INTRODUCTION
Acute extensive thrombosis of the portal vein system is a rare but severe condition characterised by the formation of blood clots in two or more blood vessels, including the portal vein, splenic vein, superior mesenteric vein (SMV), and inferior mesenteric vein. The condition manifests as a critical acute abdomen with complex clinical symptoms. The incidence rate is relatively low (0.6%-1.0%), with the disease accounting for 5%-15% of intestinal vascular diseases[1]. According to the American Association of Hepatology Guidelines for the Diagnosis and Treatment of Hepatic Vascular Disease[2], patients with persistent abdominal pain for > 24 h should be evaluated for portal vein thrombosis, irrespective of the presence of fever or intestinal obstruction. The high rates of misdiagnosis can lead to significant treatment challenges and, in part, account for the high mortality of this condition.
Mesenteric vein thrombosis (MVT) constitutes only 2%-10% of all acute mesenteric ischaemia cases, with an incidence of ~0.1% in Europe and the United States[3]. MVT is primarily seen in older adults, occurring rarely in younger individuals among which 36% have unidentified mechanisms and causes[4].
Half of the deaths from acute mesenteric infarction (AMI) are attributed to superior mesenteric artery (SMA) occlusion, divided equally between non-occlusive infarction and inferior mesenteric artery occlusion/MVT[5]. The incidence rate of AMI ranges from 4.3 to 10 per 10000 individuals[6-9]; as a percentage of all acute surgical admissions, the incidence of AMI increases with age, ranging from 0.09% to 0.2%. If left untreated, the mortality rate is between 50% and 90%. Timely diagnosis and intervention can reduce the mortality rate by half. Like MVT, AMI mainly impacts older adults, with a mortality rate estimated between 68% and 75%, which rises in parallel with age. Conversely, AMI is uncommon in individuals under 40. While the exact incidence in this age group is uncertain, the mortality rate for AMI in younger patients is between 45% and 74.8% and age < 60 is linked to better survival rates than in older age groups[9]. Early diagnosis is often challenging, but if the condition goes unrecognised, it can lead to fatal outcomes, even in younger patients. Here we present our experience in the diagnosis and treatment of a 27-year-old patient with sub-mesenteric vein embolism, aiming to encourage clinicians to consider acute mesenteric ischaemia when assessing unexplained abdominal pain in young patients.
CASE PRESENTATION
Chief complaints
Persistent abdominal discomfort for 3 d along with nausea and vomiting lasting 5 h.
History of present illness
On February 29, 2024, a 27-year-old man presented to the emergency department of our hospital with persistent abdominal discomfort for 3 d along with nausea and vomiting lasting 5 h prior to admission.
History of past illness
The patient’s overall health status was good and he had no prior surgical interventions or traumatic events.
Personal and family history
The patient denied any family history of genetic disorders.
Physical examination
The initial assessment revealed stable vital signs but localised tenderness in various abdominal regions (upper, periumbilical, and lower right abdomen), without evidence of rebound tenderness or muscular rigidity. Left kidney percussion elicited discomfort while the bowel sounds were diminished, without any other notable abnormalities.
Laboratory examinations
Subsequent laboratory investigations revealed an elevated white blood cell (WBC) count (11.39 × 109/L) and an increased C-reactive protein (CRP) level (10.31 mg/L). The urinalysis findings included urinary protein (1+), urocholinogen (1+), urobilirubin (2+), and specific gravity > 1.030, as well as an elevated WBC count (11/µL), without detectable red blood cells. Amylase and lipase levels were within the normal range. These findings effectively excluded acute pancreatitis but suggested a urinary tract infection rather than the presence of calculi. Nevertheless, the patient complained of intense abdominal pain. He was administered phloroglucinol and scopolamine, which provided temporary spasm relief but the pain persisted.
