confined areas where arteries of interest are located; and (3) using multidimensional RF pulses to directly label the arteries of interest[31]. Currently, the most applicable technique is regional perfusion imaging based on anatomy-driven spatially selective slabs in which magnetic resonance angiography (MRA) is used for prescribing the position of the labeling slabs[32].
Most clinical applications of territorial ASL may be found in diagnosis and prognosis of cerebrovascular disease[32]. In acute stroke, delineation of individual perfusion territories may demonstrate collateral contributions to the ischemic penumbra and may allow for differentiation between thromboembolic and hemodynamic etiologies[32]. In chronic cerebrovascular disease, TASL may help in the evaluation of the actual territorial contribution of individual collateral arteries, particularly in patients with extra-cranial steno-occlusive disease[32]. Further knowledge of the cerebral perfusion territories may explain differences in clinical outcomes and potentially expand treatment options for both acute and chronic cerebrovascular disease[32].
Chng et al[34] compared TASL with DSA in the evaluation of collateral circulation in patients with cerebral arterial steno-occlusive disease and found that TASL/MRA was able to demonstrate anatomy, anatomic variants, as well as collateral circulation at the level of the circle of Willis. The authors concluded that combined TASL/MRA can be an alternative to evaluate collateral perfusion and may supplement or replace DSA in the clinical assessment of patients with cerebrovascular disease[34].
According to the rather short duration of the ASL bolus, which decays with T1 relaxation of blood, regions with long transit delays may appear dark resulting in apparent no perfusion or a false underestimation of perfusion[34]. Obtaining TASL at different time points may demonstrate the arrival of delayed bolus. However, with loss of labeling signal, the potential failure to detect severely delayed antegrade or collateral flow remains a problem[33,34].
Typically, ASL measurements are conducted at a single TI between labeling and image acquisition[10]. As discussed previously, the effects of arterial arrival time (AAT) or arterial transit time on CBF estimation is difficult to evaluate and can cause errors in calculated perfusion values[20]. This effect is especially true for patients with carotid steno-occlusive disease for whom the labeled blood flowing via the collateral vessels can cause delayed AAT in the affected area[20]. One approach to solve this transit time problem is based on measuring AAT in addition to CBF by performing multiple ASL experiments at various inversion times between labeling and image acquisition instead of just minimizing its effect[20]. There is evidence that AAT provides additional information to characterize collateral flow and it may potentially be used to identify hemodynamically impaired regions[10,35]. The main drawback of this technique is the considerably long scan time, often rendering it impractical especially in sick patients[20,35].
It was demonstrated that CBF from a cerebral hemisphere ipsilateral to an internal carotid artery (ICA) occlusion can be calculated with ASL at multiple TIs[35,36]. Bokkers et al[37] found that in patients with symptomatic ICA occlusion, CBF values obtained with ASL at multiple TIs correlated significantly with H215O PET, although there was some overestimation of CBF by ASL-MRI.
Functional magnetic resonance imaging (fMRI) is a non-invasive and widely available technique that provides physiological imaging of the living brain. It measures the hemodynamic response related to neural activity. The most commonly used fMRI technique is blood-oxygen-level-dependent (BOLD) fMRI. BOLD signal is the result of complex changes in CBF, CBV, and the cerebral metabolic rate of oxygen uptake (CMRO2) associated with neural activity[5].
BOLD effects are measured using rapid volumetric acquisition of T2* weighted images and BOLD fMRI signal depends on the difference in the magnetic properties between oxyhemoglobin (oxy-Hb) and deoxyhemoglobin (deoxy-Hb). The paramagnetic deoxy-Hb produces a susceptibility induced field shift or field distortion manifested as a reduction in T2* signal[38]. With regional brain activation, there is an increase in local cerebral blood flow (CBF). This increase in blood flow is not clearly understood. It is assumed that a regional CBF increase is required to supply oxygen and nutrients in response to functional activation, but some studies of brain energy metabolism have not conclusively supported this idea. An alternative possibility is that regional blood flow increases to remove toxic waste products of metabolism, such as lactate. Despite this controversy, what is well known is that there is a local increase in CBF that exceeds metabolic oxygen demands. As a result, deoxy-Hb is progressively washed out, which causes an increase in BOLD T2* signal. Because this signal is primarily due to an intravascular deoxy-Hb reduction it may be observed in the overlying venous structures[38]. Task-specific BOLD signal changes are not directly quantifiable in physiological units, but rather are expressed as a percentage signal change or as a significance level based on a statistical model[38].
ASL perfusion can also be used as fMRI contrast to localize task activation as BOLD contrast or as a measure of brain function independent of any specific sensorimotor or cognitive task[11]. While BOLD contrast primarily detects changes in T2* that indirectly reflect changes in CBF, ASL perfusion techniques directly quantify CBF[38]. Changes in CBF are believed to be linked to neuronal activity rather than BOLD changes, which reflect a complex function of a number of physiological variables[12]. Several studies have shown that ASL measures can exhibit decreased inter-subject and inter-session variability as compared to BOLD, possibly reflecting this more direct relation between CBF and neural activity[12].
In contrast to BOLD fMRI, ASL perfusion fMRI uses a freely diffusible tracer that can exchange with tissue water and directly measure the amount of arterial blood that has been delivered to the capillary bed. In addition, the decay time of this tracer corresponds to the T1 relaxation time, which is approximately 1-2 s, precluding significant tracer accumulation in venous structures even in if the tracer remains intravascular. Therefore, signal changes in perfusion fMRI are not observed over veins, resulting in better localization of signal over activated cortex[38].
Because the BOLD signal is based on the susceptibility contrast, it is also very sensitive to static susceptibility effects, leading to signal loss or distortion at tissue air and tissue bone interfaces such as the orbital frontal cortex and inferior temporal lobe[39]. Another advantage of ASL perfusion methods is the possibility of using sequences (e.g. spin-echo) that are insensitive to susceptibility MRI artifacts[12].
One of the major disadvantages of the ASL perfusion fMRI technique is the lower signal difference, which is usually only 0.5%-1.5%. The typical BOLD response consists of a 0.5%-5% change in regional image intensity, which increases at higher magnetic field strengths. BOLD signal changes may approach 25% with sensorimotor tasks at 4.0T[38].
The temporal resolution of ASL perfusion fMRI is also inherently poor because of the necessity to form tag and control images and to allow time for blood to be delivered from the tagging region to the imaging slice. In a typical PASL experiment, one tag and control image pair is acquired every 4 s, while in a BOLD fMRI experiment, acquisition time is typically 1 to 2 s and can be as low as 100 ms for specialized applications[12].
The necessity of the subtraction procedure also makes ASL less favorable for event-related fMRI than BOLD, necessitating the use of complex reordering procedures[20].
At the present, ASL approaches in fMRI usually have less coverage. The number of slices that can be acquired depends on the acquisition time for each slice, the time TR-TI that is available for slice acquisition, and the need to acquire slices before the difference signal has decayed away because of the longitudinal relaxation of blood. Because of these considerations, ASL studies typically result in acquisition of a smaller number of slices (3-15) than whole-brain BOLD studies (30-40 slices), and with thicker slices (5-8 mm) than BOLD studies (3-4 mm)[12].
Nevertheless, ASL CBF more strongly correlates with changes of behavioral state than do BOLD signals when the changes occur over periods longer than a few minutes. As described earlier, ASL methods are quantitative, stable over time and less variable across subjects. These properties make ASL an especially useful noninvasive method to measure CBF in longitudinal and treatment studies[40]. ASL perfusion fMRI is also well suited to examining neural responses to pharmacological agents and abstinence states as these are sustained effects lasting hours or longer. The utility of ASL perfusion fMRI in drug development and validation is currently being explored.
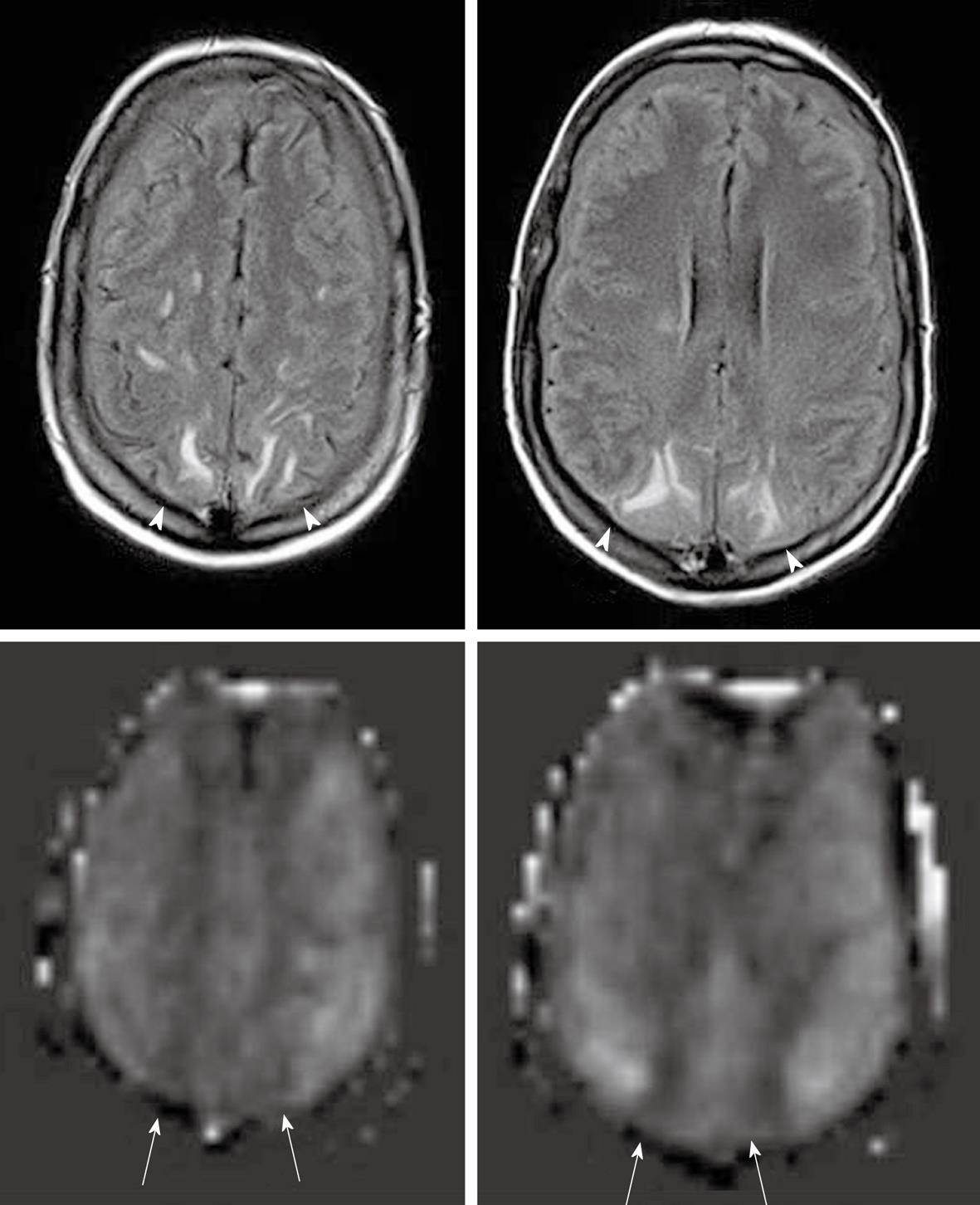
As described earlier, the ASL sequence frequently uses EPI for rapid image acquisition. A disadvantage of EPI is the presence of susceptibility artifacts in the region containing blood, calcification, metal surgical materials, and at the portion of the brain adjacent to the skull base[6]. The susceptibility effects create artifactual dark signal in perfusion images, which may mimic areas of reduced perfusion[3] (Figures 1 and 2).
