Published online Oct 28, 2024. doi: 10.4329/wjr.v16.i10.528
Revised: August 29, 2024
Accepted: September 6, 2024
Published online: October 28, 2024
Processing time: 145 Days and 1.6 Hours
Breast cancer morbidity has been increasing worldwide, but treatments are improving. The therapeutic response depends on the stage at which the disease is diagnosed. Therefore, early diagnosis has never been more essential for successful treatment and a reduction in mortality rates. Radiology plays a pivotal role in cancer detection, and advances in ultrasound (US) palpation have shown promi
To evaluate the clinical applications of 2D-SWE US in breast cancer detection and its combination with other imaging modalities.
The 200 consecutive female patients aged 50-80 were examined to evaluate palpa
Combining B-mode and shear wave US imaging with X-ray mammography revealed 100% of the suspicious lesions, resulting in greater sensitivity, specificity, and negative predictive value. The result improves compared to either B-mode or 2D-SWE alone (P = 0.02).
Combining 2D-SWE with conventional US and X-ray techniques improves the chance of early cancer detection. Including 2D-SWE in regular breast imaging routines can reduce the need for biopsies and improve the chance of early cancer detection and survivability with the proper line of therapy.
Core Tip: Breast cancer imaging benefits from integrating two-dimensional-shear wave elastography (2D-SWE) with traditional bright mode (B-mode) ultrasound (US) and X-ray mammography. Our retrospective study showed that this combination identified 100% of suspicious lesions, enhancing sensitivity, specificity, and negative predictive value compared to using B-mode or 2D-SWE alone. Incorporating 2D-SWE into regular breast imaging protocols can reduce the need for biopsies and improve early cancer detection rates. This approach enhances the chances of successful treatment and survivability, underscoring the clinical potential of combining advanced US techniques with conventional imaging methods.
- Citation: Chervenkov L, Georgiev A, Doykov M, Velikova T. Breast cancer imaging-clinical experience with two-dimensional-shear wave elastography: A retrospective study. World J Radiol 2024; 16(10): 528-536
- URL: https://www.wjgnet.com/1949-8470/full/v16/i10/528.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i10.528
As the most common type of malignancy in women worldwide, breast cancer is a cause of global health concern[1,2]. Early detection of breast cancer is vital for efficient control, successful treatment, and mortality rate reduction. Although the overall incidence rate has increased, breast cancer mortality has decreased, primarily ascribed to the availability of early diagnosis and efficient systemic therapies[3,4]. Breast cancer treatment can be highly productive, with 90% or even higher survival probabilities if the disease is detected early[5,6]. Diagnosing breast cancer poses significant challenges due to its diverse presentation and the limitations of current screening methods. Early-stage detection is often difficult, as symptoms may be subtle or non-existent. Additionally, distinguishing between benign and malignant tumors requires precise imaging and biopsy techniques, which can lead to false positives or negatives. Clinically, imaging plays a crucial role in diagnosing, treating, and managing breast cancer[5]. Two-dimensional-shear wave elastography (2D-SWE) is an advanced ultrasound (US) technique that measures tissue stiffness by generating and detecting shear waves. This non-invasive method provides detailed, real-time images, aiding in diagnosing and assessing various conditions, including liver fibrosis and tumors[6]. The 2D-SWE offers several benefits, including its non-invasive nature, providing a safe alternative to traditional biopsies. It allows for real-time imaging, delivering immediate results that facilitate quick diagnoses. Additionally, it provides precise, quantitative measurements of tissue stiffness and has a wide range of applications, instrumental in diagnosing conditions like liver fibrosis and tumors[6].
However, there are limitations to consider. The technique is highly operator-dependent, requiring skilled technicians to ensure accurate results. It has limited penetration capabilities, making it less effective in obese patients or for deep tissue imaging. The method is also susceptible to motion artifacts, which can affect the accuracy of the results. Moreover, the high cost of equipment and maintenance can be a significant drawback. Still, those problems are present to some degree in all US imaging techniques, including B mode. All US manufacturers have come up with solutions to minimize operator-dependent mistakes or inconsistencies. As the technique becomes mainstream, more solutions are expected, as well as better standardization in image acquisition. With all new technologies, there is a related cost, but with time and advancements, expenses are getting lower. Compared to magnetic resonance imaging, the cost of US is meager. Despite the many drawbacks, 2D-SWE has a viable place in the radiologist’s arsenal. Compared with the other currently applicable methods, 2D-SWE provides unique information about the stiffness of human tissues, both standard and pathological. This study evaluates the clinical applications of 2D-SWE US. We seek to determine its role in enhancing diagnostic precision and improving patient outcomes. The study explores how integrating 2D-SWE with traditional imaging methods can provide a more comprehensive and non-invasive approach to breast cancer screening and diagnosis.
