Published online Sep 28, 2025. doi: 10.4329/wjr.v17.i9.112983
Revised: August 20, 2025
Accepted: September 9, 2025
Published online: September 28, 2025
Processing time: 46 Days and 1.5 Hours
Intracerebral hemorrhage (ICH) comprises 9%-27% of stroke patients. Hematoma expansion (HE) occurs in approximately 20% of patients following ICH, typically within the first 24 hours. HE increases mortality and long-term disability in these patients and is being investigated as a therapeutic target to improve the outcome in these patients by limiting HE. Non-contrast computed tomography (NCCT) has potential in predicting HE, which can identify the individuals at risk.
To evaluate NCCT markers for predicting HE in patients with ICH and to develop a simple, practical grading system for risk stratification.
This prospective observational study evaluated 192 patients with spontaneous ICH who underwent a baseline NCCT within four hours of admission, followed by a follow-up scan after six hours or earlier if there was clinical deterioration. Hematoma volumes and imaging characteristics that predicted HE were evaluated. A simple five-point grading system score was created to predict HE. In this scoring system, five imaging parameters were evaluated, with each parameter assigned a score of either 0 or 1. The parameters included: (1) Baseline hematoma volume ≥ 30 mL vs < 30 mL; (2) Presence or absence of intraventricular hemorrhage; (3) Presence or absence of the island sign; (4) Presence or absence of the black hole sign; and (5) Presence or absence of the swirl sign.
Of the 192 patients studied, HE was seen in 106 (55.2%). The mean baseline hematoma volume was significantly greater among patients in the HE group (44.1 mL) compared to those in the non-HE group (12.2 mL), with a P-value < 0.05. Additionally, imaging biomarkers such as the island sign, swirl sign, and black hole sign were observed with significantly higher frequency in the HE group relative to the non-HE cohort (all P-values < 0.05). The island sign was strongly associated with HE [odds ratio (OR) 13.7; 95% confidence interval (CI): 10.15-16.37; P < 0.001]. Similarly, the black hole sign (OR 9.4; 95%CI: 7.4-11.62; P < 0.001) and the swirl sign (OR 5.2; 95%CI: 3.72-6.53; P < 0.001) emerged as significant predictors of HE. Initial hematoma volume ≥ 30 mL also showed a sig
The five variables demonstrated statistically significant associations with HE. This simple and practical 5-point prediction score can enable identification of patients at elevated risk of HE based on baseline NCCT findings. This can facilitate timely recognition of high-risk individuals who may benefit from targeted anti-expansion therapy.
Core Tip: Hematoma expansion (HE) in spontaneous intracerebral hemorrhage (ICH) is a major predictor of short-term mortality and long-term morbidity. Early identification of patients at risk for HE allows timely initiation of targeted anti-expansion therapies to limit hematoma growth. In this study, we evaluated non-contrast computed tomography (NCCT) markers for predicting HE after ICH and developed a simple five-point predictive scoring system. This score, based solely on NCCT findings, is practical, easy to use, and particularly valuable in resource-limited settings for guiding the manage
- Citation: Parry AH, Fatima SA, Wani M, Jehangir M, Farooq O, Wagay MI, Ashraf O, Hakeem AA. Predicting hematoma growth in spontaneous intracerebral hemorrhage: A simplified non-contrast computed tomography based five-point grading approach. World J Radiol 2025; 17(9): 112983
- URL: https://www.wjgnet.com/1949-8470/full/v17/i9/112983.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i9.112983
Stroke ranks as the second most common cause of morbidity and mortality globally, preceded only by ischemic heart disease[1]. According to estimates, 9%-27% of all strokes worldwide are due to spontaneous intracerebral hemorrhage (ICH), and the rest are caused by ischemia. The morbidity and mortality are higher in ICH compared to ischemic stroke[2].
Hematoma expansion (HE) typically occurs within the first 24 hours after ICH and affects approximately 20% of ICH patients[2]. HE causes mechanical damage to brain tissue and also elicits an inflammatory response in the surrounding brain parenchyma, which further increases the severity of neuronal damage[3,4]. HE has been shown to predict the risk of long-term neurological disability and death. Owing to its potential role in predicting death and disability, there has been an increased focus on improving patient outcome by limiting the growth of hematoma.
The vital statement ‘time is brain’ holds true not only in ischemic stroke but is equally true for ICH. As such, the first few hours after ICH offer a critical window where timely intervention aimed at limiting HE can potentially improve patient outcomes.
ICH patients are typically managed by controlling blood pressure, stopping anticoagulants, and using supportive measures. However, recent research has shown the promising role of antifibrinolytic agents like tranexamic acid to contain the growth of hematoma[5]. The Tranexamic Acid for Hyperacute Primary ICH randomized controlled trial evaluated 2325 patients with ICH, assigning them to either a tranexamic acid group or a placebo group. The study demonstrated a statistically significant reduction in HE in the tranexamic acid group compared with placebo (P = 0.0300) with reduced 7-day mortality in the tranexamic acid group[6]. Another potential anti-expansion therapy under investigation is recombinant activated factor VII (rFVIIa). The Factor VII for Acute Hemorrhagic Stroke trial, a phase 3 study involving 841 patients treated within four hours of symptom onset with doses of 80 μg/kg and 20 μg/kg of rFVIIa. The results showed a significant reduction in HE among patients receiving rFVIIa compared with placebo[7].
ICH related to anticoagulant use represents a distinct patient group that requires urgent management through immediate cessation of the anticoagulant and prompt reversal of its effects. The American Heart Association and American Stroke Association strongly advise initiating reversal therapy without waiting for the INR result. According to current guidelines, vitamin K antagonists like warfarin should be reversed with prothrombin complex concentrate. For dabigatran, a thrombin inhibitor, idarucizumab is the reversal agent of choice, whereas; factor Xa inhibitors (rivaroxaban, apixaban, edoxaban) should be treated with andexanet alfa[8].
The presence of a “spot sign” on computed tomography (CT) angiography-indicating ongoing bleeding-has been linked to a higher likelihood of HE[9]. CT angiography is seldom used in routine cases of ICH, which diminishes its role in predicting HE. CT angiography is employed only in scenarios where there is suspicion of vascular anomaly. However, non-contrast CT (NCCT) scans are commonly performed in these cases. Recognition of various signs on NCCT has demonstrated potential for predicting HE[9-11]. These NCCT markers are being vigorously studied in an attempt to devise a simple algorithm based on these markers to identify patients at risk of HE who can potentially benefit from anti-expansion therapy[12-15].