Motion is the most commonly seen artifact in clinical MRI examinations, particularly in hospitalized patients. A well-known motion-related pattern is a peripheral ring of high signal intensity[3].
Circulating gadolinium-based contrast agents cause T1 shortening in all tissues in both control and label conditions. This T1 shortening minimizes the measurable differences between tag and control images, thus producing maps with almost no usable signal intensity. Therefore, in clinical protocols, ASL data must be acquired before gadolinium administration[3].
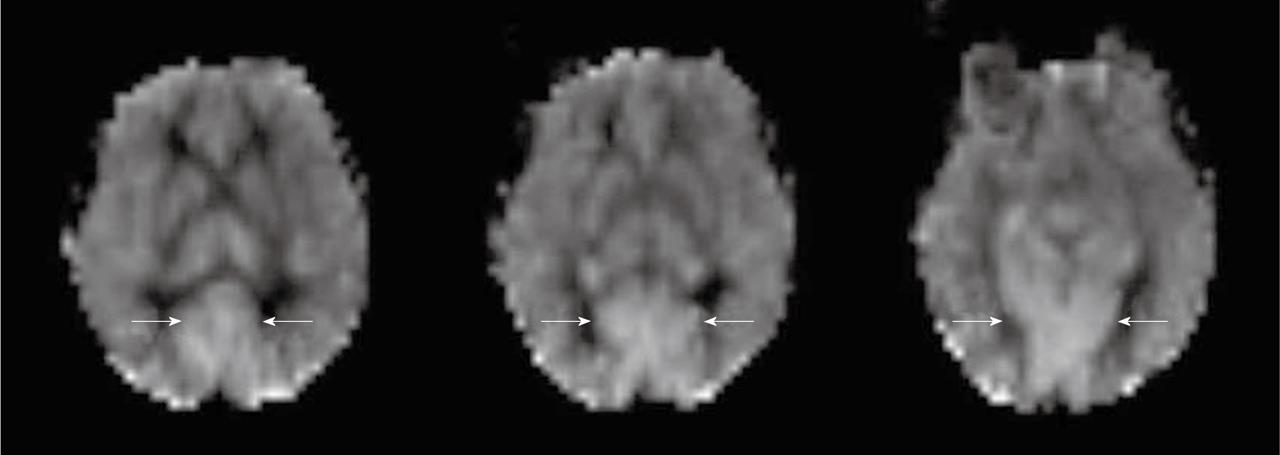
Physiologic regional hyperperfusion: Regional increased signal intensity may occur in both occipital lobes corresponding to visual cortex activation[3] (Figure 3). A hyperfrontal pattern of regional CBF distribution has also been described with various perfusion methods. It is believed to be a normal finding in young and middle aged patients and may decrease with normal aging[3].
Age-dependent variability of cerebral perfusion: ASL can demonstrate age dependent cerebral perfusion if a quantitative measurement is used[3,6]. In pediatric patients undergoing ASL, a consistent pattern of increased SNR, as well as globally elevated absolute CBF, has been observed compared with adults. This is possibly because there is a pediatric higher baseline CBF, faster mean transit time, and increased T1 values in blood and tissue. Pediatric CBF measurements begin at a low level in the perinatal period, increase to a peak at 3-8 years of age and then gradually decrease to adult levels. Decreased susceptibility artifacts at the skull base from immature paranasal sinuses also improves the image quality[41,42]. After approximately age 30 years, there is a gradual decline in gray matter perfusion. Age-dependent decreases in perfusion signal intensity are well documented and must be accounted for when interpreting ASL perfusion in older patients[20]. If bipolar “crusher” gradients are used in elderly patients, anterior and posterior watershed territories may appear relatively hypoperfused because of the physiological prolonged transit time in these regions. A longer delay between labeling and imaging acquisition may improve this apparent hypoperfusion[6].
White matter signal: It has been acknowledged that ASL does not allow for reliable detection of the white matter (WM) perfusion signal, probably due to technical issues[43]. Recently, at least two studies investigated ASL and WM perfusion. van Gelderen et al[44] studied WM perfusion using flow-sensitive alternating recovery ASL (FAIR-ASL, which is a PASL technique) at 3.0T. The authors demonstrated that WM perfusion is significantly weaker than GM and close to the noise level. They believe that poor sensitivity and heterogeneous transit time limit the applicability of ASL for measurement of WM perfusion. However, van Osch et al[43] used PCASL with background suppression at 3T to study WM perfusion in normal volunteers. The authors concluded that, except within deep white matter, ASL is sensitive enough to detect WM perfusion signal and perfusion deficits. In this study, 35 averages were necessary to detect significant WM signal, but 150 averages were needed to detect signal in deep WM. According to these studies, at 3.0T scans, PCASL with background suppression may be more sensitive in the measurement of WM perfusion than PASL.
Differences between blood and tissue T1 relaxation times also lead to errors in ASL quantification. According to the original quantification model of cerebral perfusion, it was assumed that the T1 relaxation time of the tissue and the blood are similar at 1.5T in the human brain. This implies that CBF quantification is more accurate in gray matter (GM) than in the WM, because the GM and blood T1s are closer than WM and blood T1s[5,26].
One of the most frequent clinical applications of perfusion imaging is the evaluation of cerebrovascular diseases[6]. Decreased cerebral perfusion is the common underlying cause of all ischemic strokes and is a predictor of recurrent stroke[45]. The perfusion-diffusion mismatch concept is widely used in MR imaging for ischemic acute stroke[46]. Potentially salvageable tissue by timely reperfusion (tissue at risk or ischemic penumbra) can be identified by perfusion imaging. By definition, the ischemic penumbra is an area of reduced perfusion without restricted diffusion[46,47].
Previous studies have shown that ASL can be applied in acute stroke, in both adult and pediatric populations[4,47]. Areas of focal or hemispheric perfusion deficits, post ischemic hyperperfusion or perfusion-diffusion mismatches can be depicted by ASL techniques[47,48] (Figure 4). Agreement between ASL perfusion maps, conventional MRI, MRA and clinical symptoms have been observed[47]. ASL also can be used to demonstrate perfusion deficits in patients with a history of transient ischemic attacks or significant extracranial carotid artery stenosis[6,45]. Studies in normal cerebral tissues demonstrate that ASL and DSC (dynamic susceptibility contrast) MR perfusion yield comparable values[4,49]. There are a few studies describing CBF maps or relative perfusion measures from ASL as correlating best with MTT (mean transit time) or TTP (time to peak) maps from DSC in the setting of significantly delayed arterial transit time in patients with severe arterial stenoses or carotid occlusions[4,6].
