Published online Oct 28, 2024. doi: 10.4329/wjr.v16.i10.552
Revised: September 8, 2024
Accepted: September 13, 2024
Published online: October 28, 2024
Processing time: 185 Days and 4.3 Hours
High complex anal fistulas are epithelialized tunnels, with the main fistula piercing above the deep external sphincter and the internal opening approaching the dentate line. Conventional surgical procedures for high complex anal fistulas remove most of the external sphincter and damage the anorectal ring. Postope
A patient with a high complex anal fistula located above the anorectal ring underwent modified TROPIS, which involved sepsis drainage and identification of the internal opening in the intersphincteric space. The patient with the high complex anal fistula recovered well postoperatively, without any postoperative complications or anal dysfunction. Anal function returned to normal after 17 months of follow-up.
The modified TROPIS procedure is the most minimally invasive surgery for anal fistulas that minimally impairs anal function. It allows the complete removal of infected anal glands and reduces the risk of postoperative complications. Modified TROPIS via the intersphincteric approach is an alternative sphincter-preserving treatment for high complex anal fistulas.
Core Tip: High complex anal fistulas are a type of perianal infectious diseases barely to spontaneously heal. Surgical procedures usually impair the anal function. Trans-anal opening of inter-sphincteric space (TROPIS) is an effective surgical procedure to treat high anal fistulas, although there exist demerits. We created a modified TROPIS via polishing up the surgical approach, identification of internal opening and management of fistulas, which greatly simplified the surgical procedures, and largely reduced postoperative complications. We believed that the modified TROPIS is suitable for treating high complex anal fistulas in Asian people with a narrower anal canal.
- Citation: Wang YQ, Wang Y, Jia XF, Yan QJ, Zheng XP. High complex anal fistula managed by the modified transanal opening of the intersphincteric space via the inter-sphincteric approach: A case report. World J Radiol 2024; 16(10): 552-560
- URL: https://www.wjgnet.com/1949-8470/full/v16/i10/552.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i10.552
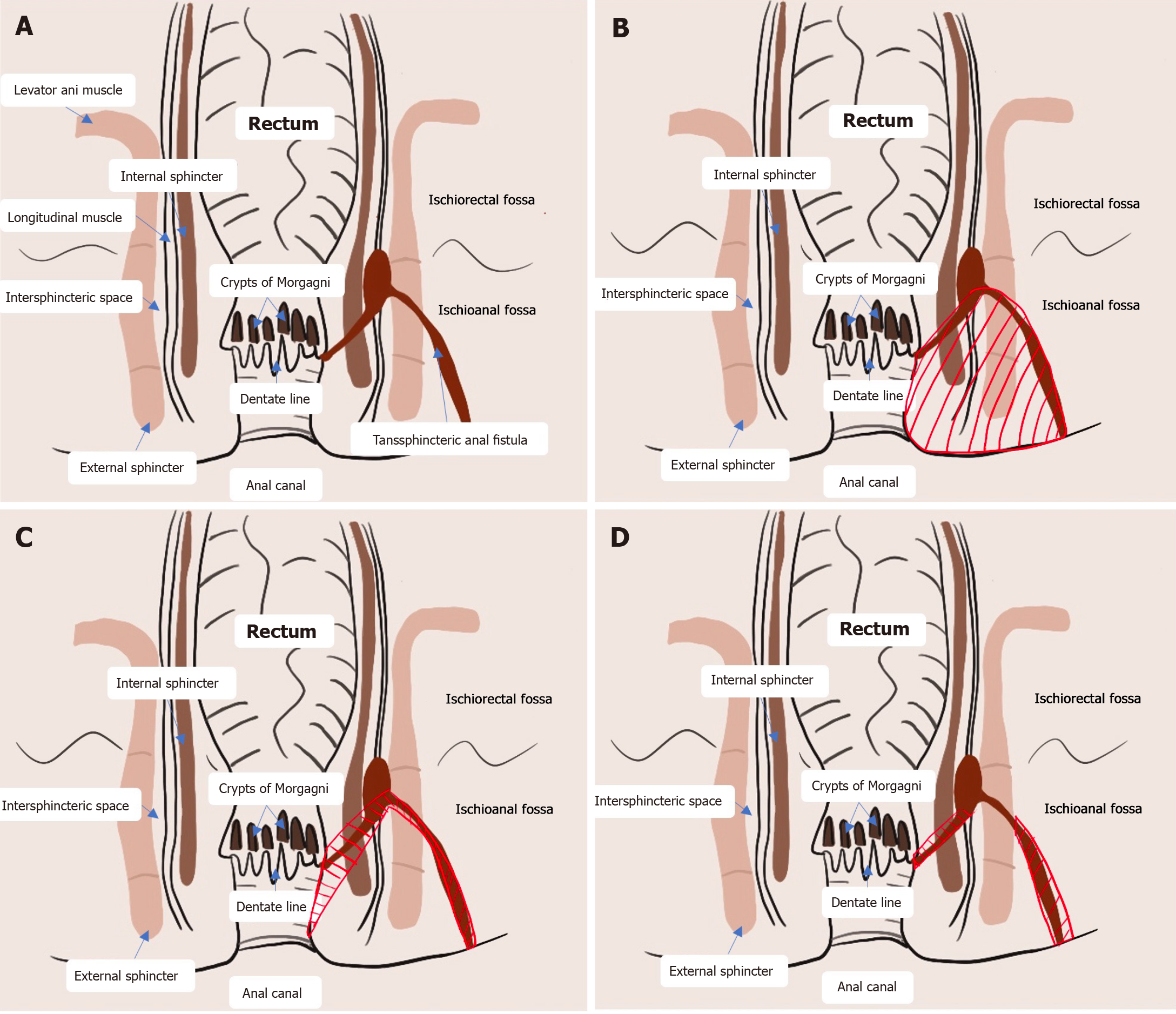
Anal fistulas are common manifestations of perianal infections that rarely heal on their own[1]. Surgical procedures are the only reliable treatment for anal fistulas[2]. High complex anal fistulas are typically complicated by their high anatomic location, the presence of branching fistulas with intricate fistula distributions and the risk of carcinogenesis[3]. Conventional surgical techniques are challenging, potentially leading to concerns of postoperative relapse and damage to anal function[4]. Fistulectomy is the conventional surgical option for anal fistulas[5] (Figure 1). Although it is recognized for its definite efficacy, fistulectomy results in large wounds, causes intense pain and is