Various radiological signs on NCCT scans-such as the swirl sign, black hole sign, blend sign, and island sign-have demonstrated significant potential in predicting HE, thereby aiding in the identification of patients at risk for hematoma growth[16-21]. The present study aimed to evaluate the association between these NCCT markers on initial imaging and HE, with the objective of developing a predictive model based on these radiological biomarkers.
The prospective observational study was conducted over a 26-month period after receiving approval from the Institutional Ethics Committee. 230 adult cases of spontaneous ICH were initially included in the study. Twenty critical patients were admitted to the intensive care unit, and 18 patients could not be reached for follow-up as they were shifted to other hospitals and were therefore excluded from the study. 192 adult (> 18-year) patients with a history of stroke and who were subsequently diagnosed with spontaneous ICH based on an NCCT performed within 4 hours of admission were enrolled in the study. These patients underwent a follow-up NCCT scan within a 6-hour period or earlier in case of cli
Secondary ICH caused by a brain tumor, trauma, or vascular malformation; hemorrhagic transformation of a cerebral infarct; ischemic stroke; or venous thrombosis; and patients with coagulopathy and/or on antiplatelet and anticoagulant medications were excluded.
A detailed medical history was obtained for all patients, with a focus on hypertension, diabetes, smoking, anticoagulant and antiplatelet medication, illicit drug use, and antihypertensive medicines. A clinical examination was performed, with an emphasis on neurological evaluation. The patients underwent routine blood tests such as a complete blood count, a kidney function test, electrolytes, and random blood sugar.
The baseline CT scan was performed within 4 hours of symptom onset, followed by a second CT within 6 hours of the baseline scan or sooner if there was clinical worsening of the patient. The CT scans were performed on a 16-slice Siemens Somatom Emotion Multidetector CT system (Erlangen, Germany) using acquisition parameters of 100-120 kVp tube voltage, 240 mAs tube current, and 5.0 mm slice thickness. Images were reconstructed at 2 mm intervals and reviewed using a window width of 80 HU and a window level of 30 HU.
Two radiologists independently assessed the CT images on an Apple Mac workstation in a satellite room. In case of disagreement, a third experienced radiologist with 20 years of experience in neuroradiology provided the final consensus report. The CT image evaluation focused on the location of hematomas in the brain, including supratentorial, infratentorial, lobar, and basal ganglia. Additional features that were evaluated included intraventricular hemorrhage (IVH), perihematomal edema, mass effect, midline shift, and subarachnoid hemorrhage. The NCCT scans were also systematically analyzed for the following specific imaging markers.
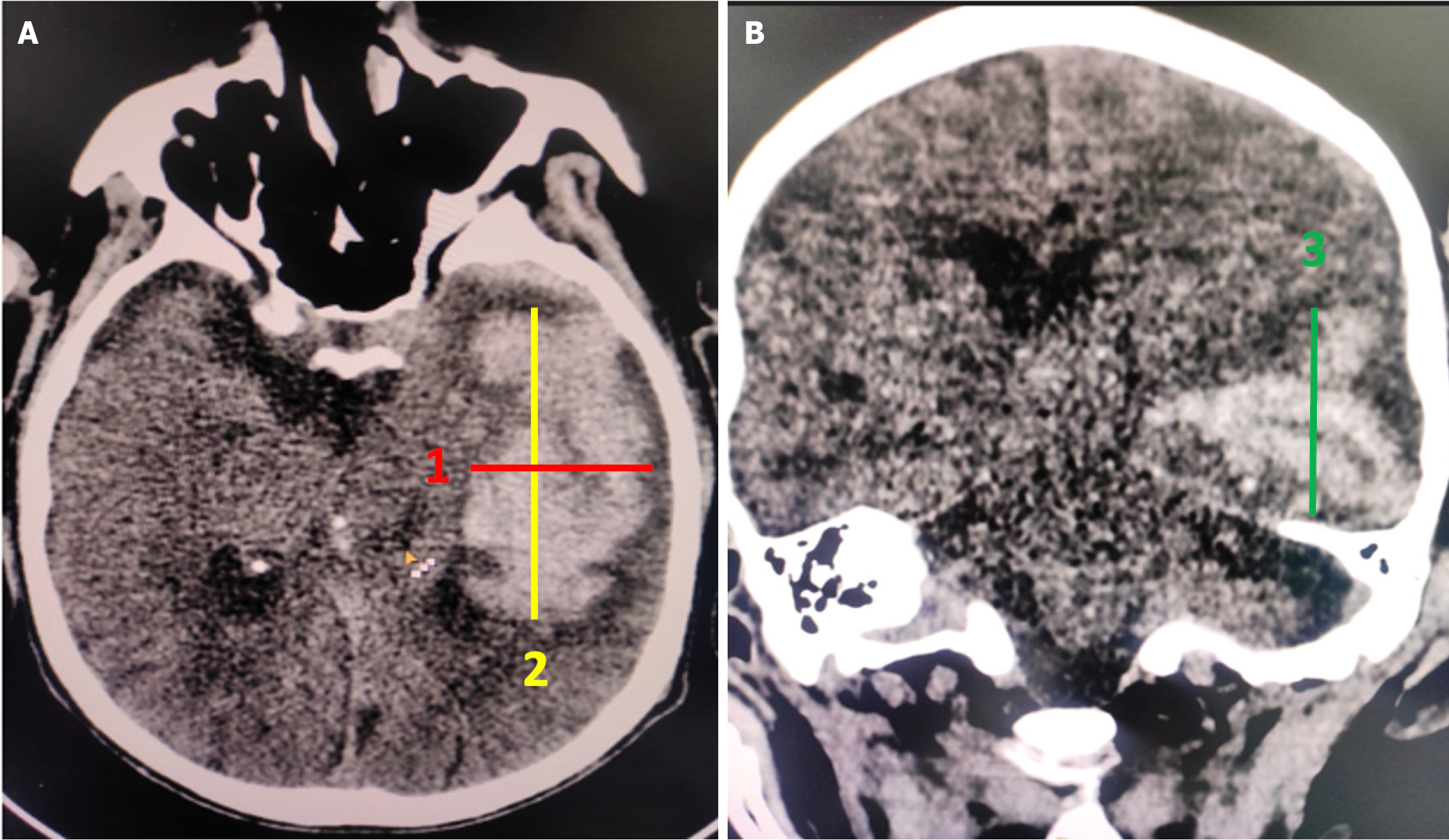
The volume of the hematoma was measured using the simplified ellipsoid formula, ABC/2[11]. First, the CT slice showing the largest hematoma size was selected. The longest dimension of the hematoma on this slice was measured as in Figure 2A, and then the dimension perpendicular to it was measured as in Figure 2A. The third measurement was acquired either by multiplying the number of slices containing the hematoma by the slice thickness or by measuring the craniocaudal dimension (as in Figure 2B) in the coronal plane (Figure 2).
