Published online May 28, 2025. doi: 10.4329/wjr.v17.i5.107141
Revised: April 12, 2025
Accepted: May 13, 2025
Published online: May 28, 2025
Processing time: 71 Days and 0.9 Hours
Indolent NK-cell lymphoproliferative disorder of the gastrointestinal tract (iNKLPD) is a rare and recently defined entity, recognized in the 2022 WHO classification of hematolymphoid tumors. iNKLPD typically exhibits a benign or slowly progressive clinical course, with disease localized to the gastrointestinal tract. Here, we present what we believe to be the first reported case of iNKLPD associated with protein-losing enteropathy (PLE), characterized by a poor response to chemotherapy and rapid clinical deterioration, culminating in death within a few months.
We report the case of a 64-year-old man who presented with bilateral lower-extremity edema and fatigue. Laboratory tests revealed marked hypoalbuminemia, while other liver function parameters remained within normal limits. Renal and cardiac function assessments were unremarkable. Histopathological examination of endoscopic biopsies confirmed a diagnosis of iNKLPD of the gastrointestinal tract. The patient was treated with oral prednisone and cyclosporine, which led to temporary improvement in both symptoms and serum albumin levels. However, disease relapse occurred during corticosteroid tapering, accompanied by worsening hypoalbuminemia and refractory diarrhea. The patient died eight months after diagnosis, likely due to disease progression or severe treatment-related complications.
iNKLPD generally exhibits an indolent course; nonetheless, the prognosis may be poor if secondary PLE is involved.
Core Tip: Indolent NK-cell lymphoproliferative disorder of the gastrointestinal tract is a rare disease characterized by indolent behavior, for which diagnosis is primarily dependent on histopathological examination. Most cases are managed conservatively with follow-up observation, allowing patients to live with the disease for prolonged periods. Herein, we report the case of a patient with gastrointestinal indolent NK cell lymphoproliferative disorder presenting with protein-losing enteropathy as a prominent manifestation. When this condition arises, the prognosis may be poor.
- Citation: Jiang S, Wang LJ, Jia CW, Zhang W, Wang W, Li HL, Sun XH, Qu X, Kang L. Indolent NK-cell lymphoproliferative disorder of the gastrointestinal tract complicated by protein-losing enteropathy: A case report. World J Radiol 2025; 17(5): 107141
- URL: https://www.wjgnet.com/1949-8470/full/v17/i5/107141.htm
- DOI: https://dx.doi.org/10.4329/wjr.v17.i5.107141
Indolent NK-cell lymphoproliferative disorder of the gastrointestinal tract (iNKLPD), previously referred to as lymphomatoid gastropathy or NK-cell enteropathy, was first reported in 2006[1]. In 2022, the disease was included in the fifth edition of the World Health Organization classification of hematolymphoid tumors as iNKLPD[2]. iNKLPD is a rare indolent condition, with fewer than 50 cases reported worldwide[3]. This disorder lacks specific clinical manifestations and abnormal laboratory findings, and no cases involving hypoalbuminemia have previously been documented. Because of the absence of indicative symptoms, diagnosis primarily relies on histopathological findings. Regarding treatment, most cases are managed through follow-up observation, and prognosis is generally favorable.
Herein, we report a case of iNKLPD with secondary protein-losing enteropathy (PLE) in a patient admitted through the geriatric department.
The patient was a 64-year-old man admitted to our institution with bilateral lower-extremity edema and fatigue persisting for five months.
The patient had first developed bilateral lower-extremity edema and fatigue five months prior to admission. Multiple hospitalizations at local hospitals revealed significantly decreased serum albumin levels (16-20 g/L; normal range, 35-52 g/L). Although albumin infusion provided temporary symptomatic relief, recurrence rapidly occurred. The patient reported regular bowel movements without associated symptoms such as nausea, vomiting, abdominal pain, bloating, or rectal bleeding.
The patient’s medical history included a Billroth II gastrectomy 30 years earlier for incomplete pyloric obstruction.
Regarding family history, the patient’s mother and siblings had histories of heart disease, although the specific details were unclear.
On admission, the patient’s vital signs were as follows: Body temperature, 36.5 ºC; blood pressure, 117/68 mmHg; heart rate, 82 beats per minute; respiratory rate, 19 breaths per minute. Mild pitting edema was present in both lower extremities, with no other notable physical findings.
