Published online Jul 28, 2025. doi: 10.4329/wjr.v17.i7.107459
Revised: May 6, 2025
Accepted: July 1, 2025
Published online: July 28, 2025
Processing time: 123 Days and 8 Hours
Obstructed defecation syndrome (ODS) is a complex defecatory disorder asso
Core Tip: This article reviews the role of various imaging modalities in evaluating pelvic floor disorders, which affect approximately 50% of women aged 50 and older. Accurate diagnosis is crucial for effective management, as undetected abnormalities can lead to a high rate of treatment failure. Through a comparative analysis of different imaging techniques, this article seeks to provide insights that can help guide more informed management strategies for pelvic floor disorders.
- Citation: Singh JP, Assaie-Ardakany S, Aleissa MA, Al-Shaer K, Chitragari G, Drelichman ER, Mittal VK, Bhullar JS. Optimizing diagnosis in obstructed defecation syndrome: A review of imaging modalities. World J Radiol 2025; 17(7): 107459
- URL: https://www.wjgnet.com/1949-8470/full/v17/i7/107459.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i7.107459
Obstructed defecation syndrome (ODS) is a defecatory disorder characterized by difficulty in passing stool despite the urge to defecate. It is associated with pelvic floor dysfunction and may involve structural abnormalities such as rectocele, enterocele, intussusception, and pelvic floor descent, or functional disorders like anismus, where the puborectalis muscle either contracts paradoxically or fails to relax adequately. Patients with ODS often present with symptoms including excessive straining, a feeling of incomplete evacuation, reliance on manual assistance, and discomfort while having bowel movements[1,2].
Pelvic floor disorders represent a highly prevalent yet frequently underdiagnosed global health problem, with prevalence rates reported as high as 46.5%. In the United States, up to 25% of healthy, non-pregnant women are affected, with prevalence exceeding 50% among women aged 50 years and older. In Japan, 46.5% of adult women report experiencing at least one type of pelvic floor dysfunction[3]. ODS affects up to 50% of patients with chronic constipation and is about five times more common in women than in men[4]. In the general female population, roughly one in five women (20%) experience ODS, and this increases to nearly one-third (32%) among women with symptoms of pelvic floor dysfunction[5]. These disorders exert a substantial burden on physical and psychological health, often resulting in significant functional impairment, social withdrawal, disruption of interpersonal relationships, and reduced participation in occupational and recreational activities[3].
Beyond individual morbidity, pelvic floor disorders also impose a considerable socioeconomic burden. In the United States alone, the annual economic burden associated with pelvic floor disorders is estimated to exceed $12 billion. Indirect costs, such as lost productivity, work absenteeism, and early retirement, further amplify the societal impact[6,7].