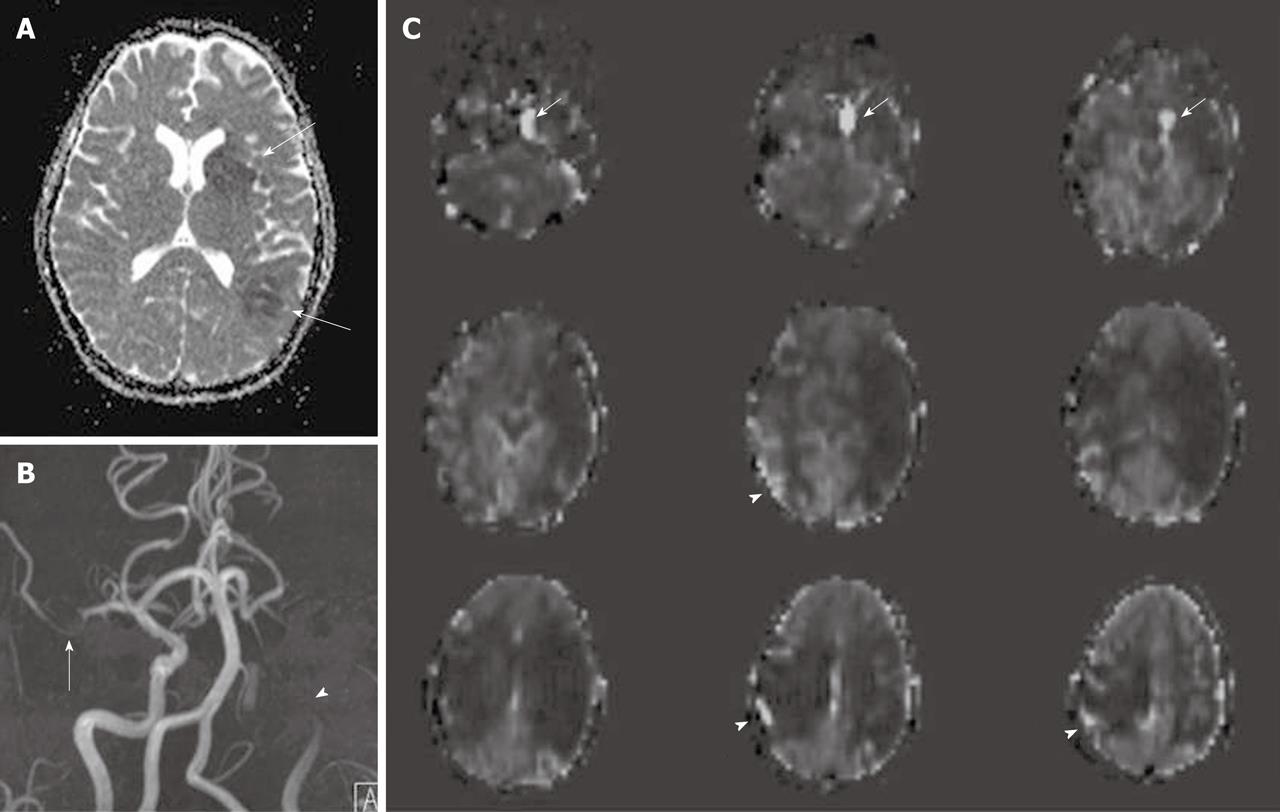
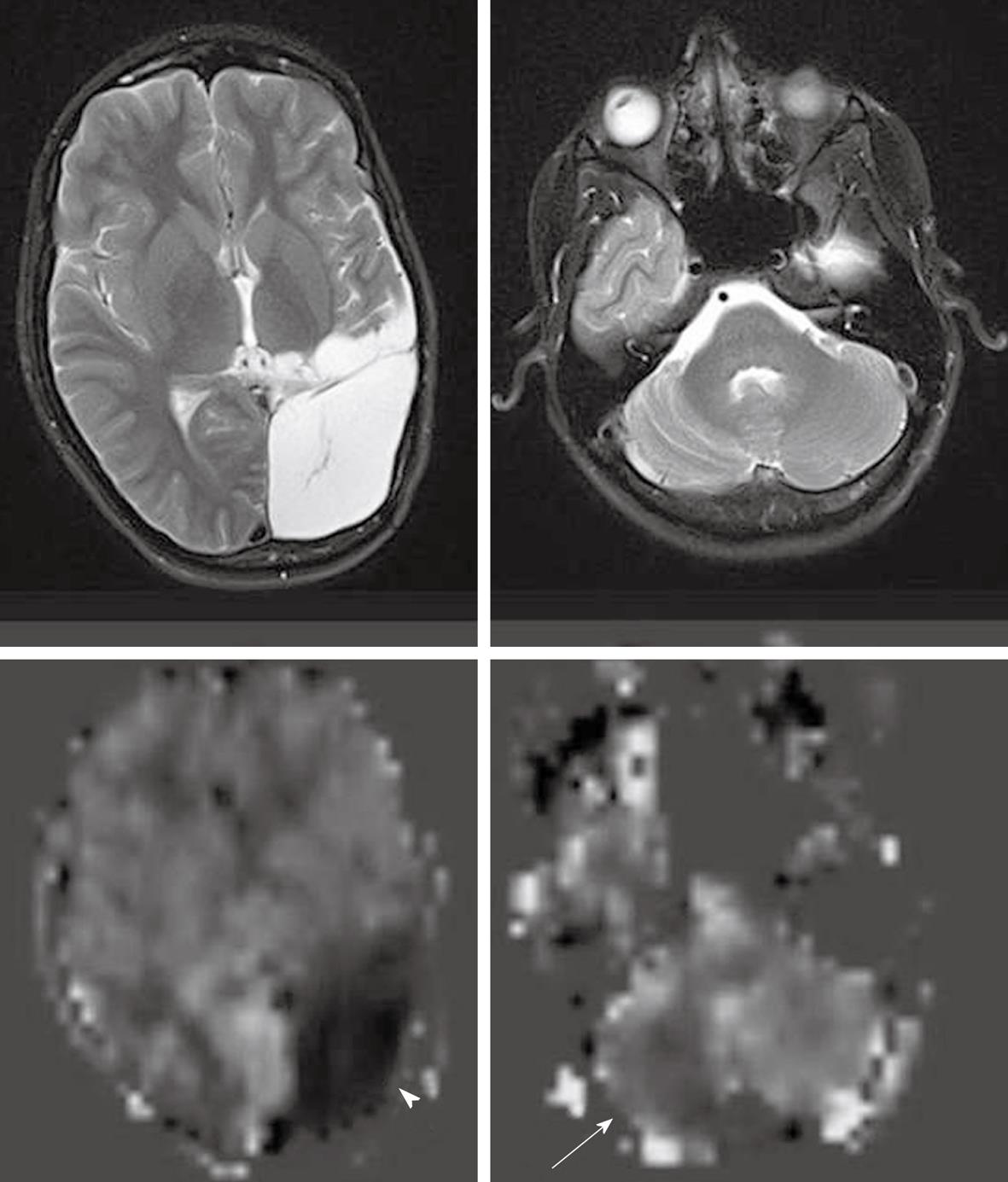
Delayed arterial transit effects (delayed arterial transit artifacts or focal intravascular signal) have been widely described in ASL studies in patients with cerebrovascular disease[4,47,50]. These effects are seen as serpiginous high signal intensity in the cortex[40] (Figure 5).This finding may represent labeled arterial blood remaining in the feeding arteries at the time of image acquisition[47,50] or presence of collateral circulation through leptomeningeal vessels resulting in prolonged transit time for spins to travel from the tagging region to the imaging plane[40,45,47,48]. Some studies found that the cortical areas where the delayed arterial transit was initially seen showed no infarction or only a small infarct on follow up images. This pattern may herald a protective effect and may predict positive clinical outcomes[45-47]. In the presence of delayed arterial transit effects, qualitative interpretation of perfusion deficits is still possible, but there are potential difficulties for the absolute quantification of CBF[4,6]. As discussed previously, some ASL techniques have been developed to minimize these effects or to calculate the arterial arrival time.
Zaharchuk et al[50] demonstrated that ASL revealed additional abnormalities in patients with normal DSC perfusion due to the sensitivity of ASL to prolonged arterial arrival times. This abnormal finding is seen as low ASL signal in the cerebral arterial watershed regions with the presence of delayed arterial transit effects on the surrounding cortical areas, and is termed “borderzone sign” (Figure 6). The authors believe that this finding represents labeled blood remaining in feeding arteries that has not yet reached capillary beds and reflects long arterial arrival times, reduced CBF or a combination of these. Possible causes include underlying reduced CBF, reduced cardiac output and/or small vessel disease.
MR perfusion studies in the setting of CNS neoplasms are commonly performed with a DSC technique, focusing on relative cerebral blood volume (rCBV)[4,51], which has been shown to correlate with tumor grade and histologic findings of increased vascularity[52]. Perfusion imaging can be used as such to assess tumor grade, heterogeneity, and to target stereotactic biopsy sites at the most malignant portion[53]. Perfusion evaluation in treated tumors also provides a noninvasive indicator of malignant progression or treatment response[51].
Several studies demonstrated that DSC and ASL perfusion are comparable for distinction between low grade (WHO grades I and II) and high grade (WHO grades III and IV) glioma[4,53]. A good correlation between ASL and DSC imaging for determination of relative tumor blood flow (rTBF) has also been observed[54]. In comparison to DSC, ASL provides quantitative CBF values that are unrelated to disruptions of the blood-brain barrier, while DSC provides information about tumor blood volume and vessel permeability[40].
To evaluate tumor response after treatment, Weber et al[55] used PASL and DSC to study metastases post stereotactic surgery. The authors found that relative tumor blood flow (TBF) measurements at 6 wk were predictive of outcome. Increased TBF predicted tumor progression while decreased TBF predicted tumor response.
Ozsunar et al[56] studied a series of 30 postoperative glioma patients treated with proton-beam therapy comparing ASL, DSC and PET to distinguish predominantly recurrence or progression from predominantly radiation necrosis. The authors used quantitative single slice ASL and compared results with the contra-lateral normal appearing brain. They found that ASL is more accurate in distinguishing predominantly recurrent high-grade glioma from radiation necrosis, especially in regions of mixed radiation necrosis, in which DSC-CBV underestimated blood volume, probably because of leakage artifacts[56].
