Published online Jul 28, 2025. doi: 10.4329/wjr.v17.i7.110394
Revised: June 24, 2025
Accepted: July 22, 2025
Published online: July 28, 2025
Processing time: 50 Days and 1.5 Hours
Coronary computed tomography angiography (CCTA) is essential for diagnosing coronary artery disease as it provides detailed images of the heart’s blood vessels to identify blockages or abnormalities. Traditionally, determining the computed tomography (CT) scanning range has relied on manual methods due to limited automation in this area.
To develop and evaluate a novel deep learning approach to automate the determination of CCTA scan ranges using anteroposterior scout images.
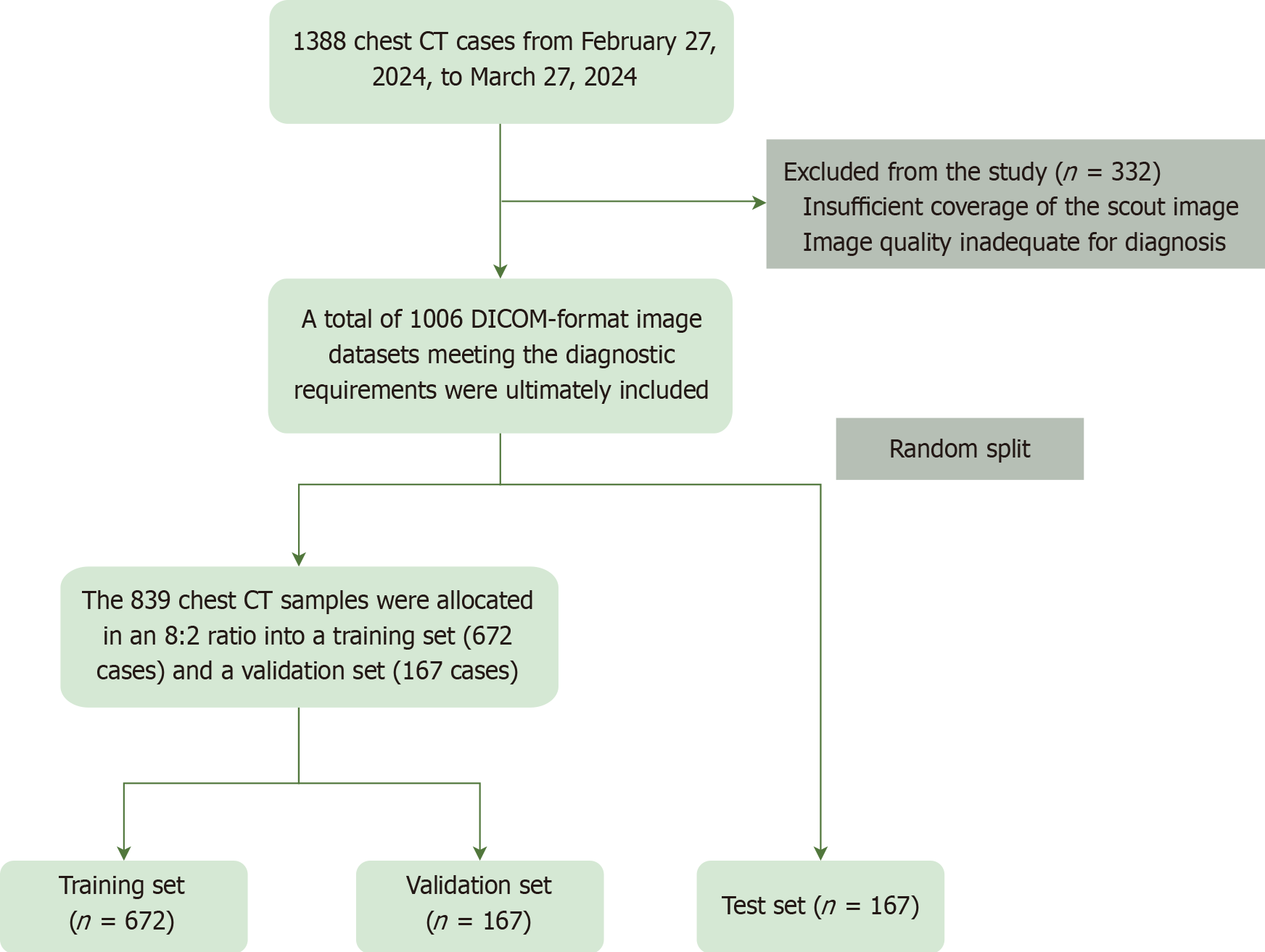
A retrospective analysis was conducted on chest CT data from 1388 patients at the Radiology Department of the First Affiliated Hospital of a university-affiliated hospital, collected between February 27 and March 27, 2024. A deep learning model was trained on anteroposterior scout images with annotations based on CCTA standards. The dataset was split into training (672 cases), validation (167 cases), and test (167 cases) sets to ensure robust model evaluation.
The study demonstrated exceptional performance on the test set, achieving a mean average precision (mAP50) of 0.995 and mAP50-95 of 0.994 for determining CCTA scan ranges.
This study demonstrates that: (1) Anteroposterior scout images can effectively estimate CCTA scan ranges; and (2) Estimates can be dynamically adjusted to meet the needs of various medical institutions.
Core Tip: Current coronary computed tomography angiography (CCTA) scanning often requires manual delineation of scan boundaries, limiting automation. This study introduces an innovative deep learning approach to automate CCTA scan range determination using anteroposterior scout images. The method provides a highly precise and adaptable solution, significantly enhancing diagnostic efficiency for coronary artery disease. This advancement overcomes the constraints of manual range selection, facilitating seamless integration across diverse medical institutions and optimizing clinical workflows.