associated with long recovery times[6,7]. High anal fistulas are complicated in that the resection of a large amount of tissues and muscles is required, which increases the risks of postoperative anal dysfunction and incontinence[8]. Multiple sphincter-preserving methods, including fistula flap surgery, fibrin glue injection, bioprosthetic plug use, ligation of the intersphincteric fistula tract and stem cell therapy, are available. Nevertheless, all these methods have drawbacks. The transanal opening of the intersphincteric space (TROPIS) was initially described by Garg[9] in 2017. It is an effective surgical procedure for the treatment of high anal fistulas, complex fistulas and horseshoe fistulas. There is increasing evidence advocating for TROPIS for anal fistulas owing to its satisfactory surgical outcomes, and the cure rate is particularly high, up to 90.4%[9]. Owing to the small wound area, patients with anal fistulas who undergo TROPIS can be discharged within 24 hours after surgery and return to their normal daily life and working shortly thereafter. Limitations of TROPIS, however, have been observed during clinical application. First, the indications for TROPIS for anal fistula are very strict. Second, the identification of the internal opening from the anus increases the difficulty of full exposure of the surgical field. Third, the relatively large incision in the rectum causes considerable bleeding. Limitations of TROPIS, however, have been observed during clinical application. We modified the TROPIS procedure by refining the surgical approach, identifying the internal opening and managing the fistulas. We performed an innovative sphincter-preserving surgical procedure involving the widening of the intersphincteric plane and therefore successfully eradicated a high complex anal fistula (Figure 1).
A 53-year-old middle-aged male was admitted in July 2022 for a 3-year history of intermittent swelling and pain of a buttock mass.
The patient presented with a swollen, painful lump on the right buttock (1 cm × 1 cm) that was found after a long ride 5 years ago. The pain subsided after 2 days of rest. However, the swelling and pain recurred and were sometimes relieved by self-medicating with cefixime. Owing to worsened swelling and pain after a long ride 3 years ago, the patient sought medical help at a local hospital and was subsequently diagnosed with a perianal abscess. The pain was relieved by topical drugs but recurred every 2 months. The abscess began to spread upwards. Six months after topical drug treatment, the symptoms of the perianal abscess began to recur more frequently, approximately 2-3 times per month, and were not relieved by conservative treatment.
The patient used to have good physical condition, and denied chronic diseases like hypertension, diabetes mellitus and coronary heart disease. Surgical history was deniable.
The patient and his family denied any history of infectious and hereditary diseases, smoking, alcohol consumption, major trauma and blood transfusion, or food or drug allergy.