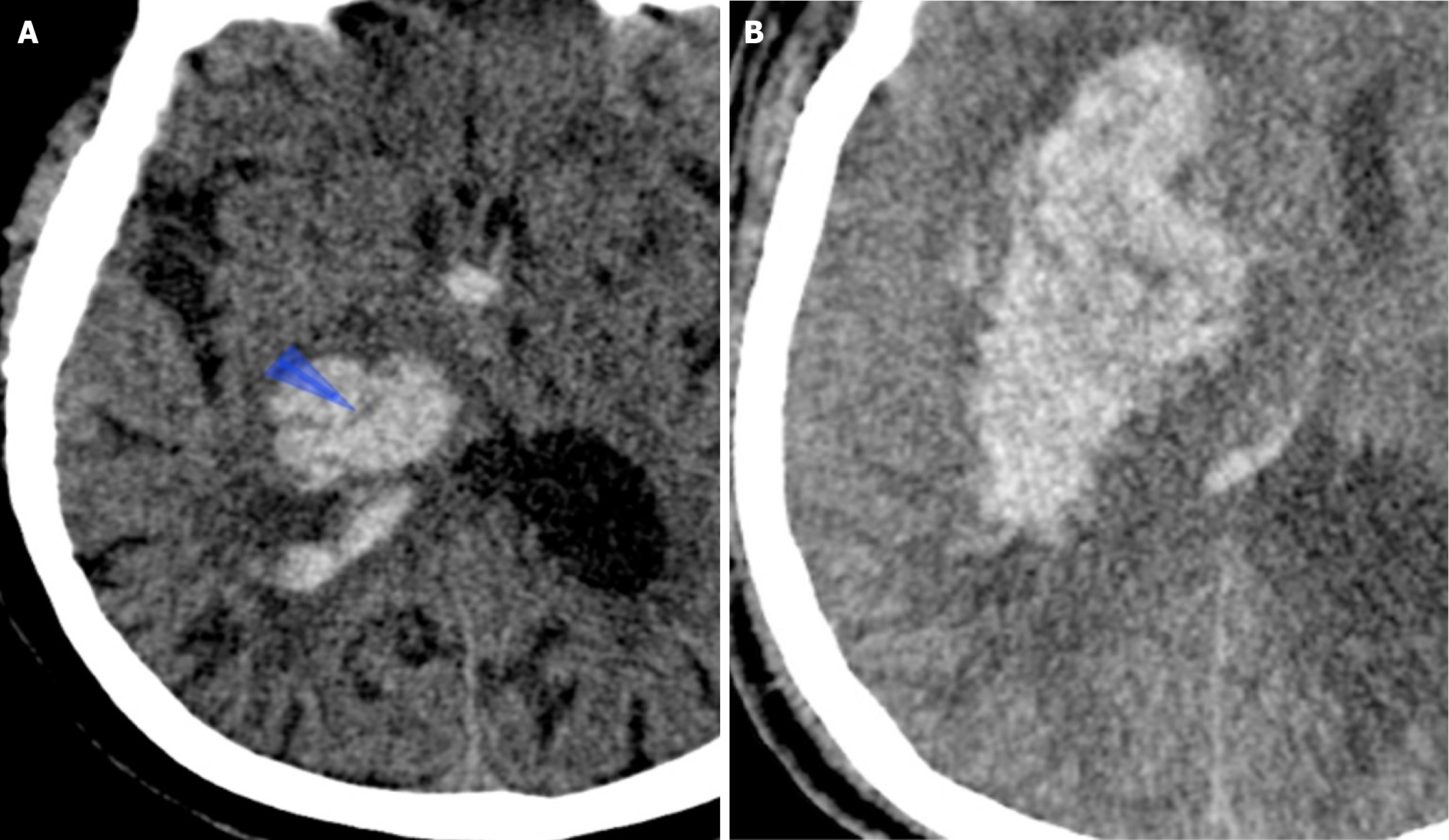
The swirl sign was defined as single or multiple areas of isoattenuation or hypoattenuation within a hyperattenuated intraparenchymal hematoma, in comparison to brain parenchyma. The shape of these regions of isoattenuation or hypoattenuation may be irregular, round, oval, or streak-like[17-19] (Figure 3).