Imaging examinations
Subsequent abdominal computed tomography (CT) scanning revealed increased density in the portal and sub-mesenteric veins as well as the presence of a fecalith in the appendix (without appendicitis). Enhanced CT scans were then performed, which ruled out intestinal obstruction and appendicitis. The patient was informed about his condition and received dezocine for pain relief due to persistent abdominal pain. He underwent portal vein ultrasound (Figure 1), mesenteric CT angiography (CTA), and a whole-abdominal enhanced CT scan in the emergency department. Multiple emboli in the main portal vein as well as in the left and right branches of the portal vein, proximal SMV, and proximal splenic vein were detected. A slight decrease in the density of the sub-mesenteric vein lumen was observed, potentially attributable to an embolus. Weak enhancement in the hepatic gallbladder bed during the arterial phase and local perfusion abnormalities were also noted.
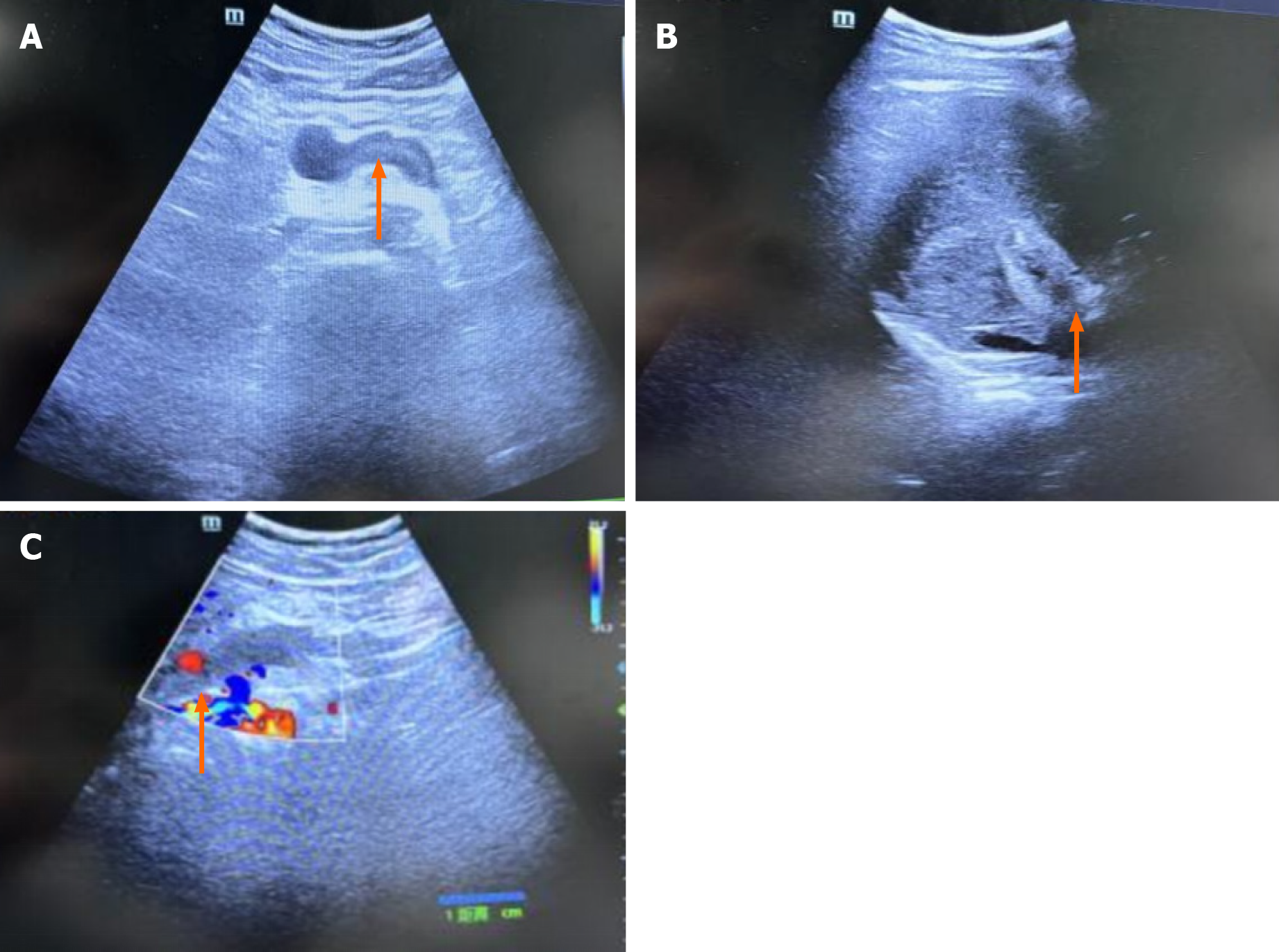
Figure 1 Ultrasonography of the abdominal and portal veins.