The patient was admitted for physical examination at the anus. The appearance of the anus was still flat. The sacrococcygeal region on the right side was 4 cm × 6 cm away from the anal truncus and a 3 cm × 3 cm hard mass was palpable, with weakly positive tenderness, normal skin temperature, and no obvious fluctuating sensation. Finger-pointing of the anus identified an anal verge at the 6 o’clock, 5 cm vertically away from the anus, located in the depression of the hard inner mouth. The right posterior rectal ring was hard without blood staining in the retracted finger.
No directly relevant laboratory tests for reference.
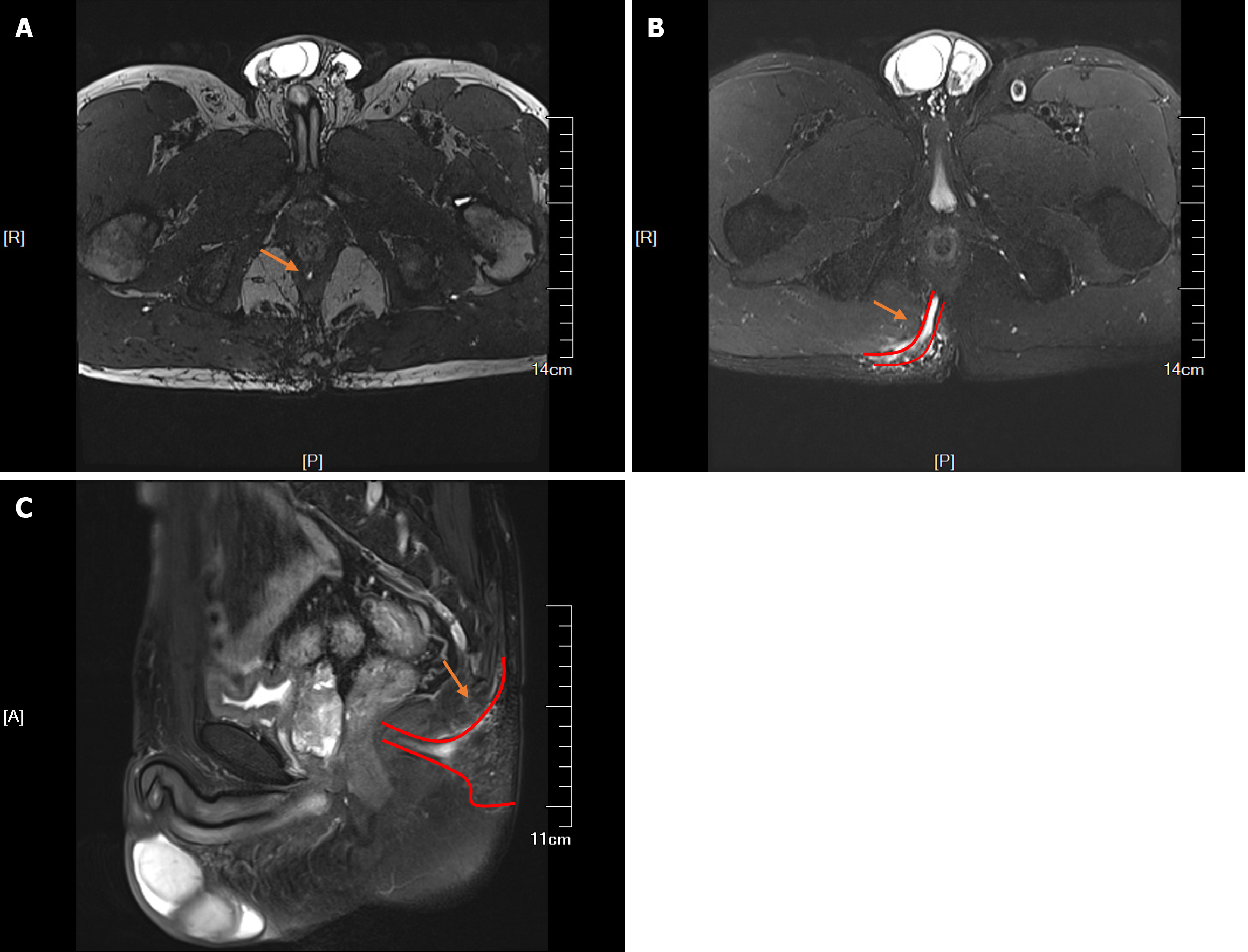
Ultrasonography of the perianal area using a high-frequency probe revealed a subcutaneous hypoechoic area on the right side of the sacrococcygeal area extending to the posterior anus, suggesting the presence of a high complex anal fistula. Hypoechogenicity was observed in the lateral sphincter at the six o'clock position of the anorectal ring, extending deep into the plane between the internal and external sphincter above the six o'clock position of the anorectal ring and spreading to the seven o'clock position on the right and five o'clock position on the left. Pelvic magnetic resonance imaging (MRI) revealed the following: (1) Strip-like high-signal shadows on T2-weighted images at the five o'clock position of the perianal area in perianal lithotomy that inferoposteriorly extended into the right ischiorectal fossa, where two branching fistulas descended posteriorly on the right side; (2) An external opening situated subcutaneously in the right buttock; (3) Flocculent and nodular shadows near the right ischiorectal fossa and a small number of flocculent shadows in the right gluteus maximus on contrast-enhanced T2WI scans with fat suppression; (4) A poorly filled bladder with thick walls; (5) A slightly larger prostate with uneven signals in the central area and multiple nodular hyperplasia and flaky cystic