Published online Jul 28, 2024. doi: 10.4329/wjr.v16.i7.247
Revised: May 10, 2024
Accepted: May 29, 2024
Published online: July 28, 2024
Processing time: 189 Days and 17.4 Hours
Hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) represent the predominant histological types of primary liver cancer, comprising over 99% of cases. Given their differing biological behaviors, prognoses, and treatment strategies, accurately differentiating between HCC and ICC is crucial for effective clinical management. Radiomics, an emerging image processing technology, can automatically extract various quantitative image features that may elude the human eye. Reports on the application of ultrasound (US)-based radiomics methods in distinguishing HCC from ICC are limited.
To develop and validate an ultrasomics model to accurately differentiate between HCC and ICC.
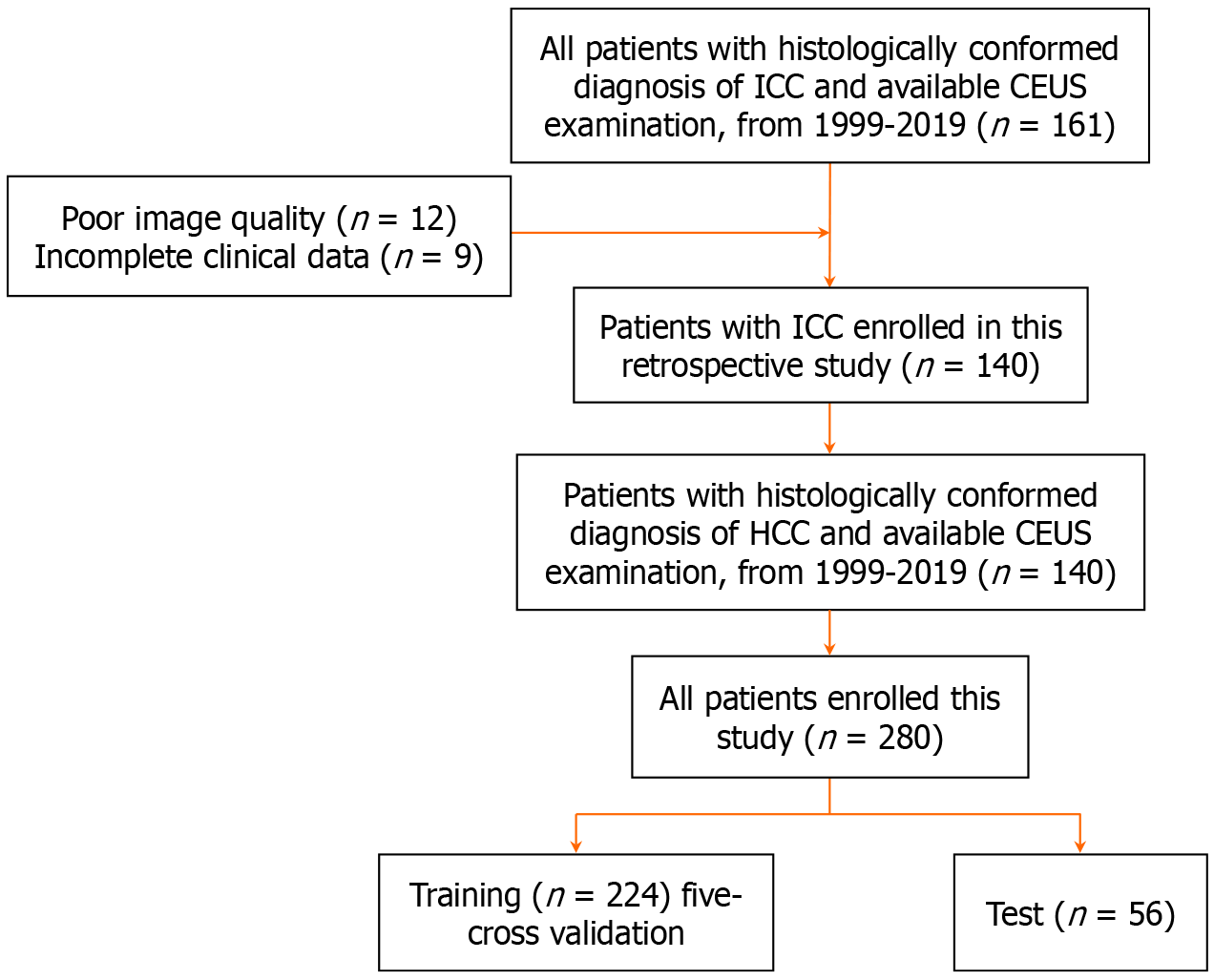
In our retrospective study, we included a total of 280 patients who were diagnosed with ICC (n = 140) and HCC (n = 140) between 1999 and 2019. These patients were divided into training (n = 224) and testing (n = 56) groups for analysis. US images and relevant clinical characteristics were collected. We utilized the XGBoost method to extract and select radiomics features and further employed a random forest algorithm to establish ultrasomics models. We compared the diagnostic performances of these ultrasomics models with that of radiologists.
