Published online Oct 28, 2024. doi: 10.4329/wjr.v16.i10.593
Revised: August 29, 2024
Accepted: September 6, 2024
Published online: October 28, 2024
Processing time: 157 Days and 23.5 Hours
Vertebral artery dissection (VAD) is a rare but life-threatening condition characterized by tearing of the intimal layer of the vertebral artery, leading to stenosis, occlusion or rupture. The clinical presentation of VAD can be heterogeneous, with common symptoms including headache, dizziness and balance problems. Timely diagnosis and treatment are crucial for favorable outcomes; however, VAD is often missed due to its variable clinical presentation and lack of robust diagnostic guidelines. High-resolution magnetic resonance imaging (HRMRI) has emerged as a reliable diagnostic tool for VAD, providing detailed visualization of vessel wall abnormalities.
A young male patient presented with an acute onset of severe headache, vomi
HR-MRI can provide precise evidence for the identification of VAD.
Core Tip: In a young male patient with headache, aphasia and dysfunction after an argument, emergency head computed tomography indicated subarachnoid hemorrhage, head magnetic resonance imaging (MRI) indicated bilateral cerebellar hemispheres and brainstem infarction, digital subtraction angiography indicated bilateral vertebral artery stenosis, but no obvious dissection was found. Finally, high-resolution MRI (HRMRI) revealed clear vertebral artery dissection (VAD). Although the patient's clinical history and initial imaging findings suggested VAD, definitive diagnosis was only achievable through HRMRI, underscoring its critical role in accurately diagnosing VAD.
- Citation: Zhang HB, Duan YH, Zhou M, Liang RC. High-resolution magnetic resonance imaging in the diagnosis and management of vertebral artery dissection: A case report. World J Radiol 2024; 16(10): 593-599
- URL: https://www.wjgnet.com/1949-8470/full/v16/i10/593.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i10.593
Vertebral artery dissection (VAD) occurs when a tear develops in the intimal layer of the vertebral artery. This tear can lead to various complications, including stenosis, occlusion or rupture. Common symptoms of VAD include dizziness, headache and balance problems[1]. Despite being less frequent than other conditions, VAD is a significant concern, particularly in younger individuals, due to its often nonspecific presenting symptoms. The lack of robust diagnostic guidelines can contribute to delays or inadequacies in treatment[2]. Fortunately, timely intervention with thrombolytic and antiplatelet therapy can be effective in managing VAD. Previously, digital subtraction angiography (DSA) was considered the gold standard for diagnosing VAD. However, it has limitations. High-resolution magnetic resonance imaging (HRMRI) has emerged as a superior diagnostic tool. HRMRI offers detailed visualization of the vessel wall and can detect intramural hematoma; a characteristic finding in VAD. This imaging technique provides a reliable foundation for prompt identification and treatment of VAD. This case report will outline the typical clinical presentation of VAD, along with the findings of DSA and HRMRI. By reviewing current literature, we aim to underscore the critical importance of early detection of VAD for achieving optimal treatment outcomes.
Headache with vomiting for 1 h after a violent argument, followed by altered consciousness for 7 h after convulsions.
Eight hours prior to admission, the patient experienced an abrupt and intense headache following a vehement argument, characterized by throbbing pain localized in the occipital region. This was followed by intermittent vomiting of a small amount of stomach contents. One hour later, the patient developed sudden limb convulsions, became unable to speak, and exhibited frothing at the mouth. During intermittent periods, the patient could communicate with family members through gestures but remained unable to speak. There were no reported instances of urinary or fecal incontinence. Emergency head computed tomography (CT) conducted at a local hospital revealed subarachnoid hemorrhage in the left leading parietal lobe. Conditioned taste aversion (CTA) of the head and neck did not detect an aneurysm or arteriovenous malformation. The patient received sedation, analgesia and antiepileptic medications before being transferred to our hospital's emergency department with a preliminary diagnosis of spontaneous subarachnoid hemorrhage.
