Published online Oct 28, 2024. doi: 10.4329/wjr.v16.i10.537
Revised: September 11, 2024
Accepted: September 23, 2024
Published online: October 28, 2024
Processing time: 267 Days and 18.3 Hours
Digital subtraction angiography (DSA), the gold standard for the diagnosis of intracranial arteriovenous malformations (AVMs), can show clean nidus resection, leading to a perceived cure. Most cases of intracranial AVM recurrence have been reported in pediatric patients. The conventional understanding indicates that AVMs arise when abnormal blood vessels develop between the fourth and eighth weeks of embryonic development, which coincides with the typical period of blood vessel formation in the brain. As such, recurrent ectopic AVM are rare in adults.
Herein, we present the case of a 31-year-old adult with a history of an intracranial AVM originally diagnosed with a symptomatic de novo cerebellar AVM forma
The clinical course of the reported patients demonstrated the possibility of ectopic AVM recurrence in adults. The median time between the diagnosis of the initial AVM and the occurrence of ectopic recurrent AVM in adults was 11 years (range: 5–20 years). Magnetic resonance imaging follow-up for more than 10 years may be required in adult AVM-treated patients.
Core Tip: This study highlights an exceptionally rare case of ectopic recurrent intracranial arteriovenous malformation (AVM) in a 31-year-old adult, diagnosed five years following initial AVM resection. this de novo cerebellar AVM demonstrated