changes; no obvious abnormalities in the bilateral seminal vesicles; and (6) Long shadows (11 mm × 16 mm) in the sacral canal at the level of the second and third sacral bones (S2-S3). MRI revealed a high complex anal fistula with local abscesses, slight oedema in the right gluteus maximus, benign prostatic hyperplasia and cystic lesions in the S2-S3 sacral canals (Figure 2).
Combined with the clinical symptoms, signs and imaging findings, the final diagnosis was high complex anal fistulae.
Considering individual patient factors, location of the high complex anal fistula and extensive spread of local infections, conventional fistulectomy may cause massive damage to the anal sphincter and perianal tissue. Moreover, daily life and work would be greatly influenced by the large wound and long recovery time. We therefore preserved the sphincter by modifying the TROPIS procedure in the present case.
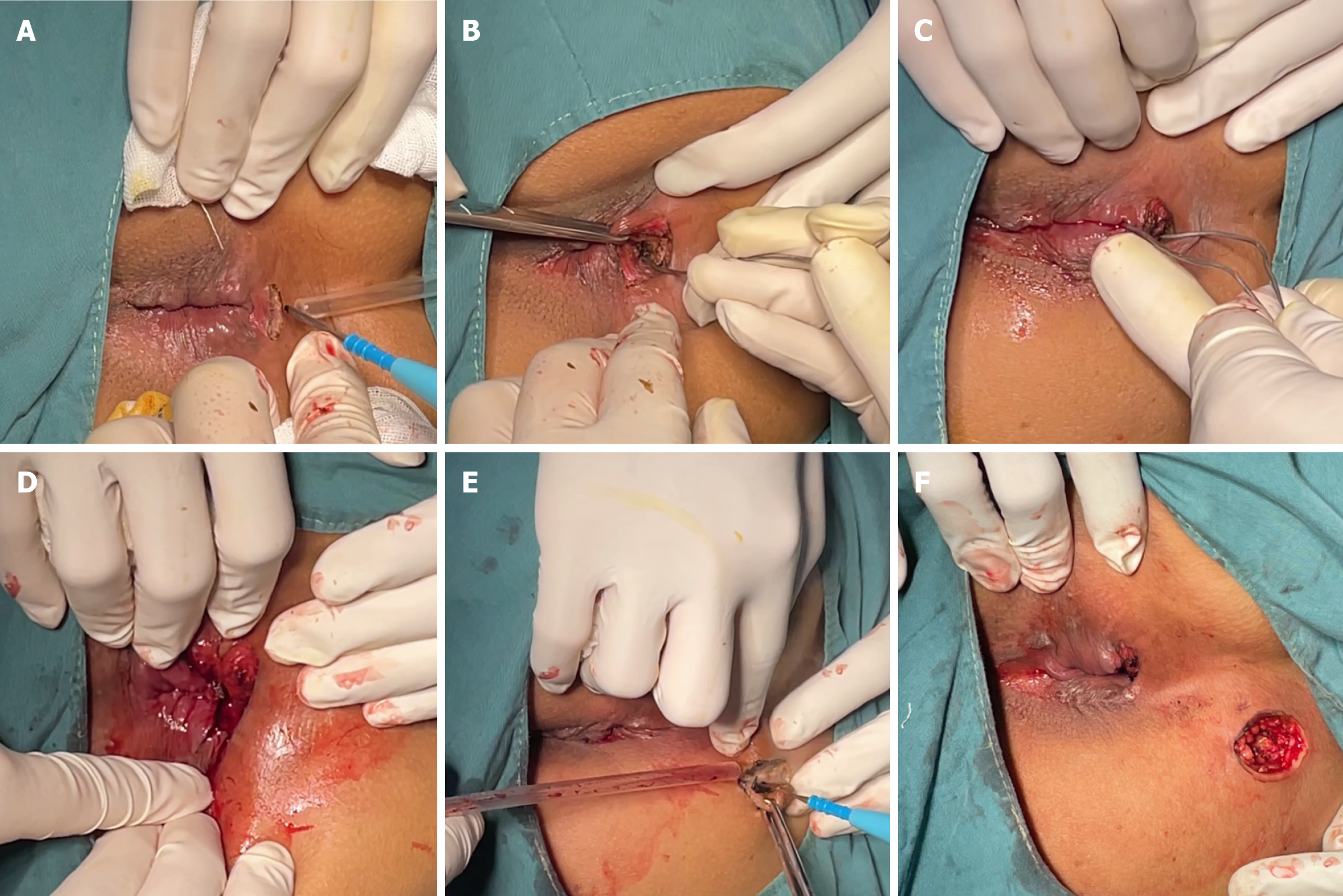
Intraspinal anesthesia was induced with the patient in the right recumbent position. An arc-shaped incision that was 1.5 cm long was made at the intersphincteric groove (Hilton white line), corresponding to the internal opening at the six o'clock position. After the sphincter space was opened superiorly, the fistula was explored superiorly until reaching the anorectal ring at the six o'clock position. With a 10-cm distance from the anal margin, the sphincter space was separated from the top of the fistula. The fistula probe was inserted through the space lying between the internal and external sphincters and the internal opening was identified 4 cm above the dentate line at the six o’clock position. The mucosa on both sides of the incision was ligated and trimmed to allow the drainage of pus. In addition, a circular incision was made on the lump (external opening) in the right-sided sacrococcygeal region, 7 cm away from the anal margin at the seven o'clock position. Fistula walls and necrotic tissues were exposed after the skin and subcutaneous tissues were cut open. Tunnel-like fistulectomy of the fistulas along the lateral wall of the external sphincter was performed and the necrotic tissues were removed. The edges of the incision were trimmed, and Vaseline gauze was used to fill the incision (Figure 3).