Previously healthy.
No significant toxic exposure was reported. The patient has a smoking history of > 5 years, at ~1 pack per day. Family history was negative for similar conditions.
Mental status: Shallow coma, Glasgow Coma Scale score was E3VTM3 = 6T, with T indicating the presence of an artificial airway due to intubation, eyes open upon call but unable to follow commands.
Respiratory system: Tracheal intubation in place.
Cranial nerves: (1) III (oculomotor): Pupils bilaterally dilated (1.5 mm) and round with sluggish light response; and (2) VII (facial): Symmetrical facial muscle tone (bilateral frontal striae and nasolabial grooves symmetrical, mouth angle not crooked).
Motor: Increased tone and weakness (limbs tingling and contraction, neck resistance with positive tone, limb muscle strength grade 1).
Reflexes: Bilateral negative Babinski sign.
Laboratory evaluation revealed normal levels of serum brain natriuretic peptide, potassium and D-dimer. However, genetic testing was not performed.
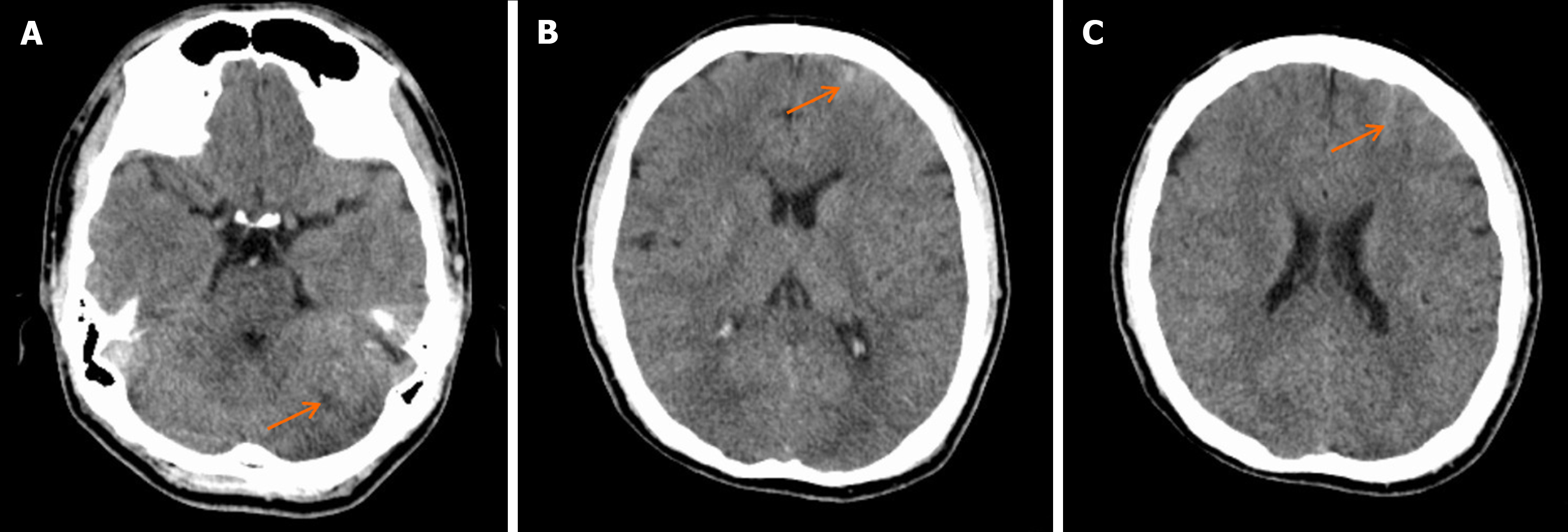
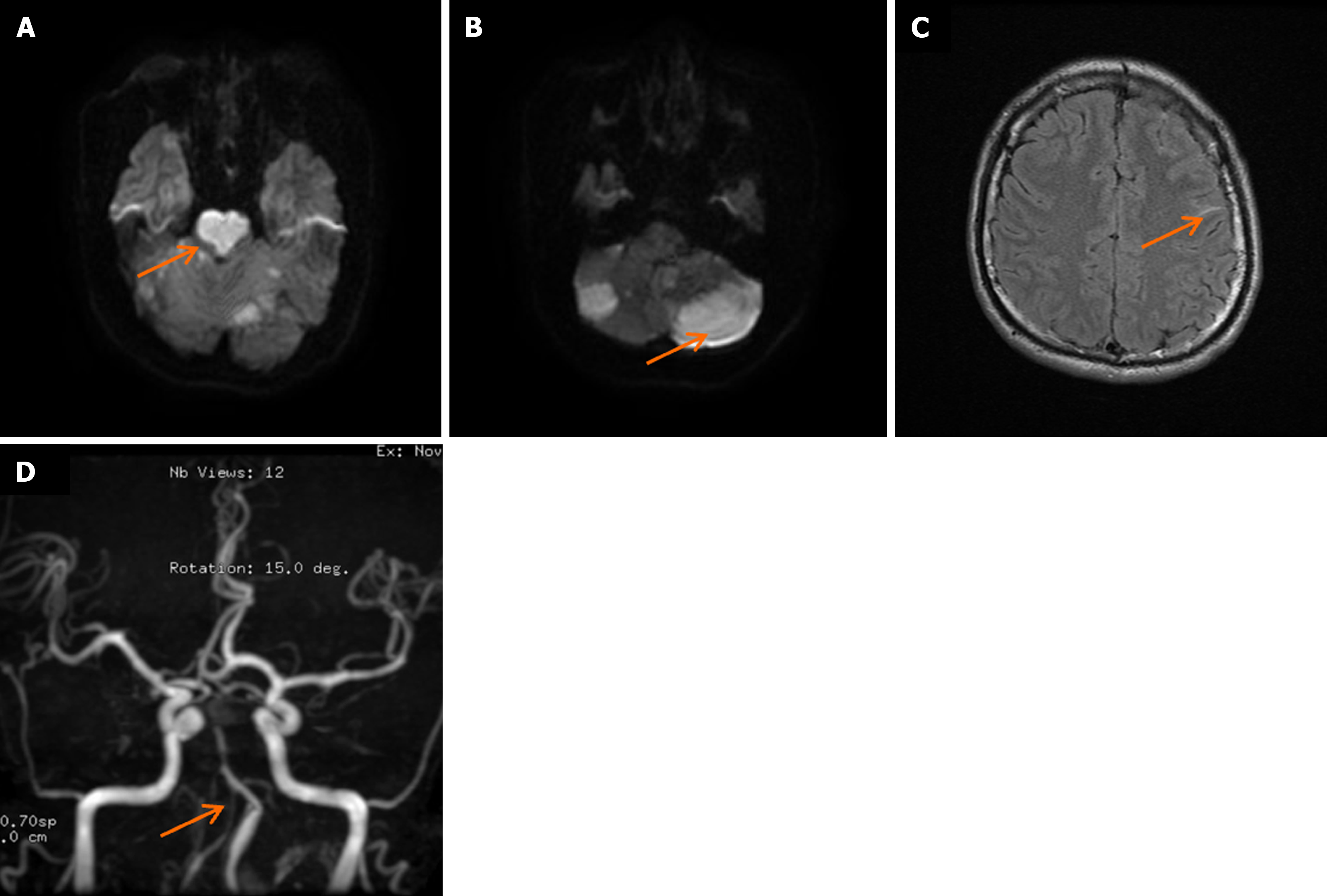
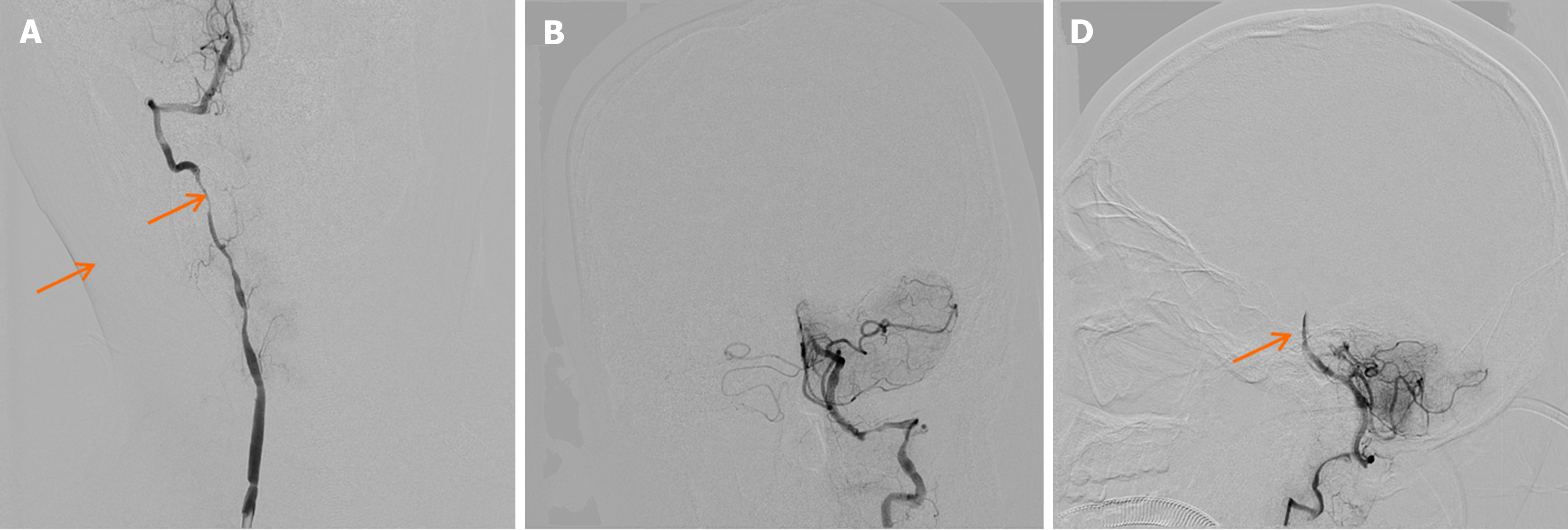
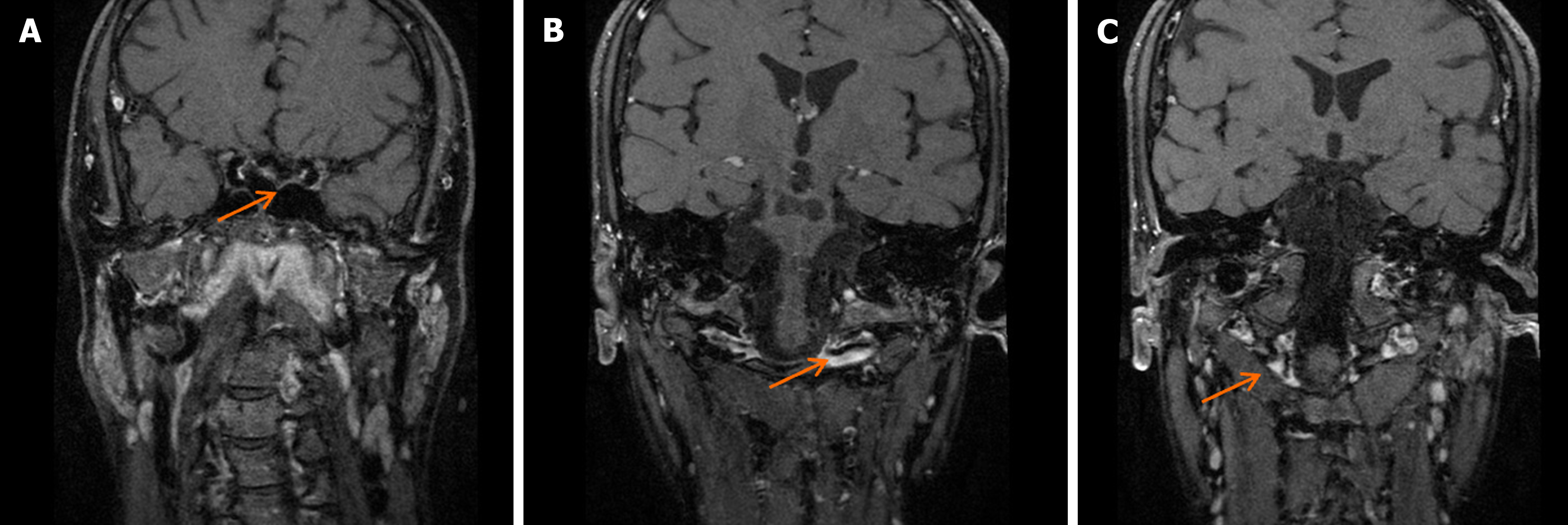
Head CT upon admission suggested potential ischemia in the brainstem and bilateral cerebellar hemispheres (Figure 1A), along with a left frontal lobe hematoma and subarachnoid hemorrhage (Figure 1B and C). Head MRI confirmed ischemic lesions in the brainstem and bilateral cerebellar hemispheres (Figure 2A and B) and left frontal subarachnoid hemorrhage (Figure 2C). Magnetic resonance angiography showed narrowing of the right and left vertebral arteries, basilar artery, and both posterior cerebral arteries (Figure 2D). DSA demonstrated segmental stenosis in both vertebral arteries (Figure 3A and B), with inconclusive visualization of the bilateral posterior cerebral arteries (Figure 3C). HRMRI confirmed bilateral VAD with thrombus formation (Figure 4A and B) and identified a thrombus in the apex of the basilar artery and right posterior cerebral artery (Figure 4C).