arterial and venous configurations distinct from typical pediatric cases. Reviewing six similar adult cases, this study explored the potential causes of such rare recurrences. This experience suggests that prolonged magnetic resonance imaging follow-up beyond 10 years may be required to detect new AVMs in previously treated patients.
- Citation: Cao WY, Li JP, Guo P, Song LX. Ectopic recurrence following treatment of arteriovenous malformations in an adult: A case report and review of literature. World J Radiol 2024; 16(10): 537-544
- URL: https://www.wjgnet.com/1949-8470/full/v16/i10/537.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i10.537
Arteriovenous malformation (AVM) is a deformity defined by the direct communication between arteries and veins, without the presence of a capillary bed[1]. The conventional understanding suggests that AVMs arise when abnormal blood vessels develop between the fourth and eighth weeks of embryonic development, coinciding with the typical period of blood vessel formation in the brain[2]. However, the mechanisms underlying AVM remain unclear. AVM has traditionally been considered a congenital disorder[2–4]; however, numerous case reports have indicated that AVM may also be acquired[5,6].
Most AVMs can be successfully treated with surgical resection, Gamma Knife radiosurgery, or embolization. However, these treatments carry a risk of recurrence. Recurrence is more commonly observed at the original site in pediatric cases, whereas ectopic recurrence in adults is exceedingly rare. Hereon, we present a case of ectopic recurrence of a cranial AVM at a different anatomical site in an adult patient, which is an exceptionally rare form of recurrence.