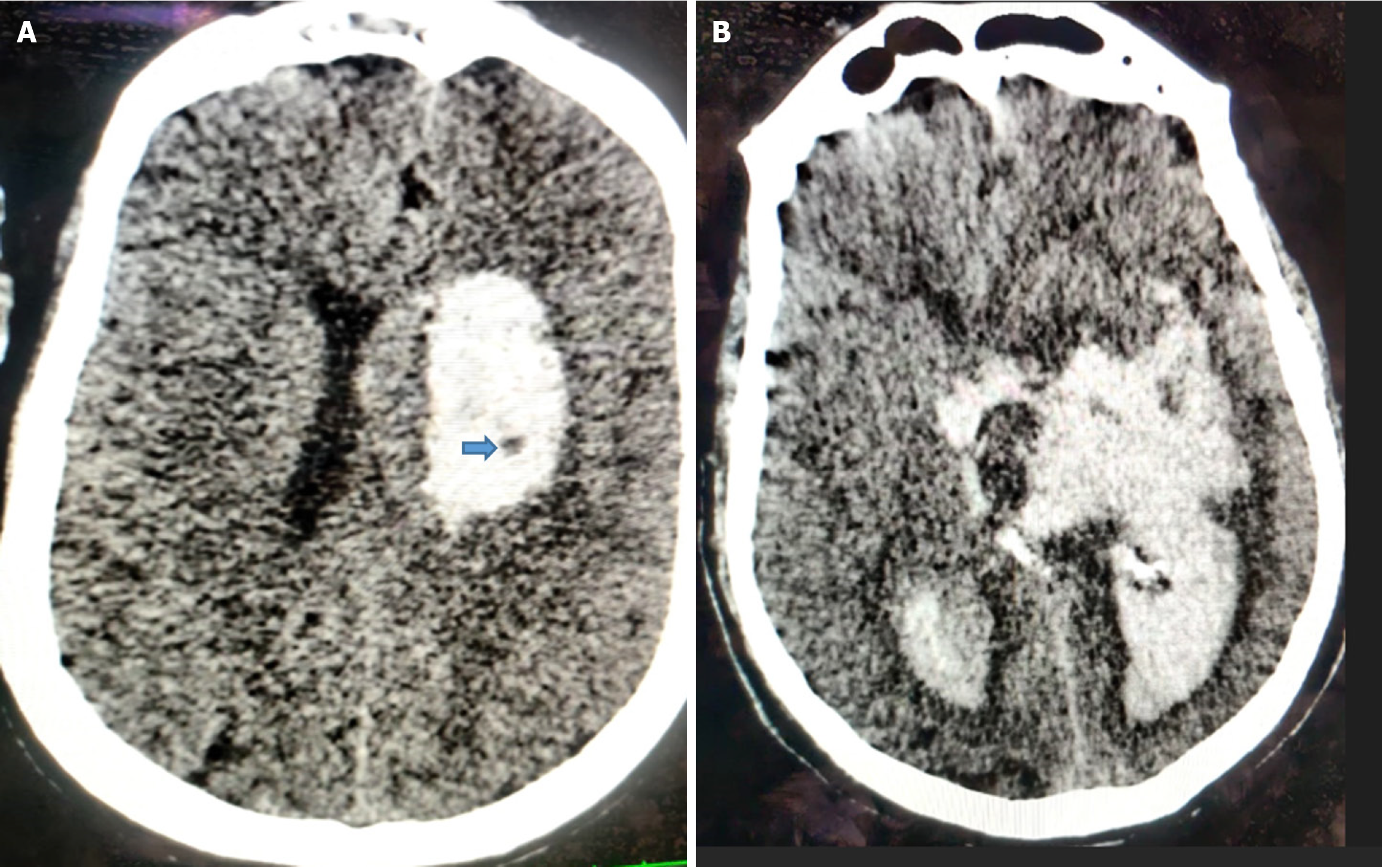
The black hole sign was considered present when an oval or round hypodense area is completely enclosed within the hyperdense hematoma, with no continuity to the adjacent brain parenchyma. It typically exhibits mixed density, signifying bleeding at different times and thus indicating a greater risk of HE. The density difference between hypoattenuated and hyperattenuated components should be at least 28 Hounsfield units for it to qualify as a black hole sign[20] (Figure 4).
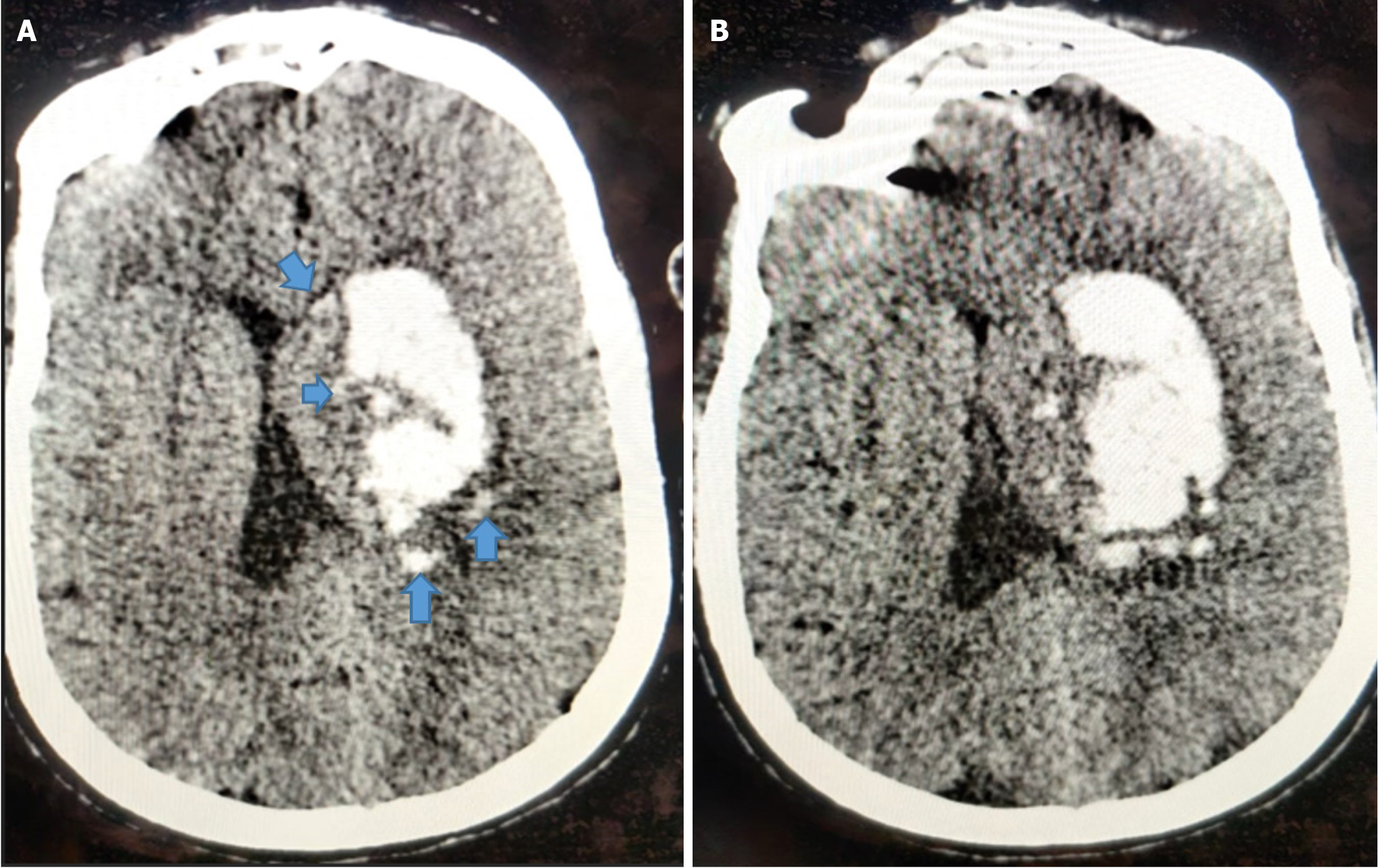
The island sign is defined as the presence of either three or more small, oval, or round hematomas separate from the main hemorrhage, or four or more sprout-like or bubble-like hematomas that may be connected to, but not lobulated with, the primary hematoma[21] (Figure 5).