Bilateral VAD, basilar artery thrombosis, multiple brain stem and cerebellar infarcts, and subarachnoid hemorrhage.
The patient underwent tracheotomy for respiratory support. A multimodal medication regimen was initiated, including aspirin for blood clot prevention, edaravone for neuroprotection, levetiracetam to prevent seizures, piperacillin–tazo
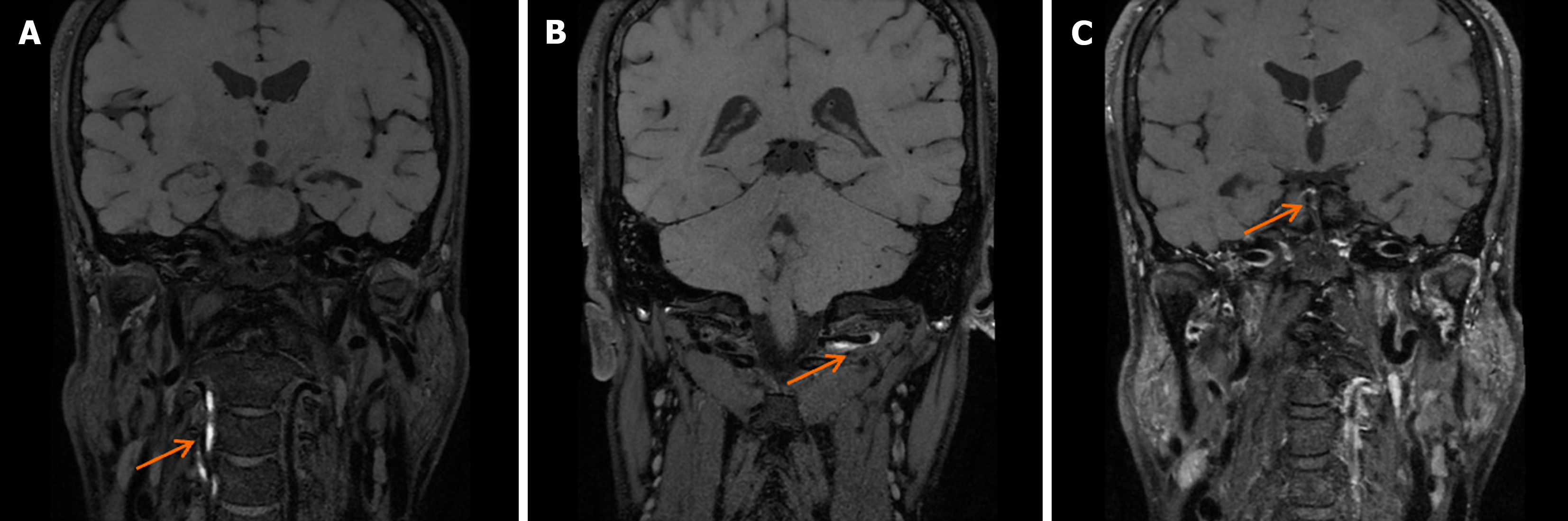
After 1 mo of treatment, the patient regained consciousness and mental clarity. After 5 mo of rehabilitation, swallowing function improved, and limb strength reached grade 3 on the muscle strength grading scale. HRMRI revealed thickening of the bilateral vertebral artery walls with narrowed lumens, suggestive of thrombus absorption and new granulation tissue formation (Figure 5).
VAD risk factors encompass a variety of elements. While atherosclerosis, genetic predisposition, and minor neck trauma are well-established[3], factors like migraine, pregnancy, infection, connective tissue disease and hypertension can also contribute. Structural