Four distinct ultrasomics models were constructed, with the number of selected features varying between models: 13 features for the US model; 15 for the contrast-enhanced ultrasound (CEUS) model; 13 for the combined US + CEUS model; and 21 for the US + CEUS + clinical data model. The US + CEUS + clinical data model yielded the highest area under the receiver operating characteristic curve (AUC) among all models, achieving an AUC of 0.973 in the validation cohort and 0.971 in the test cohort. This performance exceeded even the most experienced radiologist (AUC = 0.964). The AUC for the US + CEUS model (training cohort AUC = 0.964, test cohort AUC = 0.955) was significantly higher than that of the US model alone (training cohort AUC = 0.822, test cohort AUC = 0.816). This finding underscored the significant benefit of incorporating CEUS information in accurately distinguishing ICC from HCC.
We developed a radiomics diagnostic model based on CEUS images capable of quickly distinguishing HCC from ICC, which outperformed experienced radiologists.
Core Tip: In this study, we successfully established a novel radiomics model that leveraged contrast-enhanced ultrasound (US) for accurate discrimination between intrahepatic cholangiocarcinoma and hepatocellular carcinoma. The refined radiomics model incorporated 21 essential features, surpassing the diagnostic accuracy of seasoned radiologists. This model excelled in diagnostic performance and ease of use, requiring only three specific time-point images and by a transparent image-acquisition protocol. Its implementation enhanced diagnostic objectivity and diminished the operator-dependence inherent in US examinations. This ultrasomics-based model can provide additional diagnostic insights to radiologists of varying levels of experience, thereby elevating overall diagnostic accuracy.
- Citation: Su LY, Xu M, Chen YL, Lin MX, Xie XY. Ultrasomics in liver cancer: Developing a radiomics model for differentiating intrahepatic cholangiocarcinoma from hepatocellular carcinoma using contrast-enhanced ultrasound. World J Radiol 2024; 16(7): 247-255
- URL: https://www.wjgnet.com/1949-8470/full/v16/i7/247.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i7.247
Primary liver cancer ranks as the sixth most common cancer globally and the third-leading cause of cancer-related mortality, with the fifth highest incidence and fourth highest mortality rates[1]. Hepatocellular carcinoma (HCC) and intrahepatic cholangiocarcinoma (ICC) represent the predominant histological types, comprising over 99% of cases[2]. Effective treatments such as resection, liver transplantation, and ablation are available for early-stage HCC or ICC. However, for lesions diagnosed at an advanced, incurable stage, systematic treatment varies significantly between these tumor types[3,4], necessitating distinct therapeutic approaches. Given their differing biological behaviors, prognoses, and treatment strategies, accurately differentiating between HCC and ICC is crucial for effective clinical management.
Over the past decade, contrast-enhanced ultrasound (CEUS) has been extensively studied across various domains, achieving notable success in characterizing focal liver lesions. Numerous studies have attempted to distinguish between ICC and HCC using CEUS, yielding promising findings. For instance, Liu et al[5] reported an area under the receiver operating characteristic (ROC) curve (AUC) of 0.808 in differentiating ICC from HCC, highlighting the high efficiency of peripheral rim-like enhancement and rapid contrast washout in these distinctions.
Despite advancements in modern imaging techniques, standard imaging modalities and qualitative image analysis often fall short of conclusively differentiating between ICC and HCC, especially in early-stage tumors[6]. Radiomics, an emerging image processing technology, can automatically extract various quantitative image features that may elude the human eye[7]. For example, Lewis et al[8] utilizing magnetic resonance imaging (MRI)-based radiomics, achieved an AUC of 0.900 in the differential diagnosis of ICC from HCC by incorporating radiomics with patient sex and Liver Imaging Reporting and Data Systems criteria.
Reports on the application of ultrasound (US)-based radiomics methods in distinguishing HCC from ICC are limited. Our research aimed to develop and validate an ultrasomics model to accurately differentiate between HCC and ICC.
All patients diagnosed with liver cancer at our institution from 1999 to 2019 underwent CEUS. Prior to analysis, all patient data were fully anonymized. The inclusion criteria were: (1) Availability of preoperative CEUS images; and (2) Surgical excision or biopsy with pathological examination. Exclusion criteria included: (1) Lack of histopathological evaluation by surgery or biopsy; (2) Incomplete clinical data (CL); and (3) Substandard CEUS image quality. Conse
We divided the cohort into two groups: 224 patients for the training set; and 56 patients for the testing set. The workflow is depicted in Figure 1. Clinical characteristics of the patients, including sex, age, hepatitis B virus (HBV) infection, alpha-fetoprotein (AFP), and carbohydrate antigen 19-9 (CA19-9), were collected from admission records, as shown in Table 1.
| Characteristics | HCC, n = 140 | ICC, n = 140 | P value |
| Age in yr | 53.39 + 11.85 | 57.46 + 11.39 | 0.58 |
| 26-79 | 33-86 | ||
| Sex | 0.311 | ||
| Male | 126 | 120 | |
| Female | 14 | 20 | |
| HBV (+) | 126 | 106 | 0.562 |
| Lesion size in cm | 66.37 + 27.38 | 69.04 + 32.68 | 0.271 |
| AFP in μg/L | 0.001 | ||
| > 20 | 91 | 18 | |
| < 20 | 49 | 122 | |
| CA19-9 in U/mL | 0.001 | ||
| > 34 | 15 | 83 | |
| < 34 | 125 | 57 |
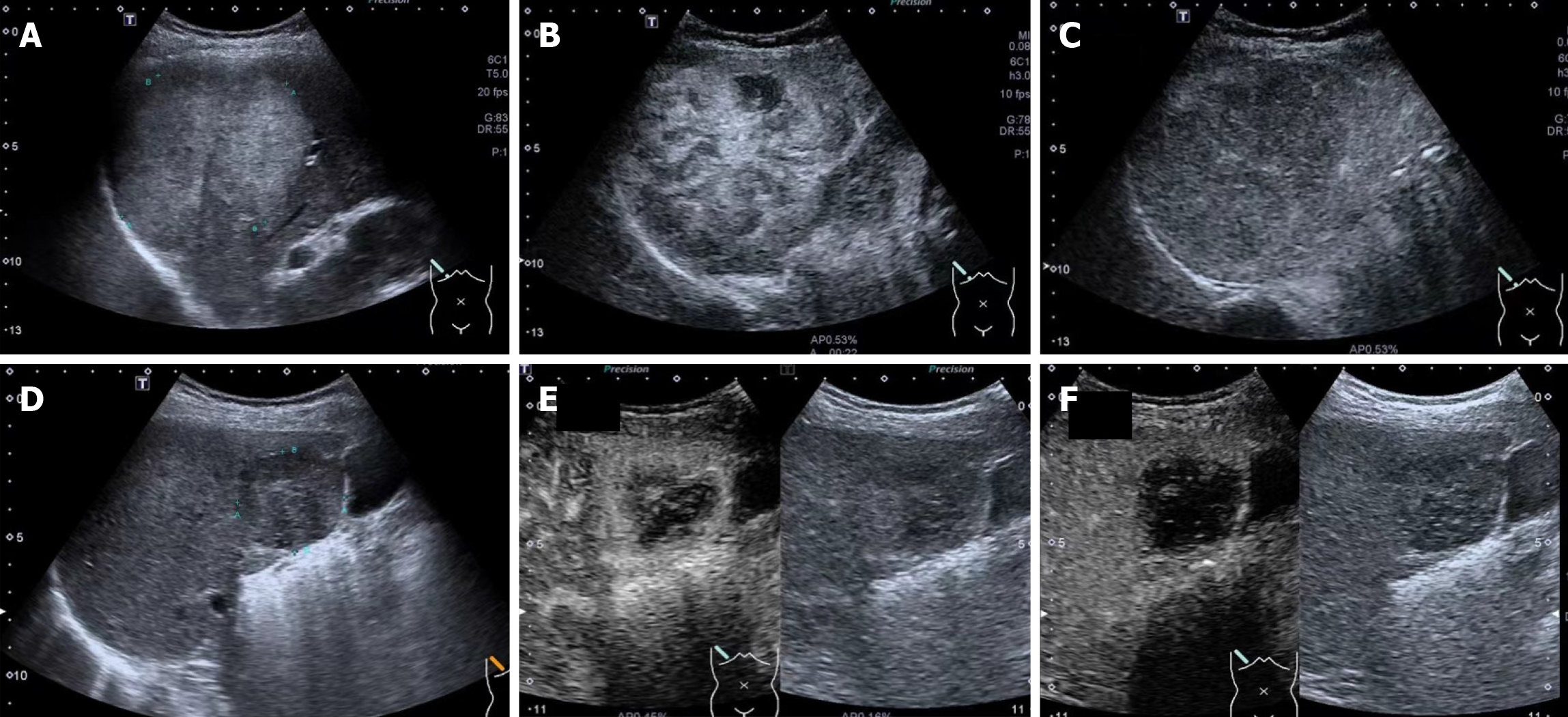
All enrolled patients underwent preoperative CEUS via convex array scanners (Canon/Supersonic Explorer/Esaote/Mindray) with a frequency range of 1.0 to 6.0 MHz. For patients with multiple liver lesions, the largest lesion was targeted. The ultrasound contrast agent, Sonovue, was administered through the antecubital vein as a bolus (within 1-2 s), followed by a 5 mL flush of 0.9% normal saline using a 20-gauge cannula. CEUS clips were recorded for 90 s following the injection of Sonovue. Additional CEUS images were captured at 120 s and subsequently every 60 s until the contrast washed out.
The ultrasound images (Figure 2) obtained included: (1) US. Baseline US image of the target lesion with optimal image depth and gain (standard plane); (2) Arterial enhancement (AE). CEUS image in the arterial phase showing peak enhancement of the target lesion in the standard plane; and (3) Portal enhancement (PE). CEUS image of the target lesion at 120 s in the standard plane.
The whole tumor segmentation was manually delineated in Labelme by an abdominal radiologist with 6 years of experience in liver CEUS and validated by a senior abdominal radiologist with 10 years of experience. Regions of interests (ROIs) were manually segmented along the tumor contour on all US images. The Image preprocessing and feature extraction were performed by Pyradiomics package.
Eight hundred and twenty-eight features were extracted from each region of interest, as listed in Table 2. The significance of each feature was calculated using XGBoost, and the top 20 significant features were selected for further analysis. The relationships among these features were assessed using the Spearman correlation coefficient. The less significant feature was discarded for radiomics features with a SCC greater than 0.8. The remaining features were chosen for subsequent analysis. A random forest algorithm was utilized to establish the radiomics model.
| Types | Features |
| Shape (n = 9) | Elongation, MajorAxisLength, MaximumDiameter, MeshSurface, MinorAxisLength, Perimeter, PerimeterSurfaceRatio, PixelSurface, Sphericity |
| First order (n = 18) | 10Percentile, 90Percentile, Energy, Entropy, InterquartileRange, Kurtosis, Maximum, MeanAbsoluteDeviation, Mean, Median, Minimum, Range, RobustMeanAbsoluteDeviation, RootMeanSquared, Skewness, TotalEnergy, Uniformity, Variance |
| GLCM (n = 22) | Autocorrelation, JointAverage, ClusterProminence, ClusterShade, ClusterTendency, Contrast, Correlation, DifferenceAverage, DifferenceEntropy, DifferenceVariance, JointEnergy, JointEntropy, Imc1, Imc2, Idm, Idmn, Id, Idn, InverseVariance, MaximumProbability, SumEntropy, SumSquares |
| GLRLM (n = 16) | GrayLevelNonUniformity, GrayLevelNonUniformityNormalized, GrayLevelVariance, HighGrayLevelRunEmphasis, LongRunEmphasis, LongRunHighGrayLevelEmphasis, LongRunLowGrayLevelEmphasis, LowGrayLevelRunEmphasis, RunEntropy, RunLengthNonUniformity, RunLengthNonUniformityNormalized, RunPercentage, RunVariance, ShortRunEmphasis, ShortRunHighGrayLevelEmphasis, ShortRunLowGrayLevelEmphasis |
| GLSZM (n = 16) | GrayLevelNonUniformity, GrayLevelNonUniformityNormalized, GrayLevelVariance, HighGrayLevelZoneEmphasis, LargeAreaEmphasis, LargeAreaHighGrayLevelEmphasis, LargeAreaLowGrayLevelEmphasis, LowGrayLevelZoneEmphasis, SizeZoneNonUniformity, SizeZoneNonUniformityNormalized, SmallAreaEmphasis, SmallAreaHighGrayLevelEmphasis, SmallAreaLowGrayLevelEmphasis, ZoneEntropy, ZonePercentage, ZoneVariance |
| GLDM (n = 14) | DependenceEntropy, DependenceNonUniformity, DependenceNonUniformityNormalized, DependenceVariance, GrayLevelNonUniformity, GrayLevelVariance, HighGrayLevelEmphasis, LargeDependenceEmphasis, LargeDependenceHighGrayLevelEmphasis, LargeDependenceLowGrayLevelEmphasis, LowGrayLevelEmphasis, SmallDependenceEmphasis, SmallDependenceHighGrayLevelEmphasis, SmallDependenceLowGrayLevelEmphasis |
| NGTDM (n = 5) | Busyness, Coarseness, Complexity, Contrast, Strength |
We developed four models and compared their performance to determine the most efficient one. These models include the US model (using US images), the CEUS model (using AE and PE images), the US + CEUS model (combining US, AE, and PE images), and the US + CEUS + CL model (integrating US, AE, and PE images with clinical characteristics).
The ROC curves were plotted, and metrics such as the AUC, accuracy, sensitivity, specificity, and precision were calculated to evaluate the predictive efficacy of each model in both training and test cohorts. Three radiologists with varying levels of experience (3, 8, and 11 years in liver CEUS) reviewed the images of all cases and made diagnoses. The diagnostic performances of the radiomic models and the radiologists were compared using the DeLong test. A decision curve was plotted to assess the clinical utility of the model by quantifying the net benefits at different risk thresholds.
Statistical analysis was conducted with SPSS 22.0 (IBM Corp., Armonk, NY, United States). Continuous variables were expressed as the means ± standard deviation. Categorical variables were reported as numbers and percentages and were compared by the χ2 test. Clinical features were analyzed for statistical differences in the training and test cohorts by Student’s t-test, Mann-Whitney U test, Wilcoxon test, χ2 test, or Fisher’s exact test, as appropriate.
The reported statistical significance levels were all two-sided, and P values of less than 0.05 were considered statistically significant. Calibration diagnostic accuracy was expressed as the AUC, and the resulting specificity, sensitivity, positive predictive value, and negative predictive value were calculated. The DeLong test was used to compare AUC values.
The final study cohort consisted of 280 patients (HCC = 140; ICC = 140) randomly divided into a training cohort (n = 224; HCC = 112; ICC = 112) and a validation cohort (n = 56; HCC = 28; ICC = 28). The primary characteristics of all patients and lesions are shown in Table 1. There was no significant difference in age, sex, HBV infection, and tumor size (P > 0.05). However, the levels of AFP and CA19-9 demonstrated significant differences between HCC and ICC (P < 0.05). Five clinical characteristics, age, sex, HBV infection, AFP, and CA19-9, were subsequently included in the radiomics model.
Independent significant features were identified for each model using XGBoost and Spearman correlation coefficient. The number of features selected was 13 for the US model, 15 for the CEUS model, 13 for the US + CEUS model, and 21 for the US + CEUS + CL. Four radiomic models were constructed using the random forest algorithm.
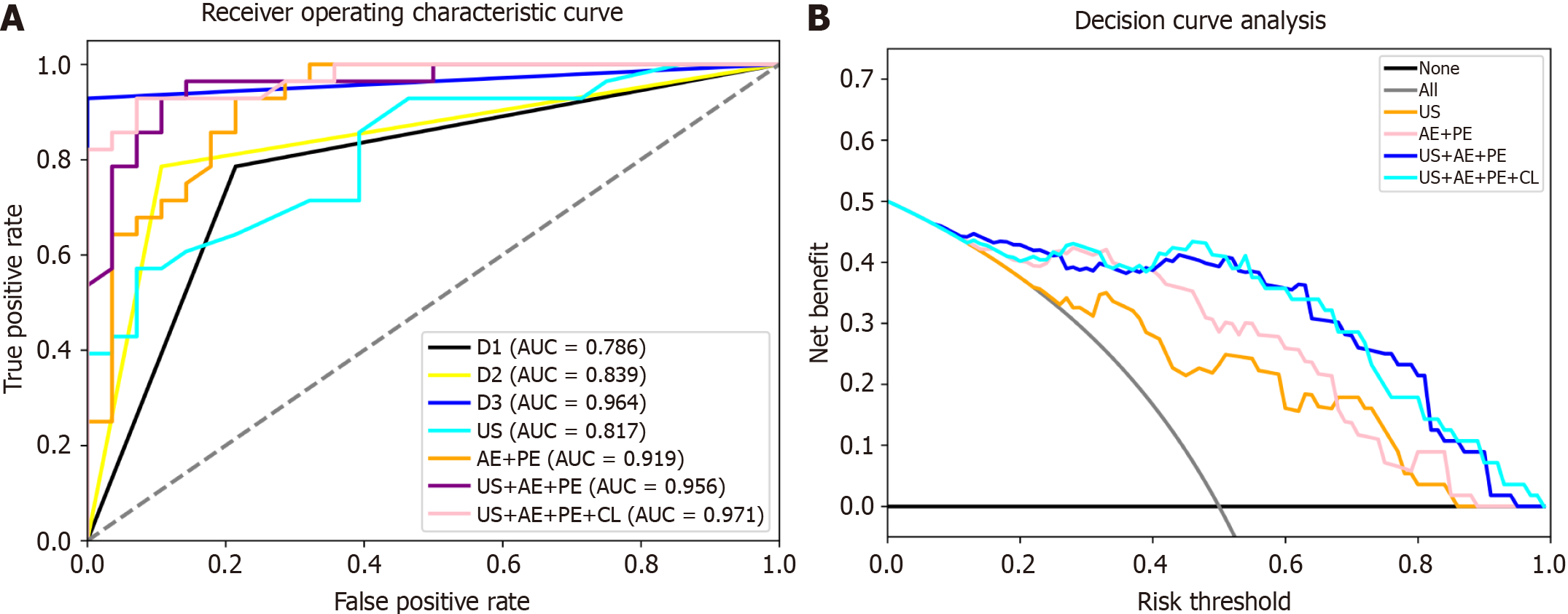
ROC analysis results are depicted in Figure 3, and a summary of the model outcomes is provided in Table 3. Our study’s radiomics model based on a single US image achieved an AUC of 0.822 in the validation cohort and 0.816 in the test cohort.
| Parameter | Training | Validation | Test | |||||||||
| US | AE + PE | US + AE + PE | US + AE + PE + CL | US | AE + PE | US + AE + PE | US + AE + PE + CL | US | AE + PE | US + AE + PE | US + AE + PE + CL | |
| AUC (95%CI) | 0.885 (0.825, 0.889) | 0.955 (0.919, 0.962) | 0.966 (0.934, 0.972) | 0.975 (0.941, 0.975) | 0.822 (0.738, 0.863) | 0.956 (0.856, 0.962) | 0.964 (0.907, 0.977) | 0.973 (0.933, 0.983) | 0.816 (0.690, 0.825) | 0.919 (0.876, 0.959) | 0.955 (0.907, 0.967) | 0.971 (0.916, 0.980) |
| Sensitivity | 0.766 | 0.933 | 0.900 | 0.911 | 0.727 | 0.909 | 0.818 | 0.863 | 0.607 | 0.714 | 0.928 | 0.892 |
| Specificity | 0.853 | 0.842 | 0.876 | 0.921 | 0.681 | 0.818 | 0.909 | 0.954 | 0.857 | 0.857 | 0.857 | 0.928 |
| Accuracy | 0.810 | 0.888 | 0.888 | 0.916 | 0.704 | 0.863 | 0.863 | 0.909 | 0.732 | 0.785 | 0.892 | 0.910 |
| PPV | 0.841 | 0.857 | 0.880 | 0.921 | 0.695 | 0.833 | 0.900 | 0.950 | 0.809 | 0.833 | 0.866 | 0.925 |
| NPV | 0.783 | 0.925 | 0.896 | 0.911 | 0.714 | 0.900 | 0.833 | 0.875 | 0.685 | 0.750 | 0.923 | 0.896 |
The CEUS model demonstrated a significant increase in AUC compared to the US model in both cohorts. In the validation cohort, the AUC for the CEUS model was 0.956, and in the test cohort the AUC was 0.919. The AUC for the US + CEUS model was higher (training cohort = 0.964, test cohort = 0.955) than that for the US model alone, indicating a significant benefit from adding CEUS information in distinguishing ICC from HCC. The AUC for the US + CEUS + CL was the highest among all models, with 0.973 in the validation cohort and 0.971 in the test cohort.
AUCs for three radiologists with different experience levels (3, 8, and 11 years in liver CEUS) were 0.786, 0.964, and 0.839, respectively. These results show that the AUC of the US + CEUS + CL was slightly higher than that of the most experienced radiologist.
The decision curve analysis is shown in Figure 3. CEUS provided a significantly increased benefit compared to US alone. Moreover, the models that included more information, such as US, CEUS, and clinical characteristics, demonstrated greater benefits.
In this study, we constructed a new radiomics model to differentiate between ICC and HCC using CEUS, achieving good diagnostic performance. The final radiomics model incorporated 21 features and demonstrated higher efficacy than experienced radiologists.
ICC is the second most common primary hepatic malignancy, following HCC. The incidence of these two malignant hepatic tumors, in parallel with their mortality, has markedly increased worldwide in recent years[9,10]. Given their distinct pathogenesis, pathological features, prognostic outcomes, and responses to adjuvant therapies, accurate discrimination between HCC and ICC is paramount for effective clinical management[11].
While pathology remains the gold standard diagnostic test for liver cancer, often requiring paracentesis or surgery, its invasive nature and associated risks, such as bleeding and tumor cell seeding, render it a less favorable option. Hence, image diagnosis occupies a pivotal role in this realm, with US serving as the primary modality for liver disease surveillance.
In recent years, CEUS has played a significant role in characterizing focal liver lesions, including the differentiation of ICC from HCC. Compared to computed tomography (CT) and MRI, CEUS is a real-time dynamic imaging modality that provides enhanced information on the blood supply of lesions, which is crucial for liver tumor discrimination. CEUS is completely radiation-free for patients. Several studies have reported that CEUS has an excellent diagnostic performance in distinguishing between ICC and HCC. However, the scanning technique and visual interpretation of US images are both operator dependent, and diagnostic accuracy varies significantly across different radiologists and CEUS centers. Therefore, developing a more reliable, efficient, and user-friendly method for differentiation is imperative.
Radiomics is a process that enables the extraction and analysis of quantitative data from medical images. It is an evolving field with numerous potential applications in medical imaging[12]. Several studies have demonstrated that textural features from CT, MRI, and US images can accurately differentiate ICC from HCC, mixed HCC-ICC, and inflammatory masses, with an AUC greater than 0.800. However, CEUS-based radiomics studies on this issue have been limited.
In our study, the AUC for the US + CEUS + CL reached 0.971 in the test cohort. This result was comparable to those obtained using CT/MRI, underscoring the capacity and reliability of CEUS in differentiating HCC from ICC. Additionally, the result was slightly higher than the diagnostic accuracy of experienced radiologists (AUC = 0.964) and significantly higher than that of less-experienced radiologists (AUC = 0.786). It is promising that the model exceeds the accuracy of experienced radiologists.
The model is highly effective in diagnostic performance and is user-friendly, requiring only three specific-time-point US + CEUS images, complemented by a clear image acquisition protocol. It enhances objectivity and mitigates the operator dependence of US examinations. A stable, radiomics-based model could be particularly valuable in regional hospitals lacking experienced radiologists and could provide additional diagnostic suggestions to less-experienced radiologists, thereby enhancing diagnostic accuracy.
The images in this study were selected based on previous research. Image acquisition is a well-known challenge in radiomics. The selection of CEUS images was crucial. In the largest CEUS study involving 819 participants, Liu et al[5] found that peripheral rim-like enhancement and rapid contrast washout were highly effective in differentiating HCC from ICC. ICCs generally exhibited lower peak intensities and tumor/tissue ratios, with earlier washout (often within 60 s) compared to HCCs, which typically showed washout after 60 s[13]. Consequently, two classic CEUS images (one in the arterial phase with peak enhancement of the target lesion and another at 120 s) were selected for feature extraction.
Four radiomics models were constructed: US; CEUS; US + CEUS; and US + CEUS + CL. The data indicated that adding CEUS information to the US model significantly enhanced the diagnostic accuracy of the radiomics model. CEUS has been omitted from the diagnostic techniques in the latest American Association for the Study of Liver Diseases guidelines due to concerns that it might lead to false-positive HCC diagnoses in patients with ICC[14-16]. Every model (training, validation, and testing) in our study demonstrated AUC values above 0.900 for the CEUS model, underscoring its substantial value in characterizing focal liver lesions.
CEUS-based artificial intelligence holds promise, particularly for liver tumors. Looking forward, developing artificial intelligence models that can predict responses to systemic treatments for liver tumors is a valuable and significant area of research, though it presents challenges that will aid clinicians in decision-making.
There were limitations to our study. First, our data originated from a single center, necessitating validation and replication of results across multiple centers to confirm their reproducibility. Second, the selection of the CEUS image in the arterial phase was made by the naked eye of an experienced doctor, introducing potential selection bias. In future studies, employing a time intensity curve could aid in image selection and enhance the robustness of the results.
We developed a radiomics diagnostic model based on CEUS images capable of quickly distinguishing HCC from ICC, which outperformed experienced radiologists. This model may serve as a diagnostic assistance tool in regional hospitals lacking CEUS expertise and provide additional diagnostic suggestions for less-experienced radiologists.
The authors thank Yan Li for supporting this study.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68390] [Article Influence: 13678.0] [Reference Citation Analysis (201)] |
| 2. | Sia D, Villanueva A, Friedman SL, Llovet JM. Liver Cancer Cell of Origin, Molecular Class, and Effects on Patient Prognosis. Gastroenterology. 2017;152:745-761. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 619] [Cited by in RCA: 867] [Article Influence: 96.3] [Reference Citation Analysis (2)] |
| 3. | Gordan JD, Kennedy EB, Abou-Alfa GK, Beg MS, Brower ST, Gade TP, Goff L, Gupta S, Guy J, Harris WP, Iyer R, Jaiyesimi I, Jhawer M, Karippot A, Kaseb AO, Kelley RK, Knox JJ, Kortmansky J, Leaf A, Remak WM, Shroff RT, Sohal DPS, Taddei TH, Venepalli NK, Wilson A, Zhu AX, Rose MG. Systemic Therapy for Advanced Hepatocellular Carcinoma: ASCO Guideline. J Clin Oncol. 2020;38:4317-4345. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 305] [Cited by in RCA: 439] [Article Influence: 73.2] [Reference Citation Analysis (1)] |
| 4. | European Association for the Study of the Liver. EASL-ILCA Clinical Practice Guidelines on the management of intrahepatic cholangiocarcinoma. J Hepatol. 2023;79:181-208. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 193] [Article Influence: 64.3] [Reference Citation Analysis (0)] |
| 5. | Liu GJ, Wang W, Lu MD, Xie XY, Xu HX, Xu ZF, Chen LD, Wang Z, Liang JY, Huang Y, Li W, Liu JY. Contrast-Enhanced Ultrasound for the Characterization of Hepatocellular Carcinoma and Intrahepatic Cholangiocarcinoma. Liver Cancer. 2015;4:241-252. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 83] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 6. | European Association for the Study of the Liver. EASL Clinical Practice Guidelines: Management of hepatocellular carcinoma. J Hepatol. 2018;69:182-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5593] [Cited by in RCA: 6397] [Article Influence: 799.6] [Reference Citation Analysis (9)] |
| 7. | Aerts HJ. The Potential of Radiomic-Based Phenotyping in Precision Medicine: A Review. JAMA Oncol. 2016;2:1636-1642. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 320] [Cited by in RCA: 493] [Article Influence: 54.8] [Reference Citation Analysis (0)] |
| 8. | Lewis S, Peti S, Hectors SJ, King M, Rosen A, Kamath A, Putra J, Thung S, Taouli B. Volumetric quantitative histogram analysis using diffusion-weighted magnetic resonance imaging to differentiate HCC from other primary liver cancers. Abdom Radiol (NY). 2019;44:912-922. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 9. | Bertuccio P, Malvezzi M, Carioli G, Hashim D, Boffetta P, El-Serag HB, La Vecchia C, Negri E. Global trends in mortality from intrahepatic and extrahepatic cholangiocarcinoma. J Hepatol. 2019;71:104-114. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 477] [Article Influence: 68.1] [Reference Citation Analysis (0)] |
| 10. | Kim SJ, Lee JM, Han JK, Kim KH, Lee JY, Choi BI. Peripheral mass-forming cholangiocarcinoma in cirrhotic liver. AJR Am J Roentgenol. 2007;189:1428-1434. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 98] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 11. | You MW, Yun SJ. Differentiating between hepatocellular carcinoma and intrahepatic cholangiocarcinoma using contrast-enhanced MRI features: a systematic review and meta-analysis. Clin Radiol. 2019;74:406.e9-406.e18. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 23] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 12. | Scapicchio C, Gabelloni M, Barucci A, Cioni D, Saba L, Neri E. A deep look into radiomics. Radiol Med. 2021;126:1296-1311. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 269] [Article Influence: 53.8] [Reference Citation Analysis (0)] |
| 13. | Li R, Yuan MX, Ma KS, Li XW, Tang CL, Zhang XH, Guo DY, Yan XC. Detailed analysis of temporal features on contrast enhanced ultrasound may help differentiate intrahepatic cholangiocarcinoma from hepatocellular carcinoma in cirrhosis. PLoS One. 2014;9:e98612. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 40] [Cited by in RCA: 52] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Galassi M, Iavarone M, Rossi S, Bota S, Vavassori S, Rosa L, Leoni S, Venerandi L, Marinelli S, Sangiovanni A, Veronese L, Fraquelli M, Granito A, Golfieri R, Colombo M, Bolondi L, Piscaglia F. Patterns of appearance and risk of misdiagnosis of intrahepatic cholangiocarcinoma in cirrhosis at contrast enhanced ultrasound. Liver Int. 2013;33:771-779. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 92] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 15. | Barreiros AP, Piscaglia F, Dietrich CF. Contrast enhanced ultrasound for the diagnosis of hepatocellular carcinoma (HCC): comments on AASLD guidelines. J Hepatol. 2012;57:930-932. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 55] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 16. | Jang JY, Kim MY, Jeong SW, Kim TY, Kim SU, Lee SH, Suk KT, Park SY, Woo HY, Kim SG, Heo J, Baik SK, Kim HS, Tak WY. Current consensus and guidelines of contrast enhanced ultrasound for the characterization of focal liver lesions. Clin Mol Hepatol. 2013;19:1-16. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 48] [Cited by in RCA: 60] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/