HE was considered present if the hematoma volume increased by 33% or more relative to baseline, or if the absolute hematoma volume increased by at least 6 mL in comparison to the initial volume[13]. After assessment of HE, the imaging features associated with HE were evaluated to determine the predictive imaging biomarkers.
A simple five-point grading system score was developed to predict HE. In this scoring system, five imaging parameters were evaluated, with each parameter assigned a score of either 0 or 1. The parameters included: (1) Baseline hematoma volume ≥ 30 mL vs < 30 mL; (2) Presence or absence of intraventricular hemorrhage; (3) Presence or absence of the island sign; (4) Presence or absence of the black hole sign; and (5) Presence or absence of the swirl sign.
Data entry was done using Microsoft Excel. Continuous variables were expressed as means with SD, whereas categorical variables were summarized as percentages. Comparison of continuous variables was performed using the t-test, whereas the χ2 test was used for categorical variables. Both univariate and multivariate logistic regression analyses were performed to identify factors associated with HE. Variables with P values less than 0.05 were regarded as statistically significant.
The study cohort comprised 192 patients with spontaneous ICH, of whom 62.5% (n = 120) were male and 37.5% (n = 72) were female. The mean patient age was 60.4 ± 10.75 years, ranging from 35 to 87 years. Hypertension emerged as the leading risk factor, present in 78.13% (n = 150) while diabetes mellitus was identified in 38.54% (n = 74). Chronic kidney disease was identified as risk factor in 35% (n = 66). Additional risk factors occurred less often. Table 1 presents the prevalence of different risk factors in the study cohort.
| Gender | Number (n) | Percentage (%) |
| Male | 120 | 62.5 |
| Female | 72 | 37.5 |
| Total | 192 | 100 |
| Male:female = 1.7:1 | ||
| Comorbidity | ||
| Hypertension | 150 | 78.1 |
| Diabetes | 74 | 38.5 |
| Chronic kidney disease | 66 | 35.1 |
| Smoking | 46 | 24 |
| Dyslipidemia | 38 | 19.8 |
| Alcohol intake | 6 | 3.1 |
| Parameter | ||
| Capsuloganglionic | 100 | 52.1 |
| Lobar | 70 | 36.5 |
| Infratentorial | 22 | 11.5 |
| IVH | ||
| Present | 108 | 56.3 |
| Absent | 84 | 43.8 |
| SAH | ||
| Present | 6 | 3.1 |
| Absent | 186 | 96.9 |
| Baseline hematoma volume | ||
| < 30 mL | 114 | 59.4 |
| ≥ 30 mL | 78 | 40.6 |
| Midline shift | ||
| Present | 148 | 77.1 |
| Absent | 44 | 22.9 |
| Island sign | ||
| Present | 62 | 32.3 |
| Absent | 130 | 67.7 |
| Swirl sign | ||
| Present | 44 | 22.9 |
| Absent | 148 | 77.1 |
| Black hole sign | ||
| Present | 58 | 30.2 |
| Absent | 134 | 69.8 |
The site of initial hematoma was the internal capsule-basal ganglia region in 100 cases (52.1%), the different lobes of cerebral hemispheres in 70 cases (36.5%), and the infratentorial fossa in 22 cases (11.5%). The baseline hematoma volume was < 30 mL in 114 patients (59.4%) and > 30 mL in 78 patients (40.6%). IVH was seen in 108 patients (56.3%), whereas SAH was noted in 6 patients (3.1%) on the initial CT scan (Table 1).
The patients with HE demonstrated significantly larger baseline hematoma volumes compared to those without HE (44.1 ± 26.34 mL vs 12.2 ± 16.32 mL, P < 0.001). The presence of IVH was also significantly higher in the HE group (77.4% vs 30.2%, P < 0.001). In contrast, the presence of SAH did not show a significant difference between the groups (P = 0.685) (Table 2).
Among the 192 patients with ICH, the island sign was identified in 32.29% (n = 62), whereas, the swirl sign was observed in 22.91% (n = 44), and black hole sign was identified in 30.20% (n = 58), in the initial CT scan. The HE was detected in 106 patients (55.2%; 106 out of 192) on follow-up imaging. Of those with HE, 56 patients (52.8%) demonstrated the island sign, compared with only 6 patients (7%) in the non-HE group (P < 0.05). The black hole sign was seen in 42 (39.6%) among the HE group compared to 16 (18.6%) in the non-HE group (P < 0.05). The swirl sign was observed in 32 (30.2%) in the HE group compared to 12 (14%) in the non-HE group (P < 0.05). Among NCCT imaging features, the island sign (P < 0.001), swirl sign (P = 0.049), and black hole sign (P = 0.026) were all significantly associated with HE.
The study found that baseline hematoma volume in patients with at least one positive NCCT marker (island sign, swirl sign, and black hole sign) was significantly higher compared to patients with the absence of any of these NCCT markers (all P values < 0.05) (Table 3).
| Variable | IS/SS/BS (+) | IS/SS/BS (-) | P value |
| Imaging characteristics as per island sign | |||
| Baseline hematoma volume (mL), mean ± SD | 40.9 ± 27.03 | 24.5 ± 26.08 | 0.005a |
| HE, n (%) | 56 (90.3) | 50 (38.5) | < 0.001a |
| Imaging characteristics as per swirl sign | |||
| Baseline hematoma volume (mL), mean ± SD | 37.9 ± 21.62 | 25.4 ± 23.97 | 0.031a |
| HE, n (%) | 32 (72.7) | 74 (50.0) | 0.049a |
| Imaging characteristics as per black hole sign | |||
| Baseline hematoma volume (mL), mean ± SD | 35.8 ± 21.98 | 26.1 ± 18.85 | 0.045a |
| HE, n (%) | 42 (72.4) | 64 (47.8) | 0.026a |
Multivariate logistic regression analysis demonstrated that patients with an initial volume of intracerebral hematoma of 30 mL or greater, the presence of intraventricular hemorrhage, or NCCT markers such as the island sign, swirl sign, and black hole sign were significantly more likely to experience HE, with odds ratios (OR) ranging from 1.9 to 13.7. Table 4 summarizes the ORs and their respective P-values for different imaging signs. Based on these significant variables of HE, a simplified scoring system was developed incorporating different NCCT based radiological signs (black hole sign, swirl sign and island sign), initial volume of hematoma, and IVH. Each parameter was given a score of 0 or 1 (Table 5). The predictive accuracy of this 5-point grading system was then assessed. Higher scores corresponded to greater risk of HE. The probability of HE increased progressively with the score: 7.4% (4/54) for score 0, 37.5% (12/32) for score 1, 75% (30/40) for score 2, 85.7% (24/28) for score 3, 93.3% (28/30) for score 4, and 100% (8/8) for score 5 (Table 6). Low scores (0-1) are associated with a low to moderate risk (7%-37%) of HE, intermediate scores (2-3) show a high risk (75%-85%), and high scores (4-5) correspond to an extremely high to certain risk (93%-100%) of HE.