In this retrospective cohort study, rigorous measures were implemented to address potential sources of bias at every stage of the research process. Firstly, during the study design, careful consideration was given to selecting study par
Two hundred consecutive female patients aged 50-80 years (all females) were referred to The Department of Diagnostic Imaging to evaluate palpable breast lesions, followed by an US-assisted biopsy. We have not excluded younger or older patients but rather selected patients of the most common ages treated in the hospital. Imaging characteristics of breast neoplasms are generally not age-dependent. This study complied with the Declaration of Helsinki. Informed consent was obtained from all participants. Exclusion criteria consisted of those with cystic lesions of the breast, history of prior biopsy, postsurgical status, or silicone breast implants. All patients underwent mammography, bright mode (B-mode), US, and 2D-SWE followed by US-guided biopsy in two consecutive sessions.
Mammography was performed with a digital mammograph. Mediolateral oblique and craniocaudal views were taken in all patients, in addition to magnification or coned-down projections when necessary.
Ultrasonography of the breast was performed with a high-frequency linear probe. Studied imaging features of lesions are size, shape, margins, orientation, internal echogenicity, vascularity, calcification, and posterior acoustic shadowing. The lesions were categorized by the standardized Breast Imaging Reporting and Data System (BI-RADS). The probe was placed over the region of interest (commonly known as ROI) for the SWE with the lesion centered on the image. At least 10 mm of normal adjacent tissue was included to assess the lesion stiffness concerning surrounding tissues—color map settings with red coded as hard and blue coded as soft were used. The images were displayed on a split screen mode with B-mode images on the right and elastographic images on the left side of the screen. Visual elasticity patterns were scored using the Tsukuba scoring system. The elasticity scores 1 and 2 were considered benign, score 3 was considered probably benign, while scores 4 and 5 were labeled as malignant. Measurements in kPa from the lesion and surrounding normal tissues have been compared to evaluate the stiffness of the finding. Three expert radiologists performed all examinations and biopsies. All specialists have over 10 years of experience in oncological and breast imaging.
The statistical analysis was done with Statistical Package for the Social Sciences (SPSS) version 27.0 (IBM Corp., Armonk, NY, United States). The normality was tested through the Shapiro-Wilk test. The categorical variables were presented as percentages. Using the receiver operating characteristic curve and area under the curve (referred to as AUC) assessed the level of agreement between imaging and biopsy in breast cancer detection. All statistical tests were two-tailed, and the results were interpreted as significant at type I error α = 0.05 (P < 0.05).
We have identified 80 benign neoplasms and 120 malignant neoplasms. The biopsy results are shown in Table 1.