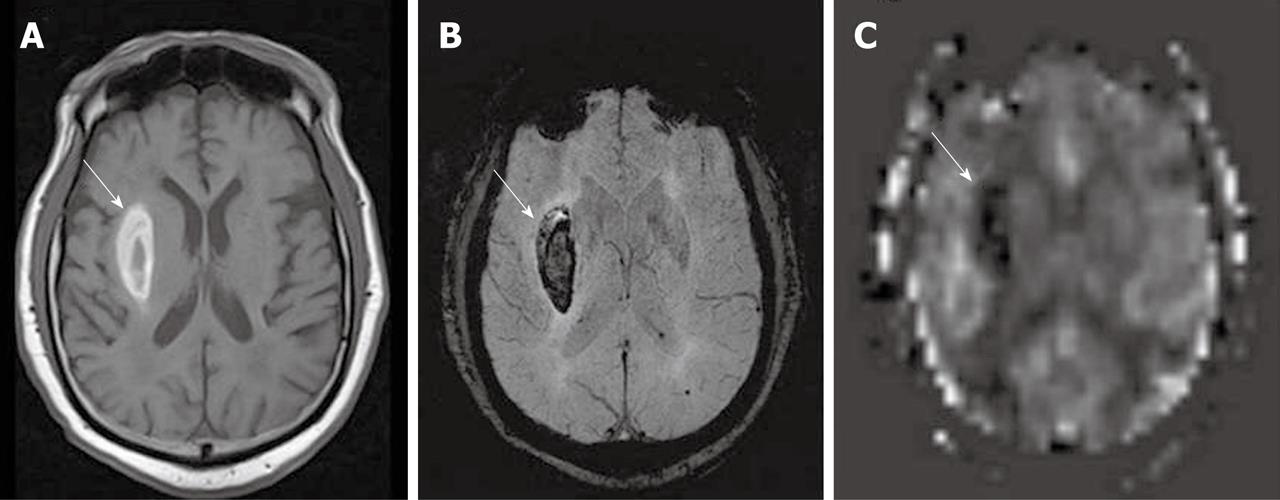
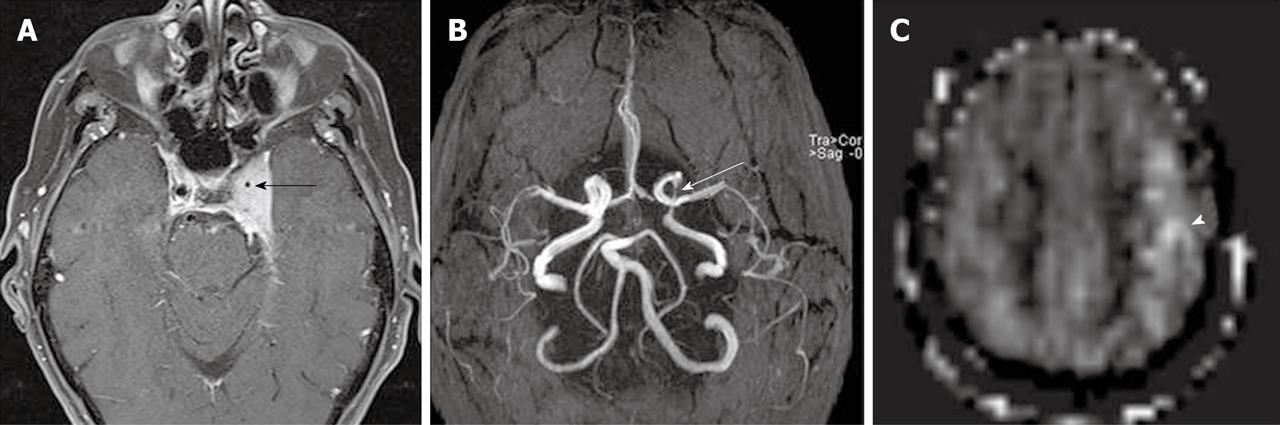
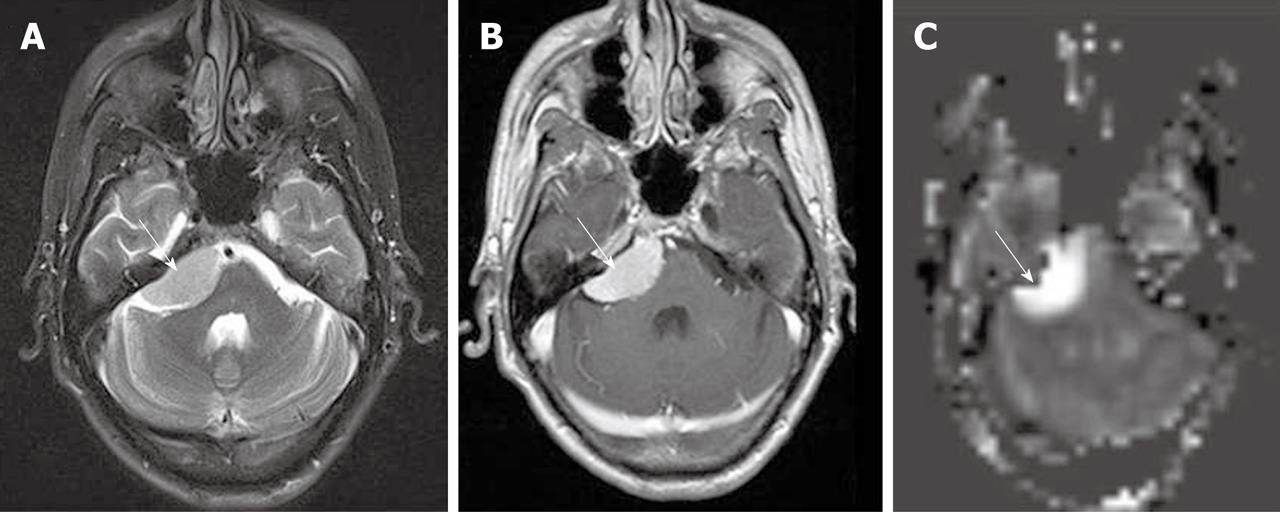
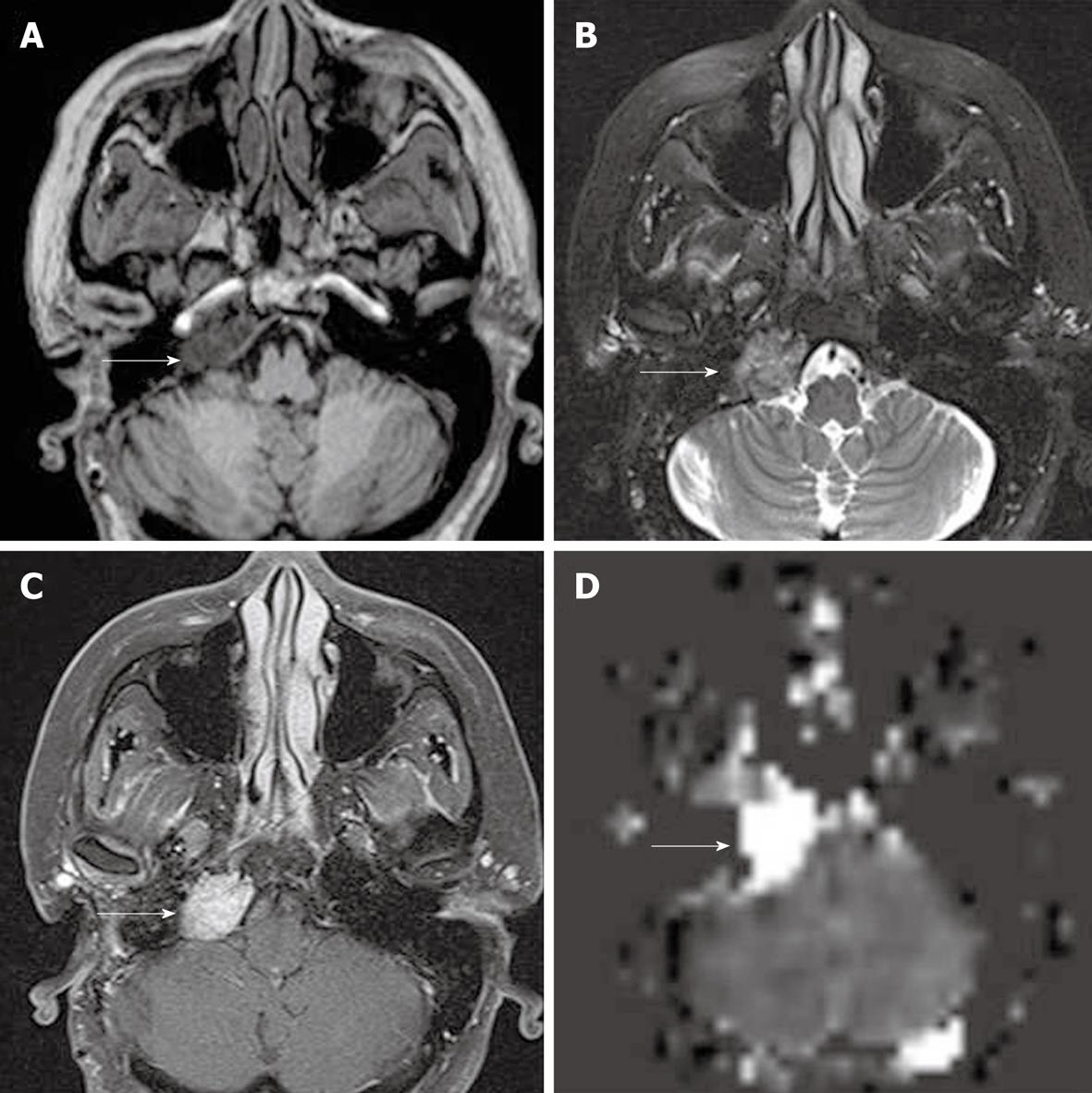
In qualitative clinical interpretations, high grade primary brain tumors usually demonstrate high perfusion on ASL maps while low grade tumors usually show hypoperfusion. As described earlier, hypoperfusion in tumors on ASL maps must also be correlated with hemorrhage, cysts and/or calcifications, which may show artificially low signal intensity[6,57]. Hyperperfusion is also observed in meningiomas (Figure 7), oligodendrogliomas, hemangioblastomas[6,58] and glomus tumors (Figure 8). Metastases can demonstrate either hypo- or hyperperfusion patterns on ASL maps[57] (Figure 9).
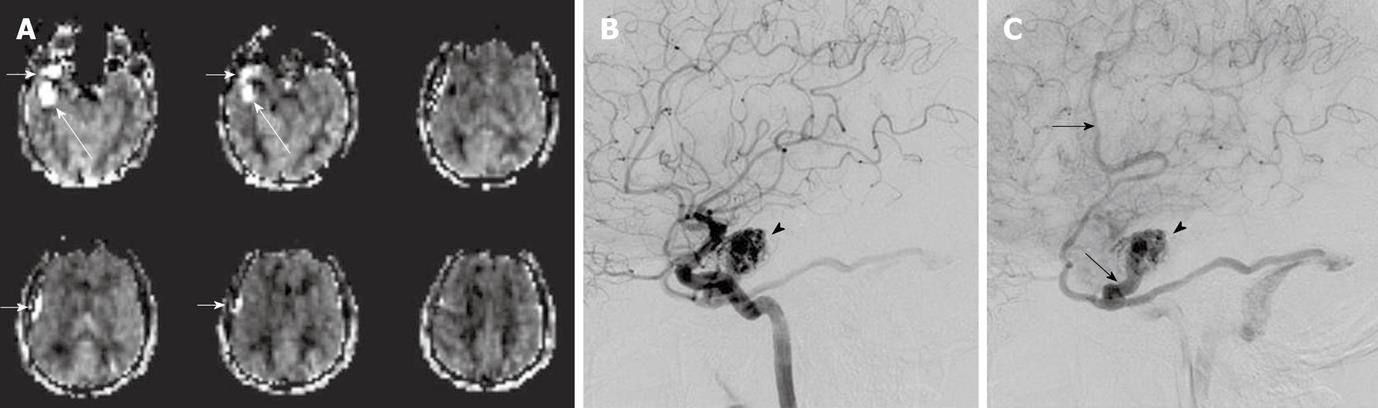
Arteriovenous malformations (AVMs) are vascular malformations with arteriovenous shunting. AVM nidus and draining veins may appear as high signal intensity on ASL perfusion maps representing AV shunting or rapid transit (Figure 10). The presence of hemorrhage or embolic material may produce dark signal due to susceptibility artifacts. Small AVMs may be difficult to detect with any other technique, particularly in an acute setting when hemorrhage is present. ASL can provide evidence of AV shunting, which may be the clue for detecting subtle lesions[59]. Perfusion abnormalities in regions adjacent to AVMs reflect various states of hyperemia and/or steal phenomenon[43,58,59].