An accurate diagnosis is essential for appropriate management, as failing to detect a relevant abnormality can result in a high rate of treatment failure[8]. The objective of this review is to assess the accuracy, advantages, and limitations of various diagnostic modalities for pelvic floor disorders, with the goal of guiding optimal clinical decision-making.
Dynamic defecography is a radiological imaging technique that captures the defecation process in real-time using fluo
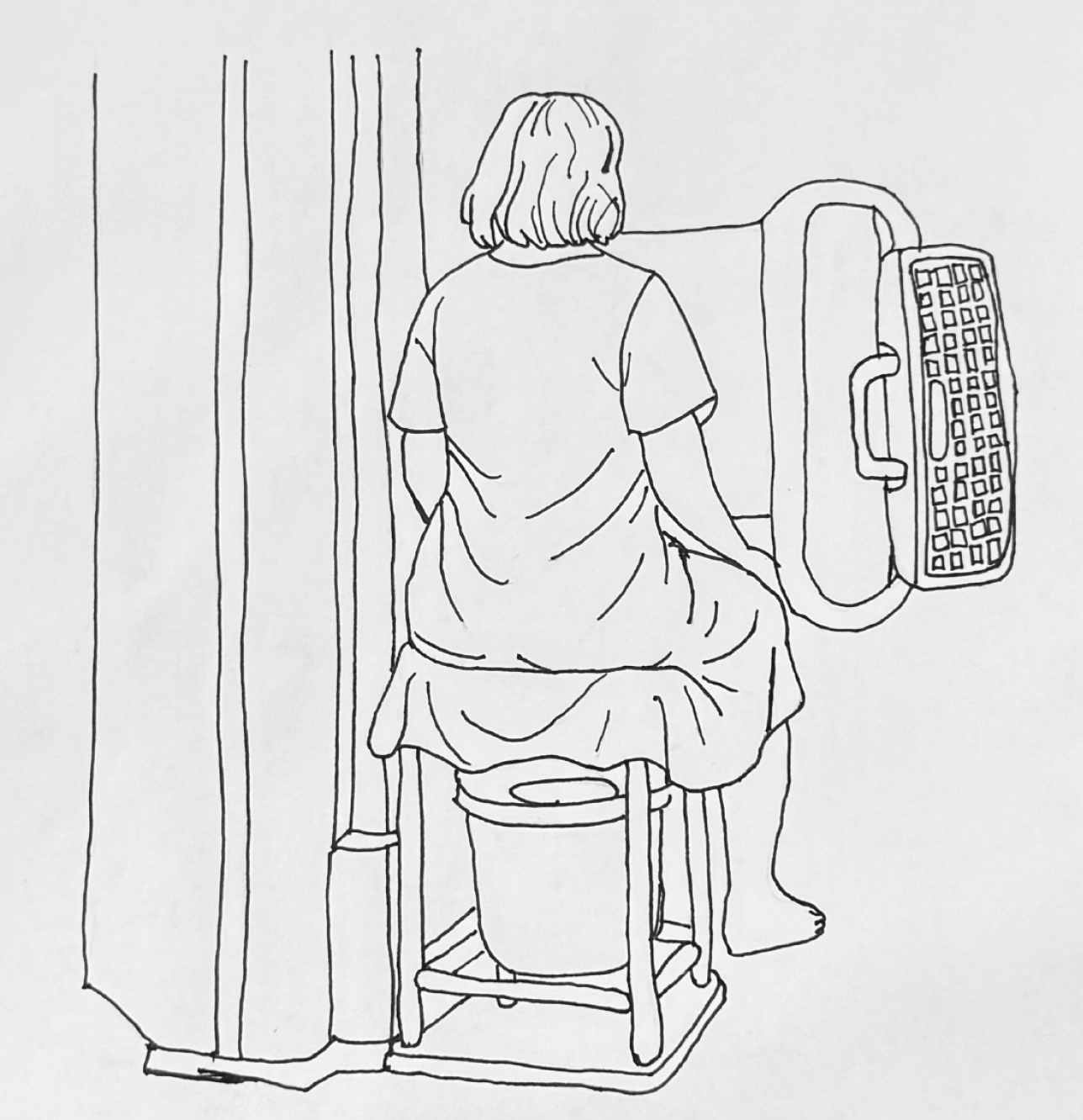
Fluoroscopic defecography (FD), also referred to as cinedefecography, fluoroscopic evacuation proctography, or evacuation proctography is a fluoroscopy-based imaging technique used to examine the anatomy and functionality of the anorectum and pelvic floor. This imaging modality evaluates the positioning and interaction of pelvic structures at rest, under increased intra-abdominal and intrapelvic pressure, and throughout the process of defecation[9]. Most notably, it provides a real-time, dynamic assessment of defecation mechanics in a natural seated position on a commode[8]. FD was first introduced by Wallden[10] in 1952 and gained recognition as a diagnostic test in the 1980s when Mahieu et al[11] refined the technique by highlighting the visualization of pelvic landmarks. Presently, FD is the most commonly per
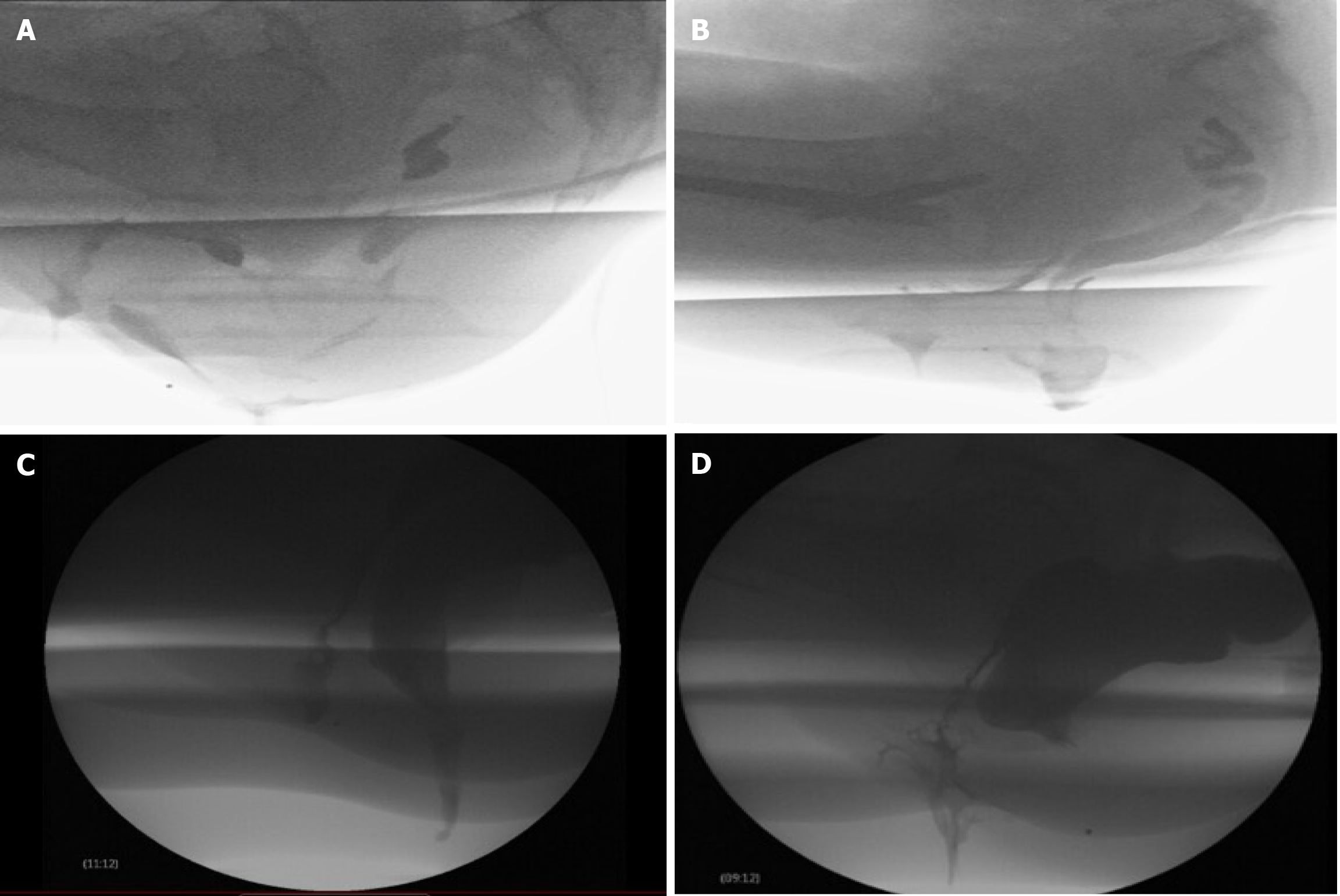
Patients receive a rectal enema before the procedure to ensure the rectum is empty. The urinary bladder should also be empty to prevent any interference with the assessment of pelvic floor or defecatory dysfunction. The patient is positioned in the left lateral decubitus position, and the rectum is filled with barium paste, which mimics the consistency of stool. Typically, 120-240 mL of barium paste is instilled, though additional volume may be required to achieve adequate rectal distension. The paste is administered until the patient experiences the urge to defecate. A radiopaque marker is usually placed on the perineal skin to help identify anatomical landmarks. Vaginal contrast is used to enhance visualization of rectoceles, cystoceles, and rectovaginal space widening, as seen in perineal hernias. This is achieved by introducing ultrasound gel mixed with iodinated contrast or barium into the vagina. While oral contrast is generally unnecessary, it may be used if the patient is being evaluated for enteroceles. Urinary bladder contrast is generally not used but can be used if looking for urinary bladder related abnormalities such as cystocele or vesicovaginal fistula. Once contrast is instilled, the patient is seated on a radiolucent commode positioned next to an upright fluoroscopy table (Figure 1). Static and dynamic imaging are then performed in three stages: Pre-evacuation, evacuation, and post-evacuation. Pelvic landmarks and specific measurements are utilized to assess structural and functional abnormalities[8]. The mean dose of radiation exposure during defecography is 0.5 to 5 millisieverts (mSv)[12,13]. Figure 2 illustrates various disease processes diagnosed using FD.