A 31-year-old man presented with sudden onset vertigo, headache, nausea, vomiting, and urinary and fecal incontinence lasting 6 hours.
On January 31, 2018, the patient presented to our neurology outpatient clinic with acute onset of vertigo, headache, nausea, vomiting, and urinary and fecal incontinence without any apparent precipitating factors. The patient denied experiencing unilateral limb numbness, weakness, or altered mental status.
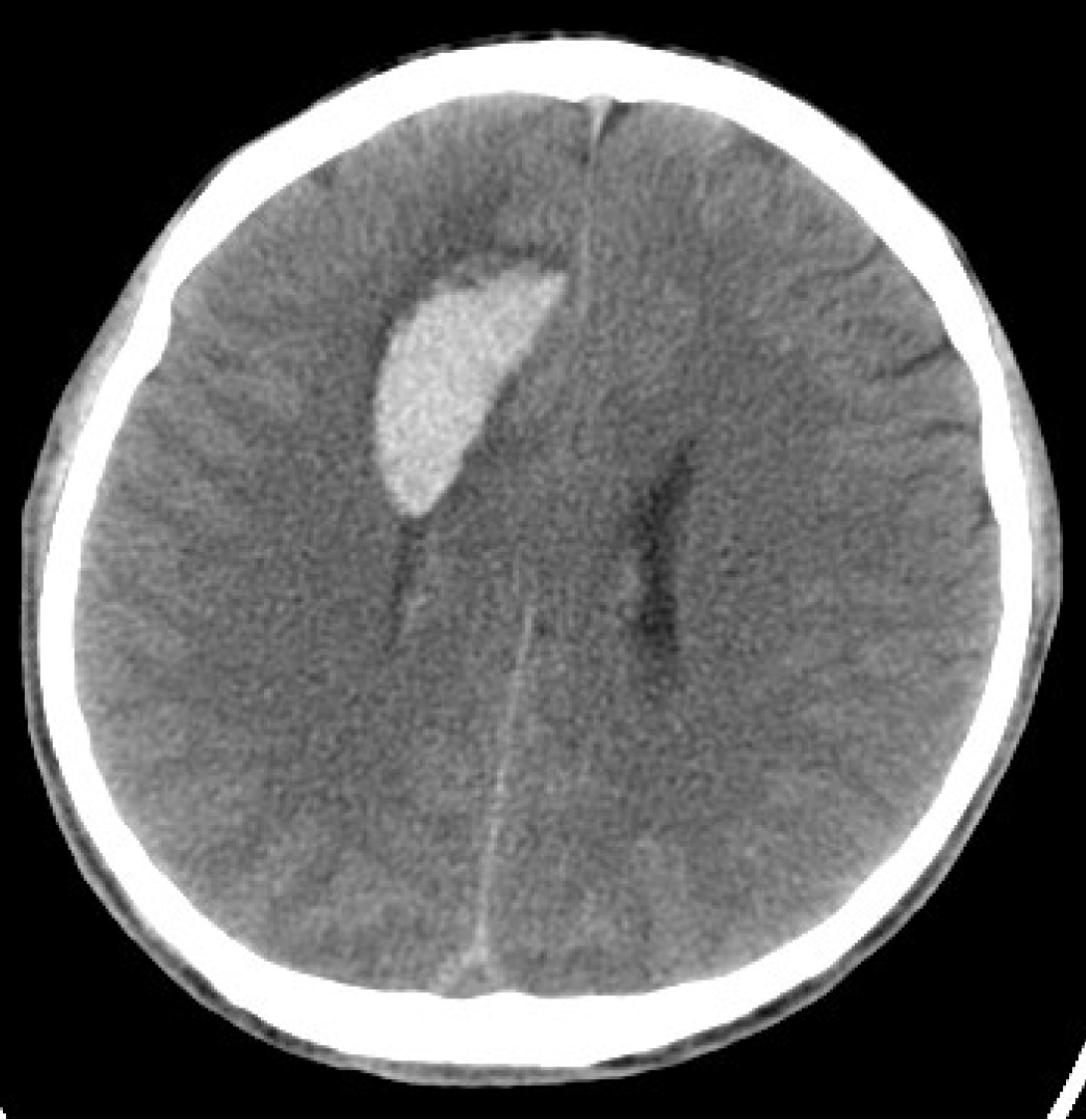
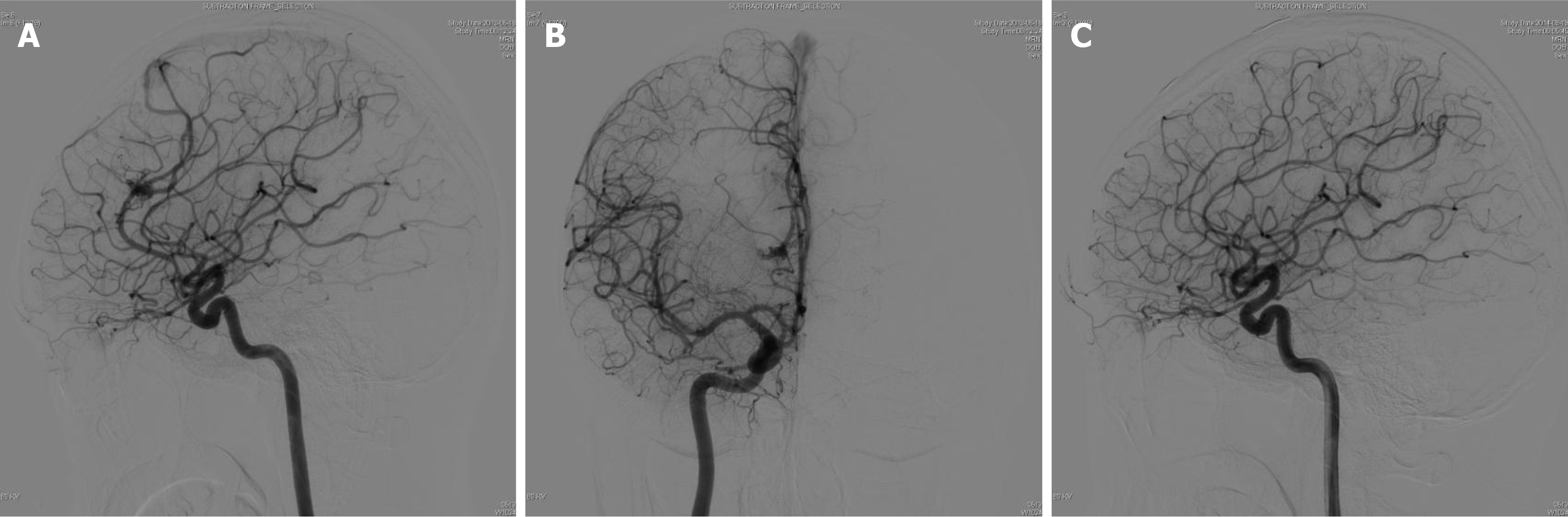
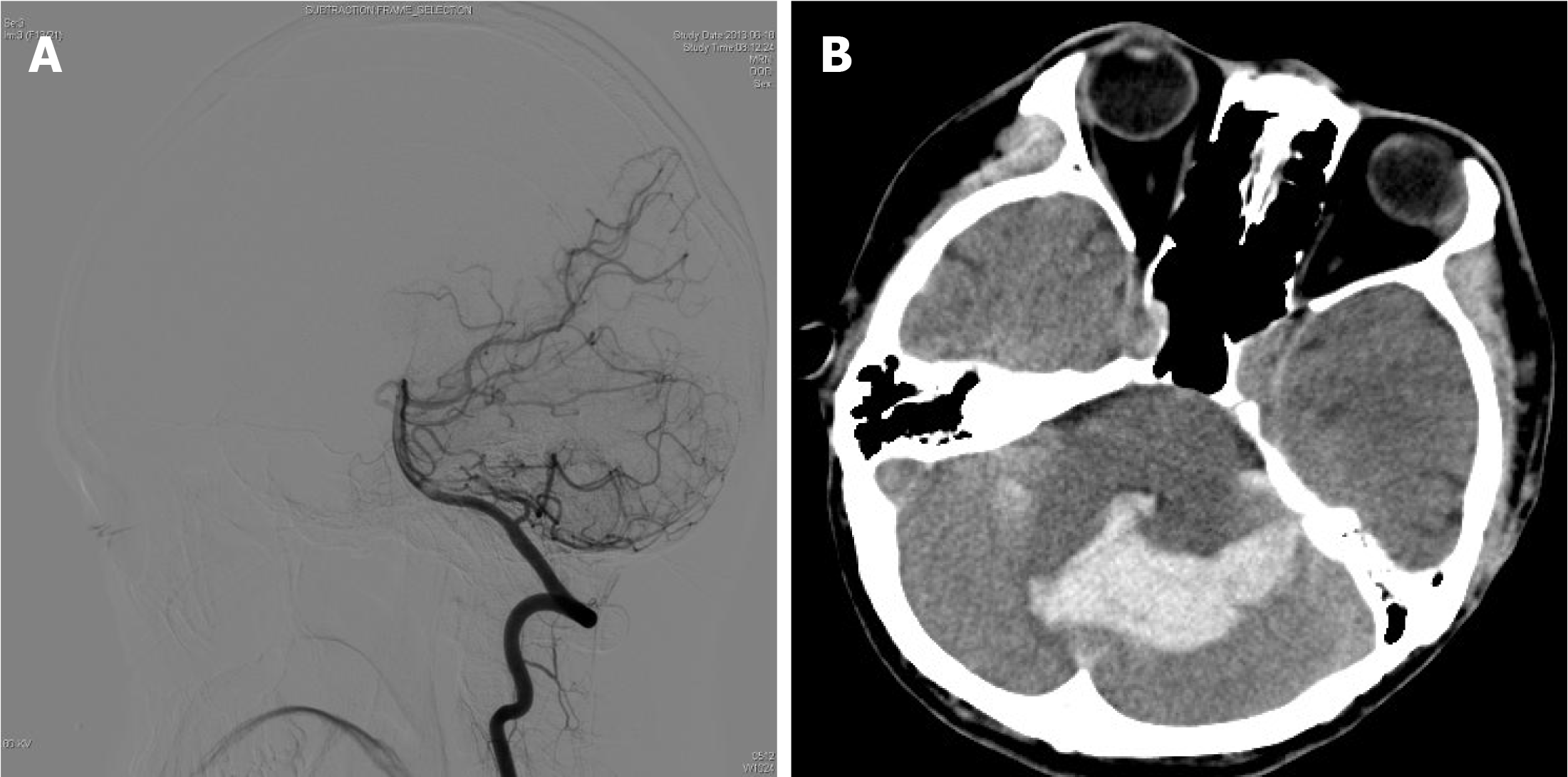
The patient had a history of seizures, and was initially admitted to the hospital after experiencing a cerebral hemorrhage in 2013, during which time a right frontal lobe AVM was identified through computed tomography (CT) and digital subtraction angiography (DSA) (Figure 1 and Figure 2). AVM resection was performed and confirmed by intraoperative pathology. Angiography performed one year postoperatively (Figure 2) revealed no residual AVM. Additionally, DSA performed prior to the initial AVM resection indicated no cerebellar abnormalities (Figure 3).
The patient’s personal and family history were unremarkable.
A neurological examination was performed on admission, which yielded normal findings.
All laboratory examinations were normal.