anomalies such as vertebral artery distortion may further elevate risk. While minor neck injuries from massage, whiplash or prolonged tension were once suspected triggers, recent research suggests minimal impact on blood flow and an uncertain link to cerebrovascular disease[4]. Similarly, levofloxacin and amphetamine use has been linked to VAD, but requires further investigation. A single-center study found no significant difference in vertebral artery tortuosity between patients experiencing single or recurrent VAD events[5]. This suggests that intrinsic causes may play a more prominent role in intracranial VAD compared to carotid artery dissection[6]. This case exemplifies the complex nature of VAD. A young patient with a slender build and above-average height (185 cm) experienced a sudden illness during an argument. While the possibility of neck injury remains unclear, the left vertebral artery displayed a twisted appearance on DSA. This highlights the multifactorial nature of VAD, where genetic predisposition likely plays a significant role, with minor neck trauma potentially acting as a trigger. Further research is necessary to fully elucidate the pathogenesis of VAD. VAD in the posterior circulation can manifest with various symptoms. Headache, dizziness, vomiting and balance problems can arise due to stenosis and ischemia. Severe cases may progress to brainstem infarction and coma. Subarachnoid hemorrhage can occur with VAD, potentially due to blood vessel wall rupture or genetic factors[7]. Research suggests that posterior communicating artery hypoplasia may be linked to subarachnoid hemorrhage from VAD, causing abnormal bleeding in the cerebral cortex[8,9]. In this case, bilateral VAD and subarachnoid hemorrhage occurred simultaneously, but the hemorrhage was located in the cerebral cortical groove, distant from the vertebrobasilar artery. During hospital consultations, Marfan syndrome or Ehler–Danlos syndrome was suspected due to the patient's presentation, but genetic testing was unavailable due to limitations.