Magnetic resonance defecography (MRD) is another form of defecography where MRI is used instead of ionizing radiation to take the images during defecation[14].
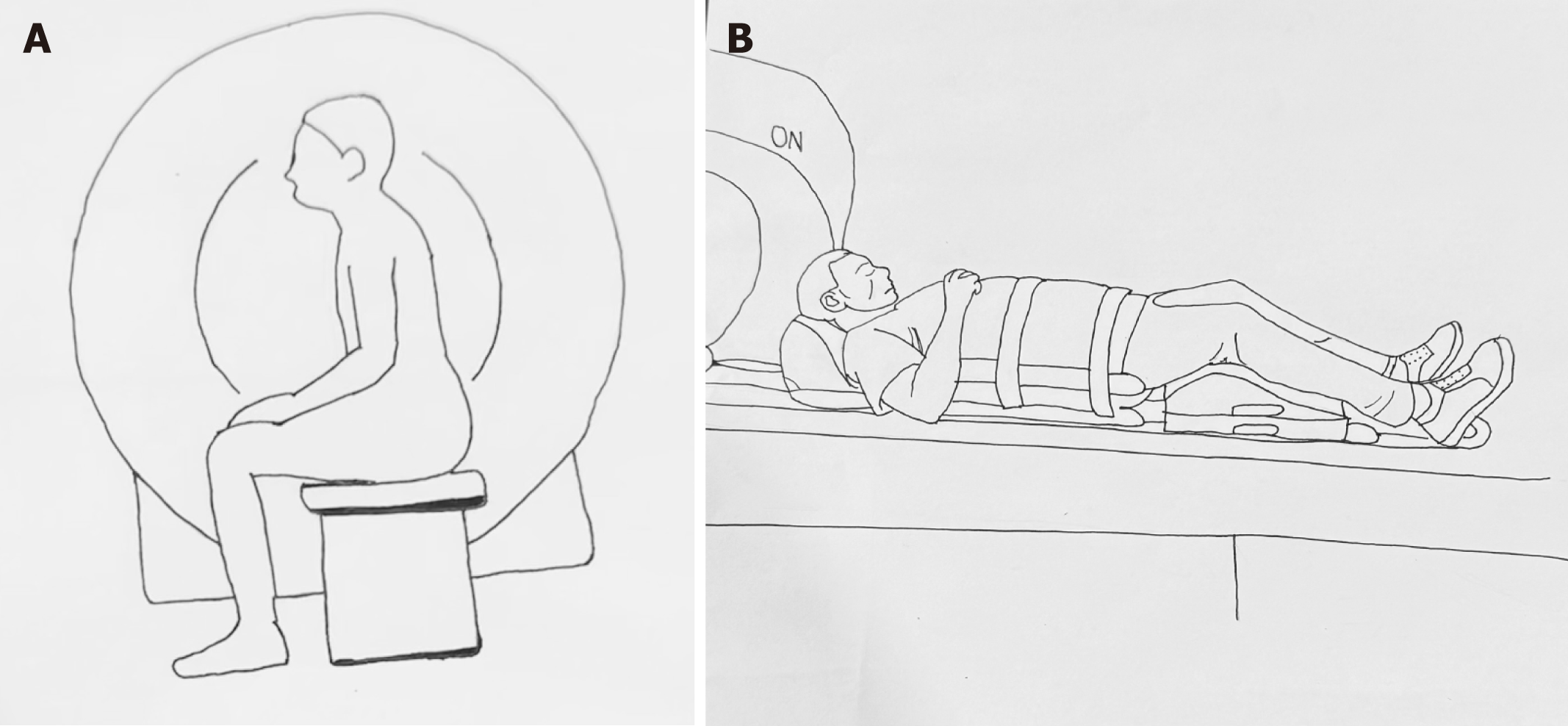
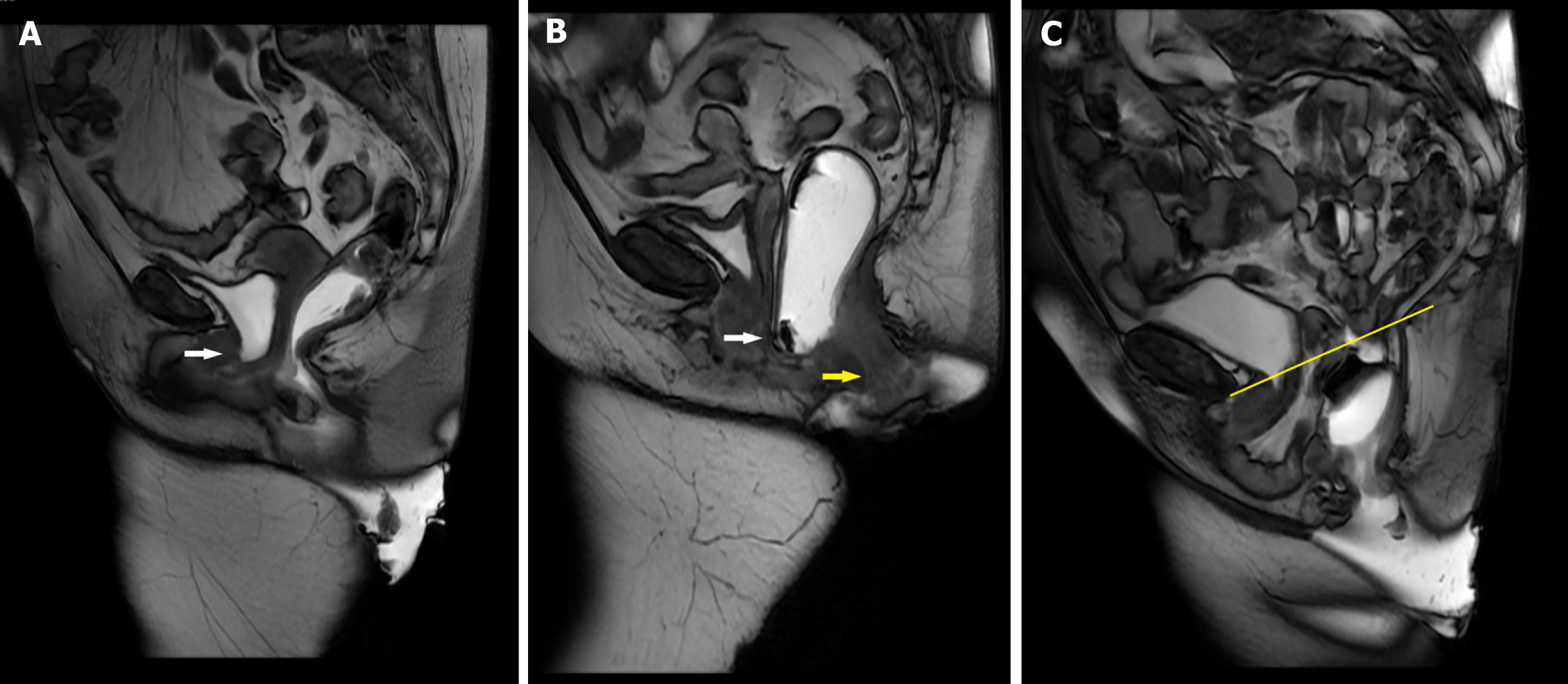
MRD is typically conducted using magnets with field strengths of 1.5 or 3.0 Tesla (T). MRD can be performed in a sitting position using an open-configuration system or in a supine position within a closed magnet system. The availability of upright MRI systems is highly limited, making supine positioning the most common approach for most MRD[14]. For preparation, around 120 mL of ultrasound gel is introduced into the rectum while the patient is positioned in the left lateral decubitus position on the scanner table. Alternatively, a potato-based starch mixture combined with gadolinium can be used. Vaginal gel is optional, while bladder contrast is not required. To prevent spillage, absorbent pads are used. Oral or intravenous contrasts are not used[14,15]. In open-configuration systems, patients are seated upright on a commode-like device specifically designed for the MRI machine, while in closed systems, the patient is positioned in a supine position with the hips and knees flexed to closely mimic a sitting posture[14,15]. Figure 3 demonstrates patient positioning during MRD procedure, with Figure 3A illustrating upright sitting position and Figure 3B depicting the supine position. The pelvis is carefully centered within the magnet bore. The static images are taken first, followed by dynamic imaging, which captures sequences during straining, squeezing, and defecation. Multiple attempts at straining and defecation are encouraged to maximize diagnostic accuracy. The defecation sequence is usually repeated at least three times to facilitate complete evacuation of the rectal gel. If evacuation remains incomplete, patients are advised to empty their rectum in the restroom. Post-evacuation images are then obtained to enhance the visualization of perineal hernias, which may otherwise be obscured by rectal distension[14]. Figure 4 illustrates various disease processes diagnosed using MRD.
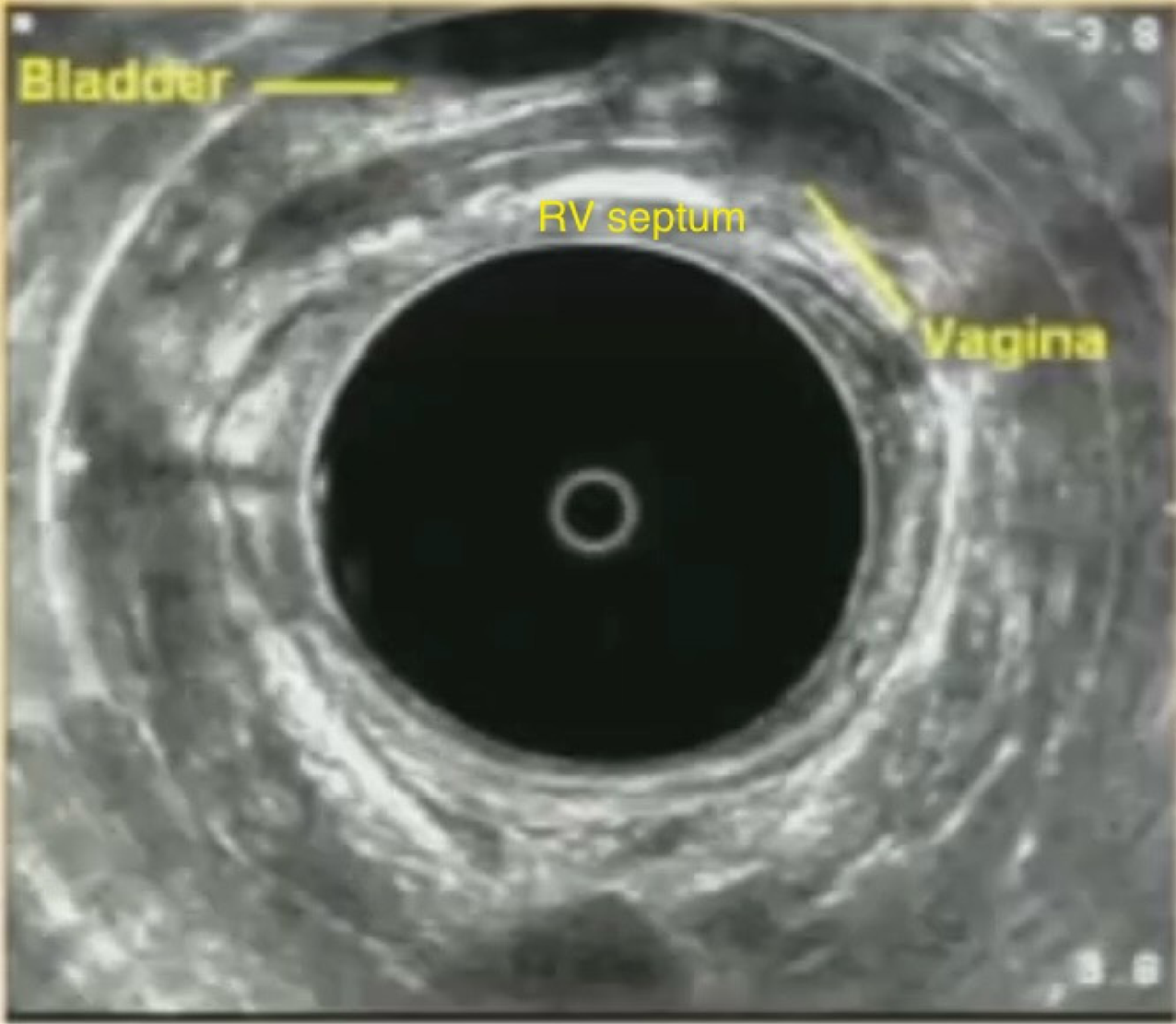