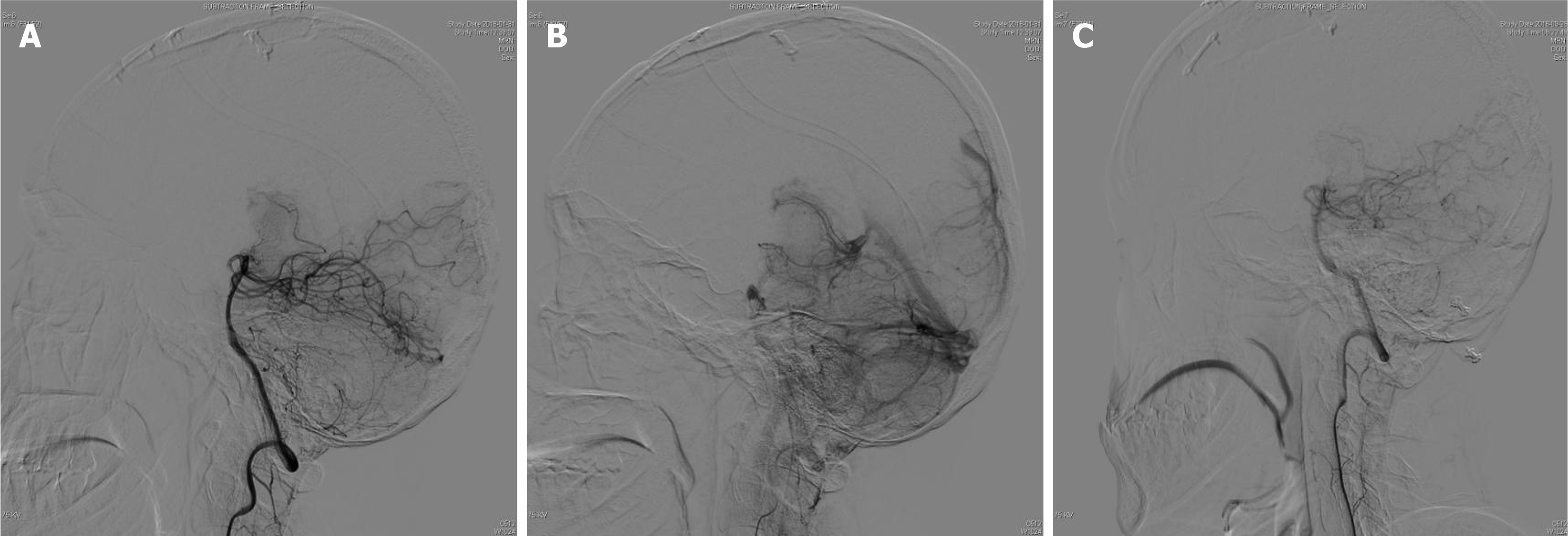
CT revealed cerebellar hemorrhage secondary to lateral ventricular hemorrhage (Figure 3), while DSA demonstrated a left cerebellar AVM fed by the left superior cerebellar artery, draining into the transverse sinus via two branches of the draining veins (Figure 4).
Hypertensive intracerebral hemorrhage (ICH) is a form of acute bleeding within the brain parenchyma or ventricles which develops in patients with a history of hypertension[7]. This condition arises from chronic hypertension, which results in arteriosclerosis and subsequent rupture of the small arteries. The most common sites of hemorrhage are the basal ganglia, pons, and cerebellum[8]. Clinically, it presents with acute onset of severe headache, altered consciousness, and focal neurological deficits. CT typically reveals a hyperdense hemorrhagic lesion, with pronounced surrounding edema. The diagnosis of hypertensive ICH was ruled out given the patient's lack of any history of hypertension.
Cerebral cavernous malformations (CCM) are composed of clusters of abnormal thin-walled blood vessels. Pathologically, it is characterized by multiple lumen formations and vascular leakage at the brain capillary level, leading to disruption of the blood-brain barrier[9]. Clinically, the symptoms are mild and primarily involve recurrent minor hemorrhages. However, in some cases, it can cause severe neurological symptoms, including seizures, focal neurological deficits, and hemorrhagic stroke[10]. magnetic resonance imaging (MRI) findings typically show "popcorn-like" or "honeycomb" lesions, often with evidence of chronic bleeding. CCM was excluded as the patient's MRI did not reveal a "popcorn" appearance, and the hemorrhage was located within the lesion.
Cerebral developmental venous anomalies (DVAs) are among the most common cerebral vascular anomalies, and are currently viewed as normal variations of the cerebral parenchymal venous angioarchitecture, rather than true malformations[11]. Most DVAs are asymptomatic, although a few patients may present with symptoms related to hemorrhage or local mass effects. On imaging, DSA reveals the characteristic "caput medusae" appearance, in which multiple small veins converge into a single large draining vein[11]. Cerebellar AVM was definitively diagnosed based on the results of DSA. Additionally, the absence of the "caput medusae" sign in the venous phase of the DSA excludes the possibility of a venous malformation.
A final diagnosis of ectopic recurrent intracranial AVM was made.