A: The portal vein lumen displays hypoechoic filling with a solid echo (orange arrows), indicating thrombosis; B: Thrombosis in the lumen of the posterior pancreatic spleen vein (orange arrows) was suspected; C: The absence of a blood flow signal in the portal vein (orange arrows) suggests thrombosis.
TREATMENT
Anticoagulation treatment with 5000 IU of low molecular weight heparin was administered subcutaneously every 12 h. Emergency mesenteric venography and catheter-directed thrombolysis were performed to prevent paralytic ileus and intestinal ischaemic necrosis (Figure 2). The treatment regimen included continuous infusion of 100000 IU of urokinase along with 50 mL of 0.9% sodium chloride at a rate of 20000 IU/h. The patient underwent additional testing, including routine nasopharyngeal polymerase chain reaction (PCR) screening, which yielded a positive result for coronavirus disease 2019 (COVID-19). The results of additional laboratory investigations for protein S, protein C, antithrombin III, coagulation factor II, coagulation factor V, histidine-rich glycoprotein, thrombomodulin, and anti-cardiolipin antibodies were negative. Genetic testing revealed a heterozygous mutation in the upstream 2-kb region of plasminogen activator inhibitor-1, potentially linked to low-dose heparin usage. A homozygous missense mutation in methylenetetrahydrofolate reductase, suggesting an increased genetic predisposition to hyperhomocysteinaemia and an elevated thrombotic risk, was identified as well. Subsequently, the patient underwent mechanical thrombectomy on March 5, 2024 (Figure 3), during which 20 mL of thrombus was removed; he was then administered 15 mg of rivaroxaban twice daily.