Pelvic floor ultrasound (PFUS) is an important diagnostic tool for evaluating pelvic floor disorders, offering real-time visualization of pelvic anatomy and function across all three compartments. PFUS encompasses several techniques, each of which can be used independently or in combination based on clinical indication. These include transperineal ultrasound (TPUS), performed with a curved transducer placed on the perineum; endovaginal ultrasound (EVUS), which uses a linear probe inserted into the vagina; and endorectal ultrasound (ERUS), involving a probe introduced into the rectum[16]. TPUS is non-invasive and can be conducted during a clinical visit, allowing for a correlation between physical examination and ultrasound findings[2,17,18]. PFUS requires specific training, but when all three modalities are combined, it offers excellent dynamic imaging, as each provides complementary views. Moreover, PFUS avoids radiation exposure and does not require bowel preparation. It also serves as a more accessible and cost-effective alternative to FD and MRD, especially in rural or underserved areas. However, limitations include potential discomfort or embarrassment for the patient, and reduced sensitivity for detecting rectal prolapse due to the supine or lateral positioning and the presence of the probe. Additionally, despite offering dynamic imaging, the absence of an evacuation phase may hinder the detection of abnormalities that only manifest during or after defecation[2,16-18]. Figure 5 shows representative image of ERUS showing the vagina, urinary bladder, and rectovaginal septum.
Echodefecography (EDF) is a three-dimensional (3D) dynamic ultrasound performed with a 3D endoprobe inserted into the rectum, providing images in the axial and midline longitudinal planes. Patients are examined in the left lateral position, and three series of scans are taken. Scan 1 is performed at rest without ultrasound gel to visualize the anatomy of the sphincter and assess the positioning of the sphincter and puborectalis at rest. Scan 2 is conducted in a rest-straining-rest sequence without gel to evaluate voluntary muscle movement during the evacuatory process, helping to identify anismus. Scan 3 is performed with 120-140 mL of ultrasound gel inserted into the rectum, and the sequence from Scan 2 is repeated[19,20].
EDF offers more detailed visualization of pelvic floor structures than the two-dimensional ultrasound in ERUS. Additionally, ERUS provides limited dynamic imaging capabilities compared to the comprehensive dynamic assessment possible with EDF[16,19,20].
EDF is a minimally invasive technique that avoids radiation exposure and is relatively well tolerated. Additionally, it clearly demonstrates the anatomical structures involved in the defecation process, which are not visible in conventional FD[19,20].