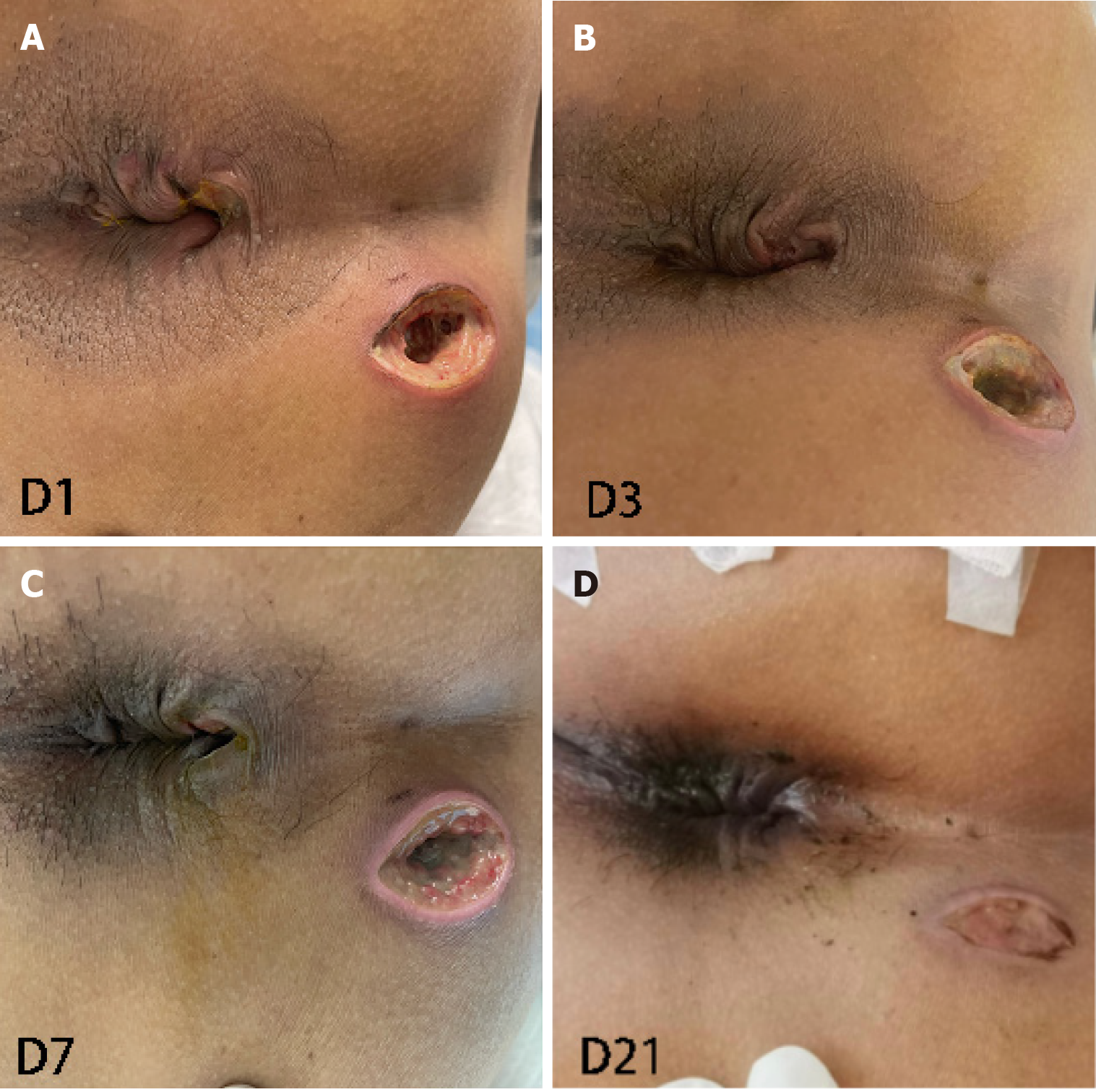
Postoperative vital signs were stable. Urinary retention was not observed. The patient could urinate at 6 hours postoperatively and perform off-bed activities 24 hours postoperatively (Figure 4). The postoperative visual analogue scale score was 2 points, indicating a minimal pain.
The wound had completely healed at the 2-month postoperative examination (Figure 5). Anal discharge and fecal incontinence were not reported. The digital rectal exam was normal, and no blood was identified after withdrawing the finger used for examination. Anal stenosis, abnormal masses or cords or abnormalities in the lower rectum were not observed.
Anal sphincter pressure was measured using water-perfused anorectal manometry at the 17-month postoperative examination. The resting pressure, squeeze pressure, functional anal canal length and other indices were all within the normal ranges (Table 1). Postoperative anal function returned to normal, indicating the safety of the modified TROPIS procedure in the treatment of high complex anal fistulas.
| Measurements | Values | References (in adults) |
| Anal resting pressure (mmHg) | 51 | 50-70 |
| Anal squeeze pressure (mmHg) | 143 | 120-170 |
| Functional anal canal length (cm) | 3.2 | 3.2-3.5 |
| Delayed defecation reflex | Reversible and pressure curve decline | Reversible and pressure curve decline |
| Rectoanal contraction reflex | + | + |
| Rectoanal inhibitory reflex | 10 mL lead out | 5-10 mL lead out |
| Anal reflex diastolic pressure (mmHg) | 35 | > 30 |
| The first rectal sensation (mL) | 20 | 10-30 |
| Volume to get an urge to defecate (mL) | 100 | 50-80 |
| Maximal tolerated volume (mL) | 170 | 110-280 |