Blood tests revealed preserved bone marrow, liver, and kidney function, with no coagulation abnormalities. Hypoalbuminemia (20 g/L) was detected, along with decreased prealbumin levels (171 mg/L; normal range, 200-400 mg/L). Immunoglobulin levels and renal and cardiac function were normal. However, the high-sensitivity C-reactive protein levels were elevated (16.65 mg/dL; normal range, 0-8 mg/dL). Epstein-Barr virus (EBV) tests were negative, as were antinuclear antibody tests. Serum tumor marker levels showed no abnormalities.
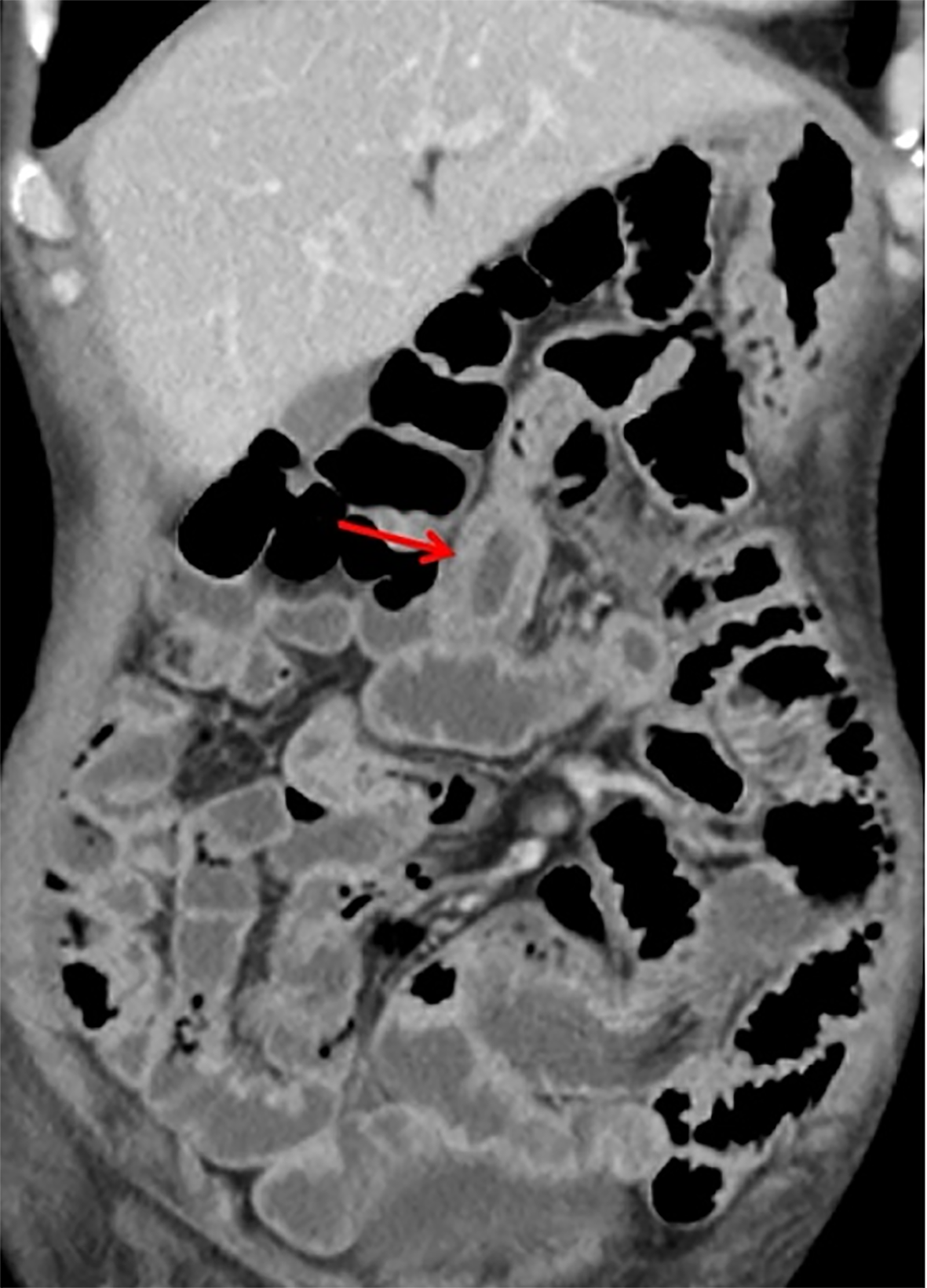
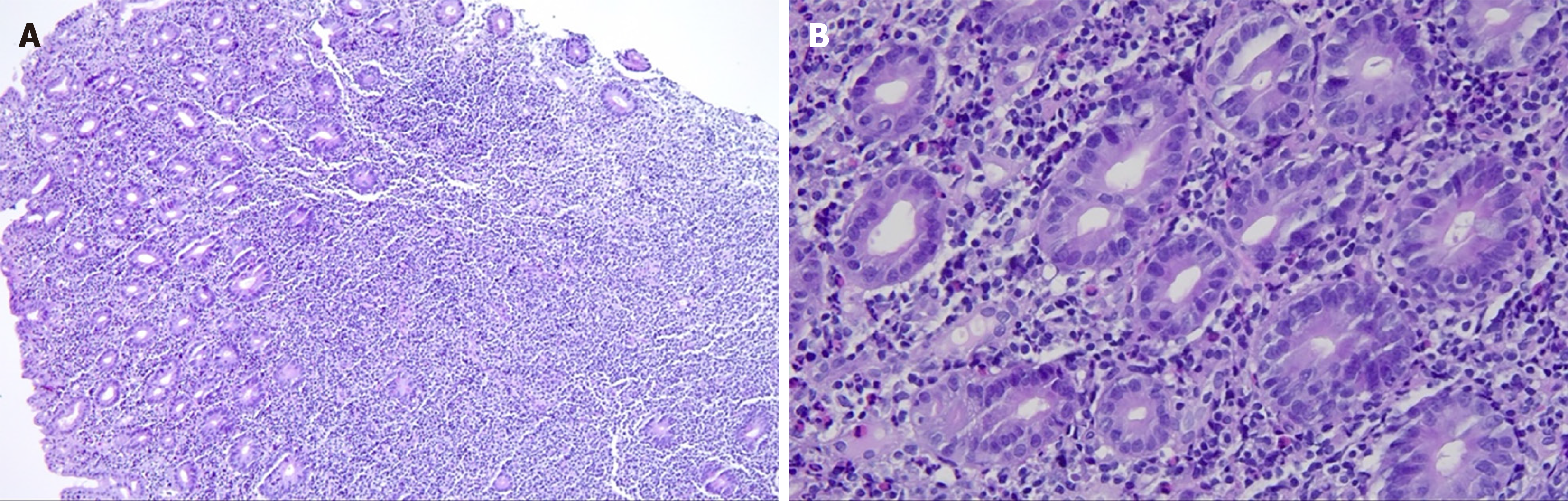
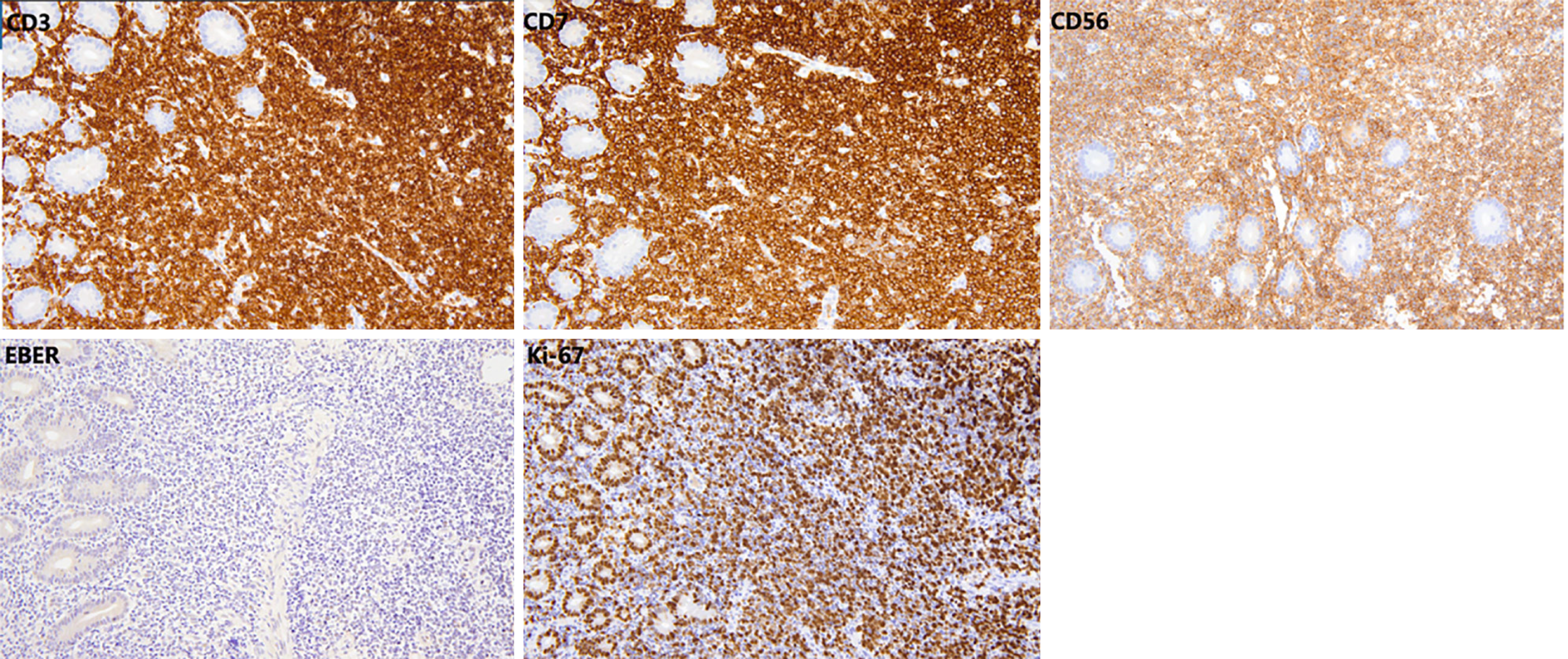
Computed tomography of the small intestine revealed diffuse thickening of the intestinal wall in the distal small intestine (jejunum) at the anastomosis site, with flattening of the intestinal folds (Figure 1). Subsequently, we performed 99Tcm-human serum albumin scintigraphy for intestinal protein loss, as follows: In the blood flow phase, imaging was initiated immediately following intravenous administration of the tracer, with no abnormal focal