| Histopathological results | Number of lesions |
| Benign | |
| Fibroadenoma | 35 (17.5) |
| Intraductal papilloma | 12 (6) |
| Fibro adenomatoid change | 10 (5) |
| Mammary duct ectasia | 7 (3.5) |
| Fibrocystic change | 5 (2.5) |
| Complex fibroadenoma | 3 (1.5) |
| Other benign lesions | 8 (4) |
| Total | 80 (40) |
| Malignant | |
| Invasive ductal carcinoma | 71 (35.5) |
| Ductal carcinoma in situ | 30 (15) |
| Invasive lobular carcinoma | 14 (7) |
| Mucinous carcinoma | 2 (1) |
| Malignant phyllodes tumor | 2 (1) |
| Angiosarcoma of the breast | 1 (0.5) |
| Total | 120 (60) |
| Total number of biopsies | 200 (100) |
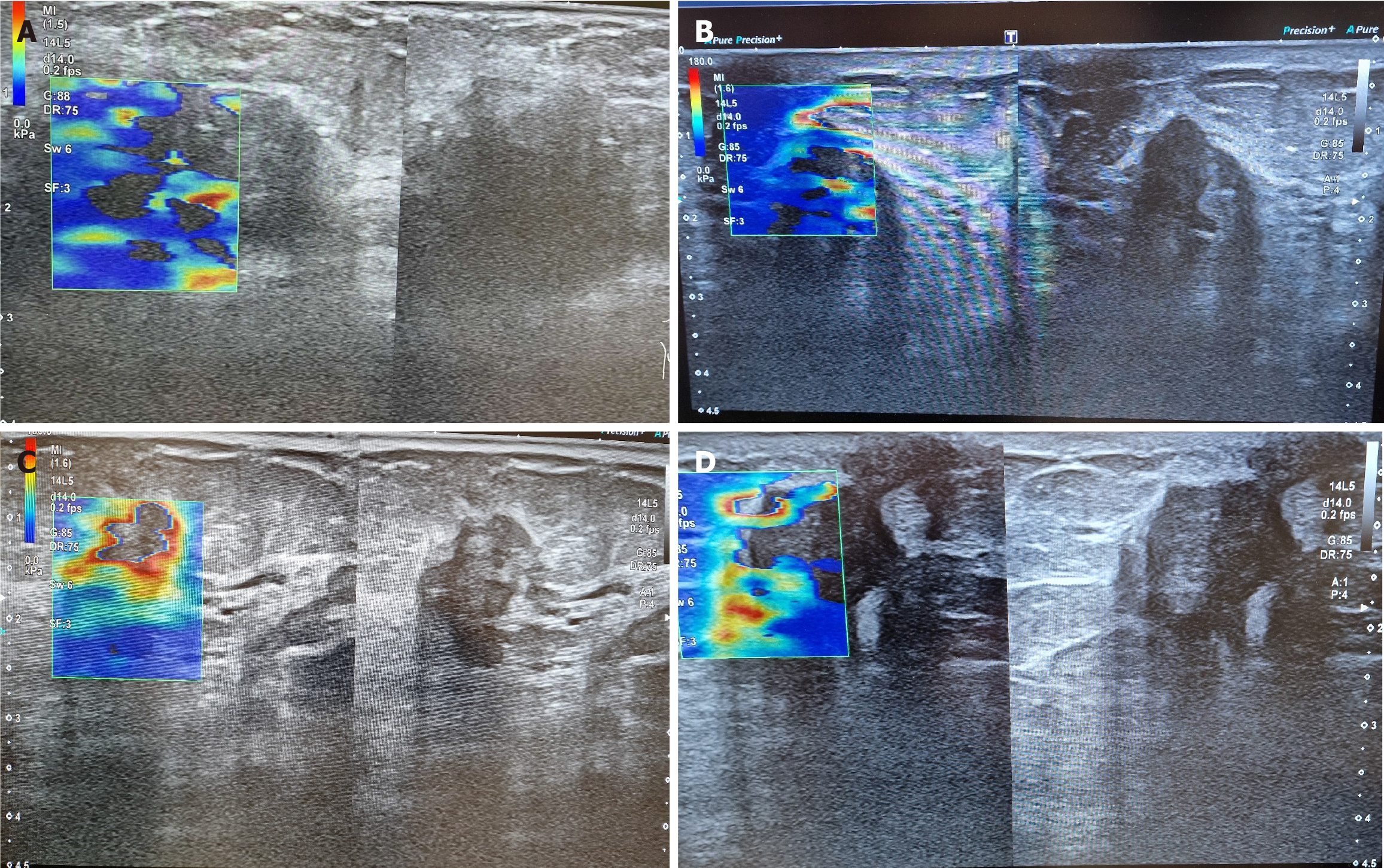
The main visual features of benign lesions were a size not more minor than 15 mm (range: 3–50 mm) and a mean stiffness value below 50 kPa in 2D-SWE imaging. Twenty of these lesions also had suspicious X-ray mammograms and B-mode images. The benign lesions most frequently misclassified as suspicious were fibroadenomas, papillomas, fibrocystic changes, or fat necrosis. Suspicious SWE findings have been seen in fibroadenomas, phyllodes neoplasms, and fibrocystic changes.