- Citation: Zhao YH, Fan YH, Wu XY, Qin T, Sun QT, Liang BH. Determining the scanning range of coronary computed tomography angiography based on deep learning. World J Radiol 2025; 17(7): 110394
- URL: https://www.wjgnet.com/1949-8470/full/v17/i7/110394.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i7.110394
Coronary heart disease, a prevalent global cardiovascular condition, presents with symptoms including chest pain, exertional dyspnea, and arrhythmias. Its high incidence and mortality rates establish it as a major public health challenge[1,2]. Therefore, early screening and treatment of the disease can effectively improve patient prognosis and survival rates, offering substantial clinical value. Coronary computed tomography angiography (CCTA), as the preferred method for noninvasive assessment of coronary artery lesions, has an essential role in coronary artery calcium scoring and cardiovascular risk assessment, and its clinical value has been validated by several multicenter studies[2-7]. Specifically, the diagnostic accuracy of CCTA has surpassed that of conventional cardiac magnetic resonance and nuclear myocardial perfusion imaging in assessing ischemic mechanisms in non-obstructive lesions, with a negative predictive value of more than 98%, thereby significantly reducing the proportion of unnecessary invasive examinations[8,9]. Determination of the CCTA scanning range remains a significant impediment to the efficiency of the examination. According to Chinese guidelines for the application of standardized cardiac or CCTA techniques, the standard scanning range should extend from 1 to 2 cm below the tracheal eminence to the diaphragmatic surface of the heart, with the left and right borders exceeding the cardiac margins by 1-2 cm[10]. This requirement underscores the importance of the technician’s spatial positioning capabilities. Furthermore, the manual outlining process is time-consuming and tedious, and has become a significant impediment to the automation process of medical image acquisition, particularly in time-sensitive scenarios[11,12].
In recent years, deep learning techniques have made significant advancements in medical imaging research. However, in the field of computer vision research for CCTA, deep learning has primarily been applied to image reconstruction and quantification[13–15]. To address the aforementioned challenges, this study aims to explore the feasibility of automatically estimating the CCTA scan range based on anteroposterior chest scout images and proposes an automated solution using the YOLO deep learning framework. This approach seeks to streamline the technician’s workflow, reduce manual intervention time, and enhance the overall efficiency of CCTA examinations. Particularly in emergency scenarios, this automated method has the potential to optimize clinical workflows, providing technical support for rapid diagnosis and treatment decisions.
The data for this study were obtained from 1388 chest computed tomography (CT) images collected from the hospital’s Picture Archiving and Communication System during the collection period of February 27, 2024, to March 27, 2024. Some scout images were initially excluded from the study. The exclusion criteria were as follows: (1) Inadequate coverage of the localized image; and (2) Image quality that was difficult to diagnose (e.g., respiratory and motion artifacts in the image; high heart rate and arrhythmia artifacts; excessive image noise, etc.)[16-18]. The image data in digital imaging and communications in medicine format from 1006 cases that met the necessary diagnostic requirements were included in the study. To ensure that all imaging sequences from the same patient were exclusively assigned to a single data subset, patient IDs were used to create a key-value mapping, preventing data leakage across subsets[19]. The dataset was divided into training, validation, and test sets using stratified random sampling to maintain a balanced distribution of patient characteristics. Specifically, 672 cases (80%) were allocated to the training set, 167 cases (20%) to the validation set, and an independent test set of 167 cases was constructed from patients who underwent CCTA examinations. This test set was used to assess the model’s performance metrics (Figure 1).
The selection of images was acquired from the SOMATOM force (Siemens Healthineers) and revolution CT (GE Healthcare). A subset of localization images was scanned in an anteroposterior direction, extending from the thoracic inlet to the diaphragmatic surface of the heart with breath-holding, employing a tube voltage of 100 kV or 120 kV.
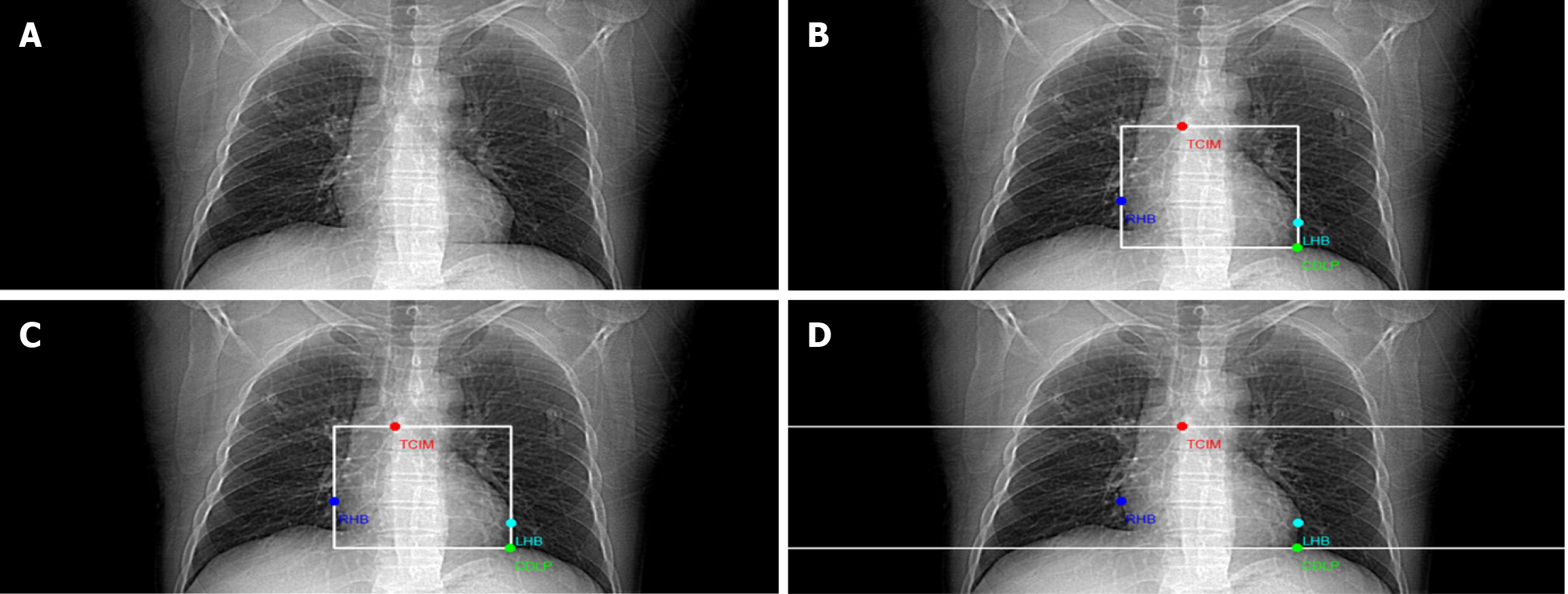
In each of the 1006 Localization images, four keypoints were approximately labeled, including the inferior border of the tracheal rongeur, the lowest point of the diaphragmatic plane of the heart, the left cardiac margin, and the right cardiac margin. All keypoints were annotated by one medical expert. The following labeling paradigm was established for the four categories of key anatomical landmarks.