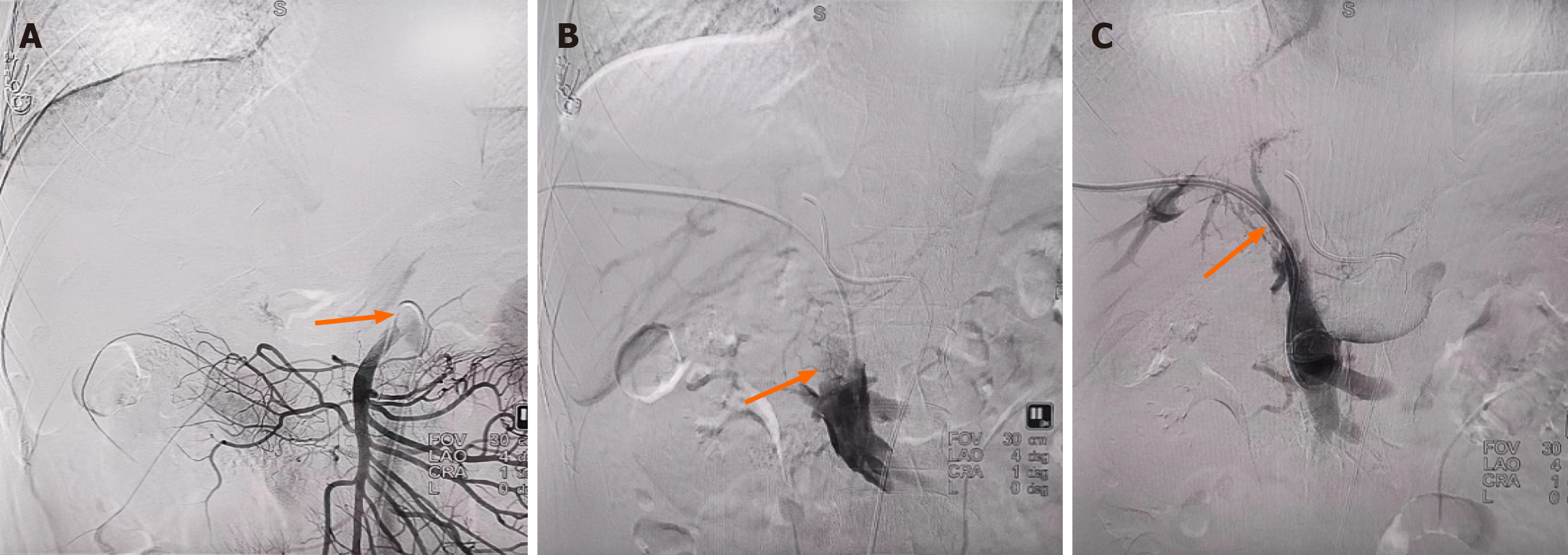
Figure 2 Indirect and direct portal venography conducted on February 29, 2024.
A: Indirect portal venography showed an underdeveloped portal vein (orange arrows); B: Direct portal vein angiography showed significant filling defects (orange arrows), indicating thrombosis; C: Partial visualisation of the portal vein (orange arrows) during intraoperative angiography after thrombolysis via an indwelling biliary drainage tube.
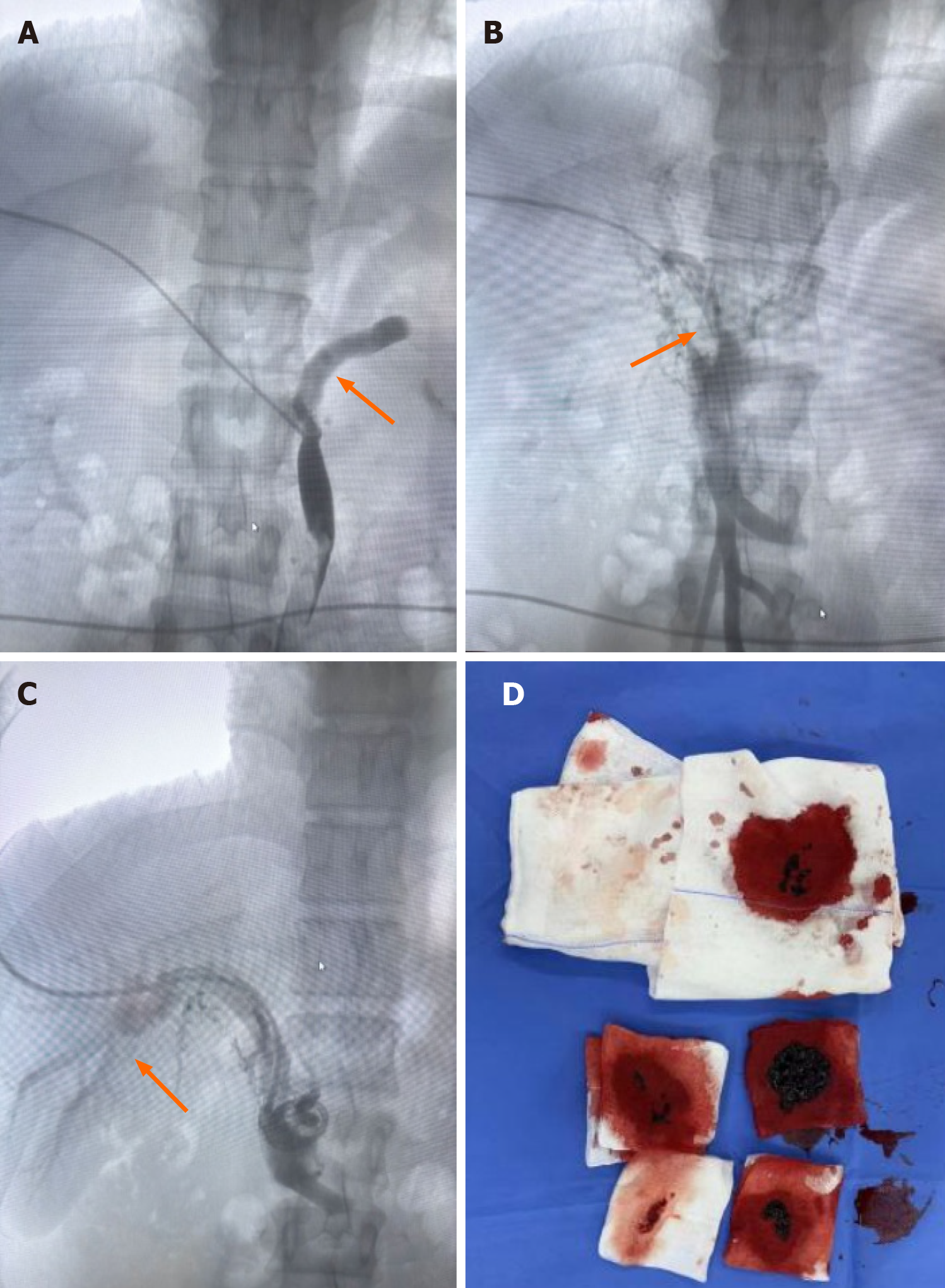
Figure 3 Portal venography and mechanical thrombectomy performed on March 5, 2024.
A: Portal vein angiography showed persistent filling defects in the inferior mesenteric and splenic veins (orange arrows), suggesting thrombosis; B: Filling defects in the superior mesenteric vein (orange arrows), indicating thrombosis; C: Successful visualisation of the left and right branches of the portal vein (orange arrows) after mechanical thrombectomy; D: Approximately 20 mL of thrombus was aspirated following mechanical thrombectomy.
On March 7, 2024, mesenteric artery and vein venography with catheter-directed thrombolysis was performed again (Figure 4). Further treatment consisted of a continuous infusion of 200000 IU of urokinase and 50 mL of 0.9% sodium chloride at a rate of 20000 IU/h, in conjunction with 20 mg rivaroxaban taken once daily.
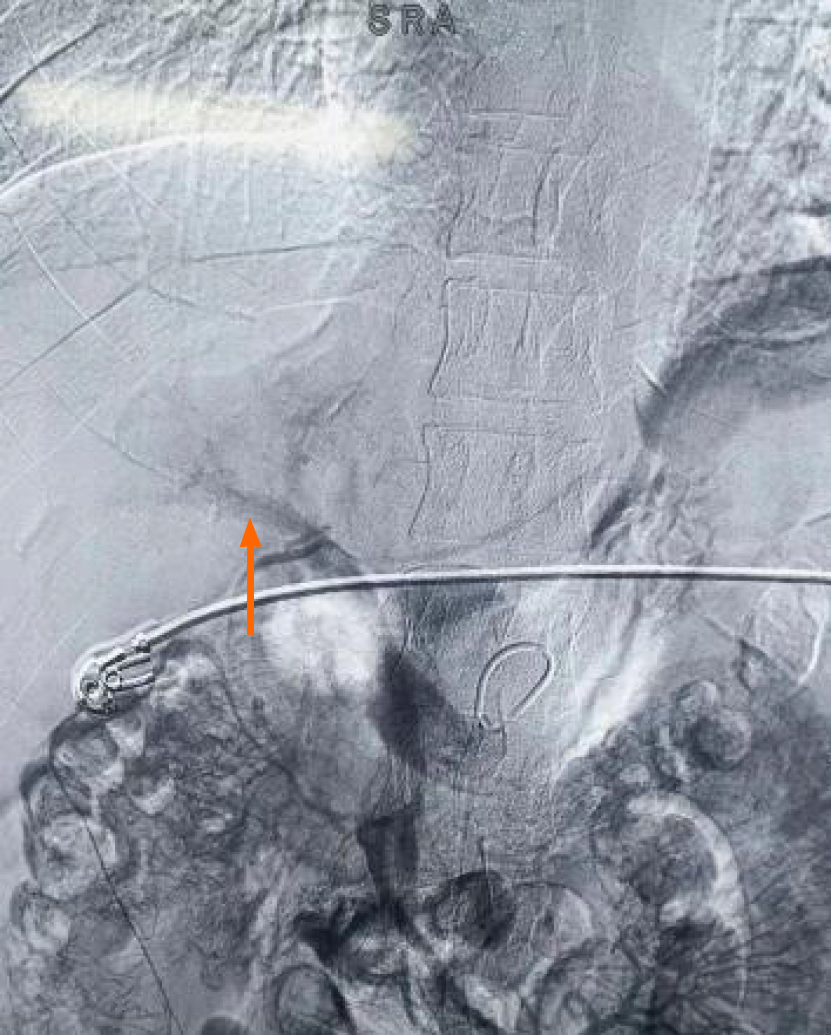
Figure 4 Indirect portal vein angiography after thrombolysis on March 7, 2024.