The average US size of ductal carcinoma in situ was 15 mm as well (range: 11–25 mm). The average mean stiffness of in situ lesions was 50 kPa (range: 27–113 kPa). The other malignant lesions had a mean size of 25 mm (range: 5–225 mm), with an average mean stiffness value of 149.5 kPa (range: 30–300 kPa). A Tsukuba score above 2 is present in most malignant cases. The 2D-SWE can visualize the invasion of the neoplasm in its surroundings by demonstrating a layer of more dense tissue. This layer usually surrounds the neoplasm like a ring or is seen as rays/tentacles spreading from the finding. That is explained by the stromal response to breast cancer, increasing the stiffness of the tumor and the surrounding tissues. Almost all cancers misclassified as negative by one modality were correctly classified as positive by another. Those cases include 75 patients, or 37.5% of all studied patients. The detailed results of the biopsy of malignant neoplasms that were misinterpreted in any modality are visible in Table 2.
| Biopsy results | |
| Invasive size in mm | |
| < 15 | 50 (66.7) |
| > 15 | 25 (33.3) |
| Histological grade | |
| I | 6 (8) |
| II | 60 (80) |
| III | 9 (12) |
| Neo vascularization | |
| Yes | 7 (9.3) |
| No | 68 (90.7) |
| Nodal status | |
| Yes | 8 (10.7) |
| No | 67 (89.3) |
| Mitotic index of proliferation (Ki-67) | |
| < 3% | 5 (6.7) |
| Between 3% and 20% | 60 (80) |
| > 20% | 10 (13.3) |
| Hormone receptor | |
| Positive | 60 (80) |
| Negative | 15 (20) |
| Human epidermal growth factor receptor 2 | |
| Positive | 40 (53.3) |
| Negative | 35 (46.7) |
| Triple-negative breast cancer | |
| Yes | 10 (13.3) |
| No | 65 (86.7) |
Most misinterpreted neoplasms (around 80%) are intermediate cancers with moderately differentiated cells and sizes below 15 mm. About 9% of the cases have high-tier cancer with low differentiation, and 6% of misinterpreted lesions are low-grade neoplasms. The results are concerning as only a tiny percentage of misinterpreted lesions by a single modality are grade I tumors. The bigger parentage, around 89, consists of higher-grade tumors with unfavorable metastatic potential.
The added benefit of 2D-SWE imaging has helped in 47 (62.6%) of the 75 (100%) misinterpreted cases. Findings of dense areas above 50 kPa or Tsukuba score above two on the elastograms were suspicious findings during the US examinations. The combination of US and X-ray mammography yielded a much better clinical result, revealing 100% of the suspicious lesions.
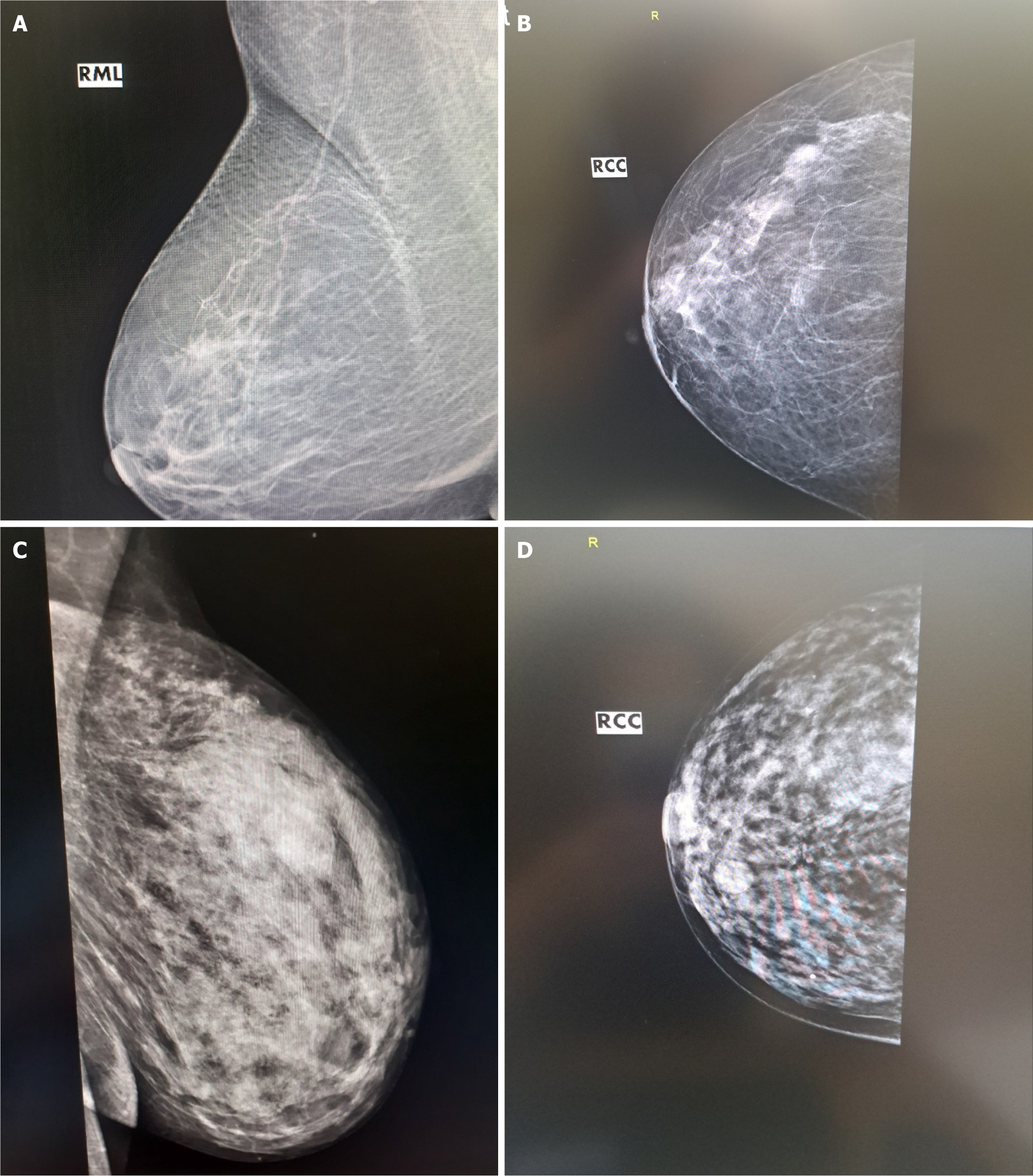
The combined power of US B-mode and 2D-SWE results in sensitivity and a negative predictive value of 100%, increasing the total diagnostic value. The result improves compared to either B-mode or 2D-SWE alone (P = 0.02). The combination of US techniques and X-ray mammography proved statistically significant and clinically superior to any methods used alone (P = 0.00). The results are visible in Table 3. The AUC of 0.98 for the combined imaging techniques demonstrates the model's discriminatory ability. In 98% of the cases, they will correctly classify a lesion as benign or malignant. Figures 1A-C show typical examples of breast cancer, found on US. Figure 1D shows the advantage of the method over mammography.