uptake detected in the abdominal cavity. In the delayed phase, serial imaging was conducted at 2, 5, 10, 15, 20, 25, and 30 minutes and 1, 2, 4, and 5 hours post-injection, to dynamically assess the distribution of abdominal radioactivity. The colon could not be distinctly visualized at any point during the imaging process. Gastroscopy showed good healing at the anastomosis site, with mucosal swelling 10-15 cm proximal to the output loop, with changes suggestive of lymphatic vessel dilation. Rapid urease testing for Helicobacter pylori was negative. Histopathological examination of a small intestine mucosal biopsy from the output loop revealed marked hyperplasia of the lymphoid tissue in the lamina propria, with nondestructive lymphocytic infiltration diffusely distributed between glandular structures without inducing damage. The infiltrating cells were small to medium in size, with mildly irregular nuclei, and some exhibited abundant pale-stained cytoplasm (Figure 2). Immunohistochemical analysis revealed CD3(++), CD7(++), CD56(++), CD20 (partially weak+), CD21 (FDC+), CD2 (partially+), CD4 (scattered+), CD5(-), CD8 (scattered+), Bcl-6(-), CD23 (FDC+), Cyclin D1(-), and a Ki-67 index of 70%. In situ hybridization revealed EBER(-), confirming that the proliferating lymphocytes were of NK-cell origin and consistent with an iNKLPD (Figure 3).
The patient exhibited no significant gastrointestinal symptoms. Endoscopic biopsy revealed lesions confined to the lamina propria, with dense infiltration of NK cells expressing CD56, CD3, and CD7. EBV infection and histologic features of high-grade lymphoma were excluded. Accordingly, a diagnosis of iNKLPD of the gastrointestinal tract was established. Comprehensive laboratory and imaging evaluations excluded other potential causes of hypoalbuminemia, including impaired protein synthesis (e.g., advanced liver disease, malnutrition), excessive urinary protein loss (e.g., nephrotic syndrome), increased protein catabolism (e.g., systemic malignancies), and altered protein distribution (e.g., capillary leakage associated with acute inflammatory states). Based on these findings, a clinical diagnosis of PLE secondary to iNKLPD was established.