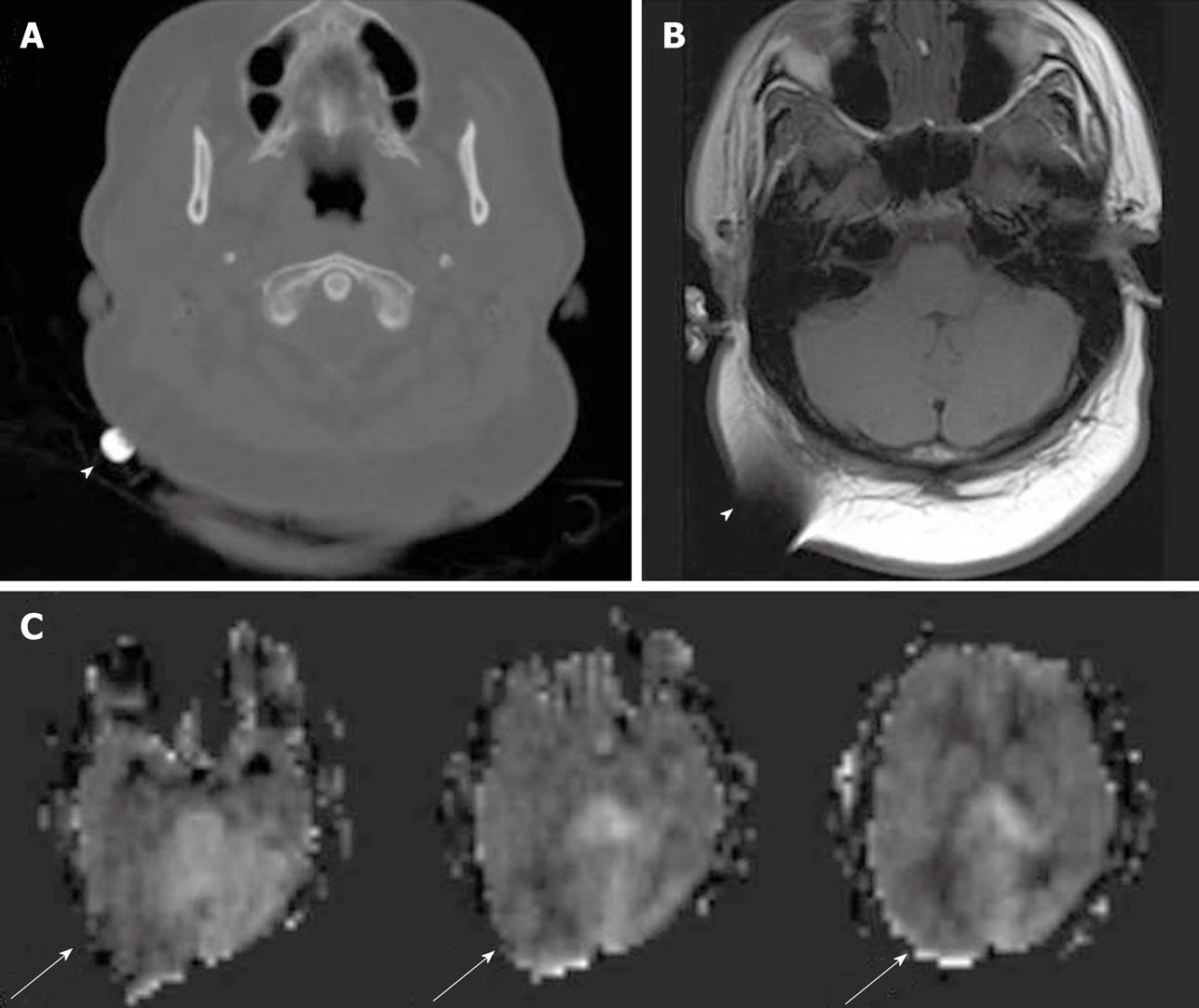
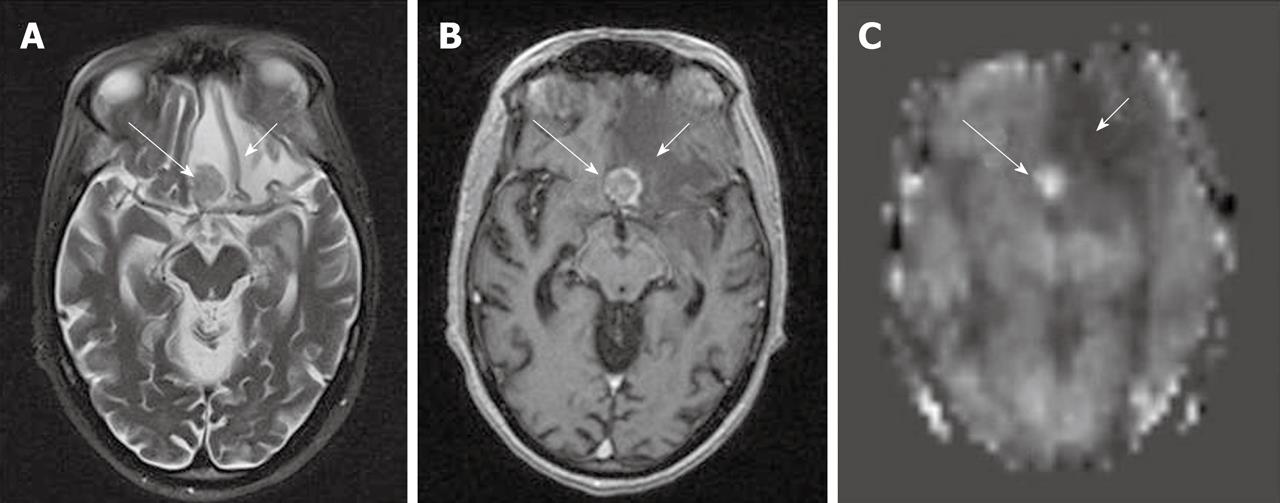
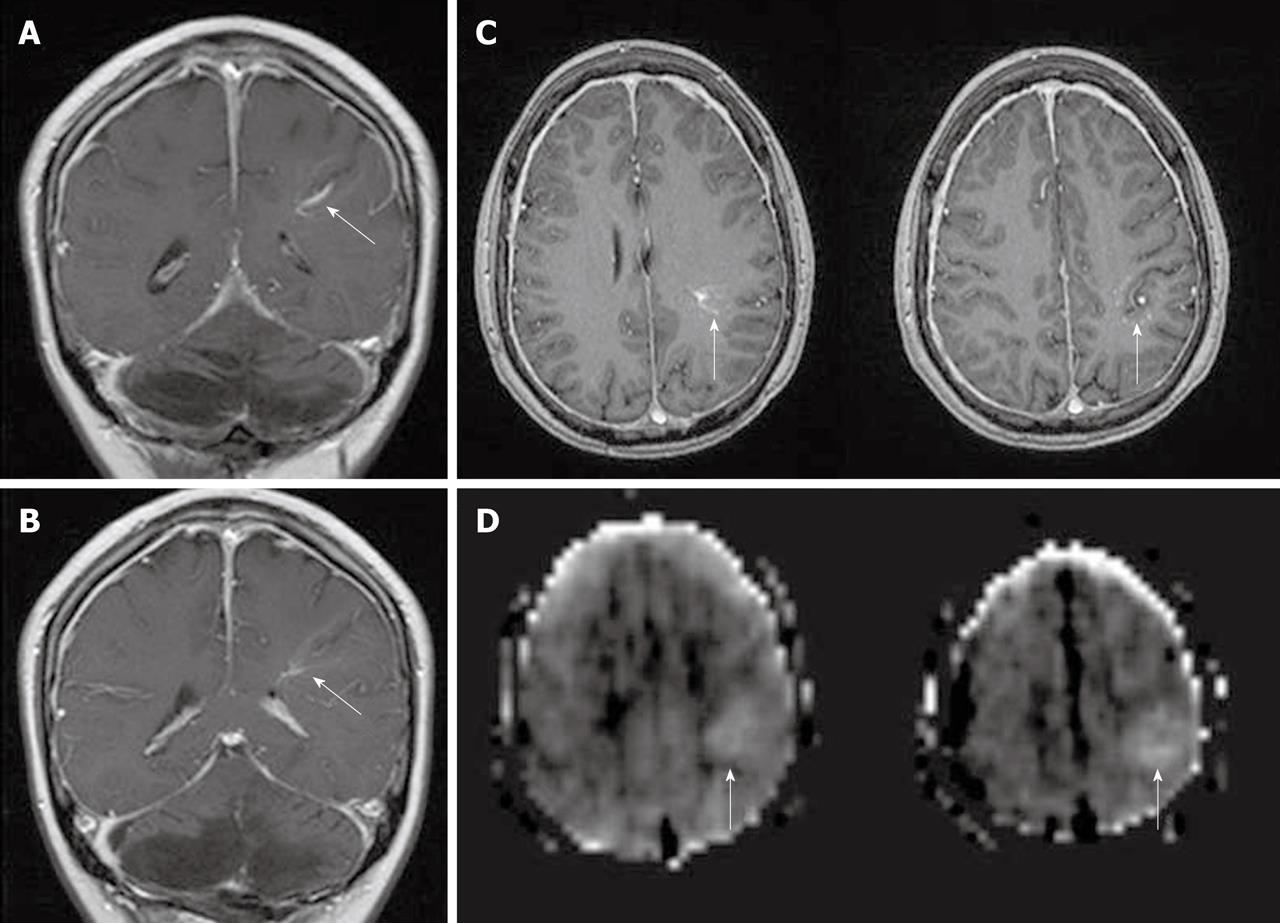
Developmental venous anomalies (DVAs) are frequently identified in contrast brain MRI. DVAs and their surrounding parenchyma may show increased CBF, CBV, MTT and TTP on DSC MRI perfusion studies[60]. Increased perfusion in the brain parenchyma adjacent to the DVAs also can be demonstrated on the ASL perfusion maps[6,58] (Figure 11).