Poncelet et al[21] compared FD with MRD in 50 patients with pelvic floor disorders and found that FD demonstrated sensitivities of 90.9% for peritoneocele, 71.4% for rectocele, 81.1% for rectal prolapse, and 63.6% for anismus. In comparison, MRD exhibited sensitivities of 86.4%, 78.6%, 62.2%, and 63.6% for these respective conditions. According to their study, no significant differences were observed between the two imaging modalities in diagnosing these abnormalities. However, the power of the study was limited due to a small sample size.
Bertschinger et al[15] reported the advantage of upright MRI in better detection of rectal intussusception. They studied 38 patients who had pelvic floor evaluation in supine as well as in upright positions. In their study, 3 patients were found to have rectal intussusception, which were identified only in the sitting position, and were missed on supine MRI. The study’s limitations included a small sample size and a lack of matching MR findings between the two groups.
A meta-analysis by van Gruting et al[2], published in 2021 in the Cochrane Database of Systematic Reviews, included 39 studies with a total of 2483 patients and compared the diagnostic performance of FD, MRD, PFUS, and EDF in patients with ODS. The meta-analysis reported that the diagnostic accuracy of FD was high, with a sensitivity of 98% and specificity of 78% for rectocele; 91% and 96% for enterocele; 89% and 92% for intussusception; 80% and 97% for anismus; and 98% and 83% for pelvic floor descent. The analysis reported that, due to its high sensitivity, FD cannot be replaced by any other test for diagnosing rectocele, enterocele, intussusception, and pelvic floor descent. Although FD had low sensitivity for anismus, it remains irreplaceable due to its high specificity.
The same meta-analysis evaluated the diagnostic accuracy of MRD and reported a sensitivity of 94% and specificity of 90% for rectocele; 85% and 99% for enterocele; 61% and 97% for intussusception; 86% and 96% for anismus; and 94% and 79% for pelvic floor descent. While MRD did not meet the criteria to replace FD, it met the criteria to be used as a rule-in test for rectocele, enterocele, and intussusception. It met the criteria for a rule-out test for anismus[2].
For TPUS, the reported sensitivity and specificity were 88% and 89% for rectocele; 84% and 98% for enterocele; 75% and 96% for intussusception; and 92% and 91% for anismus. Pelvic floor descent was not assessed with TPUS. While TPUS did not meet the criteria to replace FD, it met the criteria to be used as a rule-in test for rectocele, enterocele, and intussusception, and as a rule-out test for anismus[2].