Tracheal carina inferior margin: In the anteroposterior projection of the localization image, the keypoint annotation for the inferior margin of the tracheal carina should be positioned at the horizontal plane of the tracheal bifurcation. This level is usually located at the height of the fourth to fifth vertebrae of the thoracic spine and is in the same plane as the aortic arch. During keypoints annotation, keypoints should be centered on the trachea’s inferior margin, which serves as the upper boundary of the annotation box.
Cardiac diaphragmatic lowest point: In the anteroposterior localization image, the keypoint annotation for the cardiac diaphragmatic lowest point (CDLP) should be positioned at the most inferior site of contact between the heart and the diaphragm. It is usually located at the interface between the apical portion of the left ventricle and the pericardium, corresponding to the lowest projection point of the diaphragmatic dome in the anteroposterior view. This keypoint annotation delineates the most inferior extent of the heart within the thoracic cavity.
Right heart border: In the anteroposterior localization image, the keypoint annotation for the right heart border (RHB) should be defined along the continuous boundary extending from the junction of the right atrium and the superior vena cava to the diaphragmatic surface of the right ventricle. During the annotation process, the point that is farthest from the sternal midline along this boundary should be selected to reflect the maximum lateral extent of the right ventricle. This point is referred to as the rightmost point of the RHB.
Left heart border: In the anteroposterior localization image, the keypoint annotation for the left heart border (LHB) should be positioned along the intersection of the outer edge of the left ventricular free wall and the medial border of the left lung. During labeling, the point farthest from the sternal midline must be selected along this boundary to designate the maximum lateral extent of the left ventricle. The selection of this keypoint reflects the extreme position of the left cardiac contour.
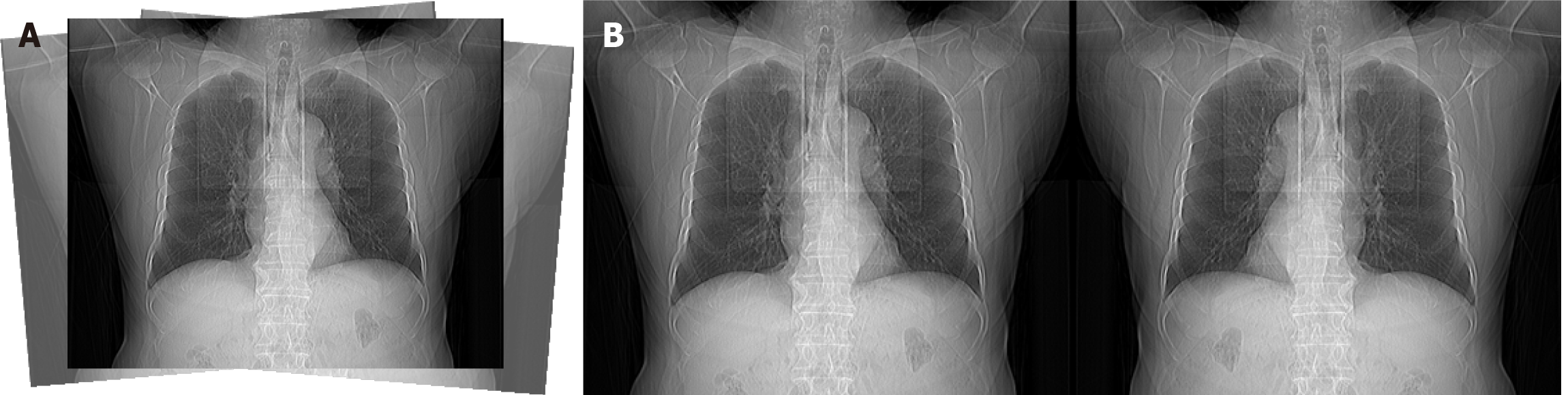
In medical image analysis, data preprocessing strategies must balance general computer vision characteristics with domain-specific features of medical imaging data. Addressing the pose estimation requirements in medical image acquisition, this study proposes a preprocessing scheme aimed at enhancing the robustness of medical images. The core parameter configurations of this scheme are as follows: Rotation angle (degrees: 5.0), perspective distortion (perspective: 0.0005) and horizontal flip probability (fliplr: 0.1). This scheme achieves a reasonable expansion of data distribution and optimization of model generalization through the following aspects.
Geometric transformation adaptive adjustment: Based on the anatomical orientation of medical images, the rotation angle is limited to ± 5° to account for slight patient positioning variations, enhancing the generalization of localization images, as shown in Figure 2A. In addition, the perspective distortion parameter is set to 0.0005 to simulate minor projection offsets from imaging equipment, improving the model’s adaptability to non-ideal angles.
Keypoint flipping augmentation: The incidence rate is extremely low for certain rare diseases (e.g., situs inversus[20,21]), yet their CT scout images differ from those of healthy individuals. Alternatively, due to specific reasons, patients may adopt unusual examination positions that affect medical images, resulting in CT images that deviate from typical conditions[22]. Therefore, the horizontal flip probability is set to 10%. Moderate flipping can still simulate mirrored variation scenarios, enhancing the model’s robustness to minor positional differences, as illustrated in Figure 2B.
The model employed in this study is based on the YOLOv8 architecture. The backbone utilizes an optimized version of CSPDarknet53, which enhances spatial feature extraction by combining 3 × 3 convolutional layers with batch normalization and the SiLU activation function, enabling more effective localization of anatomical landmarks in medical images[23,24].