| Feature | Shear wave elastography + bright mode | X-ray mammography | Ultrasound + X-ray mammography |
| True positive | 115 | 114 | 120 |
| True negative | 39 | 15 | 13 |
| False positive | 25 | 24 | 10 |
| False negative | 0 | 2 | 0 |
| Sensitivity | 1.00 (1.00-1.00) | 0.87 (0.81–0.92) | 1.00 (1.00–1.00) |
| Specificity | 0.62 (0.52–0.76) | 0.90 (0.83–0.95) | 0.95 (0.83–0.95) |
| Positive predictive value | 0.83 (0.78–0.92) | 0.76 (0.80–0.92) | 0.92 (0.85-0.95) |
| Negative predictive value | 1.00 (1.00–1.00) | 0.996 (0.99–1.00) | 1.00 (1.00–1.00) |
| Accuracy | 87 | 91 | 98 |
A 'mammogram' is a term used to describe a 2D X-ray image of the breast that can identify suspicious findings in breast cancer, such as masses, asymmetric calcifications, and architectural deformation. A standard screening mammogram consists of two views of each breast: Craniocaudal and mediolateral oblique views[3,4]. A diagnostic mammogram may include additional features such as spot compression, magnification, rolled, and actual lateral views of the tissue under investigation to characterize local abnormalities. Breast US is an imaging technique that employs US waves to assess the morphology, orientation, internal structure, and margins of lesions in fatty breasts and dense granular structures[7-9]. US imaging helps physicians determine whether a breast abnormality is solid, fluid-filled, or cystic and decide whether a detected mass is malignant or benign. Doppler functionality may visualize abnormal blood flow and malignant angiogenesis. Breast US elastography is a relatively new imaging modality developed to provide a non-invasive assess
Currently, there are three ways to perform SWE that contribute to improvement in differentiation between benign and malignant breast lesions for a more reliable clinical diagnosis. The options are transient elastography (TE), point SWE (pSWE), and 2D-SWE. TE uses an external device to generate vibrations, representing the earliest attempt at SWE[7]. Both pSWE and 2D-SWE utilize the generation of internal shear waves in tissues and calculate their lateral propagation speed[8]. This effect is created by an acoustic force emanating from the transducer. PSWE is obtained as a single value from a small ROI, and 2D-SWE is calculated for each pixel in a larger area-given field of view. The distribution of shear wave velocities at each pixel directly relates to Young's modulus-a measurement of the elastic properties of the matter[9,14]. Shear wave images are fused with standard B-mode images, providing colored elastograms. In addition, 2D-SWE has real-time imaging capabilities displaying live B-mode images and elastograms[8,9]. The elasticity of the soft tissues ranges from 1-100 kPa[3]. Typically, the stiffness of fibroadenomas is twice that of