Following discussion, the patient underwent a transoccipital submedial craniotomy to address the intracranial hematoma and allow excision of the malformed vascular mass. The hematoma cavity was surgically accessed through the left superior vermis of the cerebellum, allowing removal of 15 mL of the chronic hematoma. Upon exploration, a vascular malformation measuring 2 mm × 1.5 mm × 1.5 mm was identified. Intraoperatively, the cerebellar AVM was fed by the left superior cerebellar artery, which drained into the transverse sinus via two branches of the draining veins. The procedure was completed successfully.
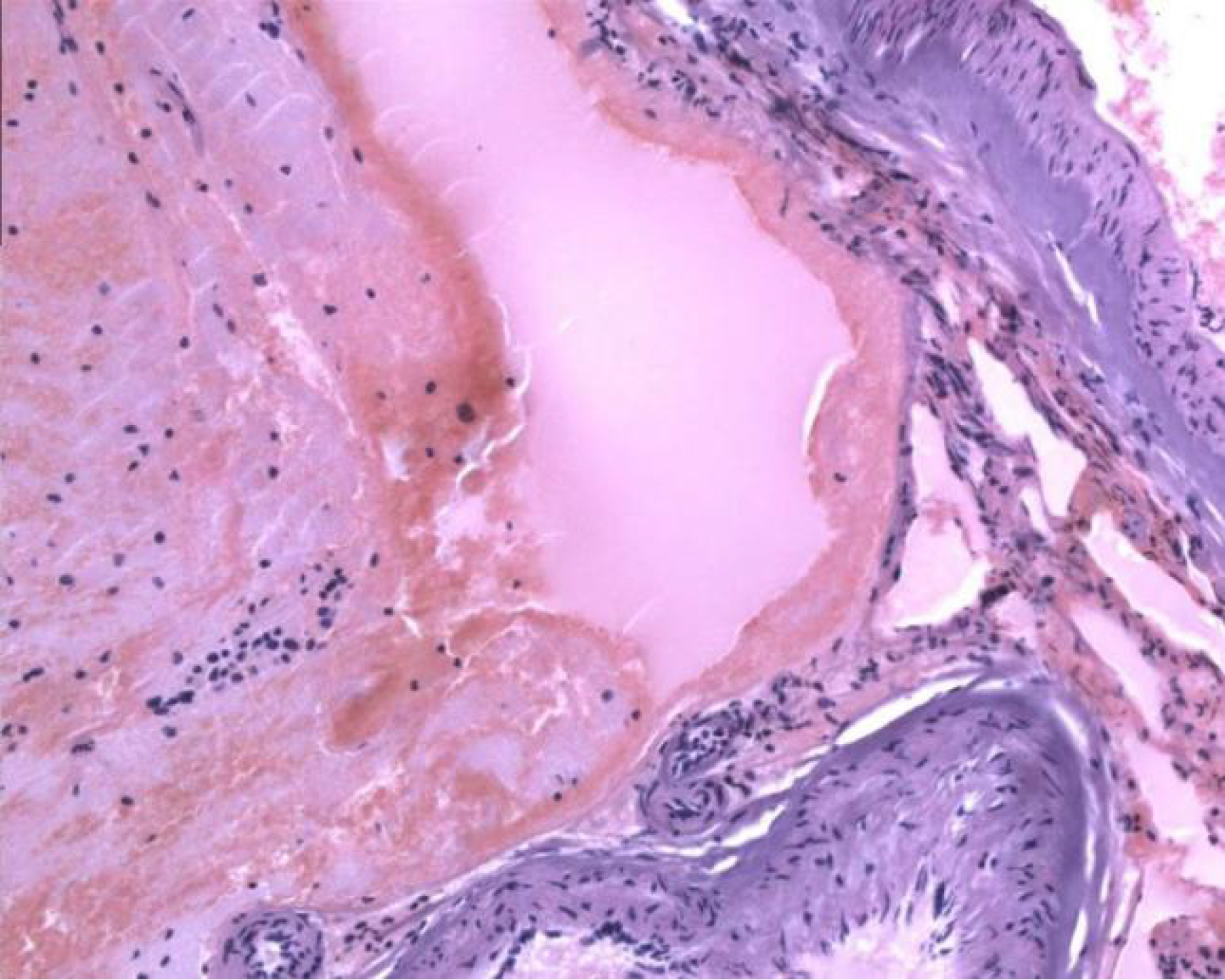
Postoperatively, the patient recovered satisfactorily, with no new neurological dysfunction. Postoperative pathological examination confirmed the presence of an AVM (Figure 5), while immunohistochemistry indicated the presence of CD34, and a final diagnosis of ectopic recurrent intracranial AVM was made.
A follow-up examination seven months postoperatively verified complete resection of the lesion (Figure 4), and the patient had recovered completely. The six-year postoperative follow-up confirmed that the patient had fully recovered neurologically, with no signs of recurrence.