| Rectoanal pressure during defecation (mmHg) | 83 | > 45 |
Minimally invasive surgical treatment of anal fistulas has become the focus of research. Sphincter-preserving surgeries performed to date protect anal function to some extent but are infrequently performed due to their association with high recurrence rates, high medical expenses and challenging surgical procedure.
Garg[10] proposed that previous surgical procedures for anal fistulas aimed to mainly repair the primary internal opening and remove the fistula and did not focus on the eradication of the intersphincteric infection (Figure 1A). The TROPIS procedure proposed by Garg et al[11] is a simple surgical procedure that is especially suitable for patients with high complex anal fistulas. A large-scale retrospective study revealed that the overall cure rate of TROPIS for anal fistulas has increased to 86%. The infection in the sphincter muscles is caused by the collection of pus in a closed space. We consistently highlighted the importance of continuous drainage of pus from the perianal abscess to promote secondary healing of the anal fistula[12]. At present, infections in the anal glands are considered the main cause of anal fistulas. Originating in the anal glands, infected pus gradually spreads into the perianal space to form an intersphincteric abscess (Figure 1A). Approximately 20% of adult anal glands are tortuous, even extending outside the external sphincter[13]. Owing to their unique anatomic characteristics, anal gland infections easily result in abscesses[14]. High anal fistulas more frequently develop in the deep space posterior to the intersphincteric space than in the deep postanal space[15,16]. The intersphincteric space is a narrow airtight space enclosed by internal and external sphincters, which are tightly circular muscular tissues. Even a small amount of pus invading the intersphincteric space via the anal opening may cause high pressure, eventually leading to the infiltration of pus into the surrounding structures[17].