Indirect portal vein angiography after repeated thrombolysis showed the development of the portal vein.
OUTCOME AND FOLLOW-UP
After 10 d of treatment, the patient's abdominal pain had significantly improved and indicators of infection had decreased notably. Repeat angiography demonstrated that blood flow in the portal vein had largely returned to normal. Due to the early diagnosis and prompt intervention, no complications, such as intestinal obstruction and bowel necrosis or shock, developed during hospitalisation. The patient was discharged on March 9, 2024. The 2-mo follow-up included an enhanced abdominal CT scan, which showed that the initial splenic vein thrombosis was no longer visible, the slightly high-density shadow in the gallbladder was absent, and there was a significant reduction in thrombosis in both the left and right branches of the portal vein and some of its tributaries. The presence of multiple enlarged, tortuous vascular shadows around the main trunk and branches of the portal vein suggested the development of collateral circulation (Figure 5).
Figure 5 Portal venography before and after treatment.
A: Computed tomography angiography (CTA) performed on February 29, 2024, revealed an underdeveloped portal vein; B: Repeat CTA of the portal vein confirmed its development, and revealed that the portal vein was visible and collateral circulation has formed.
DISCUSSION
Causes of abdominal pain
There are two main types of mesenteric ischaemia: Non-occlusive and occlusive. The latter is subdivided into acute mesenteric artery embolism, acute mesenteric arterial thrombosis, and MVT. Mesenteric ischaemia is classified as acute or chronic based on disease severity. In 2017, the European Society for Vascular Surgery (ESVS)[10] defined AMI as a sudden cessation of blood flow to the small intestine, colon, or both, accompanied by symptoms that may last a few minutes (due to embolism) or a few hours (due to atherosclerosis). The European Guidelines for Chronic Mesenteric Ischemia, developed with research collaboration from seven professional societies, describe the symptoms of chronic mesenteric ischaemia as recurrent abdominal pain that intensifies after a meal, typically beginning 10-30 min after eating and lasting 1-2 h[11]. After a meal, the demand for oxygen to carry out digestion increases markedly, resulting in a rise in mesenteric blood flow of 30%-150%. MVT due to mechanical obstruction accounts for 5%-10% of ischaemia cases, while arterial ischaemia is primarily the result of obstruction (60%-70%) or low blood flow (20%-30%)[12]. A previous study[13] showed that patients with SMA embolism are at increased risk of developing an embolus in other solid organs, such as the spleen, liver, kidneys, and even brain. Chronic obstruction of the SMA is an uncommon cause of acute abdomen, and its diagnosis is often complicated by subtle symptoms, such that it may take several days for the condition to manifest clearly. However, the delayed onset of precursor symptoms and AMI are linked to survival outcomes, described as an ‘acute-chronic’ syndrome[14].