In cognitive disorders, imaging modalities are becoming increasingly important for differential diagnosis, for monitoring disease progression, and as surrogate markers in treatment trials[61]. Besides PET and SPECT, several MRI techniques, including anatomic or volumetric imaging, BOLD fMRI, MR perfusion and diffusion tensor imaging, are currently being used for these purposes[4,61].
Several studies have demonstrated the ability of ASL in detecting regional hypoperfusion in frontotemporal dementia (FTD), Alzheimer’s disease (AD), and mild cognitive impairment (MCI), consistent with previous PET and SPECT studies[4,62].
A preliminary study of AD using PASL demonstrated decrease parieto-occipital and temporo-occipital perfusion compared with controls[63]. A subsequent ASL study showed significant temporal, parietal, frontal and posterior cingulate hypoperfusion in ASL subjects[64]. More recently, PASL revealed hypoperfusion in AD patients bilaterally in the inferior parietal and inferior frontal cortex, as well as the posterior cingulate[65]. Although areas of hypoperfusion in these studies differ, likely related to differences in brain coverage, they are consistent with previous PET findings as well as the pathophysiology and neuropsychological deficits characteristic of AD[22]. ASL perfusion techniques may also predict cognitive decline and conversion from MCI to dementia, and may be useful for identifying candidates for AD treatment trials[66]. According to Du et al[62], regional cerebral hypoperfusion, as detected by ASL, may help in the differentiation of FTD from AD.
Epileptic foci have been studied extensively using nuclear medicine techniques. These methods require an ictal and a subsequent interictal injection of radiotracer[67]. Assessment of tissue metabolism is standard practice in epilepsy pre-surgical work-up with the assumption that the epileptogenic focus is abnormal tissue that has a decreased metabolic rate and blood flow between seizures and increased metabolism and blood flow during seizure activity[38,68]. The hypoperfusion interictal pattern is probably related to neuronal loss[40,68], but the mechanism of ictal hyperperfusion is not completely understood. It may be related to transient loss of autoregulatory function in the surrounding vasculature or to the release of excitatory neurostimulators in areas of increased neuronal activity[58]. Controversy exists as to whether the interictal hypoperfused region or the ictal hyperperfused region is more sensitive in identifying seizure foci[57].
Changes in perfusion have also been demonstrated using ASL techniques[38]. Most patients who have clinical seizures have been imaged in the interictal state and unless they have epileptic activity, have shown hypoperfusion in the involved cortex[6]. In the rare instance of post ictal imaging, hyperperfusion has been shown with ASL studies up to 1 h after seizure onset[6].
As described earlier, the inferior temporal lobe may be difficult to image using EPI ASL perfusion techniques because of the susceptibility artifacts caused by the skull base. However, Wolf et al[39] reported asymmetry in interictal mesial temporal lobe function in patients with temporal lobe epilepsy using EPI CASL perfusion MRI. Furthermore, other non-EPI sequences are available for ASL perfusion studies. Liu et al[69] detected perfusion abnormalities in temporal lobe epilepsy using PASL perfusion with a pulse sequence that is insensitive to susceptibility effects (FAIR-HASTE). Both studies also found a significant correlation between the ASL results and PET measurements of CBF[69] or glucose PET[39] rate.
ASL perfusion techniques can complement the traditional evaluation of seizures. When compared with nuclear medicine studies ASL is completely noninvasive and involves no radiation exposure. It also can be performed multiple times during and after the ictal event and provides superior temporal resolution due to the very short half time of the spin tag[57].
Little has been reported about ASL perfusion and CNS infections. In a recent review of ASL clinical applications, the authors found that most CNS infections demonstrate ASL hypoperfusion, but they also found some infections presented with hyperperfusion, such as herpes encephalitis[6]. One diagnostic dilemma in immunocompromised patients is differentiating toxoplasmosis from lymphoma. In the same study, the authors found that ASL hypoperfusion was seen in toxoplasmosis while hyperperfusion was seen in lymphoma[6].
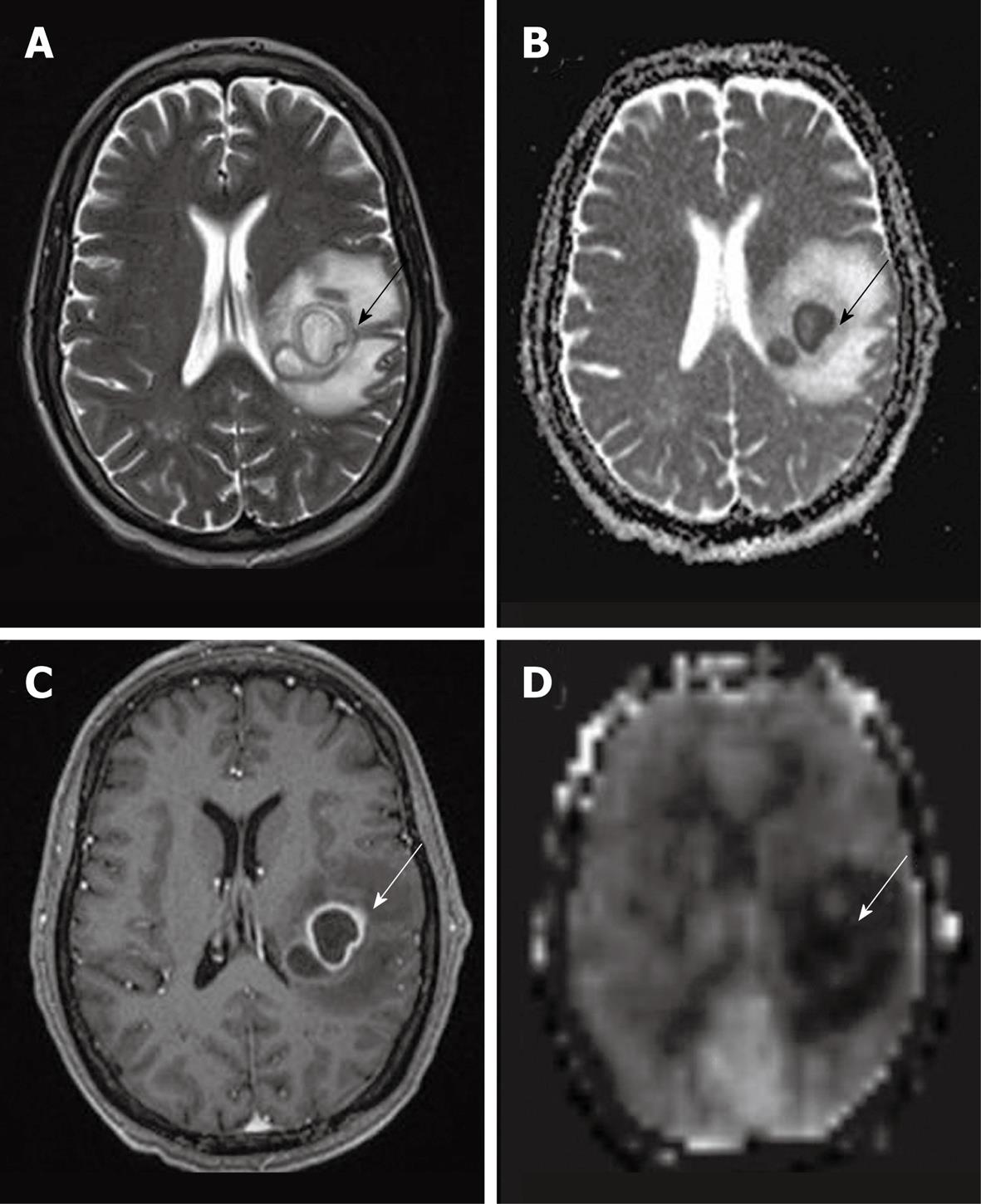
It is commonly accepted that cerebral abscesses show decreased perfusion in the lesion itself as well as in the surrounding edema, however the enhancing rim can demonstrate increased perfusion signal intensity[57] (Figure 12). Cortical hyperperfusion was also reported in epidural abscesses[6]. This may be secondary to compression of the cortical venous outflow or malfunction of the local cerebral regulatory mechanisms secondary to infection.