Chemotherapy comprising prednisone 60 mg daily and cyclosporine 75 mg twice daily was initiated in October 2023, along with a diet rich in medium-chain triglycerides and high-quality proteins. One month later, the patient's lower limb edema had largely resolved, and serum albumin levels stabilized at > 24 g/L. The treatment plan involved tapering prednisone by 5 mg weekly, while increasing cyclosporine to 100 mg twice daily. However, after prednisone reduction to 25 mg once daily, the patient was readmitted with severe diarrhea, hypoalbuminemia (18-20 g/L), and pulmonary infection, along with decreased lymphocyte count and reduced immunoglobulin G levels. Increasing prednisone to 30 mg once daily stabilized serum albumin at 24 g/L. After maintaining this dose for one month, the dose was further tapered by 2.5 mg every two weeks. However, at 27.5 mg daily, the patient experienced recurrent diarrhea and hypoproteinemia. Restoring prednisone to 30 mg daily, at which point the cyclosporine concentration was 342 μg/L, did not significantly relieve diarrhea, and cyclosporine was discontinued. Concurrently, serum albumin dropped to 16 g/L, accompanied by electrolyte disturbances. The prednisone dose was subsequently increased to 40 mg daily, while cyclosporine 75 mg twice daily was initiated; however, the patient continued to experience recurrent diarrhea and hypoproteinemia. Subsequently, cyclosporine was replaced with Tacrolimus 1 mg twice daily, while prednisolone was gradually tapered. Despite this adjustment, the patient showed an inadequate treatment response.
During treatment, the patient developed cytomegalovirus viremia, deep venous thrombosis of the lower extremity, and gastrointestinal bleeding. The patient ultimately passed away in the eighth month of chemotherapy.
iNKLPD is a rare proliferative disorder of NK cells primarily confined to the gastrointestinal tract. Recent reports have described its involvement in the nasopharynx, esophagus, gallbladder, female reproductive tract, and lymph nodes[4-6]. Because of the rarity of this condition, most cases have been documented in isolated reports. As such, there are no comprehensive cohort studies currently available. According to three larger case series, the median age of patients is 55 years, with a male-to-female patient ratio of 1:2[7-9]. Although the exact pathogenesis of iNKLPD remains unclear, it may be linked to activation of the STAT5 pathway due to JAK3 mutations[7]. The clinical presentation of iNKLPD is nonspecific. Some patients experience upper abdominal discomfort, vomiting, bloating, abdominal pain, and diarrhea; whereas others are asymptomatic and incidentally diagnosed through gastrointestinal endoscopy. Laboratory findings are typically unremarkable. Diagnosis relies primarily on endoscopic examination, biopsy pathology, and immunohistochemistry[3]. Endoscopic findings may include erythema, erosion, ulceration, or flat elevated lesions. Microscopically, atypical lymphocytes diffusely infiltrate the lamina propria, and typically exhibit mild atypia[8]. Atypical cells express cytoplasmic CD3, CD7, CD56, and cytotoxic markers (granzyme B, TIA1, perforin)[4]. T-cell lineage markers such as CD4, CD5, and CD8 are negative, as are the B-cell markers CD20 and CD79α and anaplastic large cell lymphoma-associated markers CD30 and ALK. The Ki-67 index is typically low (< 40%), although cases with indices as high as 90% have previously been reported[7]. This disease should be differentiated from extranodal NK/T-cell lymphoma, gastrointestinal indolent T-cell lymphoma, enteropathy-associated T-cell lymphoma, and mucosa-associated lymphoid tissue lymphoma[10-13].