Given the potentially gradual progression of intestinal damage, diagnosing and treating AMI can present significant challenges, due to its diverse clinical presentations and non-specific initial symptoms, particularly in patients with arterial blockage. However, the incidence of AMI is low, although it increases exponentially with age and is similar for both men and women[15]. In older adults, AMI occurs more frequently than ruptured abdominal aortic aneurysm, appendicitis, and other causes of acute abdominal pain. Our patient presented with recurrent abdominal pain for a 3-d duration. Considering his medical history and current auxiliary examinations, his abdominal pain was likely to be due to obstructive mesenteric ischaemia caused by MVT, with clinical manifestations resembling an ‘acute-chronic’ syndrome (duration of symptoms lasting up to 3 d). Abdominal pain caused by MVT accounts for only a small fraction of ischaemia cases and is extremely rare, particularly in the younger population. A primary cause of mesenteric ischaemia in young adults, besides susceptibility due to hypercoagulable states or underlying vasculitis, is cocaine abuse. Heavy smoking may also increase the risk of mesenteric arterial occlusive disease and mesenteric ischaemia in young patients[9].
Diagnosis of the cause of abdominal pain (MVT)
Early enhanced CT scans are critical in patients with suspected acute mesenteric ischaemia, to determine treatment strategies. Dynamic enhanced CT, with a sensitivity of 90%, is the preferred diagnostic method for suspected cases of MVT. CT is more sensitive in detecting venous thrombosis than other forms of acute mesenteric ischaemia, and can specifically reveal venous filling defects[16]. Case reports suggest that, in the diagnosis of AMI, repeated echocardiographic assessments of the presence of the ‘aquarium sign’, caused by the entry of hepatic portal venous gas into the right atrium and ventricle, can be valuable. Point-of-care ultrasound may also be a useful diagnostic tool for AMI in patients in whom there is active suspicion of AMI based on detection of the aquarium sign[17]. At present, no individual test or combination of tests has demonstrated consistently reliable sensitivity or specificity for diagnosing AMI[4]. Leukocyte count, serum amylase, lactate dehydrogenase, D-dimer, and lactate levels may be informative in the differential diagnosis of AMI. However, since CRP and lactate levels may be normal in the early stages of AMI, normal values of these indicators do not rule out the diagnosis. About half of the patients with AMI have elevated levels of troponin T, and D-dimer levels may increase during SMA embolism[18]. The ESVS guidelines recommend D-dimer as an exclusion test for AMI but stress its ineffectiveness in cases of acute to chronic mesenteric ischaemia[15]. In our patient, CRP and WBC levels were elevated, while troponin T, D-dimer, and lactate levels remained normal. Abdominal CT indicated increased density in the portal and inferior mesenteric veins, leading to a consideration of embolism. The diagnosis was confirmed using colour Doppler ultrasound and enhanced CT.
Factors contributing to MVT susceptibility
The patient had no personal or family history of thromboembolic events. After admission, diagnostic tests were performed to determine the potential causes of MVT, including acquired (e.g., tumours) or hereditary (e.g. genetic mutations or deficiencies) thrombophilia. His laboratory tests yielded negative results for protein S, protein C, antithrombin III, coagulation factor II, coagulation factor V, histidine-rich glycoprotein, thrombomodulin, and anti-cardiolipin antibodies. Genetic testing identified a homozygous missense mutation in methylenetetrahydrofolate reductase, indicating an increased genetic predisposition to hyperhomocysteinaemia and a heightened thrombotic risk. Additionally, routine nasopharyngeal PCR screening yielded a positive result for COVID-19. Interactions between hereditary thrombophilia factors and severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection may thus have played a role in triggering MVT. Viral-infection-induced thrombosis can be attributed to inflammation triggered by the infection, leading to platelet activation and clot formation as platelets adhere to damaged cells and activate clotting factors[19]. The development of MVT is associated with a broad range of factors[17], including hypercoagulable states (deficiencies in protein C, protein S, or antithrombin III, factor V Leiden mutation), anticardiolipin antibodies, malignancy, oestrogen influences, pregnancy, peripheral deep vein thrombosis, and conditions such as portal hypertension, intra-abdominal inflammation, sepsis, postoperative status, and trauma. Prothrombin mutation, protein C deficiency, protein S deficiency, antithrombin deficiency, impaired fibrinolysis (reduced levels of anti-tissue plasminogen activator), hyperviscosity syndrome, pancreatitis, various blood disorders, and the use of oral contraceptives can lead to secondary intestinal oedema due to venous thrombosis and increased vascular resistance; the resulting reduction in arterial blood flow leads to intestinal ischaemia[10]. Antithrombin-III deficiency is a significant factor in ‘primary’ MVT[20,21]. In individuals with inherited antithrombin-III deficiency, the activity of this protein usually decreases by around 50%, despite normal results in standard coagulation or fibrinolysis tests. The higher risk of venous thrombosis often manifests in adulthood.