A search of the PubMed and Web of Science databases covering the period from 2000 to October 2023 was conducted to identify all English-language articles describing adult patients with ectopic recurrent AVM (Table 1). The final search was completed on October 30, 2023. The following subject headings and keywords were used: AVM, recurrence, ectopic, and de novo. The first author assessed 20 potentially eligible articles, of which 14 were excluded, including cases with recurrence occurring in situ, those with no difference in the supply artery or draining vein between the two AVMs, and those in which the patient was underaged at the time of developing recurrent ectopic AVMs.
| Ref. | Sex | Initial AVM | Recurrent AVM in adult | Duration | ||||
| Age | Location | Feeding vessels | Age | Location | Feeding vessels | |||
| Nagm et al[15], 2015 | Female | 5 | Right frontal | MCA, ACA | 24 | Cortex of the right frontal | MCA, ACA (different branch) | 19 |
| Akimoto et al[12], 2003 | Female | 10 | Splenium, left occipital | 27 | Cingulate gyrus, corpus callosum | Bilateral pericallosal arteries | 17 | |
| MusLuman et al[16], 2010 | Female | 30 | Left occipital | MCA | 35 | Left parietal-occipital | MCA, PCA | 5 |
| Kawashima et al[13], 2019 | Female | 8 | Cerebellum | SCA, PICA, PCA | 28 | Cerebellum | SCA, PCA | 20 |
| Shi et al[6], 2018 | Male | 61 | Left temporal | 72 | Right temporal-occipital | MCA | 11 | |
| Torres-Quinones et al[14], 2019 | Male | 70 | Cerebellum | SCA | 80 | Left posterior thalamus | SCA, PCA | 10 |
| Present case | Male | 26 | Right frontal | ACA | 31 | Cerebellum | SCA | 5 |
Our literature search finally revealed six previously-published case reports[6,12–16]; after including the present case report, seven patients were included in the analysis. The patients’ demographic characteristics, AVM features, and recurrence times were extracted. Among the seven patients, three were male and four were female. Of the new oc
Studies have shown that AVM recurrence predominantly occurs in pediatric patients. Specifically, 82% of AVM recurrences have been reported in this age group[17]. Analysis has further revealed that the average age at initial AVM diagnosis is 13.8 years, with a mean recurrence interval of 4.2 years[17]. Additionally, research has shown that the risk of AVM recurrence decreases by 14% for every annual increase in age at initial surgical resection[18]. These data indicate that the incidence of AVM recurrence is substantially higher among children than adults.
This case study and literature review identified seven adult patients with ectopic recurrent AVM, all of whom were supported by clinical evidence. Although intracranial AVM are relatively rare, they often lead to hemorrhagic stroke in children and young adults[4]. However, the pathogenesis of this malformation remains unclear[2]. AVM recurrence is more common in pediatric patients, and some authors have suggested that abnormal vascular growth may continue into childhood, resulting in the eventual development of an AVM[6,16,19]. Studies have further indicated that genetic and hereditary factors associated with cerebrovascular malformations may play a role in angiogenesis[3,4,20]. For example, the association between AVM and systemic genetic disorders, such as Osler-Weber-Rendu disease, Sturge-Weber disease, and Wyburn-Mason syndrome, has been reported[20,21].
This case report describes the case of a man who developed ectopic recurrence at a different anatomical site following initially AVM resection in adulthood, a rarely reported scenario. Six similar cases were further analyzed. Some authors have suggested that postoperative complications such as hematoma, edema, or thrombus in the residual AVM may result in imaging opacities, creating a compartment adjacent to the primary lesion[13,15,16,22,23]. However, this hypothesis is less applicable in cases in which imaging does not reveal any abnormalities following the initial surgery[24-25].
Moreover, in four of the seven analyzed cases, the new AVM was located close to the primary site, with differences in arteries or draining veins, challenging the idea of a hardened compartment[15]. In the present case, the recurrent AVM did not develop adjacent to the initial AVM, thereby reducing the applicability of such an inference. Harris et al[26] demonstrated the role of environmental factors and angiogenic processes in AVM formation, supporting the hypothesis that abnormal genes or vasculature coupled with environmental factors activate dormant angiogenesis and form a new AVM mass at a different site[3,24-26].
Some researchers have suggested that the existence of a single genetic abnormality or vascular abnormality increases the risk of AVMs[4]. The presence of external stimuli may further induce neovascularization in a phenomenon known as the “second strike” theory[2,3,20,27]. These external stimuli may include systemic diseases, seizures, radiation exposure, and surgical procedures[2,5,6,14,24,25], and can upregulate environmental proangiogenic factors through multiple pathways, thereby activating otherwise quiescent angiogenic mechanisms[5,14,25].
At the genetic level, research has shown that both germline and somatic mutations can play key roles in the development of AVMs. Indeed, a 2018 study by Nikolaev et al[28] revealed that AVM development may be closely associated with KRAS-induced activation of the MAPK-ERK signaling pathway in brain endothelial cells[28,29]. However, a recent study noted that somatic mutations in KRAS are not detected in all patients, and that a single mutation may not be sufficient to trigger AVM, which could require multiple mutations in the same signaling pathway[30]. In addition, signaling pathways such as BMP/TGF-β, PDGF-B/PDGFR-β, and VEGF/VEGFR, as well as microRNA processing, have been indicated to play important roles in the pathogenesis of AVM[29]. Notably, KRAS was the only gene consistently associated with recurrent somatic mutations[30]. Gao et al[30] further identified G12V and G12D mutations in KRAS as common recurrent mutations by identifying 24 relevant somatic mutations in the MAPK pathway[30].
In addition, external stimuli may induce AVM formation by altering the local environment via other mechanisms. For example, seizures may upregulate angiogenic factors via a hypoxic mechanism, while radiation exposure may promote neoangiogenesis by damaging vascular endothelial cells[3,5]. Related studies have further shown that the expression level of Ki-67 is significantly elevated in pathological sections with recurrence following radiation therapy[13]. These findings indicate that AVM may be characterized by acquired characteristics, and that recurrence may occur as a result of the combined influence of genetic, environmental, and other factors.
The interval between the first AVM treatment and the rediscovery of AVMs in seven patients ranged from 5 to 20 years, with a median of 11 years. Approximately 70% of patients experienced ectopic recurrence more than 10 years after treatment, suggesting a significant potential for ectopic AVM recurrence in adulthood. These results suggest that extended MRI follow-up beyond 10 years may be significant for patients who have undergone complete AVM treatment in both childhood and adulthood.
Herein, we report a case of ectopic recurrent intracranial AVM in an adult at a different anatomical site, and summarize similar cases to speculate on its pathogenesis, thereby increasing the likelihood of acquired AVM occurrence. The clinical course of these patients demonstrates the possibility of ectopic intracranial AVM recurrence in adults. MRI follow-up for more than 10 years in adult AVM-treated patients may be significant.
| 1. | Boone CE, Caplan JM, Yang W, Ye X, Colby GP, Coon AL, Tamargo RJ, Huang J. Hemorrhage risk and clinical features of multiple intracranial arteriovenous malformations. J Clin Neurosci. 2016;23:51-57. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 2. | Nagai Y, Anan M, Fujiki M. Cerebral Arteriovenous Malformations as Acquired Lesions: Case Reports and Review of the Literature. J Stroke Cerebrovasc Dis. 2020;29:105157. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 3. | Rodrigues de Oliveira LF, Castro-Afonso LH, Freitas RK, Colli BO, Abud DG. De Novo Intracranial Arteriovenous Malformation-Case Report and Literature Review. World Neurosurg. 2020;138:349-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 4. | Tasiou A, Tzerefos C, Alleyne CH Jr, Boccardi E, Karlsson B, Kitchen N, Spetzler RF, Tolias CM, Fountas KN. Arteriovenous Malformations: Congenital or Acquired Lesions? World Neurosurg. 2020;134:e799-e807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 5. | Dogan SN, Bagcilar O, Mammadov T, Kizilkilic O, Islak C, Kocer N. De Novo Development of a Cerebral Arteriovenous Malformation: Case Report and Review of the Literature. World Neurosurg. 2019;126:257-260. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 6. | Shi S, Gupta R, Moore JM, Griessenauer CJ, Adeeb N, Motiei-Langroudi R, Thomas AJ, Ogilvy CS. De novo AVM formation following venous sinus thrombosis and prior AVM resection in adults: report of 2 cases. J Neurosurg. 2018;128:506-510. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 14] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Yu Z, Tao C, Xiao A, Wu C, Fu M, Dong W, Liu M, Yu X, You C. Chinese multidisciplinary guideline for management of hypertensive intracerebral hemorrhage. Chin Med J (Engl). 2022;135:2269-2271. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 8. | Sessa M. Intracerebral hemorrhage and hypertension. Neurol Sci. 2008;29 Suppl 2:S258-S259. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 12] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Kim J. Introduction to cerebral cavernous malformation: a brief review. BMB Rep. 2016;49:255-262. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 26] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Eren Gozel H, Kök K, Ozlen F, Isler C, Pence S. A novel insight into differential expression profiles of sporadic cerebral cavernous malformation patients with different symptoms. Sci Rep. 2021;11:19351. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 11. | San Millán Ruíz D, Gailloud P. Cerebral developmental venous anomalies. Childs Nerv Syst. 2010;26:1395-1406. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 32] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 12. | Akimoto H, Komatsu K, Kubota Y. Symptomatic de novo arteriovenous malformation appearing 17 years after the resection of two other arteriovenous malformations in childhood: case report. Neurosurgery. 2003;52:228-31; discussion 231. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 13. | Kawashima M, Hasegawa H, Kurita H, Suzuki K, Shin M, Ikemura M, Saito N. Ectopic Recurrence of Arteriovenous Malformation After Radiosurgery: Case Report and Insight Regarding Pathogenesis. World Neurosurg. 2020;135:63-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 14. | Torres-Quinones C, Koch MJ, Raymond SB, Patel A. Left Thalamus Arteriovenous Malformation Secondary to Radiation Therapy of Original Vermian Arteriovenous Malformation: Case Report. J Stroke Cerebrovasc Dis. 2019;28:e53-e59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 15. | Nagm A, Horiuchi T, Ichinose S, Hongo K. Unique double recurrence of cerebral arteriovenous malformation. Acta Neurochir (Wien). 2015;157:1461-1466. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 16. | Musluman AM, Cavusoglu H, Yilmaz A, Aydin Y. Recurrent cerebral arteriovenous malformation with a posterior inferior cerebellar artery aneurysm. Turk Neurosurg. 2011;21:674-679. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 17. | Sorenson TJ, Brinjikji W, Bortolotti C, Kaufmann G, Lanzino G. Recurrent Brain Arteriovenous Malformations (AVMs): A Systematic Review. World Neurosurg. 2018;116:e856-e866. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 47] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Copelan A, Drocton G, Caton MT, Smith ER, Cooke DL, Nelson J, Abla AA, Fox C, Amans MR, Dowd CF, Halbach VV, Higashida RT, Lawton MT, Kim H, Fullerton HJ, Gupta N, Hetts SW; UCSF Center For Cerebrovascular Research and UCSF Pediatric Brain Center. Brain Arteriovenous Malformation Recurrence After Apparent Microsurgical Cure: Increased Risk in Children Who Present With Arteriovenous Malformation Rupture. Stroke. 2020;51:2990-2996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 19. | Robert T, Blanc R, Botta D, Ciccio G, Smajda S, Redjem H, Fahed R, Piotin M. Management of multiple cerebral arteriovenous malformations in a non-pediatric population. Acta Neurochir (Wien). 2016;158:1019-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 6] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 20. | Leblanc GG, Golanov E, Awad IA, Young WL; Biology of Vascular Malformations of the Brain NINDS Workshop Collaborators. Biology of vascular malformations of the brain. Stroke. 2009;40:e694-e702. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 183] [Cited by in RCA: 168] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 21. | Achrol AS, Guzman R, Varga M, Adler JR, Steinberg GK, Chang SD. Pathogenesis and radiobiology of brain arteriovenous malformations: implications for risk stratification in natural history and posttreatment course. Neurosurg Focus. 2009;26:E9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Pellettieri L, Svendsen P, Wikholm G, Carlsson CA. Hidden compartments in AVMs--a new concept. Acta Radiol. 1997;38:2-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 45] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 23. | Utsuki S, Kurata A, Miyasaka Y, Takano M, Ootaka H, Fujii K. Multiple arteriovenous malformations with hemorrhage. Acta Neurochir (Wien). 2002;144:97-101. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 24. | Jiang W, Zuo Q, Liu J. Developmental venous anomaly with radiation-induced arteriovenous malformation. Neurol Sci. 2022;43:6601-6603. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 25. | Lo Presti A, Rogers JM, Assaad NNA, Rodriguez ML, Stoodley MA, Morgan MK. De novo brain arteriovenous malformation after tumor resection: case report and literature review. Acta Neurochir (Wien). 2018;160:2191-2197. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 26. | Harris OA, Chang SD, Harris BT, Adler JR. Acquired cerebral arteriovenous malformation induced by an anaplastic astrocytoma: an interesting case. Neurol Res. 2000;22:473-477. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 22] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 27. | Florian IA, Beni L, Moisoiu V, Timis TL, Florian IS, Balașa A, Berindan-Neagoe I. 'De Novo' Brain AVMs-Hypotheses for Development and a Systematic Review of Reported Cases. Medicina (Kaunas). 2021;57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 20] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Nikolaev SI, Vetiska S, Bonilla X, Boudreau E, Jauhiainen S, Rezai Jahromi B, Khyzha N, DiStefano PV, Suutarinen S, Kiehl TR, Mendes Pereira V, Herman AM, Krings T, Andrade-Barazarte H, Tung T, Valiante T, Zadeh G, Tymianski M, Rauramaa T, Ylä-Herttuala S, Wythe JD, Antonarakis SE, Frösen J, Fish JE, Radovanovic I. Somatic Activating KRAS Mutations in Arteriovenous Malformations of the Brain. N Engl J Med. 2018;378:250-261. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 266] [Cited by in RCA: 366] [Article Influence: 45.8] [Reference Citation Analysis (0)] |
| 29. | Barbosa Do Prado L, Han C, Oh SP, Su H. Recent Advances in Basic Research for Brain Arteriovenous Malformation. Int J Mol Sci. 2019;20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 40] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Gao S, Nelson J, Weinsheimer S, Winkler EA, Rutledge C, Abla AA, Gupta N, Shieh JT, Cooke DL, Hetts SW, Tihan T, Hess CP, Ko N, Walcott BP, McCulloch CE, Lawton MT, Su H, Pawlikowska L, Kim H. Somatic mosaicism in the MAPK pathway in sporadic brain arteriovenous malformation and association with phenotype. J Neurosurg. 2022;136:148-155. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 22] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/