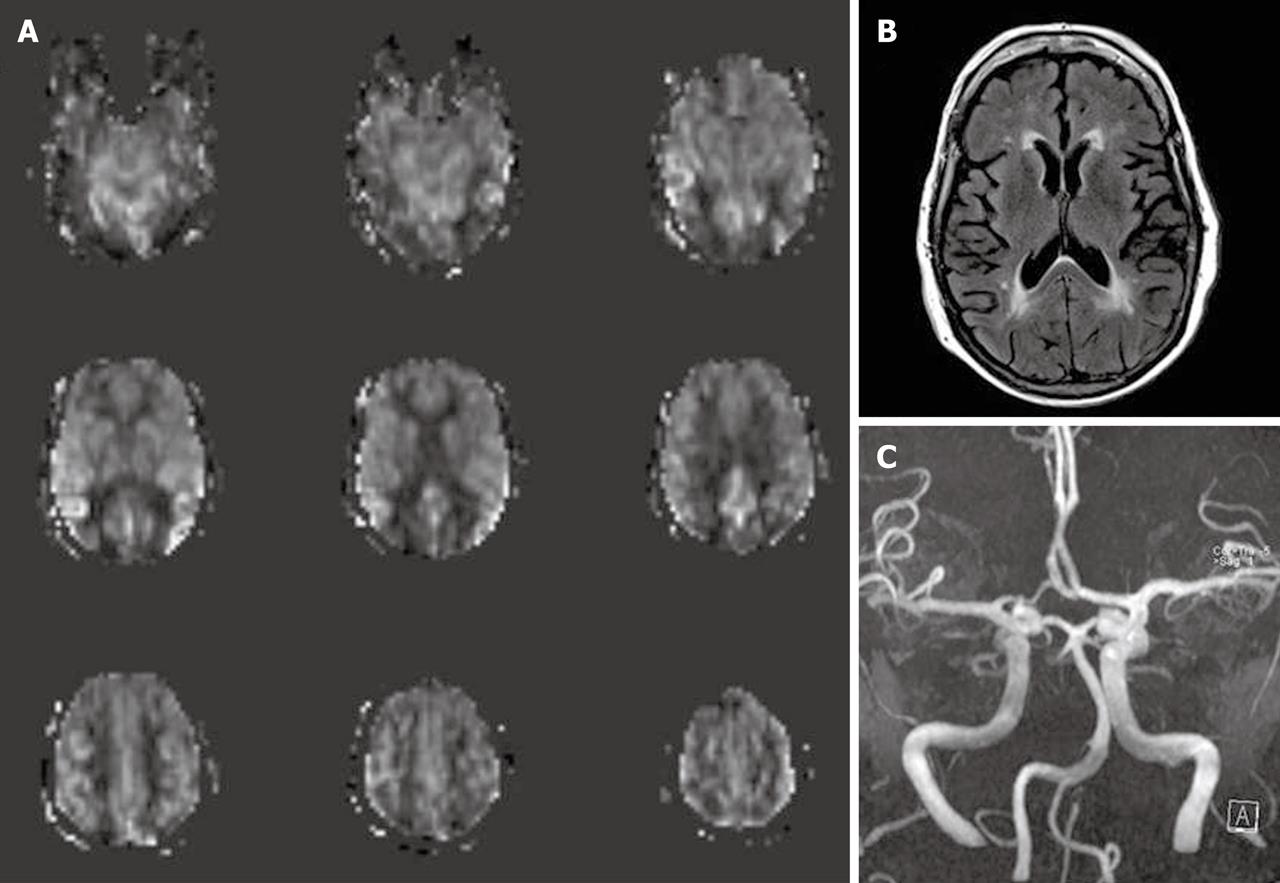
Posterior reversible encephalopathy syndrome (PRES) can have variety of appearances on perfusion studies that may be related to the time course of the disease. Patients who are imaged acutely show hyperperfusion in occipital and frontal regions while those imaged in the subacute phase show hypoperfusion in the aforementioned areas[6] (Figure 13).
Crossed cerebellar diaschisis, or reduced perfusion in the cerebellum contralateral to acute cerebral infarction, is a condition mostly reported by PET but has also been demonstrated with DSC MRI perfusion showing prolonged TTP and diminished CBF[70]. This condition can be detected by ASL perfusion as well[31] (Figure 14).
ASL provides an important method for measuring cerebral perfusion. The feasibility of measuring tissue perfusion with ASL has clearly been demonstrated in several clinical neuroimaging studies. A number of investigations have demonstrated that ASL perfusion is comparable with other more invasive methods such as DSC MRI perfusion and PET. These characteristics make ASL likely to become widely used in research and clinical practice.
| 1. | Rosen BR, Belliveau JW, Vevea JM, Brady TJ. Perfusion imaging with NMR contrast agents. Magn Reson Med. 1990;14:249-265. |
| 2. | Detre JA, Zhang W, Roberts DA, Silva AC, Williams DS, Grandis DJ, Koretsky AP, Leigh JS. Tissue specific perfusion imaging using arterial spin labeling. NMR Biomed. 1994;7:75-82. |
| 3. | Deibler AR, Pollock JM, Kraft RA, Tan H, Burdette JH, Maldjian JA. Arterial spin-labeling in routine clinical practice, part 1: technique and artifacts. AJNR Am J Neuroradiol. 2008;29:1228-1234. |
| 4. | Wolf RL, Detre JA. Clinical neuroimaging using arterial spin-labeled perfusion magnetic resonance imaging. Neurotherapeutics. 2007;4:346-359. |
| 5. | Petersen ET, Lim T, Golay X. Model-free arterial spin labeling quantification approach for perfusion MRI. Magn Reson Med. 2006;55:219-232. |
| 6. | Pollock JM, Tan H, Kraft RA, Whitlow CT, Burdette JH, Maldjian JA. Arterial spin-labeled MR perfusion imaging: clinical applications. Magn Reson Imaging Clin N Am. 2009;17:315-338. |
| 7. | Petersen ET, Zimine I, Ho YC, Golay X. Non-invasive measurement of perfusion: a critical review of arterial spin labelling techniques. Br J Radiol. 2006;79:688-701. |
| 8. | Alsop DC, Detre JA. Multisection cerebral blood flow MR imaging with continuous arterial spin labeling. Radiology. 1998;208:410-416. |
| 9. | Williams DS, Detre JA, Leigh JS, Koretsky AP. Magnetic resonance imaging of perfusion using spin inversion of arterial water. Proc Natl Acad Sci USA. 1992;89:212-216. |
| 10. | MacIntosh BJ, Filippini N, Chappell MA, Woolrich MW, Mackay CE, Jezzard P. Assessment of arterial arrival times derived from multiple inversion time pulsed arterial spin labeling MRI. Magn Reson Med. 2010;63:641-647. |
| 11. | Detre JA, Wang J, Wang Z, Rao H. Arterial spin-labeled perfusion MRI in basic and clinical neuroscience. Curr Opin Neurol. 2009;22:348-355. |
| 12. | Liu TT, Brown GG. Measurement of cerebral perfusion with arterial spin labeling: Part 1. Methods. J Int Neuropsychol Soc. 2007;13:517-525. |
| 13. | Wong EC, Cronin M, Wu WC, Inglis B, Frank LR, Liu TT. Velocity-selective arterial spin labeling. Magn Reson Med. 2006;55:1334-1341. |
| 14. | Duyn JH, Tan CX, van Gelderen P, Yongbi MN. High-sensitivity single-shot perfusion-weighted fMRI. Magn Reson Med. 2001;46:88-94. |
| 15. | Hernandez-Garcia L, Lee GR, Vazquez AL, Yip CY, Noll DC. Quantification of perfusion fMRI using a numerical model of arterial spin labeling that accounts for dynamic transit time effects. Magn Reson Med. 2005;54:955-964. |
| 16. | Golay X, Petersen ET. Arterial spin labeling: benefits and pitfalls of high magnetic field. Neuroimaging Clin N Am. 2006;16:259-268, x. |
| 17. | Buxton RB, Frank LR, Wong EC, Siewert B, Warach S, Edelman RR. A general kinetic model for quantitative perfusion imaging with arterial spin labeling. Magn Reson Med. 1998;40:383-396. |
| 18. | Fernández-Seara MA, Wang Z, Wang J, Rao HY, Guenther M, Feinberg DA, Detre JA. Continuous arterial spin labeling perfusion measurements using single shot 3D GRASE at 3 T. Magn Reson Med. 2005;54:1241-1247. |
| 19. | Fernández-Seara MA, Wang J, Wang Z, Korczykowski M, Guenther M, Feinberg DA, Detre JA. Imaging mesial temporal lobe activation during scene encoding: comparison of fMRI using BOLD and arterial spin labeling. Hum Brain Mapp. 2007;28:1391-1400. |
| 20. | Golay X, Hendrikse J, Lim TC. Perfusion imaging using arterial spin labeling. Top Magn Reson Imaging. 2004;15:10-27. |
| 21. | Wu WC, Fernández-Seara M, Detre JA, Wehrli FW, Wang J. A theoretical and experimental investigation of the tagging efficiency of pseudocontinuous arterial spin labeling. Magn Reson Med. 2007;58:1020-1027. |
| 22. | Talagala SL, Kam AW. Multi-slice perfusion MRI of the kidney using SPDI-CASL. Proc Intl Soc Mag Reson Med. 2000;8:705. |
| 23. | Silva AC, Zhang W, Williams DS, Koretsky AP. Multi-slice MRI of rat brain perfusion during amphetamine stimulation using arterial spin labeling. Magn Reson MBed. 1995;33:209-214. |
| 24. | Zhang W, Silva AC, Williams DS, Koretsky AP. NMR measurement of perfusion using arterial spin labeling without saturation of macromolecular spins. Magn Reson Med. 1995;33:370-376. |
| 25. | Edelman RR, Siewert B, Darby DG, Thangaraj V, Nobre AC, Mesulam MM, Warach S. Qualitative mapping of cerebral blood flow and functional localization with echo-planar MR imaging and signal targeting with alternating radio frequency. Radiology. 1994;192:513-520. |
| 26. | Barbier EL, Lamalle L, Décorps M. Methodology of brain perfusion imaging. J Magn Reson Imaging. 2001;13:496-520. |
| 27. | Kwong KK, Chesler DA, Weisskoff RM, Donahue KM, Davis TL, Ostergaard L, Campbell TA, Rosen BR. MR perfusion studies with T1-weighted echo planar imaging. Magn Reson Med. 1995;34:878-887. |
| 28. | Kim SG. Quantification of relative cerebral blood flow change by flow-sensitive alternating inversion recovery (FAIR) technique: application to functional mapping. Magn Reson Med. 1995;34:293-301. |
| 29. | Wong EC. Quantifying CBF with pulsed ASL: technical and pulse sequence factors. J Magn Reson Imaging. 2005;22:727-731. |
| 30. | Wu WC, Wong EC. Intravascular effect in velocity-selective arterial spin labeling: the choice of inflow time and cutoff velocity. Neuroimage. 2006;32:122-128. |
| 31. | Paiva FF, Tannús A, Silva AC. Measurement of cerebral perfusion territories using arterial spin labelling. NMR Biomed. 2007;20:633-642. |
| 32. | van Laar PJ, van der Grond J, Hendrikse J. Brain perfusion territory imaging: methods and clinical applications of selective arterial spin-labeling MR imaging. Radiology. 2008;246:354-364. |
| 33. | Wu B, Wang X, Guo J, Xie S, Wong EC, Zhang J, Jiang X, Fang J. Collateral circulation imaging: MR perfusion territory arterial spin-labeling at 3T. AJNR Am J Neuroradiol. 2008;29:1855-1860. |
| 34. | Chng SM, Petersen ET, Zimine I, Sitoh YY, Lim CC, Golay X. Territorial arterial spin labeling in the assessment of collateral circulation: comparison with digital subtraction angiography. Stroke. 2008;39:3248-3254. |
| 35. | Bokkers RP, van Laar PJ, van de Ven KC, Kapelle LJ, Klijn CJ, Hendrikse J. Arterial spin-labeling MR imaging measurements of timing parameters in patients with a carotid artery occlusion. AJNR Am J Neuroradiol. 2008;29:1698-1703. |
| 36. | Hendrikse J, van Osch MJ, Rutgers DR, Bakker CJ, Kappelle LJ, Golay X, van der Grond J. Internal carotid artery occlusion assessed at pulsed arterial spin-labeling perfusion MR imaging at multiple delay times. Radiology. 2004;233:899-904. |