No transfer learning was used; the model was trained from random initialization to ensure that feature representations were learned specifically from medical imaging data. The training was conducted using the PyTorch framework (v2.2.2) and Python (v3.11.8) on a workstation equipped with an NVIDIA RTX 3090 GPU. Key hyperparameters included a batch size of 8, initial learning rate of 0.0007, and input image size of 640 × 640 pixels. The loss function comprised a combi
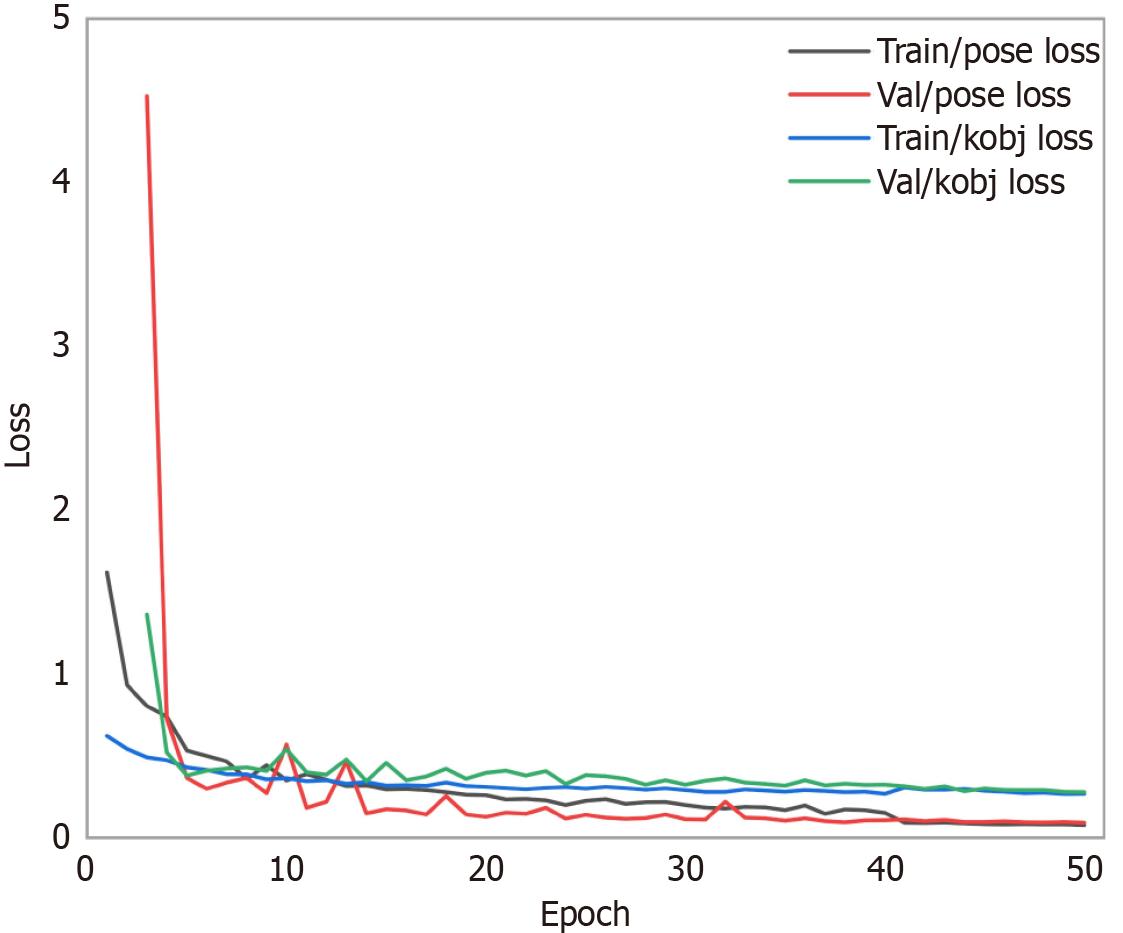
In this study, we evaluated the performance of the keypoint detection model on the validation set by analyzing the loss function and mAP metrics over 50 training epochs, shedding light on the model’s learning behavior (epochs refers to the number of times a machine learning model iterates over the entire training dataset). The training and validation loss curves (Figure 3) include pose loss (train/pose loss and val/pose loss) and keypoint loss (train/kobj loss and val/kobj loss). The training pose loss starts at an initial value of 1.62, rapidly decreases to 0.35 within the first 10 epochs, and stabilizes at 0.08 by the 50th epoch. The validation pose loss decreases from 4.53 at the 3rd epoch (the earliest epoch with available data) to 0.57 by the 10th epoch, further converging to 0.09 by the 50th epoch. Similarly, the training keypoint loss drops from an initial value of 0.62 to 0.36 by the 10th epoch and stabilizes at 0.27 by the 50th epoch. The validation keypoint loss rapidly declines from 1.36 at the 3rd epoch to 0.54 by the 10th epoch, converging to 0.28 by the 50th epoch. The gap between training and validation losses significantly narrows after the 10th epoch, indicating improved consistency and convergence.