| Component | Points |
| Baseline hematoma volume (mL) | |
| ≥ 30 | 1 |
| < 30 | 0 |
| IVH | |
| Present | 1 |
| Absent | 0 |
| Island sign | |
| Present | 1 |
| Absent | 0 |
| Black hole sign | |
| Present | 1 |
| Absent | 0 |
| Swirl sign | |
| Present | 1 |
| Absent | 0 |
| Total score | No. of patients | HE | Risk of HE (%) |
| 0 | 54 | 4 | 7.4 |
| 1 | 32 | 12 | 37.5 |
| 2 | 40 | 30 | 75.0 |
| 3 | 28 | 24 | 85.7 |
| 4 | 30 | 28 | 93.3 |
| 5 | 8 | 8 | 100 |
Spontaneous ICH remains a major global contributor to morbidity and mortality. Among the various predictors of poor outcomes, HE has emerged as a particularly important and potentially modifiable factor for outcomes. Recent advancements have identified a number of NCCT imaging signs that are valuable in predicting HE, including the black hole sign, blend sign, island sign, and swirl sign[22-25]. The swirl sign, black hole sign, and island sign are imaging surrogate markers that suggest ongoing active bleeding or extravasation within a hematoma. Each of these NCCT imaging signs reflects underlying dynamic changes going on within a hematoma, caused by continuous leakage of fresh blood into the clot. Recognition of these signs is clinically important, as they help identify a subset of patients who are at a greater risk for HE. Although these signs are detected on NCCT, they are considered indirect correlates of the CT angiographic spot sign, which provides direct evidence of active contrast extravasation and is a well-established predictor of HE. In this study, it was observed that several NCCT-based characteristics are significantly associated with early HE, consistent with findings in previous literature. Subsequently, we developed a predictive model for HE based on these NCCT markers.
Patients with HE demonstrated significantly larger baseline hematoma volumes compared to those without HE (44.1 ± 26.34 mL vs 12.2 ± 16.32 mL, P < 0.001). This aligns with the hypothesis that a larger initial hematoma may intensify the mechanical disruption of vessels and exacerbate bleeding through vascular shearing.
The presence of IVH was also significantly higher in the HE group (77.4% vs 30.2%, P < 0.001). Among NCCT imaging features, the island sign (P < 0.001), swirl sign (P = 0.049), and black hole sign (P = 0.026) were all significantly associated with HE. In contrast, the presence of SAH did not show a significant difference between the groups (P = 0.685). The presence of a statistically significant association of these NCCT markers with HE reinforces their predictive potential, as previously suggested in the literature.
In the multivariate logistic regression analysis, several significant predictors of HE were identified. A hematoma volume of ≥ 30 mL at baseline was associated with a 1.9-fold increased risk of expansion. The presence of IVH increased the odds by 4.5 times. Radiological signs such as the island sign, swirl sign, and black hole sign were strongly predictive, with ORs of 13.7, 5.2, and 9.4, respectively. Among these, the island sign and black hole sign emerged as the most powerful predictors. All predictors demonstrated statistical significance (P < 0.05). Our findings align with previous studies demonstrating the predictive value of NCCT signs. The island, swirl, and black hole signs have each been shown in prior literature to possess moderate to high sensitivity and specificity for predicting HE, although values vary across cohorts and imaging protocols.
The 5-point NCCT-based predictive score showed potential clinical utility in early risk stratification by predicting risk of HE. Based on the 5-point predictive model, patients with low scores (0-1) were associated with a low to moderate risk (7%-37%) of HE. Intermediate scores (2-3) showed a high risk (75%-85%) for HE. High scores (4-5) corresponded to an extremely high to certain risk (93%-100%) of HE. This grading system is fairly predictive, with high scores strongly correlating with a greater risk of HE. Patients with a score of 4 or 5 are at critical risk of HE and may require urgent clinical interventions.
Cai et al[12] evaluated 414 patients; 63 exhibited the blend sign, 45 had the black hole sign, 36 demonstrated the island sign, and 34 presented the swirl sign. HE was observed in 88 patients (21.26%). Of the four imaging markers, the blend sign demonstrated the highest sensitivity (25%), while the swirl sign exhibited the highest specificity (92.02%) in predicting HE.
Ducroux et al[13] included 124 patients in their study, of whom 51 (41%) experienced HE. The sensitivity of the various markers for predicting revised HE ranged from 4% to 78%, while specificity varied between 37% and 97%. The positive likelihood ratios ranged from 0.41 to 7.16, and the negative likelihood ratios spanned from 0.49 to 1.06. Univariable logistic regression analysis identified five markers that were significantly associated with HE: Black hole [OR = 8.66, 95% confidence interval (CI): 2.15-58.14, P = 0.007], hypodensity (OR = 3.18, 95%CI: 1.49-6.93, P = 0.003), blend (OR = 2.90, 95%CI: 1.08-8.38, P = 0.04), satellite (OR = 2.84, 95%CI: 1.29-6.61, P = 0.01), and Barras shape (OR = 2.41, 95%CI: 1.17-5.10, P = 0.02). In multivariable analysis, the sole imaging parameter that demonstrated significant association with HE was black hole sign with OR of 5.62 (95%CI: 1.23-40.23, P = 0.03).
Various studies have utilized a combination of clinical and radiological markers to predict HE in patients with ICH[22,24,25]. A study conducted by Morotti et al[16] wherein they developed a 5-point BAT score. The assigned score was 2 points for hypodensity within a hematoma and a scan performed within 2 and a half hours of symptom onset and 1 point for the blend sign. The model showed a fair discrimination with a c-statistic of 0.77 (95%CI: 0.70-0.83) in the derivation cohort and moderate performance in two validation cohorts [c-statistics: 0.65 (95%CI: 0.61-0.68) and 0.70 (95%CI: 0.64-0.77)]. A dichotomized BAT score (≥ 3) predicted HE with 50% sensitivity and 89% specificity.
Brouwers et al[26] created a 9-point scoring system for prediction of HE after ICH, incorporating four key factors: Detection of the spot sign on CT angiography, current use of warfarin, time from symptom onset to initial CT (> 6 hours vs ≤ 6 hours), and baseline hematoma volume (< 30 mL, 30-60 mL, or > 60 mL). In their study, they found that higher scores corresponded to a greater likelihood of HE.