Our patient exhibited severe hypoalbuminemia, which improved temporarily following the intravenous administration of human albumin, but repeatedly relapsed. Laboratory and imaging examinations ruled out insufficient albumin production due to hepatic dysfunction or malnutrition, excessive albumin consumption as observed in malignancies or tuberculosis, and kidney protein loss. The result of the 99Tcm-labeled human serum albumin scintigraphy for intestinal protein loss, which has a sensitivity and specificity of 87% and 62%, respectively[14], was negative. Common causes of false-negative results include blockage and image overlap caused by the liver, kidneys, and large blood vessels. Chiu et al[15] emphasized the significance of 24-hour continuous scanning in the detection of PLE, as 26% of patients with positive findings only exhibited detectable protein loss on delayed imaging 24 hours post-injection, indicating intermittent intestinal protein loss. Accordingly, several studies have suggested that using 99Tcm-labeled human serum albumin with delayed continuous scanning for up to 24 hours could enhance the reliability and accuracy of identifying protein loss sites[16]. Therefore, after excluding other common causes of hypoproteinemia, a clinical diagnosis of PLE was established. However, no reports in the literature have documented an association between iNKLPD and PLE. Based on pathophy
There are currently no established treatment guidelines for iNKLPD. Most cases are managed conservatively with regular follow-up. Although most patients demonstrate favorable prognosis and prolonged survival, some cases exhibit progression or transformation into malignant lymphomas. When managing our patient, in addition to optimizing nutritional support, the primary focus was on controlling PLE, as this condition affects quality of life. The primary approach for managing PLE is to correct the underlying conditions. Guidelines for common and relevant gastrointestinal diseases fall short of addressing PLE. PLE secondary to lymphoma or autoimmune diseases, such as systemic lupus erythematosus (SLE), has been more commonly reported[19-21]. The treatment regimen for PLE secondary to lymphoma primarily involves chemotherapy targeting the underlying malignancy; however, this approach was not suitable in our patient. Notably, this is the first reported case of PLE secondary to iNKLPD; as such, there were no prior cases available for reference. Given that PLE is predominantly immune-mediated, treatment strategies for secondary PLE associated with immunosuppressive conditions were considered, emphasizing a regimen of corticosteroid therapy combined with immunosuppressive agents[21]. A multidisciplinary consultation involving hematology and gastroenterology specialists was conducted, and a combination regimen of corticosteroids and cyclosporine was initiated. During the initial treatment phase, the patient’s condition showed partial improvement, with serum albumin levels stabilizing at > 24 g/L. However, disease relapse occurred upon corticosteroid tapering, resulting in severe hypoalbuminemia and refractory diarrhea. Given the patient’s dependence on glucocorticoids and his suboptimal response to corticosteroids combined with cyclosporine, tacrolimus was introduced. Tacrolimus exerts a stronger immunosuppressive effect than cyclosporine, and has demonstrated greater efficacy in glucocorticoid-dependent or refractory SLE, severe inflammatory bowel disease, and transplant-associated PLE[22-24]. In the later disease stages, the patient developed complications, including cytomegalovirus bloodstream infection, deep vein thrombosis of the lower extremities, and gastrointestinal bleeding. Due to poor tolerance for invasive procedures, such as gastrointestinal endoscopy and bone marrow biopsy, it was not possible to determine whether the disease had progressed or even transformed into invasive lymphoma. Furthermore, the potential contribution of severe chemotherapy-related adverse effects to the patient’s deterioration and eventual mortality should be considered.
This case highlights a previously unreported association between iNKLPD and PLE, raising important questions regarding the underlying pathophysiological mechanisms and optimal management strategies of this rare disease. Future research should focus on investigating the pathophysiological link between iNKLPD and PLE, particularly the role of chronic inflammation, cytokine dysregulation, and lymphatic dysfunction in driving protein loss. Additionally, given the rarity of iNKLPD and the absence of established guidelines for its treatment when complicated by PLE, future research should explore treatment strategies. Potential therapies may include targeted immunosuppressive therapy (e.g., cal
iNKLPD is an indolent disease that primarily affects the gastrointestinal tract. Its clinical manifestations are non-specific, and diagnosis therefore primarily relies on endoscopic findings and histopathological examination. Although most patients have a favorable prognosis, our patient did not survive. This is the first reported case of iNKLPD complicated by PLE that was refractory to chemotherapy. Due to disease progression and chemotherapy-related adverse effects, the patient survived for only approximately one year from disease onset. Notably, this is the only reported fatal case of iNKLPD to date, suggesting that iNKLPD-associated PLE may be linked to poor prognosis and underscoring the need for further investigation into optimal treatment strategies.