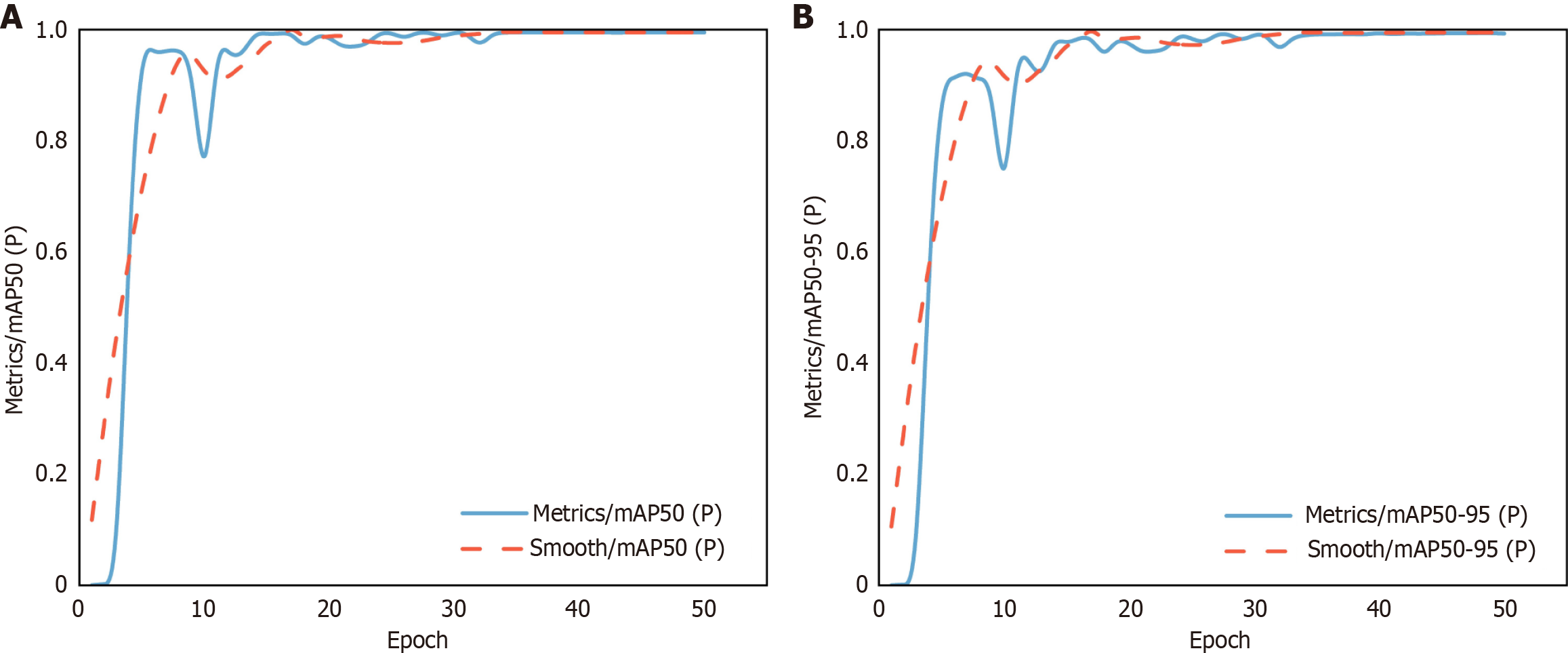
As shown in Figure 4A, the mAP50 reaching a stable level close to 1.0 after approximately 20 epochs. Figure 4B further analyzes the mAP50-95, which demonstrates a pronounced increase within the initial 10 epochs, followed by a period of stability between 0.9 and 1.0.
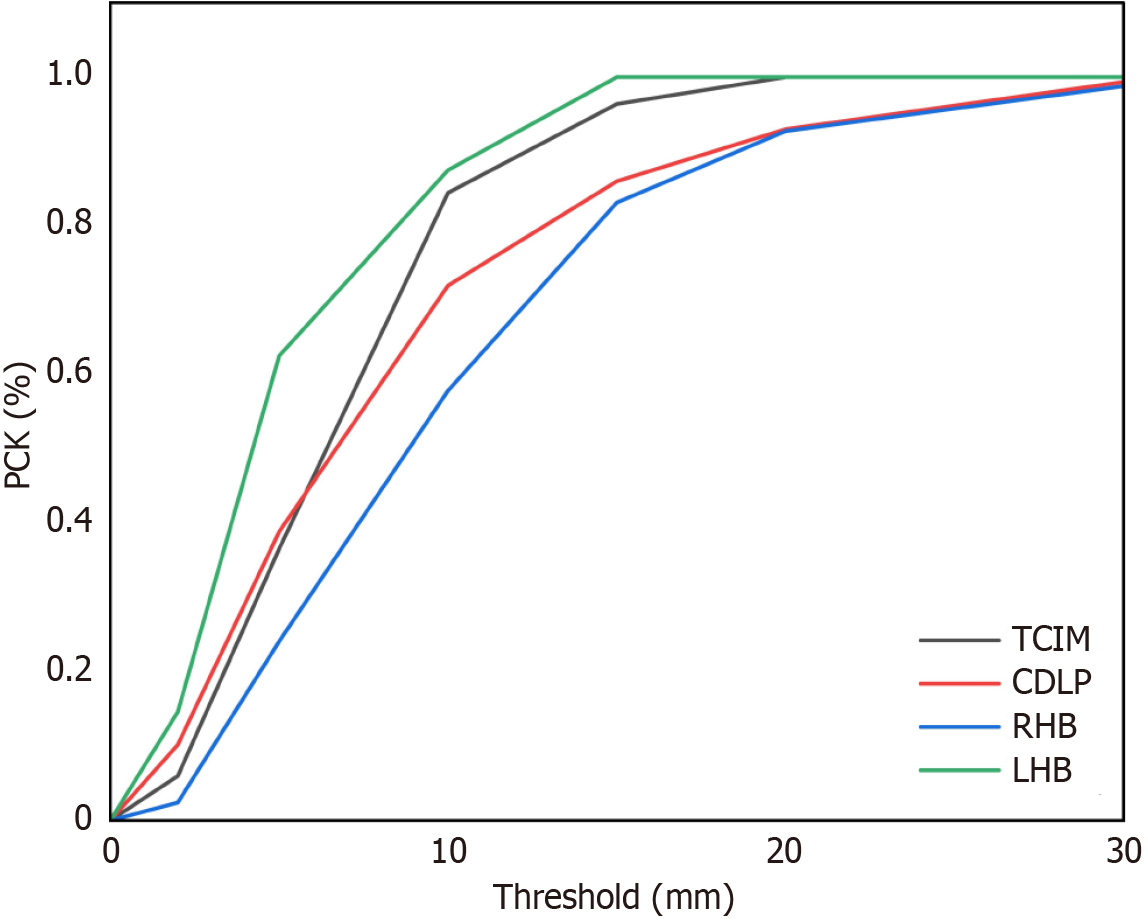
The model exhibited exceptional proficiency in pose estimation of the test set, achieving precision and recall of 1.0, with mAP50 and mAP50-95 scores of 0.995 and 0.994, respectively. On the validation set, the performance metrics are similarly impressive, with Precision close to 1, Recall at 1, and mAP50 and mAP50-95 also nearing 1, as illustrated in Table 1. Additionally, the model achieved percentage of correct keypoints (PCK) scores of 0.84 for the tracheal carina inferior margin (TCIM) point and 0.88 for the LHB point at a threshold of 1 cm (Figure 5).