VAD can be challenging to diagnose due to a spectrum of presentations. Subtle symptoms like mild headaches may be easily overlooked, while severe cases requiring imaging such as CTA, DSA or MRI/HRMRI often present with hea
In contrast, HRMRI is successful, with its unparalleled ability to penetrate through thrombi and reveal the intricate multilayered structure of the vessel wall, including any hematoma or thrombus formation within the dissection. This exceptional imaging power renders HRMRI a more precise and dependable diagnostic tool for VAD, particularly in instances where thrombosis complicates the picture. Therefore, whenever VAD is suspected and DSA findings remain ambiguous, HRMRI emerges as the indispensable supplementary examination. It ensures that the diagnostic process is both comprehensive and accurate, and is the premier modality for visualizing the intricate details of VAD with unparalleled clarity[10]. This case highlights the difficulty encountered with atypical presentations on DSA. However, identifying vertebral artery stenosis in young individuals raises a red flag for VAD. HRMRI can definitively diagnose VAD by revealing "double-tube signs" and intramural thrombus. Unfortunately, by the time of admission in our case, the therapeutic window for thrombolysis had already passed, precluding this treatment option. Therefore, timely and accurate diagnosis of VAD is critical for effective management, with HRMRI demonstrating superior sensitivity and accuracy.
The therapeutic window for thrombolytic therapy in the context of VAD is limited to 4.5 h. Administration of thrombolytic therapy beyond this time may prove ineffective and carry a heightened risk of bleeding. Endovascular therapy, encompassing stent insertion or clot removal, can be considered. However, it carries a substantial risk of thrombus shedding during stent implantation and presently lacks substantiated evidence supporting its efficacy in enhancing the prognosis of VAD. A study in Thailand found that microvascular bypass surgery successfully treated VAD[11]. VAD can lead to vascular narrowing and stroke. Treatment includes antiplatelet therapy, anticoagulation, reducing brain swelling, and lowering cholesterol. Patients with brainstem infarction require close monitoring and preventative measures for complications such as infection and blood clots. In this case, a patient with bilateral VAD presented with subarachnoid hemorrhage. Thrombolytic therapy was precluded due to temporal limitations. Consequently, neither endovascular nor thrombolytic therapy was selected. Instead, aspirin antiplatelet therapy was administered. Aspirin has been found to be just as effective as vitamin K antagonists in treating VAD, with a high level of safety[12]. It is important to evaluate the possibility of administering thrombolysis to patients who arrive at the hospital within 4.5 h with both VAD and subarachnoid hemorrhage. If VAD affects vital areas like the brainstem, using thrombolytic therapy could worsen the hemorrhage. Recognizing the risks and benefits is crucial in this situation. The patient had VAD affecting the brainstem and cerebellum, leading to various complications. A study found that long-term oral antiplatelet medications, particularly aspirin, can reduce the risk of stroke recurrence in VAD patients[13].
VAD frequently affects young people and can be precipitated by activities such as neck massage or emotional stress, leading to sudden symptoms including headache, neck pain, dizziness and vomiting. DSA is capable of detecting blood flow direction and vertebral artery stenosis, but the presence of a thrombus within the dissection can complicate the diagnosis of VAD. Conversely, HRMRI can reveal distinctive signs such as a double lumen, intramural hematoma and an intimal flap. In this specific instance, although the optimal window for thrombolytic and endovascular therapies was missed, medical treatment still provided some benefits. Nevertheless, it is recognized that this study has its limitations, and further research with a larger sample size is required to investigate improved treatment strategies for VAD.