COVID-19 not only affects the respiratory system but also induces thrombotic ischaemia, including in the gastrointestinal tract. Emerging evidence[22] links SARS-CoV-2 with endothelins, which can cause endothelial damage, inflammatory cell infiltration, and a systemic prothrombotic state triggered by excessive inflammation and elevated cytokine levels. COVID-19 is also associated with an elevated risk of diverse thromboembolic complications[23-25], including AMI, deep vein thrombosis, and pulmonary embolism. A hypercoagulable state may be induced by systemic inflammation, endothelial activation, and hypoxia. The virus's affinity for vascular endothelial cells can be attributed to their expression of ACE2, the receptor that facilitates viral entry. The resulting endothelial injury triggers the release of substantial amounts of von Willebrand factor, further increasing the risk of thrombus formation, which in turn can induce hemodynamic changes and direct injury to intestinal cells, ultimately resulting in ischaemia and necrosis of the small intestine. However, the pathogenesis of COVID-19-associated AMI has not been extensively studied, although it may involve direct viral mucosal damage, intestinal ischaemia, and the atrophy of lymphoid follicles accompanied by increased mucosal permeability[26].
MVT treatment
Non-randomised trials of patients with acute thrombotic occlusion have demonstrated[27-31] a reduced need for bowel resections and lower short- and long-term mortality with endovascular interventions rather than open surgery. The ESVS guidelines[19] state that the 30-d mortality rate after endovascular intervention is 17.2% (367/2131), while for open surgery it is 38.5%. A recent meta-analysis[32] determined a 94% technical success rate for endovascular intervention, with a 40% re-operation rate post-treatment. Various endovascular procedures for the management of portal vein thrombosis have been developed[33,34], such as transjugular intrahepatic portosystemic shunt combined with mechanical thrombectomy[35] and direct thrombolysis[36,37], percutaneous transhepatic mechanical thrombectomy[38], percutaneous transhepatic thrombolysis[39,40], SMA thrombolysis[41], and percutaneous SMV catheter placement for thrombolysis[42]. A study by Kärkkäinen et al[15] found that the mortality rate from acute mesenteric ischaemia can reach 80% without prompt surgery to restore blood flow, but early intervention can reduce mortality by 50%. In rare cases, partial intestinal resection with anticoagulant therapy may be the most effective. In unavoidable laparotomy situations, open thrombectomy, surgical bypass, or hybrid stent placement can quickly restore intestinal blood flow. Medical treatment includes the use of antibiotics, vasodilators, thrombolytic agents, and anticoagulants. Experimental drugs for primary ischaemia and reperfusion injury are also under investigation. Anticoagulation is the primary treatment for MVT, and the early administration of heparin can increase survival[30]. However, heparin cannot be the sole treatment for acute thrombosis in patients with antithrombin III deficiency, as it can lead to varying degrees of heparin resistance, potentially worsening the thrombotic condition, due to heparin's diverse effects[24].