| Precision | Recall | mAP50 | mAP50-95 | |
| Validation set | 0.9997 | 1.0000 | 0.9950 | 0.9947 |
| Test set | 1.0000 | 1.0000 | 0.9950 | 0.9940 |
PCK is calculated using the actual physical distance, a method that facilitates the integration of multimodal infor
The model that was trained has demonstrated an ability to accurately predict the required scan range for delineating the anteroposterior scout image. While the scan range for CCTA may exhibit slight variations across different countries, regions, and even local medical institutions, these differences have a negligible impact on the model and its subsequent automation (Figure 6).
In this study, a deep learning model system based on keypoint prediction was developed, which achieves high-precision scan range prediction using only the anatomical keypoints from anteroposterior scout images. The prediction results can be dynamically adjusted according to the standards of different medical institutions, offering the potential to simplify the workflow of technicians during CCTA scanning. The test set comprises 167 images. The model demonstrated exceptional performance in the pose estimation task on the test set, achieving Precision and Recall values of 1.0, with mAP50 and mAP50-95 values also approaching 1.0. These metrics indicate that the model’s performance on the test set is outstanding. Specifically, a Precision value of 1.0 suggests that the model produced no false positives during pose detection, while a Recall value of 1.0 indicates that the model successfully identified all true pose targets. Furthermore, the values of mAP50 and mAP50-95 reinforce that the model maintained high detection and localization accuracy across different Object Keypoint Similarity thresholds.
The performance evaluation on the validation set shows that Precision is close to 1, Recall is 1, and both mAP50 and mAP50-95 are also near 1. These results are highly consistent with the performance on the test set, highlighting the model’s excellent performance on the validation set.
The keypoint prediction model instead of a traditional image segmentation model was used to predict the CCTA scan range based on anteroposterior chest scout images. In both CCTA and routine chest CT scans, determining the scan range typically relies on identifying key anatomical landmarks, which are often manifested as specific points (TCIM, CDLP, RHB and LHB) on the anteroposterior scout image. The advantage is the ability to directly locate discrete anatomical landmarks without requiring pixel-level classification or segmentation of the entire image region, which avoids computational redundancy when dealing with large background areas and greatly improves prediction efficiency. In contrast, image segmentation models are more suited for tasks requiring the delineation of continuous layered organ images. Their typical applications in CCTA include vessel segmentation of coronary arteries and structural segmentation of myocardial tissue[25–29]. When applied to CCTA scanning range determination, its complexity and long processing time are points to consider[30].
Compared to traditional frameworks such as Faster R-CNN and RetinaNet, YOLO-based models demonstrate superior efficiency and accuracy in keypoint detection tasks. Fang et al[31] reported that YOLOv3 outperformed Faster R-CNN in both F1-score and mAP when applied to pose estimation, with better real-time performance. Ding et al[32] further improved the YOLO-Pose model by incorporating lightweight modules and attention mechanisms, achieving higher localization accuracy and significantly faster inference under complex and occluded conditions. The findings of the present study lend support to the selection of YOLO as a more suitable framework for applications requiring both speed and precision.
Although CCTA scout images may include dual scout images, the scan range is typically delineated using the anteroposterior scout image. Neither the Society of Cardiovascular Computed Tomography nor the Chinese Medical Asso
The keypoint prediction model showed high accuracy and efficiency in determining the CCTA scanning range, but several limitations exist. Firstly, the study data were sourced from a single center potentially limiting the model’s generalization ability to other populations or institutions. Although we acknowledge the value of external validation, access to appropriate multi-center CCTA datasets was not available during the current study period. This remains a planned direction for future work. Secondly, complex cases were not included, such as those involving severe anatomical deformities or comorbidities, which may pose challenges to model performance due to compromised CT scout image quality. Therefore, future efforts should focus on further multicenter evaluations and enhancing the model’s robustness in handling complex cases.
During the CT scanning process, one significant challenge in automating the scanning procedure is that technicians manually delineate the CT scan range using scout images. To address this issue, we propose a deep learning algorithm based on YOLO, utilizing 1388 chest CT anteroposterior scout images to train, validate, and test the model. Based on chest anteroposterior scout images, the model demonstrates high accuracy in predicting the scan range for CCTA. Each image contains a set of keypoints: TCIM, CDLP, RHB and LHB. We found that: (1) The anteroposterior scout image can be used to estimate the scan range for CCTA; and (2) The estimation results can be dynamically adjusted according to the requirements of different medical institutions.