| 1. | Keser Z, Chiang CC, Benson JC, Pezzini A, Lanzino G. Cervical Artery Dissections: Etiopathogenesis and Management. Vasc Health Risk Manag. 2022;18:685-700. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 2. | Clark M, Unnam S, Ghosh S. A review of carotid and vertebral artery dissection. Br J Hosp Med (Lond). 2022;83:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 10] [Reference Citation Analysis (0)] |
| 3. | Gupta R, Siroya HL, Bhat DI, Shukla DP, Pruthi N, Devi BI. Vertebral artery dissection in acute cervical spine trauma. J Craniovertebr Junction Spine. 2022;13:27-37. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 7] [Reference Citation Analysis (0)] |
| 4. | Moser N, Mior S, Noseworthy M, Côté P, Wells G, Behr M, Triano J. Effect of cervical manipulation on vertebral artery and cerebral haemodynamics in patients with chronic neck pain: a crossover randomised controlled trial. BMJ Open. 2019;9:e025219. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 21] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 5. | Narrett JA, Aldridge CM, Garrett J, Abdalla B, Donahue J, Worrall BB, Southerland AM. Vertebral Artery Tortuosity and Morphometric Characteristics of Patients with Recurrent Cervical Artery Dissection. J Stroke Cerebrovasc Dis. 2022;31:106346. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 6. | Zhang X, Han J, Wang J, Yu S. A Comparative Study of the Etiology of Intracranial Vertebral Artery Dissection and Carotid Artery Dissection. Neurologist. 2023;28:281-286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 7. | Bae MJ, Ha SY. Subsequent Subarachnoid Hemorrhage from Clinically Unrelated Vertebral Artery Dissection after Thrombolytic Therapy. Neurointervention. 2022;17:54-57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 8. | Ariyada K, Shibahashi K, Fujika N, Sakakura Y, Hanakawa K, Murao M. Posterior Communicating Artery Hypoplasia: A Risk Factor for Vertebral Artery Dissection Causing Subarachnoid Hemorrhage. J Stroke Cerebrovasc Dis. 2022;31:106224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 9. | Korai M, Kanematsu Y, Yamaguchi I, Yamaguchi T, Yamamoto Y, Yamamoto N, Miyamoto T, Shimada K, Satomi J, Hanaoka M, Matsuzaki K, Satoh K, Takagi Y. Subarachnoid Hemorrhage Due to Rupture of Vertebral Artery Dissecting Aneurysms: Treatments, Outcomes, and Prognostic Factors. World Neurosurg. 2021;152:e86-e93. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 10. | Almohammad M, Dadak M, Götz F, Donnerstag F, Tryc AB, Mahmoudi N, Wattjes MP. The potential role of diffusion weighted imaging in the diagnosis of early carotid and vertebral artery dissection. Neuroradiology. 2022;64:1135-1144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 11. | Durongwatana N, Sriamornrattanakul K, Wongsuriyanan S, Akharathammachote N. Microsurgical Treatment of Vertebral Artery Dissection: Surgical Strategies and Treatment Outcomes. World Neurosurg. 2022;159:e375-e388. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 12. | Engelter ST, Traenka C, Gensicke H, Schaedelin SA, Luft AR, Simonetti BG, Fischer U, Michel P, Sirimarco G, Kägi G, Vehoff J, Nedeltchev K, Kahles T, Kellert L, Rosenbaum S, von Rennenberg R, Sztajzel R, Leib SL, Jung S, Gralla J, Bruni N, Seiffge D, Feil K, Polymeris AA, Steiner L, Hamann J, Bonati LH, Brehm A, De Marchis GM, Peters N, Stippich C, Nolte CH, Christensen H, Wegener S, Psychogios MN, Arnold M, Lyrer P; TREAT-CAD investigators. Aspirin versus anticoagulation in cervical artery dissection (TREAT-CAD): an open-label, randomised, non-inferiority trial. Lancet Neurol. 2021;20:341-350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 29] [Cited by in RCA: 106] [Article Influence: 21.2] [Reference Citation Analysis (0)] |
| 13. | Markus HS, Levi C, King A, Madigan J, Norris J; Cervical Artery Dissection in Stroke Study (CADISS) Investigators. Antiplatelet Therapy vs Anticoagulation Therapy in Cervical Artery Dissection: The Cervical Artery Dissection in Stroke Study (CADISS) Randomized Clinical Trial Final Results. JAMA Neurol. 2019;76:657-664. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 117] [Cited by in RCA: 211] [Article Influence: 35.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/