We thank the patient and his family for granting permission for this case report.
| 1. | Vega F, Chang CC, Schwartz MR, Preti HA, Younes M, Ewton A, Verm R, Jaffe ES. Atypical NK-cell proliferation of the gastrointestinal tract in a patient with antigliadin antibodies but not celiac disease. Am J Surg Pathol. 2006;30:539-544. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 41] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 2. | Alaggio R, Amador C, Anagnostopoulos I, Attygalle AD, Araujo IBO, Berti E, Bhagat G, Borges AM, Boyer D, Calaminici M, Chadburn A, Chan JKC, Cheuk W, Chng WJ, Choi JK, Chuang SS, Coupland SE, Czader M, Dave SS, de Jong D, Du MQ, Elenitoba-Johnson KS, Ferry J, Geyer J, Gratzinger D, Guitart J, Gujral S, Harris M, Harrison CJ, Hartmann S, Hochhaus A, Jansen PM, Karube K, Kempf W, Khoury J, Kimura H, Klapper W, Kovach AE, Kumar S, Lazar AJ, Lazzi S, Leoncini L, Leung N, Leventaki V, Li XQ, Lim MS, Liu WP, Louissaint A Jr, Marcogliese A, Medeiros LJ, Michal M, Miranda RN, Mitteldorf C, Montes-Moreno S, Morice W, Nardi V, Naresh KN, Natkunam Y, Ng SB, Oschlies I, Ott G, Parrens M, Pulitzer M, Rajkumar SV, Rawstron AC, Rech K, Rosenwald A, Said J, Sarkozy C, Sayed S, Saygin C, Schuh A, Sewell W, Siebert R, Sohani AR, Tooze R, Traverse-Glehen A, Vega F, Vergier B, Wechalekar AD, Wood B, Xerri L, Xiao W. The 5th edition of the World Health Organization Classification of Haematolymphoid Tumours: Lymphoid Neoplasms. Leukemia. 2022;36:1720-1748. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 320] [Cited by in RCA: 2417] [Article Influence: 604.3] [Reference Citation Analysis (0)] |
| 3. | Wang XG, Yin WH, Wang HY. Indolent T-Cell/Natural Killer-Cell Lymphomas/Lymphoproliferative Disorders of the Gastrointestinal Tract-What Have We Learned in the Last Decade? Lab Invest. 2024;104:102028. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 4. | Yi H, Li A, Ouyang B, Da Q, Dong L, Liu Y, Xu H, Zhang X, Zhang W, Jin X, Gu Y, Wang Y, Liu Z, Wang C. Clinicopathological and molecular features of indolent natural killer-cell lymphoproliferative disorder of the gastrointestinal tract. Histopathology. 2023;82:567-575. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 12] [Reference Citation Analysis (0)] |
| 5. | Krishnan R, Ring K, Williams E, Portell C, Jaffe ES, Gru AA. An Enteropathy-like Indolent NK-Cell Proliferation Presenting in the Female Genital Tract. Am J Surg Pathol. 2020;44:561-565. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 17] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 6. | Dargent JL, Tinton N, Trimech M, de Leval L. Lymph node involvement by enteropathy-like indolent NK-cell proliferation. Virchows Arch. 2021;478:1197-1202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 7. | Xiao W, Gupta GK, Yao J, Jang YJ, Xi L, Baik J, Sigler A, Kumar A, Moskowitz AJ, Arcila ME, Raffeld M, Pittaluga S, Dogan A, Jaffe ES. Recurrent somatic JAK3 mutations in NK-cell enteropathy. Blood. 2019;134:986-991. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 50] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 8. | Mansoor A, Pittaluga S, Beck PL, Wilson WH, Ferry JA, Jaffe ES. NK-cell enteropathy: a benign NK-cell lymphoproliferative disease mimicking intestinal lymphoma: clinicopathologic features and follow-up in a unique case series. Blood. 2011;117:1447-1452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 134] [Article Influence: 8.4] [Reference Citation Analysis (0)] |
| 9. | Takeuchi K, Yokoyama M, Ishizawa S, Terui Y, Nomura K, Marutsuka K, Nunomura M, Fukushima N, Yagyuu T, Nakamine H, Akiyama F, Hoshi K, Matsue K, Hatake K, Oshimi K. Lymphomatoid gastropathy: a distinct clinicopathologic entity of self-limited pseudomalignant NK-cell proliferation. Blood. 2010;116:5631-5637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 101] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 10. | Sánchez-Romero C, Bologna-Molina R, Paes de Almeida O, Santos-Silva AR, Prado-Ribeiro AC, Brandão TB, Carlos R. Extranodal NK/T cell lymphoma, nasal type: An updated overview. Crit Rev Oncol Hematol. 2021;159:103237. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 65] [Article Influence: 13.0] [Reference Citation Analysis (0)] |