On the basis of our experience in the clinical management of anal fistulas via the TROPIS procedure, a series of limitations have surfaced. First, the transanal approach used in the TROPIS procedure (green arrow in Figure 1C) precluded complete exposure of the surgical field. The fistula tract in the intersphincteric space was widened, greatly influencing the efficacy of subsequent procedures. Second, the TROPIS procedure involves the widening of the intersphincteric space through the internal opening close to the dentate line; therefore, it was not recommended for anal fistula patients whose internal opening was not clearly identified. Third, a large wound made on the friable rectal mucosa with an enriched microvascular network increased the risk of bleeding. Moreover, the space for hemostasis was limited. Postoperative bleeding of the wound in anal fistula patients usually requires high-grade hemostatic techniques such as ultrasonic cutting with a hemostatic knife and Liga Sure tissue fusion. Fourth, only scraping out the fistulas was not sufficient to completely remove the necrotic and epithelialized tissues, resulting in a high risk of postoperative recurrence. Fifth, keeping the intersphincteric space open in Asian people with narrower anal canals was unfavorable[18].
The modified TROPIS procedure created by our research team has been key to circumventing the abovementioned disadvantages. We first modified the surgical approach by replacing the transanal approach with the intersphincteric approach (green arrow in Figure 1D)[9]. An arc-shaped incision was made at the intersphincteric groove below the internal opening preoperatively, as identified by pelvic MRI and/or perianal ultrasonography. The length of the incision, ranging from 1 cm to 2 cm, was determined on the basis of individual factors. Through this skin incision, the inter
Overall, three modifications were made to the TROPIS procedure to markedly enhance its efficacy and safety for high complex anal fistulas. The intersphincteric approach allowed the widening of the infection foci lying in the intersphin
The modified TROPIS procedure via the intersphincteric approach has unique merits in the surgical treatment of high complex anal fistulas. The intersphincteric approach allows the identification of the internal opening and drainage, thus simplifying the surgical procedure. Tunnel-like fistulectomy was performed from the external opening to the external sphincter, lowering the risk of postoperative anal dysfunction. A smaller wound results in less pain and faster recovery. We believe that the modified TROPIS procedure is especially suitable for the treatment of high complex anal fistulas in Asian people with narrower anal canals. On the basis of enhanced recovery after surgery and patient-centered care, the modified TROPIS procedure largely reduces the risk of postoperative complications, shortens the length of stay and enhances the utilization of health care resources. We strongly recommend the modified TROPIS procedure for the treatment of high complex anal fistulas and the preservation of the sphincter because of its low cost and focus on improved patient outcomes (Table 2).
| Fistulectomy | TROPIS | Modified TROPIS | |
| Surgical procedure | Remove the fistula, and surrounding muscles, fat and connective tissues | Identify the internal opening. Lay open the fistula tract in the intersphincteric plane through the transanal route. Resect the lower part of the internal sphincter. Scrap out the remaining branching fistulas | Widen the intersphincteric plane through the transanal route Identify the internal opening. A thorough drainage of fistulas passing through the intersphincteric plane. Tunnel-like fistulectomy of fistulas lateral to the external sphincter |
| Merits | Definite efficacy | High cure rate; small wound area; fast recovery | Easy to identify the internal opening and favor to the drainage and surgical procedure. Low risks of postoperative anal dysfunction and recurrence. Small wound area. Less pain. Fast recovery |
| Demerits | Large wound area; intensive pain; long recovery | Unable to clearly expose the surgical field. Not suitable for anal fistulas without a clearly identified internal opening. Not suitable for Asian people. High risks of bleeding and postoperative recurrence | Less popular. Lack of long-term follow-up data. Lack of a comparative group |
We appreciate the patient to voluntarily participate in this clinical study, and all the medical staff involved in the surgery and treatment of the patient.