Additionally, we acknowledge the existence of other predictive models, including both imaging-only frameworks and those incorporating clinical parameters (e.g., blood pressure, anticoagulant use, Glasgow Coma Scale). While some models rely solely on NCCT features, others have integrated laboratory and clinical data to enhance predictive perfor
Yang et al[27] developed and validated a novel risk prediction score, integrating NCCT markers and clinical factors, to predict growth of hematoma in patients with ICH. The GIVE score is derived from a total of individual points (ranging from 0 to 6) based on specific parameters: The Glasgow Coma Scale (2 points for scores ≤ 11), the presence of the island sign (1 point), detection of IVH (1 point), and a CT scan performed within 2 and a half hours from symptom onset (2 points) and the time from symptom onset to NCCT scan (2 points for ≤ 2.5 hours). In the development cohort, the c-statistic was 0.72 (95%CI: 0.66-0.78), and in the validation cohort, it was 0.73 (95%CI: 0.63-0.82), indicating moderate predictive accuracy.
Our NCCT-based model maintains a favorable balance between simplicity and accuracy. Given the widespread use of NCCT for stroke evaluation and the ease of performing this simple NCCT-based scoring, it can be used by any hospital for swift risk stratification. This supports its use in clinical decision-making, especially in resource-limited settings where advanced imaging may not be readily available. The ease of use and practicality of this grading system represent key strengths of the model.
This study has several limitations that warrant consideration. The study is based on a limited sample of patients from a single center. This study focused on individuals who were scanned within 4 hours of developing symptoms of stroke. Although, the likelihood of HE diminishes after 4-6 hours, it needs to be seen whether this predictive model can yield similar results when employed beyond this time period.
Many anti-expansion agents are being explored for use in spontaneous ICH, and the evidence to date is encouraging. The agents that have shown promising results to limit HE include tranexemic acid and rFVIIa. However, as the evidence is still emerging and supporting data is preliminary, there are no explicit guidelines from any major international society regarding their routine use in the management of ICH. At present the decision to treat ICH patients with either tranexemic acid or rFVIIa to limit HE is based on a combination of imaging findings (spot sign, if CTA is done), black hole, swirl sign, island sign, etc., the time elapsed since symptom onset, and the overall clinical picture. As the research continues to evolve, it is anticipated that clear, evidence-based guidelines will be established by major neurological societies regarding their use in ICH. Until then the use of these agents will remain on a case-by-case basis, guided primarily by the clinical judgment and expertise of the treating physician.
Further studies, including large patient samples focused on integrating radiological and clinical parameters, are required to develop effective predictive models for HE.
Our study highlights the importance of initial hematoma size, IVH, and specific NCCT markers-particularly the island, swirl, and black hole signs-as reliable predictors of HE. The development of a concise, 5-point prediction model offers a practical and effective tool to aid in early risk stratification and management of patients with ICH. The predictive model developed herein holds promise as a rapid, imaging-based tool to guide early clinical decision-making and identify patients who may benefit from anti-expansion. Future studies with larger, multicentric cohorts are warranted to validate this model and assess its applicability in diverse clinical environments.
| 1. | GBD 2015 Neurological Disorders Collaborator Group. Global, regional, and national burden of neurological disorders during 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Neurol. 2017;16:877-897. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1613] [Cited by in RCA: 1588] [Article Influence: 176.4] [Reference Citation Analysis (0)] |
| 2. | Morotti A, Boulouis G, Dowlatshahi D, Li Q, Shamy M, Al-Shahi Salman R, Rosand J, Cordonnier C, Goldstein JN, Charidimou A. Intracerebral haemorrhage expansion: definitions, predictors, and prevention. Lancet Neurol. 2023;22:159-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 135] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 3. | Voigt S, Wermer MJH. Stopping haematoma growth: the search for the right time, place, and agent. Lancet Neurol. 2024;23:547-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 4. | Fandler-Höfler S, Murthy SB. Hematoma Expansion in Intracerebral Hemorrhage: Time Is the Enemy. Stroke. 2025;56:848-849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 5. | Pszczolkowski S, Sprigg N, Woodhouse LJ, Gallagher R, Swienton D, Law ZK, Casado AM, Roberts I, Werring DJ, Al-Shahi Salman R, England TJ, Morgan PS, Bath PM, Dineen RA. Effect of Tranexamic Acid Administration on Remote Cerebral Ischemic Lesions in Acute Spontaneous Intracerebral Hemorrhage: A Substudy of a Randomized Clinical Trial. JAMA Neurol. 2022;79:468-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 14] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 6. | Sprigg N, Flaherty K, Appleton JP, Al-Shahi Salman R, Bereczki D, Beridze M, Christensen H, Ciccone A, Collins R, Czlonkowska A, Dineen RA, Duley L, Egea-Guerrero JJ, England TJ, Krishnan K, Laska AC, Law ZK, Ozturk S, Pocock SJ, Roberts I, Robinson TG, Roffe C, Seiffge D, Scutt P, Thanabalan J, Werring D, Whynes D, Bath PM; TICH-2 Investigators. Tranexamic acid for hyperacute primary IntraCerebral Haemorrhage (TICH-2): an international randomised, placebo-controlled, phase 3 superiority trial. Lancet. 2018;391:2107-2115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 423] [Cited by in RCA: 352] [Article Influence: 44.0] [Reference Citation Analysis (0)] |
| 7. | Tanaka K, Toyoda K. Clinical Strategies Against Early Hematoma Expansion Following Intracerebral Hemorrhage. Front Neurosci. 2021;15:677744. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 8. | Greenberg SM, Ziai WC, Cordonnier C, Dowlatshahi D, Francis B, Goldstein JN, Hemphill JC 3rd, Johnson R, Keigher KM, Mack WJ, Mocco J, Newton EJ, Ruff IM, Sansing LH, Schulman S, Selim MH, Sheth KN, Sprigg N, Sunnerhagen KS; American Heart Association/American Stroke Association. 2022 Guideline for the Management of Patients With Spontaneous Intracerebral Hemorrhage: A Guideline From the American Heart Association/American Stroke Association. Stroke. 2022;53:e282-e361. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1189] [Cited by in RCA: 899] [Article Influence: 224.8] [Reference Citation Analysis (0)] |