| 37. | Bokkers RP, Bremmer JP, van Berckel BN, Lammertsma AA, Hendrikse J, Pluim JP, Kappelle LJ, Boellaard R, Klijn CJ. Arterial spin labeling perfusion MRI at multiple delay times: a correlative study with H(2)(15)O positron emission tomography in patients with symptomatic carotid artery occlusion. J Cereb Blood Flow Metab. 2010;30:222-229. |
| 38. | Detre JA, Wang J. Technical aspects and utility of fMRI using BOLD and ASL. Clin Neurophysiol. 2002;113:621-634. |
| 39. | Wolf RL, Alsop DC, Levy-Reis I, Meyer PT, Maldjian JA, Gonzalez-Atavales J, French JA, Alavi A, Detre JA. Detection of mesial temporal lobe hypoperfusion in patients with temporal lobe epilepsy by use of arterial spin labeled perfusion MR imaging. AJNR Am J Neuroradiol. 2001;22:1334-1341. |
| 40. | Brown GG, Clark C, Liu TT. Measurement of cerebral perfusion with arterial spin labeling: Part 2. Applications. J Int Neuropsychol Soc. 2007;13:526-538. |
| 41. | Wang J, Licht DJ, Jahng GH, Liu CS, Rubin JT, Haselgrove J, Zimmerman RA, Detre JA. Pediatric perfusion imaging using pulsed arterial spin labeling. J Magn Reson Imaging. 2003;18:404-413. |
| 42. | Epstein HT. Stages of increased cerebral blood flow accompany stages of rapid brain growth. Brain Dev. 1999;21:535-539. |
| 43. | van Osch MJ, Teeuwisse WM, van Walderveen MA, Hendrikse J, Kies DA, van Buchem MA. Can arterial spin labeling detect white matter perfusion signal? Magn Reson Med. 2009;62:165-173. |
| 44. | van Gelderen P, de Zwart JA, Duyn JH. Pittfalls of MRI measurement of white matter perfusion based on arterial spin labeling. Magn Reson Med. 2008;59:788-795. |
| 45. | Detre JA, Alsop DC, Vives LR, Maccotta L, Teener JW, Raps EC. Noninvasive MRI evaluation of cerebral blood flow in cerebrovascular disease. Neurology. 1998;50:633-641. |
| 46. | Harris AD, Coutts SB, Frayne R. Diffusion and perfusion MR imaging of acute ischemic stroke. Magn Reson Imaging Clin N Am. 2009;17:291-313. |
| 47. | Chen J, Licht DJ, Smith SE, Agner SC, Mason S, Wang S, Silvestre DW, Detre JA, Zimmerman RA, Ichord RN. Arterial spin labeling perfusion MRI in pediatric arterial ischemic stroke: initial experiences. J Magn Reson Imaging. 2009;29:282-290. |
| 48. | Chalela JA, Alsop DC, Gonzalez-Atavales JB, Maldjian JA, Kasner SE, Detre JA. Magnetic resonance perfusion imaging in acute ischemic stroke using continuous arterial spin labeling. Stroke. 2000;31:680-687. |
| 49. | Weber MA, Günther M, Lichy MP, Delorme S, Bongers A, Thilmann C, Essig M, Zuna I, Schad LR, Debus J. Comparison of arterial spin-labeling techniques and dynamic susceptibility-weighted contrast-enhanced MRI in perfusion imaging of normal brain tissue. Invest Radiol. 2003;38:712-718. |
| 50. | Zaharchuk G, Bammer R, Straka M, Shankaranarayan A, Alsop DC, Fischbein NJ, Atlas SW, Moseley ME. Arterial spin-label imaging in patients with normal bolus perfusion-weighted MR imaging findings: pilot identification of the borderzone sign. Radiology. 2009;252:797-807. |
| 51. | Wolf RL, Wang J, Wang S, Melhem ER, O'Rourke DM, Judy KD, Detre JA. Grading of CNS neoplasms using continuous arterial spin labeled perfusion MR imaging at 3 Tesla. J Magn Reson Imaging. 2005;22:475-482. |
| 52. | Lacerda S, Law M. Magnetic resonance perfusion and permeability imaging in brain tumors. Neuroimaging Clin N Am. 2009;19:527-557. |
| 53. | Tourdias T, Rodrigo S, Oppenheim C, Naggara O, Varlet P, Amoussa S, Calmon G, Roux FX, Meder JF. Pulsed arterial spin labeling applications in brain tumors: practical review. J Neuroradiol. 2008;35:79-89. |
| 54. | Warmuth C, Gunther M, Zimmer C. Quantification of blood flow in brain tumors: comparison of arterial spin labeling and dynamic susceptibility-weighted contrast-enhanced MR imaging. Radiology. 2003;228:523-532. |
| 55. | Weber MA, Thilmann C, Lichy MP, Günther M, Delorme S, Zuna I, Bongers A, Schad LR, Debus J, Kauczor HU. Assessment of irradiated brain metastases by means of arterial spin-labeling and dynamic susceptibility-weighted contrast-enhanced perfusion MRI: initial results. Invest Radiol. 2004;39:277-287. |
| 56. | Ozsunar Y, Mullins ME, Kwong K, Hochberg FH, Ament C, Schaefer PW, Gonzalez RG, Lev MH. Glioma recurrence versus radiation necrosis? A pilot comparison of arterial spin-labeled, dynamic susceptibility contrast enhanced MRI, and FDG-PET imaging. Acad Radiol. 2010;17:282-290. |
| 57. | Deibler AR, Pollock JM, Kraft RA, Tan H, Burdette JH, Maldjian JA. Arterial spin-labeling in routine clinical practice, part 2: hypoperfusion patterns. AJNR Am J Neuroradiol. 2008;29:1235-1241. |
| 58. | Deibler AR, Pollock JM, Kraft RA, Tan H, Burdette JH, Maldjian JA. Arterial spin-labeling in routine clinical practice, part 3: hyperperfusion patterns. AJNR Am J Neuroradiol. 2008;29:1428-1435. |
| 59. | Wolf RL, Wang J, Detre JA, Zager EL, Hurst RW. Arteriovenous shunt visualization in arteriovenous malformations with arterial spin-labeling MR imaging. AJNR Am J Neuroradiol. 2008;29:681-687. |
| 60. | Camacho DL, Smith JK, Grimme JD, Keyserling HF, Castillo M. Atypical MR imaging perfusion in developmental venous anomalies. AJNR Am J Neuroradiol. 2004;25:1549-1552. |
| 61. | Schmidt R, Havas D, Ropele S, Enzinger C, Fazekas F. MRI in dementia. Neurol Clin. 2009;27:221-236, ix. |
| 62. | Du AT, Jahng GH, Hayasaka S, Kramer JH, Rosen HJ, Gorno-Tempini ML, Rankin KP, Miller BL, Weiner MW, Schuff N. Hypoperfusion in frontotemporal dementia and Alzheimer disease by arterial spin labeling MRI. Neurology. 2006;67:1215-1220. |
| 63. | Sandson TA, O'Connor M, Sperling RA, Edelman RR, Warach S. Noninvasive perfusion MRI in Alzheimer's disease: a preliminary report. Neurology. 1996;47:1339-1342. |
| 64. | Alsop DC, Detre JA, Grossman M. Assessment of cerebral blood flow in Alzheimer's disease by spin-labeled magnetic resonance imaging. Ann Neurol. 2000;47:93-100. |
| 65. | Johnson NA, Jahng GH, Weiner MW, Miller BL, Chui HC, Jagust WJ, Gorno-Tempini ML, Schuff N. Pattern of cerebral hypoperfusion in Alzheimer disease and mild cognitive impairment measured with arterial spin-labeling MR imaging: initial experience. Radiology. 2005;234:851-859. |
| 66. | Chao LL, Buckley ST, Kornak J, Schuff N, Madison C, Yaffe K, Miller BL, Kramer JH, Weiner MW. ASL perfusion MRI predicts cognitive decline and conversion from MCI to dementia. Alzheimer Dis Assoc Disord. 2010;24:19-27. |
| 67. | Wang J, Licht DJ. Pediatric perfusion MR imaging using arterial spin labeling. Neuroimaging Clin N Am. 2006;16:149-167, ix. |
| 68. | Engelhorn T, Doerfler A, Weise J, Baehr M, Forsting M, Hufnagel A. Cerebral perfusion alterations during the acute phase of experimental generalized status epilepticus: prediction of survival by using perfusion-weighted MR imaging and histopathology. AJNR Am J Neuroradiol. 2005;26:1563-1570. |
| 69. | Liu HL, Kochunov P, Hou J, Pu Y, Mahankali S, Feng CM, Yee SH, Wan YL, Fox PT, Gao JH. Perfusion-weighted imaging of interictal hypoperfusion in temporal lobe epilepsy using FAIR-HASTE: comparison with H(2)(15)O PET measurements. Magn Reson Med. 2001;45:431-435. |
| 70. | O'Gorman RL, Siddiqui A, Alsop DC, Jarosz JM. Perfusion MRI demonstrates crossed-cerebellar diaschisis in sickle cell disease. Pediatr Neurol. 2010;42:437-440. |
Peer reviewers: Owen Carmichael, PhD, Assistant Professor, Neurology Department, University of California, Davis, 1544 Newton Court, Davis, CA 95618-4859, United States; Daniel J Kelley, PhD, Medical Scientist Training Program Fellow, University of Wisconsin School of Medicine and Public Health, 5015 Sheboygan Ave # 104, Madison, WI 53705, United States
S- Editor Cheng JX L- Editor Lutze M E- Editor Zheng XM