| 11. | Perry AM, Warnke RA, Hu Q, Gaulard P, Copie-Bergman C, Alkan S, Wang HY, Cheng JX, Bacon CM, Delabie J, Ranheim E, Kucuk C, Hu X, Weisenburger DD, Jaffe ES, Chan WC. Indolent T-cell lymphoproliferative disease of the gastrointestinal tract. Blood. 2013;122:3599-3606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 126] [Cited by in RCA: 144] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 12. | Zettl A, deLeeuw R, Haralambieva E, Mueller-Hermelink HK. Enteropathy-type T-cell lymphoma. Am J Clin Pathol. 2007;127:701-706. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 67] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 13. | Raderer M, Kiesewetter B, Ferreri AJ. Clinicopathologic characteristics and treatment of marginal zone lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). CA Cancer J Clin. 2016;66:153-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 131] [Cited by in RCA: 183] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 14. | Khalesi M, Nakhaei AA, Seyed AJ, Treglia G, Zakavi SR, Sadeghi R, Kianifar HR. Diagnostic accuracy of nuclear medicine imaging in protein losing enteropathy : systematic review and meta-analysis of the literature. Acta Gastroenterol Belg. 2013;76:413-422. [PubMed] |
| 15. | Chiu NT, Lee BF, Hwang SJ, Chang JM, Liu GC, Yu HS. Protein-losing enteropathy: diagnosis with (99m)Tc-labeled human serum albumin scintigraphy. Radiology. 2001;219:86-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 59] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 16. | Hsu YJ, Lin SH, Lin YF, Yu FC, Jaiteh LE. Pitfalls of technetium-99m-labeled human serum albumin scintigraphy for protein-losing enteropathy. Kidney Int. 2009;76:911; author reply 911-911; author reply 912. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 11] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 17. | Ozen A, Lenardo MJ. Protein-Losing Enteropathy. N Engl J Med. 2023;389:733-748. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 41] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 18. | Matnani R, Ganapathi KA, Lewis SK, Green PH, Alobeid B, Bhagat G. Indolent T- and NK-cell lymphoproliferative disorders of the gastrointestinal tract: a review and update. Hematol Oncol. 2017;35:3-16. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 53] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 19. | Minemura T, Kikuchi S, Mihara H, Kamihara Y, Wada A, Fuchino M, Nanjo S, Noguchi A, Minamisaka T, Murakami J, Yasuda I, Sato T. Protein-losing Enteropathy Complicated with Primary Intestinal Follicular Lymphoma. Intern Med. 2022;61:2051-2055. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 20. | Ferrante M, De Hertogh G, Penninckx F, Van Assche G. Protein-losing enteropathy in Crohn's disease. Clin Gastroenterol Hepatol. 2005;3:A25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 5] [Article Influence: 0.2] [Reference Citation Analysis (0)] |
| 21. | Chen Z, Li MT, Xu D, Yang H, Li J, Zhao JL, Zhang HH, Han SM, Xu T, Zeng XF. Protein-losing enteropathy in systemic lupus erythematosus: 12 years experience from a Chinese academic center. PLoS One. 2014;9:e114684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 23] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 22. | Zheng Z, Zhang H, Peng X, Zhang C, Xing C, Xu G, Fu P, Ni Z, Chen J, Xu Z, Zhao MH, Li S, Huang X, Miao L, Chen X, Liu B, He Y, Li J, Liu L, Kadeerbai H, Liu Z, Liu Z. Effect of Tacrolimus vs Intravenous Cyclophosphamide on Complete or Partial Response in Patients With Lupus Nephritis: A Randomized Clinical Trial. JAMA Netw Open. 2022;5:e224492. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 56] [Cited by in RCA: 46] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 23. | Li H, Zhang X, Chen J. Successful treatment of steroid-refractory systemic lupus erythematosus-associated protein-losing enteropathy using combination therapy with tacrolimus and steroid. Lupus. 2011;20:1109-1111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 11] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 24. | Younes BS, Ament ME, McDiarmid SV, Martin MG, Vargas JH. The involvement of the gastrointestinal tract in posttransplant lymphoproliferative disease in pediatric liver transplantation. J Pediatr Gastroenterol Nutr. 1999;28:380-385. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 43] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
Open Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/