| 9. | Demchuk AM, Dowlatshahi D, Rodriguez-Luna D, Molina CA, Blas YS, Dzialowski I, Kobayashi A, Boulanger JM, Lum C, Gubitz G, Padma V, Roy J, Kase CS, Kosior J, Bhatia R, Tymchuk S, Subramaniam S, Gladstone DJ, Hill MD, Aviv RI; PREDICT/Sunnybrook ICH CTA study group. Prediction of haematoma growth and outcome in patients with intracerebral haemorrhage using the CT-angiography spot sign (PREDICT): a prospective observational study. Lancet Neurol. 2012;11:307-314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 493] [Article Influence: 35.2] [Reference Citation Analysis (1)] |
| 10. | Lv XN, Deng L, Yang WS, Wei X, Li Q. Computed Tomography Imaging Predictors of Intracerebral Hemorrhage Expansion. Curr Neurol Neurosci Rep. 2021;21:22. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Hillal A, Ullberg T, Ramgren B, Wassélius J. Computed tomography in acute intracerebral hemorrhage: neuroimaging predictors of hematoma expansion and outcome. Insights Imaging. 2022;13:180. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 27] [Reference Citation Analysis (0)] |
| 12. | Cai J, Zhu H, Yang D, Yang R, Zhao X, Zhou J, Gao P. Accuracy of imaging markers on noncontrast computed tomography in predicting intracerebral hemorrhage expansion. Neurol Res. 2020;42:973-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 14] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 13. | Ducroux C, Nehme A, Rioux B, Panzini MA, Fahed R, Gioia LC, Létourneau-Guillon L. NCCT Markers of Intracerebral Hemorrhage Expansion Using Revised Criteria: An External Validation of Their Predictive Accuracy. AJNR Am J Neuroradiol. 2023;44:658-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Yang WS, Liu JY, Shen YQ, Xie XF, Zhang SQ, Liu FY, Yu JL, Ma YB, Xiao ZS, Duan HW, Li Q, Chen SX, Xie P. Quantitative imaging for predicting hematoma expansion in intracerebral hemorrhage: A multimodel comparison. J Stroke Cerebrovasc Dis. 2024;33:107731. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 4] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 15. | Liu Y, Zhao F, Niu E, Chen L. Machine learning for predicting hematoma expansion in spontaneous intracerebral hemorrhage: a systematic review and meta-analysis. Neuroradiology. 2024;66:1603-1616. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 16. | Morotti A, Dowlatshahi D, Boulouis G, Al-Ajlan F, Demchuk AM, Aviv RI, Yu L, Schwab K, Romero JM, Gurol ME, Viswanathan A, Anderson CD, Chang Y, Greenberg SM, Qureshi AI, Rosand J, Goldstein JN; ATACH-II, NETT, and PREDICT Investigators. Predicting Intracerebral Hemorrhage Expansion With Noncontrast Computed Tomography: The BAT Score. Stroke. 2018;49:1163-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 114] [Article Influence: 14.3] [Reference Citation Analysis (0)] |
| 17. | Ng D, Churilov L, Mitchell P, Dowling R, Yan B. The CT Swirl Sign Is Associated with Hematoma Expansion in Intracerebral Hemorrhage. AJNR Am J Neuroradiol. 2018;39:232-237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 18. | Xiong X, Li Q, Yang WS, Wei X, Hu X, Wang XC, Zhu D, Li R, Cao D, Xie P. Comparison of Swirl Sign and Black Hole Sign in Predicting Early Hematoma Growth in Patients with Spontaneous Intracerebral Hemorrhage. Med Sci Monit. 2018;24:567-573. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 31] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 19. | Selariu E, Zia E, Brizzi M, Abul-Kasim K. Swirl sign in intracerebral haemorrhage: definition, prevalence, reliability and prognostic value. BMC Neurol. 2012;12:109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 105] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 20. | Li Q, Zhang G, Xiong X, Wang XC, Yang WS, Li KW, Wei X, Xie P. Black Hole Sign: Novel Imaging Marker That Predicts Hematoma Growth in Patients With Intracerebral Hemorrhage. Stroke. 2016;47:1777-1781. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 159] [Cited by in RCA: 226] [Article Influence: 22.6] [Reference Citation Analysis (0)] |
| 21. | Li Q, Liu QJ, Yang WS, Wang XC, Zhao LB, Xiong X, Li R, Cao D, Zhu D, Wei X, Xie P. Island Sign: An Imaging Predictor for Early Hematoma Expansion and Poor Outcome in Patients With Intracerebral Hemorrhage. Stroke. 2017;48:3019-3025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 135] [Cited by in RCA: 219] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 22. | Huang Y, Zhang Q, Yang M. A reliable grading system for prediction of hematoma expansion in intracerebral hemorrhage in the basal ganglia. Biosci Trends. 2018;12:193-200. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 26] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 23. | Dowlatshahi D, Demchuk AM, Flaherty ML, Ali M, Lyden PL, Smith EE; VISTA Collaboration. Defining hematoma expansion in intracerebral hemorrhage: relationship with patient outcomes. Neurology. 2011;76:1238-1244. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 512] [Cited by in RCA: 531] [Article Influence: 35.4] [Reference Citation Analysis (0)] |
| 24. | Boulouis G, Morotti A, Brouwers HB, Charidimou A, Jessel MJ, Auriel E, Pontes-Neto O, Ayres A, Vashkevich A, Schwab KM, Rosand J, Viswanathan A, Gurol ME, Greenberg SM, Goldstein JN. Association Between Hypodensities Detected by Computed Tomography and Hematoma Expansion in Patients With Intracerebral Hemorrhage. JAMA Neurol. 2016;73:961-968. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 191] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 25. | Chen S, Zhao B, Wang W, Shi L, Reis C, Zhang J. Predictors of hematoma expansion predictors after intracerebral hemorrhage. Oncotarget. 2017;8:89348-89363. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35] [Cited by in RCA: 50] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 26. | Brouwers HB, Chang Y, Falcone GJ, Cai X, Ayres AM, Battey TW, Vashkevich A, McNamara KA, Valant V, Schwab K, Orzell SC, Bresette LM, Feske SK, Rost NS, Romero JM, Viswanathan A, Chou SH, Greenberg SM, Rosand J, Goldstein JN. Predicting hematoma expansion after primary intracerebral hemorrhage. JAMA Neurol. 2014;71:158-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 283] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 27. | Yang TN, Lv XN, Wang ZJ, Hu X, Zhao LB, Cheng J, Li Q. Predicting the risk of hematoma expansion in acute intracerebral hemorrhage: the GIVE score. BMC Neurol. 2025;25:21. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/