| 1. | Martin SS, Aday AW, Allen NB, Almarzooq ZI, Anderson CAM, Arora P, Avery CL, Baker-Smith CM, Bansal N, Beaton AZ, Commodore-Mensah Y, Currie ME, Elkind MSV, Fan W, Generoso G, Gibbs BB, Heard DG, Hiremath S, Johansen MC, Kazi DS, Ko D, Leppert MH, Magnani JW, Michos ED, Mussolino ME, Parikh NI, Perman SM, Rezk-Hanna M, Roth GA, Shah NS, Springer MV, St-Onge MP, Thacker EL, Urbut SM, Van Spall HGC, Voeks JH, Whelton SP, Wong ND, Wong SS, Yaffe K, Palaniappan LP; American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Committee. 2025 Heart Disease and Stroke Statistics: A Report of US and Global Data From the American Heart Association. Circulation. 2025;151:e41-e660. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 509] [Cited by in RCA: 425] [Article Influence: 425.0] [Reference Citation Analysis (1)] |
| 2. | Nurmohamed NS, van Rosendael AR, Danad I, Ngo-Metzger Q, Taub PR, Ray KK, Figtree G, Bonaca MP, Hsia J, Rodriguez F, Sandhu AT, Nieman K, Earls JP, Hoffmann U, Bax JJ, Min JK, Maron DJ, Bhatt DL. Atherosclerosis evaluation and cardiovascular risk estimation using coronary computed tomography angiography. Eur Heart J. 2024;45:1783-1800. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 48] [Article Influence: 24.0] [Reference Citation Analysis (0)] |
| 3. | Pagano G, Sastre L, Blasi A, Brugaletta S, Mestres J, Martinez-Ocon J, Ortiz-Pérez JT, Viñals C, Prat-Gonzàlez S, Rivas E, Perea RJ, Rodriguez-Tajes S, Muxí Á, Ortega E, Doltra A, Ruiz P, Vidal B, Martínez-Palli G, Colmenero J, Crespo G. CACS, CCTA and mCAD-LT score in the pre-transplant assessment of coronary artery disease and the prediction of post-transplant cardiovascular events. Liver Int. 2024;44:1912-1923. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 14] [Reference Citation Analysis (0)] |
| 4. | Meijboom WB, Meijs MF, Schuijf JD, Cramer MJ, Mollet NR, van Mieghem CA, Nieman K, van Werkhoven JM, Pundziute G, Weustink AC, de Vos AM, Pugliese F, Rensing B, Jukema JW, Bax JJ, Prokop M, Doevendans PA, Hunink MG, Krestin GP, de Feyter PJ. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol. 2008;52:2135-2144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 936] [Cited by in RCA: 952] [Article Influence: 52.9] [Reference Citation Analysis (0)] |
| 5. | Budoff MJ, Dowe D, Jollis JG, Gitter M, Sutherland J, Halamert E, Scherer M, Bellinger R, Martin A, Benton R, Delago A, Min JK. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiography of Individuals Undergoing Invasive Coronary Angiography) trial. J Am Coll Cardiol. 2008;52:1724-1732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1568] [Cited by in RCA: 1615] [Article Influence: 89.7] [Reference Citation Analysis (0)] |
| 6. | Vrints C, Andreotti F, Koskinas KC, Rossello X, Adamo M, Ainslie J, Banning AP, Budaj A, Buechel RR, Chiariello GA, Chieffo A, Christodorescu RM, Deaton C, Doenst T, Jones HW, Kunadian V, Mehilli J, Milojevic M, Piek JJ, Pugliese F, Rubboli A, Semb AG, Senior R, Ten Berg JM, Van Belle E, Van Craenenbroeck EM, Vidal-Perez R, Winther S; ESC Scientific Document Group. 2024 ESC Guidelines for the management of chronic coronary syndromes. Eur Heart J. 2024;45:3415-3537. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1612] [Cited by in RCA: 1358] [Article Influence: 679.0] [Reference Citation Analysis (0)] |
| 7. | Koilpillai P, Aggarwal NR, Mulvagh SL. State of the Art in Noninvasive Imaging of Ischemic Heart Disease and Coronary Microvascular Dysfunction in Women: Indications, Performance, and Limitations. Curr Atheroscler Rep. 2020;22:73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 8. | Peterson PG, Berge M, Lichtenberger JP 3rd, Hood MN, Ho VB. Cardiac Imaging Modalities and Appropriate Use. Prim Care. 2018;45:155-168. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 9. | Sun B, Chen Z, Duan Q, Xue Y, Chen L, Zhang Z, An J. A direct comparison of 3 T contrast-enhanced whole-heart coronary cardiovascular magnetic resonance angiography to dual-source computed tomography angiography for detection of coronary artery stenosis: a single-center experience. J Cardiovasc Magn Reson. 2020;22:40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 10. | Chinese Medical Association Radiology Society Cardiac-chest Group. [Chinese guidelines for the application of standardized cardiac or coronary CT angiography techniques]. Zhonghua Fangshexue Zazhi. 2017;51:732-743. |
| 11. | Zhang Y, Yu B, Han Y, Wang J, Yang L, Wan Z, Zhang Z, Chen Y, Fu X, Gao C, Li B, Chen J, Wu M, Ma Y, Zhao X, Chen Y, Yan H, Xiang D, Fang W, Mehta S, Naber CK, Ge J, Huo Y. Protocol of the China ST-segment elevation myocardial infarction (STEMI) Care Project (CSCAP): a 10-year project to improve quality of care by building up a regional STEMI care network. BMJ Open. 2019;9:e026362. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Marano R, Rovere G, Savino G, Flammia FC, Carafa MRP, Steri L, Merlino B, Natale L. CCTA in the diagnosis of coronary artery disease. Radiol Med. 2020;125:1102-1113. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Catapano F, Lisi C, Savini G, Olivieri M, Figliozzi S, Caracciolo A, Monti L, Francone M. Deep Learning Image Reconstruction Algorithm for CCTA: Image Quality Assessment and Clinical Application. J Comput Assist Tomogr. 2024;48:217-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 8] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 14. | Lin A, Manral N, McElhinney P, Killekar A, Matsumoto H, Kwiecinski J, Pieszko K, Razipour A, Grodecki K, Park C, Otaki Y, Doris M, Kwan AC, Han D, Kuronuma K, Flores Tomasino G, Tzolos E, Shanbhag A, Goeller M, Marwan M, Gransar H, Tamarappoo BK, Cadet S, Achenbach S, Nicholls SJ, Wong DT, Berman DS, Dweck M, Newby DE, Williams MC, Slomka PJ, Dey D. Deep learning-enabled coronary CT angiography for plaque and stenosis quantification and cardiac risk prediction: an international multicentre study. Lancet Digit Health. 2022;4:e256-e265. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 214] [Article Influence: 53.5] [Reference Citation Analysis (0)] |