EVUS showed a sensitivity of 69% and specificity of 76% for rectocele; 68% and 97% for enterocele; 63% and 93% for intussusception; and 84% and 90% for anismus. Pelvic floor descent was not evaluated using EVUS. Despite its limitations, EVUS was considered acceptable as a rule-in test for rectocele, enterocele, and intussusception, and as a rule-out test for anismus.
ERUS demonstrated a sensitivity of 75% and specificity of 88% for rectocele; 74% and 97% for enterocele; 61% and 93% for intussusception; and 93% and 74% for pelvic floor descent. Anismus was not assessed with ERUS. Like EVUS, ERUS did not meet the criteria to replace FD but was suitable as a rule-in test for rectocele, enterocele, and intussusception.
Lastly, EDF demonstrated high diagnostic performance with a sensitivity of 96% and specificity of 89% for rectocele; 71% and 97% for enterocele; 89% and 92% for intussusception; and 87% and 93% for anismus. Pelvic floor descent was not assessed using EDF. EDF met the criteria to substitute FD for diagnosing intussusception. It also qualified as a rule-in test for rectocele and enterocele and a rule-out test for anismus[2].
The meta-analysis by van Gruting et al[2] concluded that FD remains the most reliable modality for assessing rectocele, enterocele, intussusception, and pelvic floor descent. MRD and TPUS serve as useful initial screening tools, where a positive result confirms the diagnosis of rectocele, enterocele, and intussusception, while a negative result effectively rules out anismus. The evidence supporting EVUS and ERUS is insufficient to recommend their use in evaluating pelvic floor disorders.
The meta-analysis by van Gruting et al[2] has several strengths, including the inclusion of a large number of studies from around the world. Of the 39 studies, 31 were prospectively designed, which enhances the reliability of the findings. Additionally, most studies employed blind comparative analysis, helping to minimize detection bias. However, there are a few limitations in the analysis. Heterogeneity existed among the included studies in terms of patient selection, imaging protocols, and diagnostic criteria, affecting the ability to make direct comparisons. Of the 39 studies, 38 were single-center studies with relatively small sample sizes; notably, 34 studies included fewer than 100 participants. Many studies also exhibited an unclear risk of bias, particularly regarding blinding and participant flow. In most studies, the imaging technique was assessed by only one examiner, introducing the potential for review bias. Furthermore, there was no consistent reference standard across studies, potentially contributing to the heterogeneity of the results. Nevertheless, the large overall patient sample size of the meta-analysis still ensures strong statistical power[2].
Pääkkö et al[22] evaluated the technical success and diagnostic capabilities of FD and MRD in a cohort of 64 patients. FD was diagnostic in 96.9% (62/64) of cases, significantly outperforming MRD, which was diagnostic in only 45.3% (29/64). FD was partially diagnostic in 1.6% (1/64) and non-diagnostic in 1.6% (1/64), whereas MRD was partially diagnostic in 32.8% (21/64) and non-diagnostic in 21.9% (14/64). FD identified enterocele in 30 patients, while MRD detected it in only 7, with moderate agreement. Intussusception was diagnosed in 53 patients using FD and in 27 using MRD, showing poor agreement. Rectocele was diagnosed in 47 patients by FD and in 29 by MRD, again with moderate agreement. Anismus was identified in 3 patients by FD and in 11 by MRD, with slight agreement. The study concluded that FD demonstrated superior technical success and diagnostic capability compared to MRD.
Hainsworth et al[23] studied the role of PFUS and compared its accuracy to MRD in 68 patients with pelvic floor disorders. They found that PFUS had a high negative predictive value (NPV) for conditions such as rectocele, enterocele, and intussusception, and a high positive predictive value (PPV) for cystocele. The reported NPV for rectocele, enterocele, and intussusception was 74%, 80%, and 82%, respectively, while the PPV for cystocele was 91%. They concluded that PFUS can serve as a useful screening tool, and if the results are normal, more expensive tests such as MRD can be avoided.
Regadas et al[20] compared EDF and FD in 86 patients with ODS and found substantial agreement between the two in diagnosing rectocele, intussusception, and anismus. EDF, however, had a limited role in detecting enterocele.
FD remains the gold standard for diagnosing pelvic floor disorders due to its high sensitivity and specificity across a spectrum of conditions, including rectocele, enterocele, intussusception, anismus, and pelvic floor descent. However, it is not without limitations. It requires a radiological environment, exposes patients to ionizing radiation, lacks soft tissue contrast, and offers limited multiplanar capability. Additionally, FD cannot demonstrate all the anatomic structures involved in defecation. Although it has high sensitivity for posterior compartment abnormalities, its specificity is lower compared to MRD. The analysis by van Gruting et al[2] reported the specificity of FD for rectocele, enterocele, and intussusception as 78%, 96%, and 92%, respectively, whereas MRD demonstrated higher specificity at 90%, 99%, and 97% for the same conditions. This leads to a higher rate of false positives and may contribute to over-diagnosis in clinical practice[2,20].
MRD, on the other hand, offers significant advantages, including multiplanar imaging, excellent soft tissue resolution, and the absence of radiation exposure. It is particularly useful in patients with multicompartmental disorders, recurrent symptoms, or in women of childbearing age, where radiation exposure should be avoided. MRD can visualize not only the pelvic organs but also supporting structures such as muscles and ligaments. However, it is more costly, less acce
Standard MRD is typically performed in the supine position, which does not simulate physiological defecation dynamics. While upright, open MRD potentially improves diagnostic yield for conditions like rectal intussusception, its use is limited by high cost, lower field strength affecting image quality, reduced soft tissue resolution, longer scan times, and restricted availability. Upright MRI systems require more space and specialized infrastructure, leading to higher installation and maintenance costs compared to closed-MRI systems. While upright MRI is valuable for specific applications such as weight-bearing spine imaging, dynamic joint assessments, and pelvic floor evaluations, it is not necessary for most routine exams, limiting its widespread use. Performing MRD in the sitting upright position requires an open-configuration system, which results in poorer image quality due to a lower signal-to-noise ratio and reduced soft tissue resolution[24]. Additionally, upright MRI scanners typically operate at lower magnetic field strengths (around 0.2-0.6 T), which can compromise spatial resolution compared to standard 1.5-3 T closed systems, potentially affecting diagnostic accuracy. These machines also tend to have longer scanning times, which can impact both efficiency and cost-effectiveness. As a result, upright MRI remains largely confined to specialized or academic centers, limiting its accessibility[24,25].