| 1. | Bakhtawar N, Usman M. Factors Increasing the Risk of Recurrence in Fistula-in-ano. Cureus. 2019;11:e4200. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 2. | Hall JF, Bordeianou L, Hyman N, Read T, Bartus C, Schoetz D, Marcello PW. Outcomes after operations for anal fistula: results of a prospective, multicenter, regional study. Dis Colon Rectum. 2014;57:1304-1308. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 3. | Koizumi M, Matsuda A, Yamada T, Morimoto K, Kubota I, Kubota Y, Tamura S, Tominaga K, Sakatani T, Yoshida H. A case report of anal fistula-associated mucinous adenocarcinoma developing 3 years after treatment of perianal abscess. Surg Case Rep. 2023;9:159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 4. | García-Botello S, Garcés-Albir M, Espi-Macías A, Moro-Valdezate D, Pla-Martí V, Martín-Arevalo J, Ortega-Serrano J. Sphincter damage during fistulotomy for perianal fistulae and its relationship with faecal incontinence. Langenbecks Arch Surg. 2021;406:2497-2505. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 5. | Ratto C, Grossi U, Litta F, Di Tanna GL, Parello A, De Simone V, Tozer P, DE Zimmerman D, Maeda Y. Contemporary surgical practice in the management of anal fistula: results from an international survey. Tech Coloproctol. 2019;23:729-741. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 43] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 6. | Limura E, Giordano P. Modern management of anal fistula. World J Gastroenterol. 2015;21:12-20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 114] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (2)] |
| 7. | Cadeddu F, Salis F, Lisi G, Ciangola I, Milito G. Complex anal fistula remains a challenge for colorectal surgeon. Int J Colorectal Dis. 2015;30:595-603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 37] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 8. | Garg P, Sodhi SS, Garg N. Management of Complex Cryptoglandular Anal Fistula: Challenges and Solutions. Clin Exp Gastroenterol. 2020;13:555-567. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 9. | Garg P. Transanal opening of intersphincteric space (TROPIS) - A new procedure to treat high complex anal fistula. Int J Surg. 2017;40:130-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 80] [Cited by in RCA: 73] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 10. | Garg P. A new understanding of the principles in the management of complex anal fistula. Med Hypotheses. 2019;132:109329. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Garg P, Kaur B, Goyal A, Yagnik VD, Dawka S, Menon GR. Lessons learned from an audit of 1250 anal fistula patients operated at a single center: A retrospective review. World J Gastrointest Surg. 2021;13:340-354. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 39] [Cited by in RCA: 34] [Article Influence: 6.8] [Reference Citation Analysis (3)] |
| 12. | Sugrue J, Nordenstam J, Abcarian H, Bartholomew A, Schwartz JL, Mellgren A, Tozer PJ. Pathogenesis and persistence of cryptoglandular anal fistula: a systematic review. Tech Coloproctol. 2017;21:425-432. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 68] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 13. | Seow-Choen F, Ho JM. Histoanatomy of anal glands. Dis Colon Rectum. 1994;37:1215-1218. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 32] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Srivastava KN, Agarwal A. A complex fistula-in-ano presenting as a soft tissue tumor. Int J Surg Case Rep. 2014;5:298-301. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Reference Citation Analysis (0)] |
| 15. | Kurihara H, Kanai T, Ishikawa T, Ozawa K, Kanatake Y, Kanai S, Hashiguchi Y. A new concept for the surgical anatomy of posterior deep complex fistulas: the posterior deep space and the septum of the ischiorectal fossa. Dis Colon Rectum. 2006;49:S37-S44. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 30] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Zhang H, Zhou ZY, Hu B, Liu DC, Peng H, Xie SK, Su D, Ren DL. Clinical Significance of 2 Deep Posterior Perianal Spaces to Complex Cryptoglandular Fistulas. Dis Colon Rectum. 2016;59:766-774. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 33] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 17. | Rojanasakul A, Pattanaarun J, Sahakitrungruang C, Tantiphlachiva K. Total anal sphincter saving technique for fistula-in-ano; the ligation of intersphincteric fistula tract. J Med Assoc Thai. 2007;90:581-586. [PubMed] |
| 18. | Nelson RL, Dollear T, Freels S, Persky V. The relation of age, race, and gender to the subsite location of colorectal carcinoma. Cancer. 1997;80:193-197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 19. | Lichtenstein GR, Loftus EV, Isaacs KL, Regueiro MD, Gerson LB, Sands BE. ACG Clinical Guideline: Management of Crohn's Disease in Adults. Am J Gastroenterol. 2018;113:481-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 612] [Cited by in RCA: 1032] [Article Influence: 129.0] [Reference Citation Analysis (0)] |
| 20. | Yamana T. Japanese Practice Guidelines for Anal Disorders II. Anal fistula. J Anus Rectum Colon. 2018;2:103-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 25] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/