| 15. | Han D, Lin A, Gransar H, Dey D, Berman DS. Influence of Coronary Artery Calcium Score on Computed Tomography-Derived Fractional Flow Reserve: A Meta-Analysis. JACC Cardiovasc Imaging. 2021;14:702-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 16. | Kondo T, Takamura K, Fujimoto S, Takase S, Sekine T, Matsutani H, Rybicki FJ, Kumamaru KK. Motion artifacts on coronary CT angiography images in patients with a pericardial effusion. J Cardiovasc Comput Tomogr. 2014;8:19-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 17. | Hirai N, Horiguchi J, Fujioka C, Kiguchi M, Yamamoto H, Matsuura N, Kitagawa T, Teragawa H, Kohno N, Ito K. Prospective versus retrospective ECG-gated 64-detector coronary CT angiography: assessment of image quality, stenosis, and radiation dose. Radiology. 2008;248:424-430. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 250] [Cited by in RCA: 242] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 18. | Kim HR, Yoo SM, Rho JY, Lee HY, White CS. MDCT evaluation of atherosclerotic coronary artery disease: what should radiologists know? Int J Cardiovasc Imaging. 2014;30 Suppl 1:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 19. | Jonas RA, Barkovich E, Choi AD, Griffin WF, Riess J, Marques H, Chang HJ, Choi JH, Doh JH, Her AY, Koo BK, Nam CW, Park HB, Shin SH, Cole J, Gimelli A, Khan MA, Lu B, Gao Y, Nabi F, Nakazato R, Schoepf UJ, Driessen RS, Bom MJ, Thompson RC, Jang JJ, Ridner M, Rowan C, Avelar E, Généreux P, Knaapen P, de Waard GA, Pontone G, Andreini D, Guglielmo M, Al-Mallah MH, Jennings RS, Crabtree TR, Earls JP. The effect of scan and patient parameters on the diagnostic performance of AI for detecting coronary stenosis on coronary CT angiography. Clin Imaging. 2022;84:149-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 20. | Chen W, Guo Z, Qian L, Wang L. Comorbidities in situs inversus totalis: A hospital-based study. Birth Defects Res. 2020;112:418-426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 22] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 21. | Sutherland MJ, Ware SM. Disorders of left-right asymmetry: heterotaxy and situs inversus. Am J Med Genet C Semin Med Genet. 2009;151C:307-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 165] [Cited by in RCA: 191] [Article Influence: 11.2] [Reference Citation Analysis (0)] |
| 22. | Valentin B, Kamp B, Henke J, Ljimani A, Appel E, Antoch G, Steuwe A. Influence of tube and patient positioning in thoracoabdominal CT examinations on radiation exposure-towards a better patient positioning. J Radiol Prot. 2023;43. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 23. | Terven J, Córdova-Esparza D, Romero-González J. A Comprehensive Review of YOLO Architectures in Computer Vision: From YOLOv1 to YOLOv8 and YOLO-NAS. Mach Learn Knowl Extr. 2023;5:1680-1716. [DOI] [Full Text] |
| 24. | Varghese R, M S. YOLOv8: A Novel Object Detection Algorithm with Enhanced Performance and Robustness. 2024 International Conference on Advances in Data Engineering and Intelligent Computing Systems (ADICS), 2024: 1-6. [DOI] [Full Text] |
| 25. | Piccinelli M, Dahiya N, Nye JA, Folks R, Cooke CD, Manatunga D, Hwang D, Paeng JC, Cho SG, Lee JM, Bom HS, Koo BK, Yezzi A, Garcia EV. Clinically viable myocardial CCTA segmentation for measuring vessel-specific myocardial blood flow from dynamic PET/CCTA hybrid fusion. Eur J Hybrid Imaging. 2022;6:4. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 26. | Wang Q, Xu L, Wang L, Yang X, Sun Y, Yang B, Greenwald SE. Automatic coronary artery segmentation of CCTA images using UNet with a local contextual transformer. Front Physiol. 2023;14:1138257. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 27. | Ghanem AM, Hamimi AH, Matta JR, Carass A, Elgarf RM, Gharib AM, Abd-Elmoniem KZ. Automatic Coronary Wall and Atherosclerotic Plaque Segmentation from 3D Coronary CT Angiography. Sci Rep. 2019;9:47. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 28. | Hong P, Du Y, Chen D, Peng C, Yang B, Xu L. A U-Shaped Network Based on Multi-level Feature and Dual-Attention Coordination Mechanism for Coronary Artery Segmentation of CCTA Images. Cardiovasc Eng Technol. 2023;14:380-392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 29. | Song A, Xu L, Wang L, Wang B, Yang X, Xu B, Yang B, Greenwald SE. Automatic Coronary Artery Segmentation of CCTA Images With an Efficient Feature-Fusion-and-Rectification 3D-UNet. IEEE J Biomed Health Inform. 2022;26:4044-4055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 27] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 30. | Zhang R, Jie B, He Y, Zhu L, Xie Z, Liu Z, Mo H, Wang J. Craniomaxillofacial Bone Segmentation and Landmark Detection Using Semantic Segmentation Networks and an Unbiased Heatmap. IEEE J Biomed Health Inform. 2023;PP. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 31. | Fang C, Zheng H, Yang J, Deng H, Zhang T. Study on Poultry Pose Estimation Based on Multi-Parts Detection. Animals (Basel). 2022;12:1322. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 8] [Reference Citation Analysis (0)] |
| 32. | Ding J, Niu S, Nie Z, Zhu W. Research on Human Posture Estimation Algorithm Based on YOLO-Pose. Sensors (Basel). 2024;24:3036. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 33. | Abbara S, Blanke P, Maroules CD, Cheezum M, Choi AD, Han BK, Marwan M, Naoum C, Norgaard BL, Rubinshtein R, Schoenhagen P, Villines T, Leipsic J. SCCT guidelines for the performance and acquisition of coronary computed tomographic angiography: A report of the society of Cardiovascular Computed Tomography Guidelines Committee: Endorsed by the North American Society for Cardiovascular Imaging (NASCI). J Cardiovasc Comput Tomogr. 2016;10:435-449. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 448] [Cited by in RCA: 816] [Article Influence: 81.6] [Reference Citation Analysis (0)] |
| 34. | Hoye J, Sharma S, Zhang Y, Fu W, Ria F, Kapadia A, Segars WP, Wilson J, Samei E. Organ doses from CT localizer radiographs: Development, validation, and application of a Monte Carlo estimation technique. Med Phys. 2019;46:5262-5272. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/