PFUS is a radiation-free modality, which has demonstrated high diagnostic accuracy for multiple pelvic floor disorders such as rectocele, enterocele, and intussusception and holds potential as a screening tool. In patients with normal PFUS findings, more resource-intensive imaging might be avoided. However, its effectiveness as a stand-alone diagnostic tool remains limited by operator dependence and the need for specialized training[2,20].
EDF approximates FD in its ability to detect rectocele and intussusception and has shown promise as a potential alternative in select clinical scenarios. Notably, it met substitution criteria for intussusception in the meta-analysis by van Gruting et al[2]. However, its limited sensitivity for enterocele and restricted availability prevent its broader application. As with PFUS, EDF requires significant expertise and is not widely accessible[20]. EVUS and ERUS may play roles in targeted assessments but currently lack the evidence to support routine, comprehensive evaluation of pelvic floor dis
Overall, despite advancements in imaging modalities, FD continues to hold a central role in the diagnostic algorithm for pelvic floor disorders. MRD and TPUS serve as valuable adjuncts or screening tools, particularly in scenarios requiring radiation avoidance or detailed soft tissue assessment. Table 1 summarizes the key differences between the various modalities.
| FD | MRD | PFUS | EDF | |
| Oral contrast | Needed for small bowel evaluation | Not needed | Not needed | Not needed |
| Ionizing radiation exposure | Yes | No | No | No |
| Position | Sitting on the commode | Mainly supine | Lithotomy | Left lateral |
| Multiplanar assessment | Limited | Excellent | Good | Good |
| Multicompartment diagnosis | Yes (limited) | Yes | Yes | Yes |
| Soft tissue assessment | No | Excellent | Good | Good |
| Cost | Relatively less expensive | More expensive | Low | Low |
Despite significant advancements in imaging techniques, several opportunities exist for future research and innovation.
The lack of uniform imaging protocols and diagnostic criteria affects reproducibility and interobserver reliability. Future efforts should focus on establishing standardized reporting guidelines.
Prospective, large cohorts, head-to-head trials comparing FD, MRD, PFUS, and EDF are necessary. These studies should evaluate diagnostic accuracy, impact on clinical decision-making, patient-reported outcomes, and cost-effectiveness.
Although upright MRD more accurately replicates physiological defecation, its widespread use is limited. Research into high-field-strength upright MRI systems and improved acquisition protocols could enhance their diagnostic utility and accessibility.
Hybrid approaches that incorporate minimally invasive modalities such as PFUS or EDF as initial screening tools followed by MRD or FD may optimize diagnostic efficiency and resource utilization. Prospective clinical trials could validate these tiered algorithms.
Artificial intelligence (AI) has the potential to enhance image interpretation, reduce variability, and improve diagnostic classification. Future research should focus on developing and validating AI algorithms.
Given the operator-dependent nature of modalities like PFUS and EDF, standardized training programs, simulation-based education, and wider dissemination of affordable equipment are essential. Continued progress in these areas will improve the accuracy, accessibility, and clinical relevance of pelvic floor imaging, ultimately enhancing patient care.
ODS is a complex condition requiring accurate diagnosis for effective management. FD remains the most sensitive test for evaluating rectocele, enterocele, intussusception, and pelvic floor descent, making it irreplaceable for these conditions. MRD, while offering superior soft tissue resolution and multiplanar imaging, is not as sensitive as FD but is preferable in cases requiring detailed evaluation of multicompartmental disorders and for women of childbearing age to avoid radiation exposure. PFUS shows promise as a cost-effective and accessible alternative for screening purposes. Despite its lower diagnostic accuracy compared to FD, it can be useful for ruling in rectocele, enterocele, and intussusception, as well as ruling out anismus. EDF also shows promise but requires extensive training and has limited availability. Ultimately, the choice of imaging modality should be guided by the patient’s clinical presentation, the need for radiation avoidance, cost considerations, and test availability. Future research should focus on standardizing imaging protocols, validating stepwise diagnostic algorithms incorporating minimally invasive tools like PFUS or EDF, and exploring the integration of AI to improve diagnostic accuracy and reproducibility.
The authors gratefully acknowledge Pamela L. Burgess, MD, FACS, from the Division of Colorectal Surgery at the University of Minnesota, for providing representative images of FD; Matthew L. Osher, MD, from the Department of Radiology at Henry Ford Providence Hospital, for images of MRD; and Amy J. Thorsen, MD, Associate Professor of Surgery at the University of Minnesota, for images of PFUS. Their valuable contributions significantly enhanced the clarity and quality of this manuscript.