normal breast parenchyma, and the elasticity of breast cancers is around 15 times stiffer than the soft tissues[10,11]. In that regard, 2D-SWE can reduce the number of excessive biopsies, acting as an ultrasonic palpation. The 2D-SWE values and B-mode anatomical images can help determine between malignant and benign breast neoplasms. The most discriminating 2D-SWE features are the color map and the peri-lesion ratio value. The peri-lesion ratio is the highest measurement of the peri-lesion area divided by the lowest measurement in healthy fatty tissue[9]. Therefore, a lesion with benign SWE features can be downgraded to a lower BI-RADS grade, allowing a follow-up imaging exam rather than a biopsy in some cases[3,11,15].
The Tsukuba elasticity score is the most critical visual elastographic parameter[3,8,16]. The Tsukuba score is a five-point color scale based on the stiffness of tissues in and around the lesion. The score is calculated based on the lesion stiffness relative to the stiffness of normal tissues[8,9]. Higher Tsukuba scores correspond to a greater probability of malignancy. Scores from 1-3 indicate a probably benign lesion, and scores from 4-5 require a biopsy. The Tsukuba score has a literature-reported sensitivity and specificity of 86.5% and 89.9%, respectively[8,11,16,17].
Everything has limitations, and SWE does not make an exception. 2D-SWE is still reserved for high-tier and premium US machines requiring a higher frame rate and processing power. A significant disadvantage of such systems for many is the prohibitive cost. The financial problem is further exacerbated in developing and underdeveloped countries. Such machines are still expensive in the refurbished and used markets as well. The 2D-SWE has other limitations, such as elastogram color mapping and scoring not being standardized and varying between manufacturers' and physicians' preferences (color mapping can be changed or reversed). Occasionally, a malignant lesion may appear soft[9,11,16]. It is challenging to characterize heterogeneous lesions with mixed cystic or necrotic regions[8,9,16,17]. Some benign lesions, such as fibrosis, fat necrosis, or hyalinized fibroadenomas, maybe as stiff as breast cancer[3,9,11]. Masses in the deeper breast (Chassaignac bursa) could be challenging to assess[8,16].
The remarkable advancement in "US palpation" and clinical breast US elastography applications will improve breast cancer detection and disease outcomes. At this stage of development, US elastography should only be used and interpreted with standard B-mode images to characterize a suspicious lesion, as stated by the World Federation for Ultrasound in Medicine and Biology. SWE improves breast cancer detection, but the combination of SWE, B-mode, and conventional X-ray mammography yields the best clinical results and reduces the need for biopsies. Additionally, the combined US/X-ray technique improves the chance of cancer survivability with more accurate diagnosis and guidance toward the proper line of therapy.
| 1. | Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J Clin. 2021;71:209-249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75126] [Cited by in RCA: 68439] [Article Influence: 13687.8] [Reference Citation Analysis (201)] |
| 2. | Kutikov A, Weinberg DS, Edelman MJ, Horwitz EM, Uzzo RG, Fisher RI. A War on Two Fronts: Cancer Care in the Time of COVID-19. Ann Intern Med. 2020;172:756-758. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 252] [Cited by in RCA: 307] [Article Influence: 51.2] [Reference Citation Analysis (0)] |
| 3. | Sigrist RMS, Liau J, Kaffas AE, Chammas MC, Willmann JK. Ultrasound Elastography: Review of Techniques and Clinical Applications. Theranostics. 2017;7:1303-1329. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 954] [Cited by in RCA: 1223] [Article Influence: 135.9] [Reference Citation Analysis (0)] |
| 4. | Gierach GL, Choudhury PP, García-Closas M. Toward Risk-Stratified Breast Cancer Screening: Considerations for Changes in Screening Guidelines. JAMA Oncol. 2020;6:31-33. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Narod SA. Personalised medicine and population health: breast and ovarian cancer. Hum Genet. 2018;137:769-778. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 32] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Heer E, Harper A, Escandor N, Sung H, McCormack V, Fidler-Benaoudia MM. Global burden and trends in premenopausal and postmenopausal breast cancer: a population-based study. Lancet Glob Health. 2020;8:e1027-e1037. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 393] [Cited by in RCA: 565] [Article Influence: 94.2] [Reference Citation Analysis (0)] |
| 7. | de Lédinghen V, Vergniol J. Transient elastography (FibroScan). Gastroenterol Clin Biol. 2008;32:58-67. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 177] [Cited by in RCA: 222] [Article Influence: 13.1] [Reference Citation Analysis (0)] |
| 8. | Evans A, Whelehan P, Thomson K, Brauer K, Jordan L, Purdie C, McLean D, Baker L, Vinnicombe S, Thompson A. Differentiating benign from malignant solid breast masses: value of shear wave elastography according to lesion stiffness combined with greyscale ultrasound according to BI-RADS classification. Br J Cancer. 2012;107:224-229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 193] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 9. | Shahzad R, Fatima I, Anjum T, Shahid A. Diagnostic value of strain elastography and shear wave elastography in differentiating benign and malignant breast lesions. Ann Saudi Med. 2022;42:319-326. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 5] [Reference Citation Analysis (0)] |
| 10. | Schaefer FK, Heer I, Schaefer PJ, Mundhenke C, Osterholz S, Order BM, Hofheinz N, Hedderich J, Heller M, Jonat W, Schreer I. Breast ultrasound elastography--results of 193 breast lesions in a prospective study with histopathologic correlation. Eur J Radiol. 2011;77:450-456. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 80] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Yılmaz E, Yılmaz A, Aslan A, Inan I, Evren MC, Tekesin K. Real-Time Elastography for Differentiation of Breast Lesions. Pol J Radiol. 2017;82:664-669. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 8] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 12. | Xue Y, Zou H, Ou Y, Li S, Zhao Y, Li Y, Li Y. Strain histograms used for differential diagnosis of breast masses according to hardness percentage. Medicine (Baltimore). 2019;98:e15125. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 1] [Article Influence: 0.1] [Reference Citation Analysis (0)] |
| 13. | Zhao QL, Ruan LT, Zhang H, Yin YM, Duan SX. Diagnosis of solid breast lesions by elastography 5-point score and strain ratio method. Eur J Radiol. 2012;81:3245-3249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 51] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 14. | Turnaoğlu H, Haberal KM, Arslan S, Yavuz Çolak M, Ulu Öztürk F, Uslu N. Interobserver and intermethod variability in data interpretation of breast strain elastography in suspicious breast lesions. Turk J Med Sci. 2021;51:547-554. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 9] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Brasier-Lutz P, Jäggi-Wickes C, Schaedelin S, Burian R, Schoenenberger CA, Zanetti-Dällenbach R. Agreement in breast lesion assessment and final BI-RADS classification between radial and meander-like breast ultrasound. BMC Med Imaging. 2021;21:104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 16. | Kanagaraju V, Dhivya B, Devanand B, Maheswaran V. Utility of Ultrasound Strain Elastography to Differentiate Benign from Malignant Lesions of the Breast. J Med Ultrasound. 2021;29:89-93. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 17. | Thomas R, Das SK, Balasubramanian G, Chandrappa A. Correlation of Mammography, Ultrasound and Sonoelastographic Findings With Histopathological Diagnosis in Breast Lesions. Cureus. 2022;14:e32318. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/