Published online Nov 28, 2024. doi: 10.4329/wjr.v16.i11.678
Revised: September 14, 2024
Accepted: October 23, 2024
Published online: November 28, 2024
Processing time: 312 Days and 5.4 Hours
Afferent loop syndrome (ALS) is a rare complication, Aoki et al reported that the incidence of distal gastrectomy in Billroth-II is 0.3%-1.0%. The clinical manifestations of ALS are atypical, which can manifest as severe abdominal pain, vomiting, obstructive jaundice, malnutrition, etc.
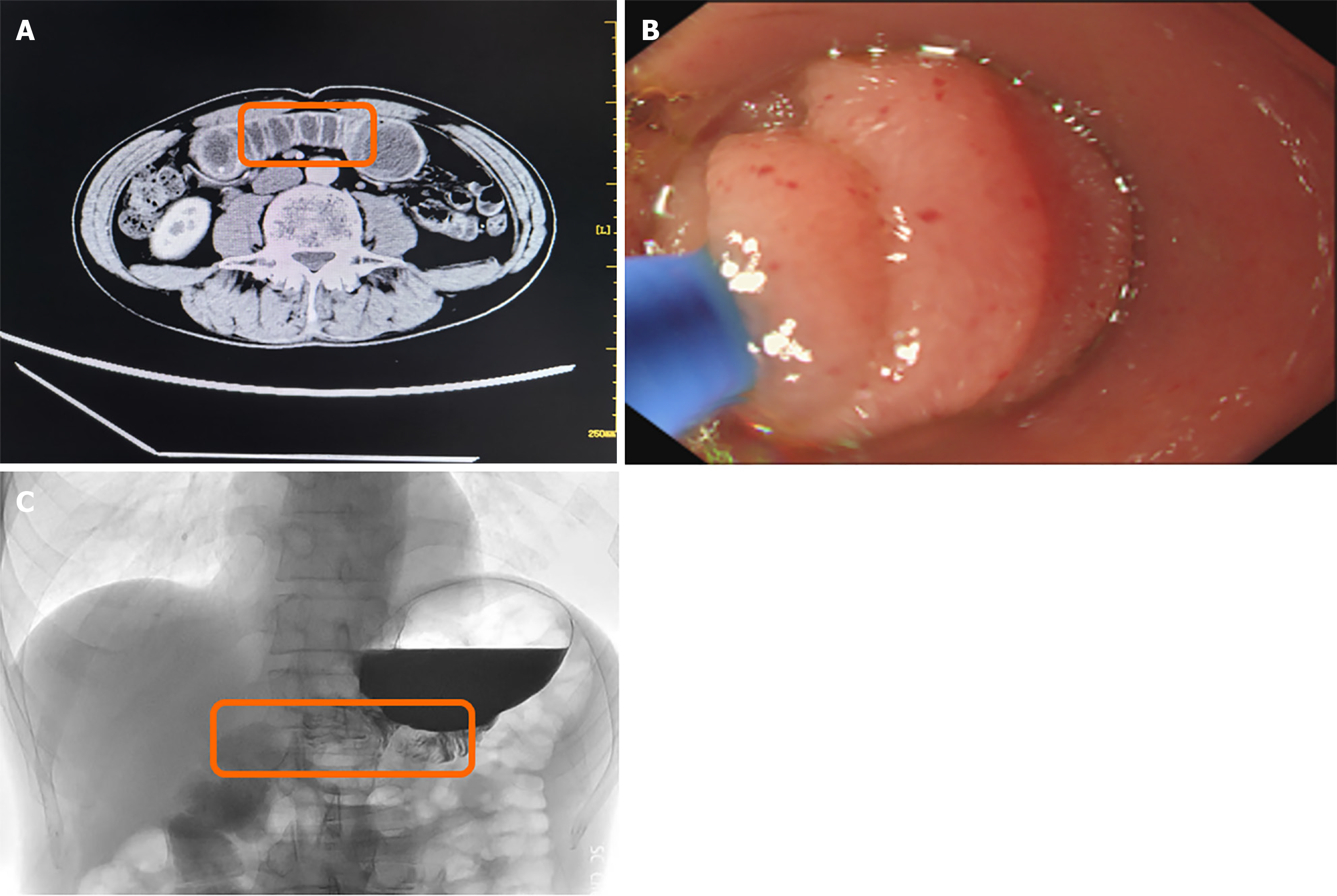
The patient was a 58-year-old man who complained of recurrent high fever for more than 1 week. Laboratory tests showed an increase in neutrophil ratio, procalcitonin, C-reactive protein, and abnormal liver function. Enhanced computed tomography scan of the abdomen showed small intestinal obstruction between the anastomosis of the gastrojejunum, bile duct, and pancreaticoduodenum. Gastroscopy revealed significant narrowing of the lumen 15 cm from the anasto
ALS is relatively rare after pancreaticoduodenectomy, and the treatment depends on the nature of the obstructive lesion. The traditional treatment method is surgery, and in recent years, endoscopy has provided a new treatment method for ALS.
Core Tip: The manifestations of the afferent loop syndrome vary, and the patient we reported presented only with recurrent fever. Instead of surgery, we managed to relieve the patient's symptoms through endoscopic and minimally invasive inter
- Citation: Yuan J, Zhang YJ, Wen W, Liu XC, Chen FL, Yang Y. Afferent loop syndrome of a patient with recurrent fever: A case report. World J Radiol 2024; 16(11): 678-682
- URL: https://www.wjgnet.com/1949-8470/full/v16/i11/678.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i11.678
Afferent loop syndrome (ALS) is a rare complication, Aoki et al[1] reported that the incidence of distal gastrectomy in Billroth-II is 0.3%-1.0%. It has been reported to occur in 0.2%-1% of patients after surgery, depending on the type of surgery and reconstruction[2]. After the afferent loop is partially or completely obstructed and a closed loop is formed, bile and intestinal secretions accumulate internally, resulting in increased intracavitary pressure and causing clinical symptoms. Termsinsuk et al[3] reported that the clinical manifestations of ALS are atypical, which can manifest as severe abdominal pain, vomiting, obstructive jaundice, malnutrition, etc.[3]. We report a case of ALS patient with recurrent fever as the main clinical manifestation after undergoing expanded pancreaticoduodenectomy for malignant tumors of the pylorus for 11 months.
His chief complaint was recurrent high fever for more than 1 week.
ALS.
Gastric malignant tumor.
Physical examination showed that the patient only had mild tenderness in the upper abdomen.
Laboratory examination results showed a white blood cell count of 6. 67 × 109/L, a neutrophil ratio of 92.7%, procalcitonin of 8.46 ng/mL, C-reactive protein of 25.8 mg/L, alanine aminotransferase of 139 U/L, and aspartate aminotransferase of 191 U/L, gamma-glutamyl transpeptidase is 141 U/L. Direct bilirubin is 11.6 μmol/L.
Based on laboratory results, the patient was preliminarily diagnosed with liver dysfunction and concurrent infection, but the infection site is unknown.
To clarify the diagnosis and search for the infection site, the patient underwent enhanced computed tomography (CT) scanning. Surprisingly, small intestine obstruction was found between the gastrojejunum, bile duct, and pancreaticoduodenal anastomosis, and the small intestine content was a non-contrast liquid (Figure 1A). On this basis, we performed gastroscopy on the patient and found significant narrowing of the lumen 15 cm from the anastomotic opening into the afferent loop (Figure 1B). Based on these findings, the patient was diagnosed with a biliary infection secondary to obstruction of the afferent loop.
ALS.
The Zebra Guidewire was implanted through the biopsy clamp, and the position was adjusted with the assistance of X-ray to make it pass the distal end of the narrow intestinal tract smoothly. The angiography catheter was implanted with the aid of the Zebra Guidewire, and the contrast agents were injected at the anastomosis. The contrast agents were slowly discharged into the afferent loop, and the narrow section of the intestinal tract was about 3 cm, and the distal intestinal tube of the afferent loop was significantly expanded (Figure 1C). Therefore, we used a balloon to perform a dilation of the digestive tract stenosis, after that we put intestinal feeding tube for the patient.
Observing the patient for 72 hours after the procedure, the patient did not experience any further fever. Five days later, the nutrition tube was removed, and a follow-up digestive tract radiography showed no gastrointestinal obstruction. We asked the patient to come to our hospital for regular follow-up visits, but the patient did not come to our hospital again because he lived in other provinces, so the long-term prognosis of the patient was unknown.
Gastrectomy combined with gastrojejunostomy is a surgical procedure in which the remaining stomach is anastomosed to the proximal jejunum after resection of the stomach, resulting in the formation of an afferent loop and an efferent loop. The afferent loop is the intestinal limb composed of duodenum and proximal jejunum, also known as the biliopancreatic limb. ALS is a rare complication, Aimoto et al[4] reported that ALS is relatively common in Billroth II gastrectomy, and is relatively rare after pancreaticoduodenectomy. It can be caused by adhesions, strictures, or internal hernias after gastrectomy. Symptoms may appear in the early and late postoperatively. ALS may worsen rapidly, and some patients may have severe complications such as digestive tract perforation. Therefore, early diagnosis and treatment are very important to reduce the mortality associated with ALS and improve the long-term quality of life of patients.
The etiology of ALS can be divided into benign and malignant causes. The benign causes of ALS include foreign bo
ALS can manifest as acute or chronic onset. The onset time of acute ALS varies, and patients may experience sudden severe abdominal pain and vomiting several hours or even years after anastomotic reconstruction[2,7,8]. Acute ALS is caused by complete obstruction of the afferent loop, which is very rare and characterized by the patient's vomit being bile free[9]. It has to be diagnosed as soon as possible, otherwise patients may develop serious complications such as intestinal perforation, intestinal necrosis, and may even lead to death.
Compared with acute ALS, the symptoms of chronic ALS are more atypical, usually manifested as periumbilical discomfort 15-30 minutes after eating, which makes patients fearful of eating, leading to malnutrition and significant weight loss. In addition, when patients with chronic ALS experience vomiting, the vomit often contains a large amount of bile[10]. However, the clinical manifestation of the patient we reported was high fever, which suggests that in patients with unexplained fever after pancreaticoduodenectomy, active imaging examination is necessary. Zissin[11], Juan et al[12] pointed out that CT is the main examination method for ALS.
The treatment of ALS depends on the nature of the obstructive lesion, the location of the obstruction, and the patency of hepatojejunostomy and pancreaticojejunostomy. Previously, the main treatment methods were re anastomosis or bypass surgery[13,14]. In recent years, endoscopy has provided new treatment methods for ALS. Cao et al[15] reported that 23 patients with ALS caused by postoperative inflammatory stenosis experienced relief in symptoms after undergoing endoscopic nasogastric decompression[15]. Kuo et al[16] reported that 11 ALS patients were successfully cured by endoscopic electrohydraulic lithotripsy for crushing fecal stones[16]. In addition, a multicenter study has shown that endoscopic ultrasound-guided enterostomy (EUS-EE) is one of the safe and effective methods for treating ALS[17]. They also point out that patients treated with EUS-EE group had higher rates of complete resolution of symptoms compared with patients who were treated with enteroscopy-assisted luminal stenting. Moreover, for patients with malignant ALS, palliative treatment is the first choice, as there is currently no data indicating a difference in survival rates between patients receiving palliative and curative treatment[11].
Our case also has limitations. One is that we should perform an endoscopic ultrasonography to better assess the nature of the lesion. The other is that we asked the patient to come to our hospital for regular follow-up visits, but the patient did not come to our hospital again because he lived in another province, so the long-term prognosis of the patient was unknown.
Patients with a history of previous gastrectomy or pancreaticoduodenectomy who experience fever, abdominal pain, vomiting, and other conditions should be alert to ALS. CT is the preferred imaging examination, and personalized treatment methods should be selected based on different etiologies during treatment.
| 1. | Aoki M, Saka M, Morita S, Fukagawa T, Katai H. Afferent loop obstruction after distal gastrectomy with Roux-en-Y reconstruction. World J Surg. 2010;34:2389-2392. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 2. | Kim DJ, Lee JH, Kim W. Afferent loop obstruction following laparoscopic distal gastrectomy with Billroth-II gastrojejunostomy. J Korean Surg Soc. 2013;84:281-286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 1.4] [Reference Citation Analysis (1)] |
| 3. | Termsinsuk P, Chantarojanasiri T, Pausawasdi N. Diagnosis and treatment of the afferent loop syndrome. Clin J Gastroenterol. 2020;13:660-668. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 24] [Article Influence: 4.0] [Reference Citation Analysis (4)] |
| 4. | Aimoto T, Uchida E, Nakamura Y, Katsuno A, Chou K, Tajiri T, Naito Z. Malignant afferent loop obstruction following pancreaticoduodenectomy: report of two cases. J Nippon Med Sch. 2006;73:226-230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 5. | Sato K, Banshodani M, Nishihara M, Nambu J, Kawaguchi Y, Shimamoto F, Sugino K, Ohdan H. Afferent loop obstruction with obstructive jaundice and ileus due to an enterolith after distal gastrectomy: A case report. Int J Surg Case Rep. 2018;50:9-12. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 6. | Spiliotis J, Karnabatidis D, Vaxevanidou A, Datsis AC, Rogdakis A, Zacharis G, Siamblis D. Acute cholangitis due to afferent loop syndrome after a Whipple procedure: a case report. Cases J. 2009;2:6339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 21] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 7. | Uriu Y, Kuriyama A, Ueno A, Ikegami T. Afferent loop syndrome of 10 years' onset after gastrectomy. Asian J Surg. 2019;42:935-937. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 8. | Ballas KD, Rafailidis SE, Konstantinidis HD, Pavlidis TE, Marakis GN, Anagnostara E, Sakadamis AK. Acute afferent loop syndrome: a true emergency. A case report. Acta Chir Belg. 2009;109:101-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Woodward ER. The pathophysiology of afferent loop syndrome. Surg Clin North Am. 1966;46:411-423. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 22] [Article Influence: 0.4] [Reference Citation Analysis (0)] |
| 10. | Blouhos K, Boulas KA, Tsalis K, Hatzigeorgiadis A. Management of afferent loop obstruction: Reoperation or endoscopic and percutaneous interventions? World J Gastrointest Surg. 2015;7:190-195. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 38] [Cited by in RCA: 44] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 11. | Zissin R. CT findings of afferent loop syndrome after a subtotal gastrectomy with Roux-en-Y reconstruction. Emerg Radiol. 2004;10:201-203. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 12. | Juan YH, Yu CY, Hsu HH, Huang GS, Chan DC, Liu CH, Tung HJ, Chang WC. Using multidetector-row CT for the diagnosis of afferent loop syndrome following gastroenterostomy reconstruction. Yonsei Med J. 2011;52:574-580. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 18] [Cited by in RCA: 19] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 13. | Pannala R, Brandabur JJ, Gan SI, Gluck M, Irani S, Patterson DJ, Ross AS, Dorer R, Traverso LW, Picozzi VJ, Kozarek RA. Afferent limb syndrome and delayed GI problems after pancreaticoduodenectomy for pancreatic cancer: single-center, 14-year experience. Gastrointest Endosc. 2011;74:295-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 71] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 14. | Katagiri H, Tahara K, Yoshikawa K, Lefor AK, Kubota T, Mizokami K. Afferent Loop Syndrome after Roux-en-Y Total Gastrectomy Caused by Volvulus of the Roux-Limb. Case Rep Surg. 2016;2016:4930354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Cao Y, Kong X, Yang D, Li S. Endoscopic nasogastric tube insertion for treatment of benign afferent loop obstruction after radical gastrectomy for gastric cancer: A 16-year retrospective single-center study. Medicine (Baltimore). 2019;98:e16475. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 15] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 16. | Kuo JY, Mo LR, Tsai CC, Chou CY, Lin RC, Chang KK. Nonoperative treatment of gastric bezoars using electrohydraulic lithotripsy. Endoscopy. 1999;31:386-388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 27] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 17. | Brewer Gutierrez OI, Irani SS, Ngamruengphong S, Aridi HD, Kunda R, Siddiqui A, Dollhopf M, Nieto J, Chen YI, Sahar N, Bukhari MA, Sanaei O, Canto MI, Singh VK, Kozarek R, Khashab MA. Endoscopic ultrasound-guided entero-enterostomy for the treatment of afferent loop syndrome: a multicenter experience. Endoscopy. 2018;50:891-895. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 44] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4. 0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/