| 1. | Rao SS, Rattanakovit K, Patcharatrakul T. Diagnosis and management of chronic constipation in adults. Nat Rev Gastroenterol Hepatol. 2016;13:295-305. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 154] [Cited by in RCA: 230] [Article Influence: 23.0] [Reference Citation Analysis (0)] |
| 2. | van Gruting IM, Stankiewicz A, Thakar R, Santoro GA, IntHout J, Sultan AH. Imaging modalities for the detection of posterior pelvic floor disorders in women with obstructed defaecation syndrome. Cochrane Database Syst Rev. 2021;9:CD011482. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 3. | Peinado-Molina RA, Hernández-Martínez A, Martínez-Vázquez S, Rodríguez-Almagro J, Martínez-Galiano JM. Pelvic floor dysfunction: prevalence and associated factors. BMC Public Health. 2023;23:2005. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 71] [Reference Citation Analysis (0)] |
| 4. | Noelting J, Eaton JE, Choung RS, Zinsmeister AR, Locke GR 3rd, Bharucha AE. The incidence rate and characteristics of clinically diagnosed defecatory disorders in the community. Neurogastroenterol Motil. 2016;28:1690-1697. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 5. | Whitcomb EL, Lukacz ES, Lawrence JM, Nager CW, Luber KM. Prevalence of Defecatory Dysfunction in Women With and Without Pelvic Floor Disorders. J Pelvic Med Surg. 2009;15:179-187. [RCA] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 6. | Wu JM, Hundley AF, Fulton RG, Myers ER. Forecasting the prevalence of pelvic floor disorders in U.S. Women: 2010 to 2050. Obstet Gynecol. 2009;114:1278-1283. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 454] [Cited by in RCA: 540] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 7. | Wu JM, Vaughan CP, Goode PS, Redden DT, Burgio KL, Richter HE, Markland AD. Prevalence and trends of symptomatic pelvic floor disorders in U.S. women. Obstet Gynecol. 2014;123:141-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 638] [Article Influence: 53.2] [Reference Citation Analysis (0)] |
| 8. | Palmer SL, Lalwani N, Bahrami S, Scholz F. Dynamic fluoroscopic defecography: updates on rationale, technique, and interpretation from the Society of Abdominal Radiology Pelvic Floor Disease Focus Panel. Abdom Radiol (NY). 2021;46:1312-1322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 15] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 9. | Kim NY, Kim DH, Pickhardt PJ, Carchman EH, Wald A, Robbins JB. Defecography: An Overview of Technique, Interpretation, and Impact on Patient Care. Gastroenterol Clin North Am. 2018;47:553-568. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 10. | Wallden L. Defecation block in cases of deep rectogenital pouch. Acta Chir Scand. 1952;103:236-238. [PubMed] |
| 11. | Mahieu P, Pringot J, Bodart P. Defecography: II. Contribution to the diagnosis of defecation disorders. Gastrointest Radiol. 1984;9:253-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 158] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 12. | Grossi U, Di Tanna GL, Heinrich H, Taylor SA, Knowles CH, Scott SM. Systematic review with meta-analysis: defecography should be a first-line diagnostic modality in patients with refractory constipation. Aliment Pharmacol Ther. 2018;48:1186-1201. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 55] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 13. | Paquette I, Rosman D, El Sayed R, Hull T, Kocjancic E, Quiroz L, Palmer S, Shobeiri A, Weinstein M, Khatri G, Bordeianou L; Members of the Expert Workgroup on Fluoroscopic Imaging of Pelvic Floor Disorders. Consensus Definitions and Interpretation Templates for Fluoroscopic Imaging of Defecatory Pelvic Floor Disorders: Proceedings of the Consensus Meeting of the Pelvic Floor Consortium of the American Society of Colon and Rectal Surgeons, the Society of Abdominal Radiology, the International Continence Society, the American Urogynecologic Society, the International Urogynecological Association, and the Society of Gynecologic Surgeons. Dis Colon Rectum. 2021;64:31-44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 14. | Kanmaniraja D, Arif-Tiwari H, Palmer SL, Kamath A, Lewis SC, Flusberg M, Kobi M, Lockhart ME, Chernyak V. MR defecography review. Abdom Radiol (NY). 2021;46:1334-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 15. | Bertschinger KM, Hetzer FH, Roos JE, Treiber K, Marincek B, Hilfiker PR. Dynamic MR imaging of the pelvic floor performed with patient sitting in an open-magnet unit versus with patient supine in a closed-magnet unit. Radiology. 2002;223:501-508. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 150] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 16. | Santoro GA, Wieczorek AP, Dietz HP, Mellgren A, Sultan AH, Shobeiri SA, Stankiewicz A, Bartram C. State of the art: an integrated approach to pelvic floor ultrasonography. Ultrasound Obstet Gynecol. 2011;37:381-396. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 175] [Cited by in RCA: 141] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 17. | Dietz HP. Pelvic Floor Ultrasound: A Review. Clin Obstet Gynecol. 2017;60:58-81. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 92] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 18. | Shobeiri SA, White D, Quiroz LH, Nihira MA. Anterior and posterior compartment 3D endovaginal ultrasound anatomy based on direct histologic comparison. Int Urogynecol J. 2012;23:1047-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 32] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 19. | Murad-Regadas SM, Regadas FS, Rodrigues LV, Silva FR, Soares FA, Escalante RD. A novel three-dimensional dynamic anorectal ultrasonography technique (echodefecography) to assess obstructed defecation, a comparison with defecography. Surg Endosc. 2008;22:974-979. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 52] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 20. | Regadas FS, Haas EM, Abbas MA, Marcio Jorge J, Habr-Gama A, Sands D, Wexner SD, Melo-Amaral I, Sardiñas C, Lima DM, Sagae UE, Murad-Regadas SM. Prospective multicenter trial comparing echodefecography with defecography in the assessment of anorectal dysfunction in patients with obstructed defecation. Dis Colon Rectum. 2011;54:686-692. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 38] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 21. | Poncelet E, Rock A, Quinton JF, Cosson M, Ramdane N, Nicolas L, Feldmann A, Salleron J. Dynamic MR defecography of the posterior compartment: Comparison with conventional X-ray defecography. Diagn Interv Imaging. 2017;98:327-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 22. | Pääkkö E, Mäkelä-Kaikkonen J, Laukkanen H, Ohtonen P, Laitakari K, Rautio T, Oikarinen H. X-ray video defaecography is superior to magnetic resonance defaecography in the imaging of defaecation disorders. Colorectal Dis. 2022;24:747-753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 23. | Hainsworth AJ, Pilkington SA, Grierson C, Rutherford E, Schizas AM, Nugent KP, Williams AB. Accuracy of integrated total pelvic floor ultrasound compared to defaecatory MRI in females with pelvic floor defaecatory dysfunction. Br J Radiol. 2016;89:20160522. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 11] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 24. | Ribas Y, Hotouras A, Chan CL, Clavé P. Imaging of pelvic floor disorders: are we underestimating gravity? Dis Colon Rectum. 2014;57:1242-1244. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 25. | Behluli E, Preuer HM, Schiefermeier-Mach N, Hornung R, Küchler M, Prokopetz M. Patient-centric comparative analysis of experiences in open upright and conventional closed MRI scanners. Radiography (Lond). 2024;30:1258-1264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |