Published online Oct 28, 2024. doi: 10.4329/wjr.v16.i10.497
Revised: September 26, 2024
Accepted: October 20, 2024
Published online: October 28, 2024
Processing time: 166 Days and 21.4 Hours
Prostate cancer (PCa) imaging forms an important part of PCa clinical manage
Core Tip: Quantitative imaging has many advantages over conventional qualitative assessment. A few parameters have also been shown to correlate with the Gleason score and can help in deciding disease prognosis and clinical management. A quantitative imaging biomarker could improve prostate cancer (PCa) detection by minimizing inter-observer variability, thereby reducing overdiagnosis of clinically insignificant PCa (Gleason score < 7). This would help avoid unnecessary biopsies and decrease the overtreatment of slow-growing PCa. In addition, with further advancement in the quantitative imaging parameters, they may be used to monitor therapeutic response or to predict response to a particular treatment.
- Citation: Dhiman A, Kumar V, Das CJ. Quantitative magnetic resonance imaging in prostate cancer: A review of current technology. World J Radiol 2024; 16(10): 497-511
- URL: https://www.wjgnet.com/1949-8470/full/v16/i10/497.htm
- DOI: https://dx.doi.org/10.4329/wjr.v16.i10.497
Prostate cancer (PCa) is the second most common malignancy and one of the main causes of cancer related mortalities in males around the globe[1]. Traditional diagnostic methods like digital rectal examination, prostate specific antigen tests, and transrectal ultrasound (US)-guided biopsy have poor sensitivity and specificity and face limitations in staging and grading of PCa. Therefore, for better prognosis effective management, and treatment planning, accurate characterization of PCa is necessary[2].
Current imaging modalities available for imaging of PCa are transrectal US, multiparametric magnetic resonance imaging (MRI), positron emission tomography-computed tomography (PET-CT) scan, and PET-MRI (PET-MRI) scan. US plays a limited role in cancer detection as very small proportion of hypoechoic lesions are revealed to be tumors upon biopsy. Thus, it is used to visualize the prostate during transrectal or trans-perineal US-guided biopsies. CT offers high specificity in detecting very high-grade tumors but is still not ideal for diagnosing PCa as it lacks soft tissue resolution and molecular insights needed for accurate PCa detection[3]. PET scan uses a tracer to target prostate-specific membrane antigen which helps to detect lymph node metastasis in men with high-risk PCa. Prostate-specific membrane antigen PET has proven to be more effective than conventional CT in accurately staging high-risk PCa patients[4]. MRI, is a no
Imaging modalities may provide structural as well as functional information, depending upon the modality and technique. However, most of the imaging assessment is qualitative i.e., based on visual inspection and thus subjected to inter-observer disagreement. To overcome this and to enhance the objectivity of reporting, quantitative imaging techniques have been recommended which may play a major role. Quantitative imaging aims to assign a numerical value to the observations enabling higher accuracy, reproducibility, standardized reporting, interpretation, and communication. Few such MRI quantitative metrics are already part of PIRADS v.2.1[6]. However, many are still under study and need further validation. This review intends to discuss the existing quantitative methods, recent developments, and novel techniques in detail.
Diffusion-weighted imaging (DWI) is an important part of multiparametric MRI and enables the assessment of changes associate with the tissue organization in progression of PCa. High cellularity tissues by virtue of reduced extracellular space restrict the Brownian movement of water molecules and thus demonstrate diffusion restriction[7]. DWI acquisition sequence can be used to obtain two sets of images: (1) Diffusion weighted imaging acquired at different b-values, where, b-value signifies diffusion sensitizing gradient’s strength with its unit being seconds/mm2[8-10]; and (2) Apparent diffusion coefficient (ADC) map generated from the diffusion weighted images acquired at least two different b-values. When the logarithm of signal intensity decay on the Y-axis is plotted against b-values on the X-axis, the slope of the line which is produced represents the ADC value[7,9,10]. A minimum of two b-values are required for the calculation of ADC. DWI acquired at more than two b-values enhance the precision of ADC estimation at the cost of increased acquisition time[7]. There is a lack of standardization of number of b-values and highest b-value to use, which leads to differences in the calculation of ADC values. Many recent studies have proven the superiority of high b-value DWI (1500-2500 s/mm2) over conventional DWI (b = 1000 s/mm2)[11-18]. However, the disadvantage of a high b value includes a reduced signal-to-noise ratio, more susceptibility artifacts, and image distortion[19]. Specific software is also required for ADC cal
Under PIRADS v.2.1, DWI is the primary sequence for assessing peripheral zone (PZ) lesions and a secondary sequence for transitional zone (TZ) lesions[22]. Low ADC values have been reported in TZ in benign stromal hyperplastic nodules, which is the reason for not using DWI as a primary sequence for TZ[22]. Few studies have demonstrated higher ADC values in stromal hyperplastic nodules as compared to PCa, however, there is no clear-cut value to distinguish both and thus the use of quantitative ADC in the diagnosis of PCa in TZ is still not validated. DWI provides both qualitative (by assessing whether the tissue in question is showing restriction or not) and quantitative assessment. Various quantification metrics which are being explored include mean ADC, ADC ratio, ADC minimum, and histogram analysis. Currently, mean ADC is the only quantitative DWI parameter included under PIRADS v.2.1. Available literature has shown that the mean ADC value has an inverse relation with histological Gleason’s score and thus it can be used to predict whether the prostate malignancy is high grade or low grade[23-25]. Lucarelli et al[5] showed that the mean ADC values decrease from International Society of Urological Pathology (ISUP) 2 to ISUP 5 showing significant differences between low, intermediate, and high-grade tumors. ADC values showed a strong positive correlation with ISUP groups in both the peripheral and transition zones; however, the correlation between ADC values and PIRADS groups is less reliable in the transition zone than in the peripheral zone[5]. One major limitation of mean ADC is averaging ADC values and masking of low ADC pixels which contain more aggressive tumor foci[26]. Also, there are no clear cut-off mean ADC values for benign and malignant lesions, as ADC value is highly influenced by many factors including the type of scanner used, b-values, physiological differences in background prostate tissue, etc[27-30]. To compensate for these differences, some authors have advised the use of ADC ratio which is defined as the ADC value of the prostatic area suspicious of malignancy divided by the ADC value in the non-malignant reference area[31-33]. There is no validated reference with respect to which ADC ratio should be calculated, different studies have used different references including normal TZ, and PZ, and some have also used urinary bladder as a reference[33,34]. The advantage of this method is that with no additional scan time, it allows lesion visualization by providing a highly diffusion-weighted image[35]. Also, there are two schools of thought on how to draw region of interest (ROI) for ADC ratio calculation. One group used ROI covering ADC minimum area[23,36] yielding ADC minimum ratio and another group advocated ROI covering whole lesion yielding ADC mean ratio[37,38]. A study compared both and found that ADC mean and the resulting ADC mean ratio was significantly better compared to ADC minimum and resulting ADC minimum ratio in discriminating high-grade from low-grade PCa[33].
ADC minimum and ADC histogram analysis have been proposed as two quantitative DWI methods which are beneficial especially in heterogeneous lesions[26,39]. ADC minimum is more sensitive to detecting aggressive tumor foci in a heterogenous mass with varying ADC values[40]. And, thus can act as a better targeting guide in prostatic biopsies. However, there is mixed data, as to whether ADC mean or ADC minimum correlates better with Gleason score (GS)[26,39,41]. The limitation of minimum ADC is being restrictive and more prone to fallacious due to artifacts[33,40,42]. Also as already discussed, ADC mean values due to averaging can mask the areas with minimum values, thus can underestimate the aggressiveness in a heterogenous lesion. To overcome this, some authors have come up with the concept of histogram analysis. Homogenous composition tumors will have the gaussian type of distribution as opposed to skewed distribution in heterogenous lesions[26]. The low centile values in the histogram have been shown to correlate better with GS[26,39,41]. However, the optimal centile that should be taken for analysis lacks standardization[36,42].
Tumor size is an important part of the decision tree of PIRADS v.2.1 and the 15 mm cut-off differentiates PIRADS 4 from PIRADS 5 lesion[6] (Figure 1). This has been independently validated by many investigators. The measurement of the single longest diameter is easy to perform and has a good positive correlation with tumor volume (TV) at radical prostatectomy[43].
TV is associated with the prognosis of PCa[44,45]. TV more than 0.5 cm3 is considered the threshold for significant disease[46]. Index lesion volume helps in deciding management, and guiding focal treatment, and is a good criterion for follow-up during active surveillance[47,48]. Focal therapy options such as high intensity focused US, cryotherapy, laser ablation, brachytherapy, electroporation, and radiofrequency ablation[49] require precise assessment of TV so that maximum energy is deposited in the cancerous tissue with minimal damage to the adjacent normal area[50,51]. Studies have found that pathological TV correlates with pathological stage and GS[43,44,52,53]. So, if TV on imaging can truly represent the pathological volume, it will also show a similar correlation with GS. A study by McNeal et al[54] de
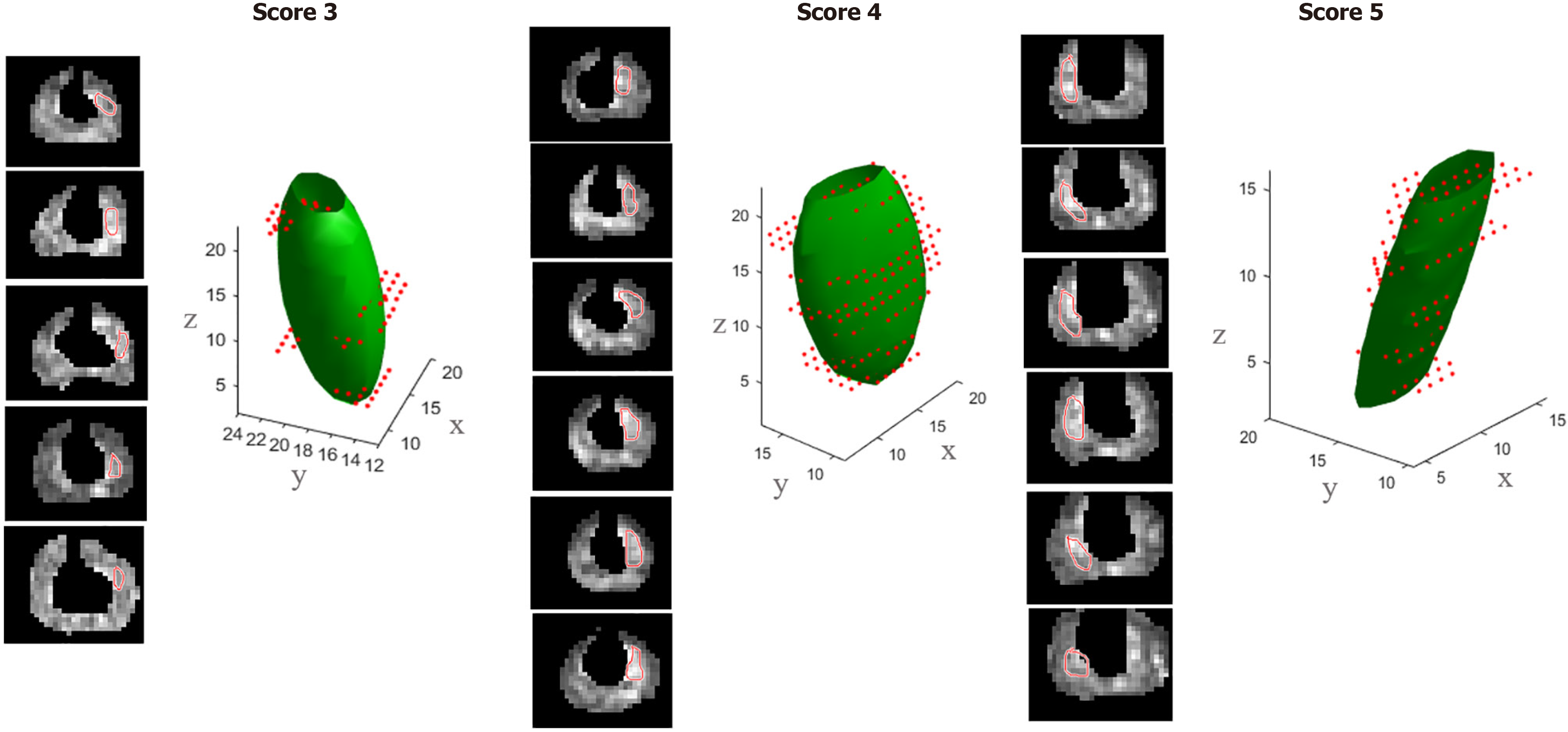
Shape analysis is one of the potential quantitative parameters, which can have an important role in staging and prognosis[59]. Currently, PIRADS V2.1 includes only qualitative shape criteria, which are separate for TZ and PZ lesions. In TZ, lenticular shape favors malignancy, while round or oval shape favors benign prostatic hyperplasia (BPH)[22,60,61]. While for PZ, wedge, linear, shape, bandlike, diffuse shape have been described to occur more frequently in benign conditions, on the other hand, round or oval shape has been associated with PCa[22,62] (Figure 3). Shape assessment is done on DWI sequence for PZ lesions[6] and on T2 sequence for TZ lesions[6]. Realizing the important role of shape analysis for PZ lesions, PIRADS v.2.1 has incorporated shape analysis of PZ lesions as well into decision making, while it was not included in the PIRADS V 2[6,22,60]. Various studies have been done to validate the importance of shape analysis in TZ and PZ lesions. In a study by Li et al[63], the ability of radiological semantics to discriminate clinically significant PCa (CsPCa) was studied on multiparametric MRI and it was found that kappa score for qualitative shape analysis showed only moderate interobserver agreement. In another shape metrics study for PZ tumors on DWI in 241 patients, the agreement of shape assessment was also moderate. The round or oval shape of the tumor was associated with a higher GS as compared to crescentic tumors and linear or wedge-shaped tumors (P = 0.011). In addition, tumors with round or oval shapes showed a greater degree of extracapsular extension (ECE) and seminal vesicle invasion[64] (extracapsular extension and seminal vesicle invasion: 70.1% and 26.0%) as compared to crescentic tumors (67.3% and 9.1%; P = 0.003) and linear or wedge-shaped tumors (40.6% and 9.4%; P = 0.008)[64]. Quantitative descriptors used for round and oval lesions were roundness and circularity, which were associated with more aggressive PCa[64]. A quantitative shape analysis study by Krishna et al[65] on TZ lesions on T2-weighted imaging compared it with subjective shape analysis. Circularity (correlating with round lesions) and convexity (correlating with lentiform lesions) shape features showed excellent inter reader agreement in differentiating TZ PCa and BPH; however, the quantitative feature representing lesion topology (number of spiculations) was not able to differentiate TZ PCa and BPH and was and thus inferior in accuracy than subjective shape analysis. In a study, it was shown that an advanced radiomic feature derived from an ADC map known as the surface area to volume ratio came out as a highly accurate and promising tool in discriminating CsPCa from non-CsPCa, and even outperforming previously described parameters such as TV and maximum diameter (5,7)[66].
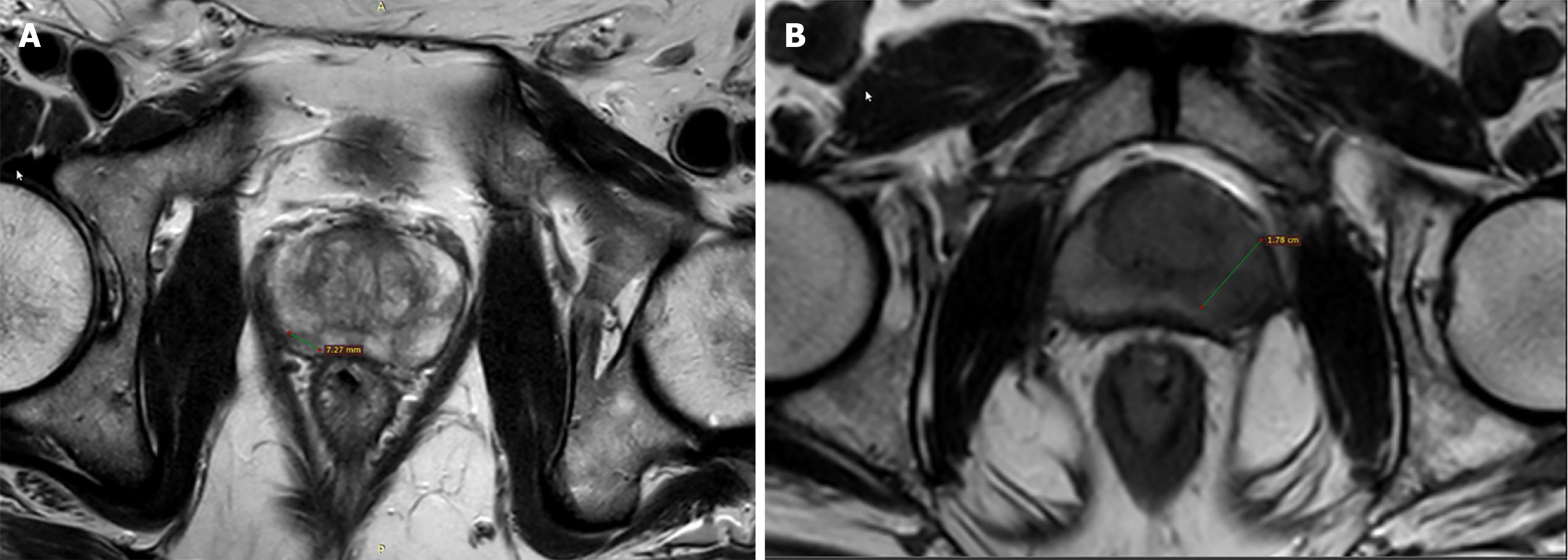
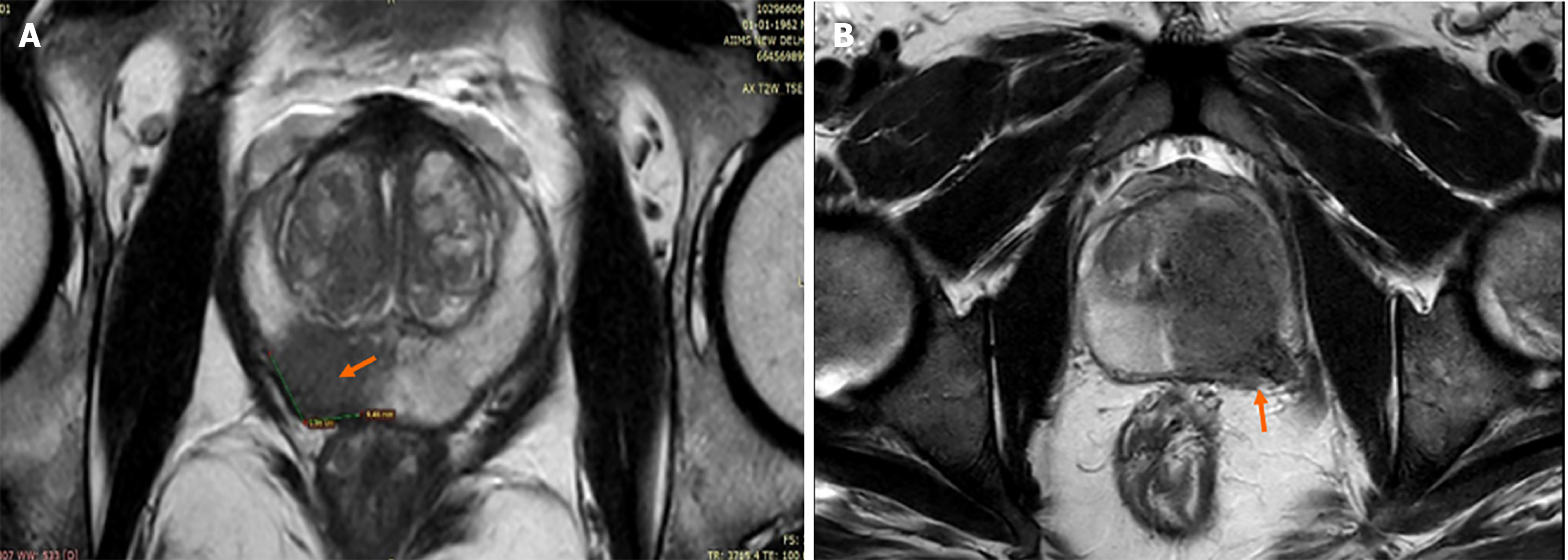
Length of capsular contact (LCC) is the length for which the prostatic tumor is in contact with the prostatic capsule[67]. It is a simple measurement, relatively independent of the radiologist’s experience, and more objective with good inter-reader reproducibility. This makes it a good quantitative criterion for diagnosing ECE, which is a crucial point in PCa staging and prognosis[68] (Figure 4). ECE in patients with T3bN0 PCa is linked with a greater risk of systemic spread of the disease and mortality. The presence of ECE is also an important treatment deciding point. If present, it upgrades the disease stage from T2 (organ-confined disease) to T3a (locally advanced disease)[68]. The curative treatment is most likely if the stage is ≤ T2c[69]. Currently, the criteria being used for diagnosing ECE in PIRADS v.2.1 include abutment or bulging of the capsule, irregularity of prostatic capsule, adjacent neurovascular bundle thickening, invasion of peri-prostatic fatty tissue and obliteration of the recto-prostatic angle[70]. However, these criteria are subjective and the reader’s experience profoundly affects ECE detection as shown in a study by Wibmer et al[68]. Also, these criteria diagnose visible macroscopic ECE, not microscopic one[70]. Thus, there should be some relatively more objective criterion such as LCC to overcome the shortcomings of above listed subjective criteria.
LCC is found to be an independent and reproducible predictor of ECE in multiple studies[67,71-73]. In a study by Baco et al[67], the logistic regression analysis revealed that the pathological-LCC (0.821) superiorly correlated with microscopic ECE as compared to the pathological cancer volume (0.685). Spearman correlation between the pathological-LCC and MRI-LCC was significant with r = 0.839 (P < 0.0001). MRI-LCC threshold of 20 mm showed superior accuracy compared to subjective analysis in diagnosing microscopic ECE (82% vs 67%, P = 0.015). This concludes that LCC can outperform subjective criteria in predicting microscopic ECE. A meta-analysis by Kim et al[74] concluded, the more the MRI-LCC value, the higher is the probability of ECE. In a cohort study[75], LCC was found to be an independent predictor of pathological lymph node, and biochemical recurrence along with pathological ECE. A retrospective study also found, LCC and ADC when used in combination, improved the diagnostic performance in demonstrating microscopic ECE[76]. Limitations of LCC are the inter-individual differences in measurement technique (by linear or curvilinear method)[72,73], no defined threshold (varying between 6-20 mm)[72,76,77], no consensus on which sequence to be used for mea
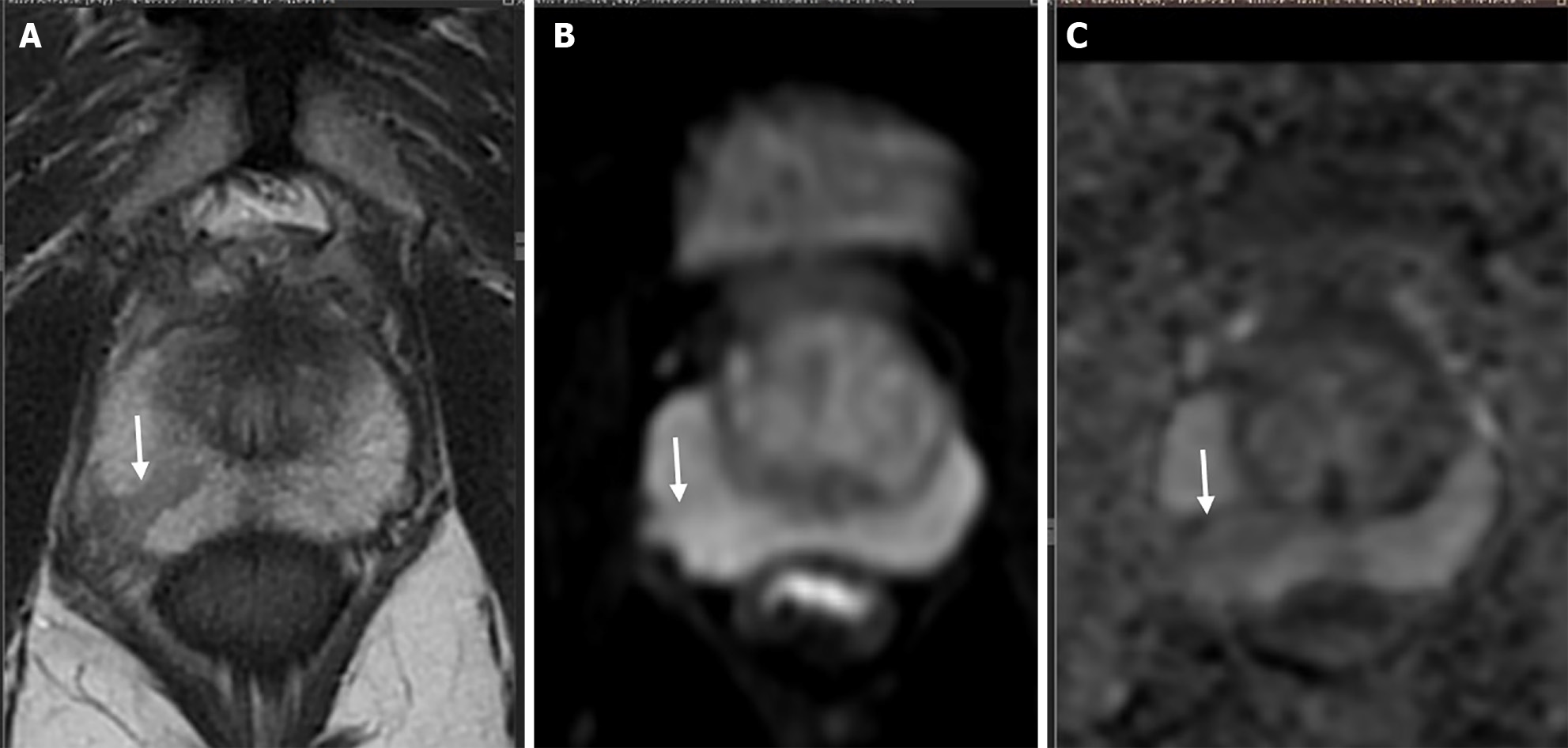
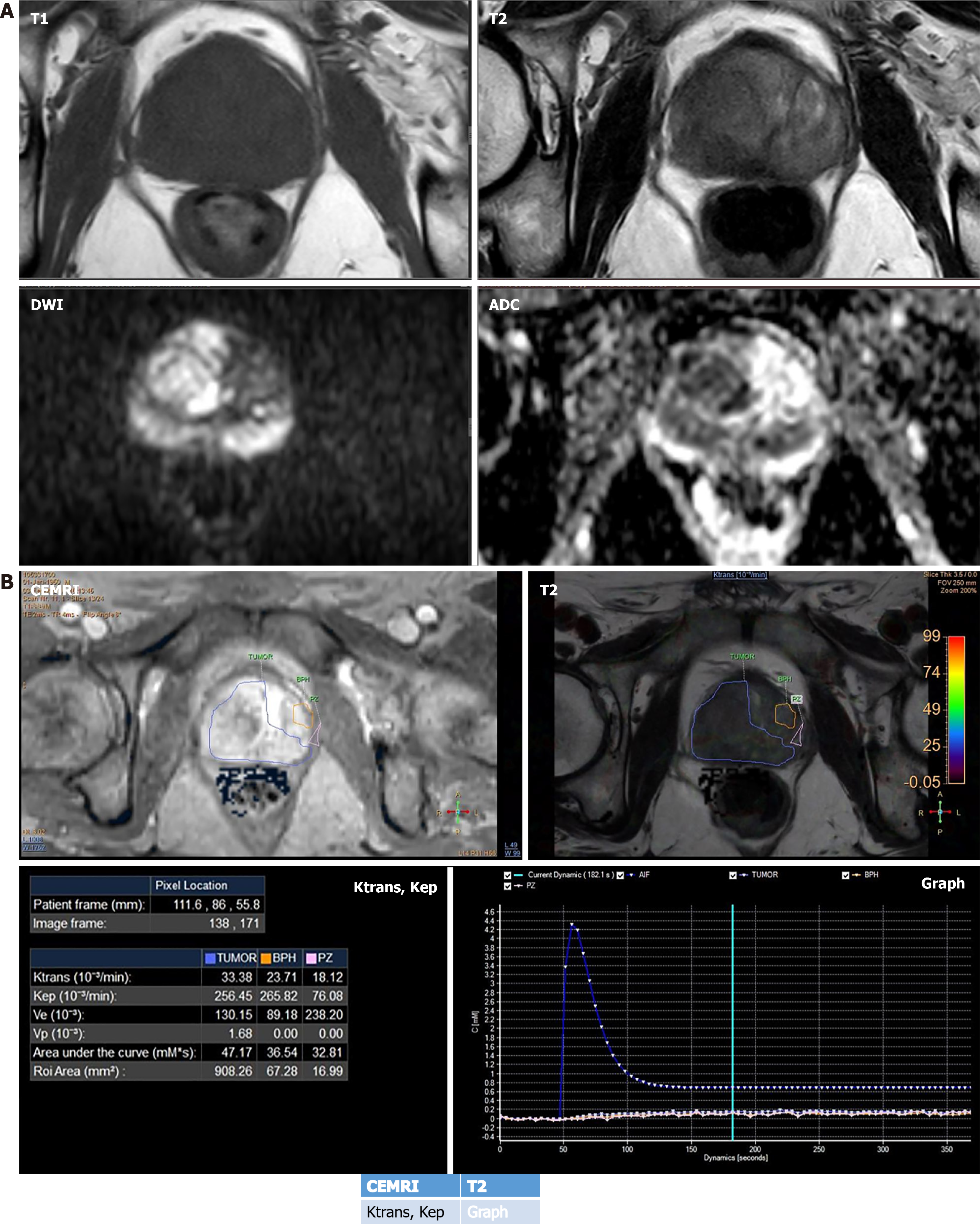
Angiogenesis is considered as a hallmark of any malignancy including PCa and dynamic contrast-enhanced (DCE) imaging enables non-invasive assessment of tissue vascularity at the cellular level. And by assessing that, it has the potential to assess the aggressiveness of the tumor[24,78-81]. In PIRADS v.2.1, DCE imaging has accessory role as compared to the primary sequences such as T2 in TZ and DWI in PZ. Its main role is in PZ PIRADS 3 lesion, positive finding on DCE MRI upgrades score to PIRADS 4[22]. Lack of quantification is one of the major reasons behind its diminished role in PIRADS and quantification can help in utilizing the full potential of this technique. DCE is a functional imaging technique that offers qualitative, semiquantitative as well as quantitative assessment of PCa[82] (Figure 5). Quantitative assessment is preferred, as it provides objective reproducible numerical values and commutation is also easy these days due to easy availability of software packages. Quantitative kinetic parameters encompass Ktrans (volume transfer constant) and Kep (rate constant). Ktrans is equal to the product of permeability and surface area (P × S) per unit volume of the tissue[82]. Kep (Ktrans/Ve), refers to the efflux of contrast from the extracellular compartment back into plasma (Ve refers to extracellular extravascular volume fraction)[82]. A retrospective study of PZ lesions at 3T found a significant correlation between Ktrans, Kep values, and aggressiveness of PCa[83]. The quantitative DCE parameters, Ktrans and Kep are highly correlated with the histopathology of the prostate tumor tissue and act as independent predictors of malignancy. Higher the Ktrans and Kep values, the lesion is more likely to be malignant. The study identified cutoff points of ± 0.2905 for Ktrans and ± 0.3365 for Kep, with sensitivities of 88.2% and 94.1%, specificities of 84.6% for both, positive predictive values of 88.2% and 88.9%, and negative predictive values (NPV) of 84.6% and 91.7%, respectively[84]. Few studies have also assessed the combined diagnostic accuracy of DCE with diffusion tensor imaging (DTI)[85] and also DCE with DWI[86] in PCa diagnosis. The combination helps in assessing different facets of the disease process, DCE assesses the micro-vessel density and permeability, while DTI and diffusion focus on cellular density. One such study by Li et al[85] in PZ lesions on 3T MRI found that DTI + DCE was significantly better than either DTI [area under the receiver operating characteristic curve (AUC) 0.93 vs 0.86, P = 0.0017] or DCE (AUC 0.93 vs 0.84, P = 0.0034) alone. On the contrary, many studies have also found an overlap of Ktrans and Kep values for benign and malignant lesions[87,88] and also there are studies that demonstrated no correlation between any of the DCE parameters and GS[89]. The study performed by Feng et al[90], showed that as a single predictor DCE [the odds ratios (OR) of Kep (0.987, P > 0.05) and Ktrans (0.794, P > 0.05) closer to 1] does not contribute to the CsPCa model. However, the diagnostic and positive predictive value of the multiparametric model were significantly higher than those of the biparametric model (prostate MRI without DCE)[90]. This concludes DCE should be interpreted always in conjunction with other MRI parameters for better results.
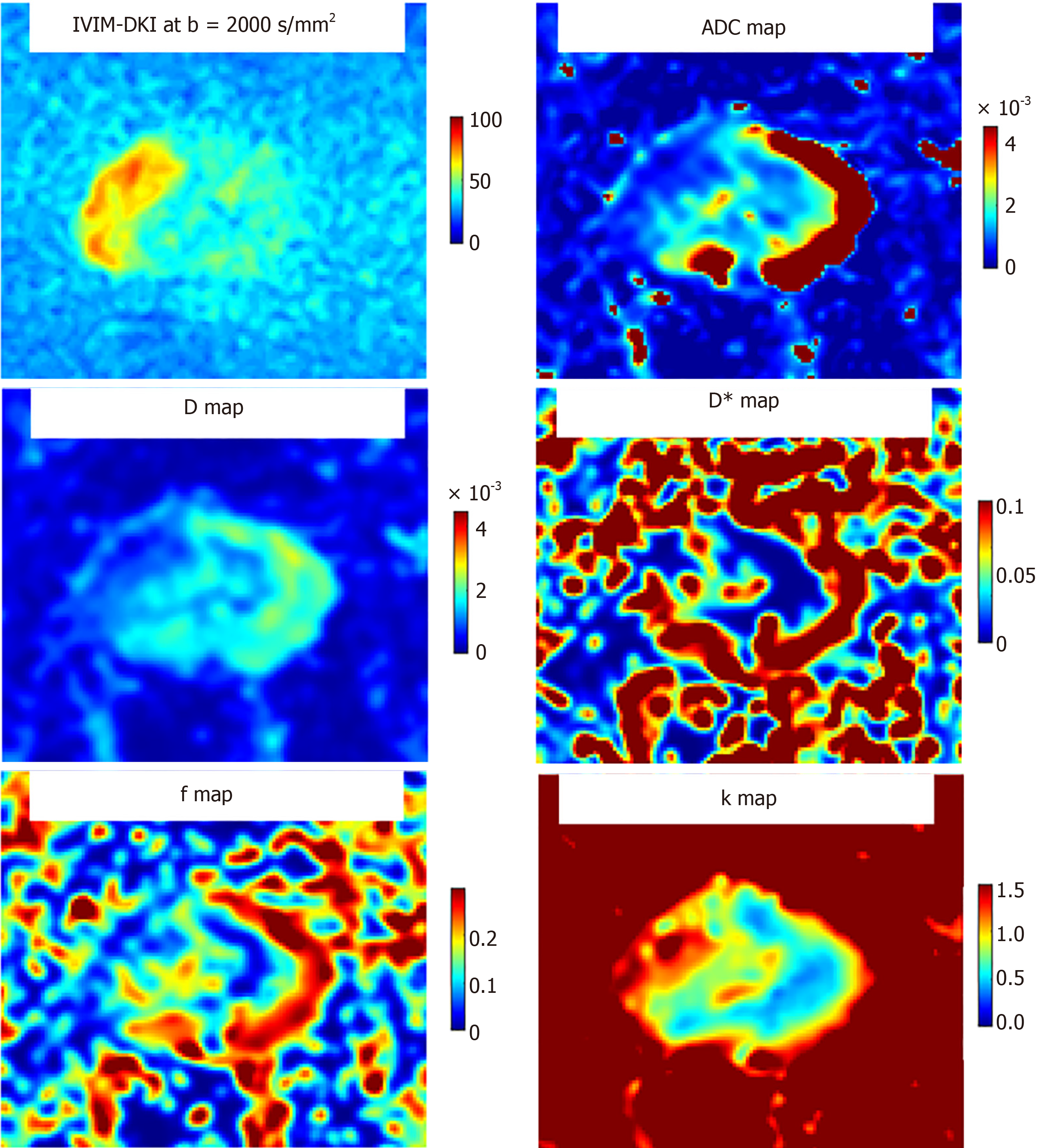
Le Bihan et al[91] and Le Bihan[92] first introduced the concept of intravoxel incoherent motion (IVIM), merging the diffusion and perfusion components, for quantitative analysis of the microstructure and microvasculature of the tissue. It is an extended version of DWI and can be used in the detection of PCa in PZ and TZ. DWI is based on the mono-exponential decay theory, assuming the gaussian distribution of Brownian motion of water molecules. However, such an assumption doesn’t always hold true in vivo, where countless numbers of barriers and inter and intracellular compart
Previous studies demonstrated that IVIM parameters were inferior to ADC in the evaluation of TZ lesions[97]; however, could increase the diagnostic yield in PZ lesions[95]. A study by Chang et al[98], found that D mean and D*kurtosis were important predictors of the postoperative ISUP high-risk group (P < 0.05). Liu et al[99] in comparison of the following models i.e., the monoexponential model (conventional DWI), IVIM, IVIM kurtosis, and kurtosis model, found that each model was useful for PCa diagnosis, however, diagnostic efficacy was similar amongst them. A recent study by Das et al[100] evaluated the role of combined IVIM-DKI and their machine-learning-based texture analysis (TA) for the detection and assessment of severity in PCa. The results of the study reported that D, f, and k computed using the IVIM-DKI model with the TV method were able to differentiate PCa from BPH and normal PZ. Texture features of combined IVIM-DKI parameters showed high accuracy and AUC in PCa detection[100]. Few studies have also demon
T1 relaxometry is a potential quantitative technique that enables the assessment of tissue properties by measuring the T1 relaxation time. T1 relaxation is an intrinsic property of any specific tissue and it is defined as the time taken by the longitudinal magnetization to recover 66.6% of its initial value[105]. Conventional sequences can quantify either T1 or T2 relaxation at one time, while the recently introduced MR fingerprinting technique enables quick and concomitant production of quantitative maps of different tissue properties including T1 and T2 relaxation time[106-109]. This technique is based on the pseudorandom variation of MRI properties like repetition time and flip angle so that unique signals are generated for each combination of tissue properties and then a chain of all possible signal evolutions is calculated for that particular sequence[110]. So far, multiple studies have been carried out to verify the role of T2 relaxation time[108,109]. In a recent study including 104 PZ cases, it was found that a combination of T1, T2 relaxometry, and ADC mapping could be useful for quantitative analysis of PCa grades and distinguishing it from benign lesions on T2-weighted images[110].
In comparison to T2 relaxometry, T1 relaxometry is relatively less studied in TZ and PZ lesions. It could be specifically more useful in differentiating prostatitis from PCa in TZ, as these both share a similar appearance on T2 images[111]. In one such study by Panda et al[111] in TZ lesions, the best results were shown by the combination of T1 and ADC in distinguishing benign and malignant lesions (AUC = 0.94) as well as in discriminating CsPCa from non-CsPCa (AUC = 0.81). ADC value lower than 0.70 × 10-3 mm2/second was highly sensitive and a T1 value lower than 1510 ms was found to be more specific for distinguishing benign and malignant lesions. There was an overlap of T2 values between the groups as expected. A study by Yu et al[112] in 2017 was the first one in the literature to study T1 differences between benign and malignant PZ lesions. In their study, T1 was significantly less in PZ Ca as compared to adjacent normal PZ. They also pointed out that the history of recent biopsy should always be elicited from the patient, as post-biopsy haemorrhage itself shortens the T1 time, resulting in fallacious results. However, the role of T1 relaxometry in PZ lesions still needs validation by larger studies.
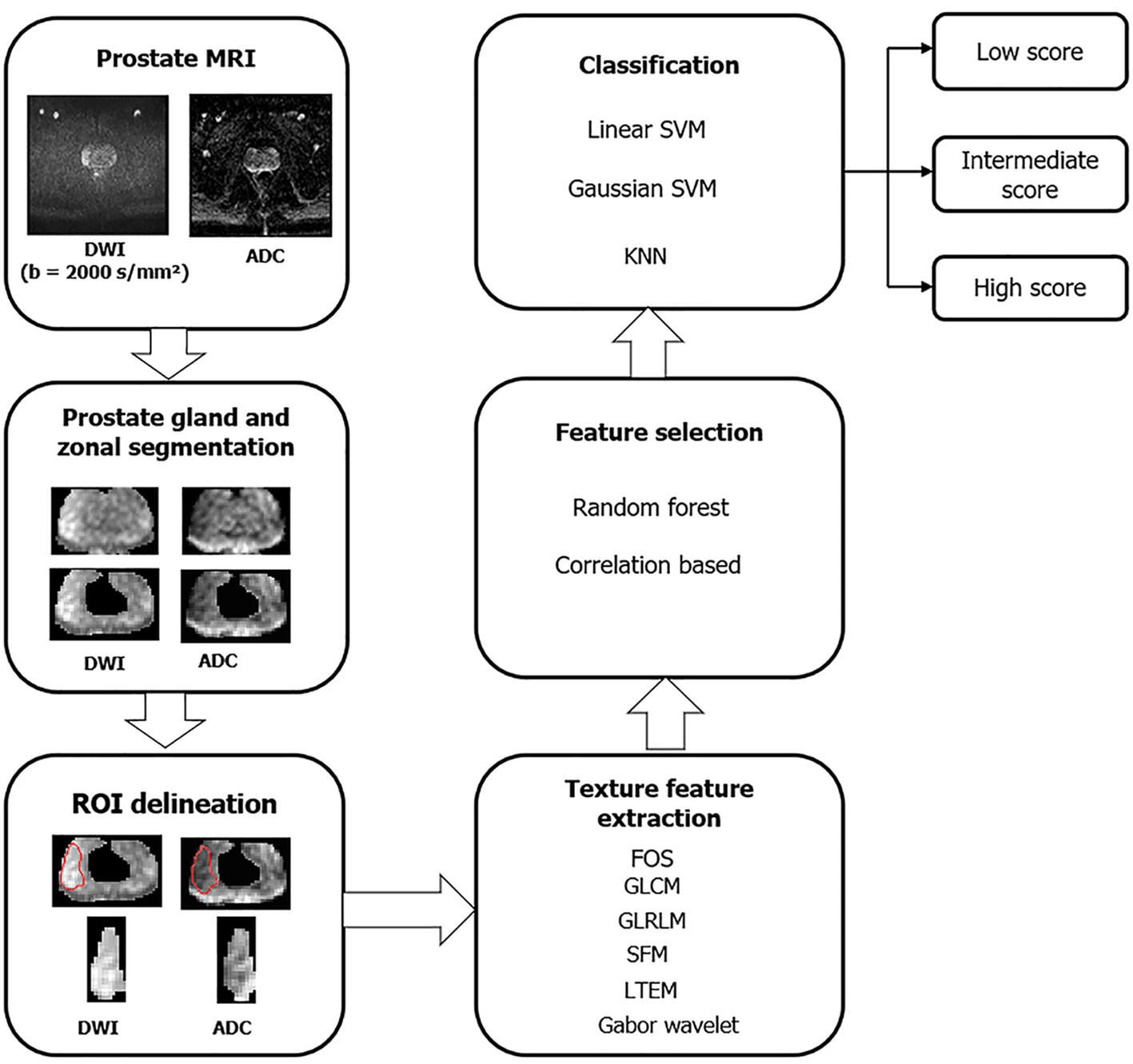
Tumour heterogeneity plays an important role in predicting a tumour’s aggressiveness. TA is a statistical tool to measure this tumor heterogeneity through a complex analysis of the spatial distribution of image pixels, grossly not recognizable on conventional sequences[113] (overview of framework for TA shown in Figure 7). Thus, TA could become a non-invasive imaging biomarker for PCa diagnosis, prognosis, and follow-up. The most widely used and verified technique of quantitative TA is the filtration-histogram technique[114]. First-order TA features are based on the extraction of pixel intensity values within a specific area of interest. Second-order statistics measure the association between two pixels while higher-order TA analyses the association among more than two pixels. Some of the texture features which are most widely studied and commonly employed in PCa are entropy (represents histogram’s randomness) and kurtosis (re
Magnetic resonance elastography (MRE) is a non-invasive method that uses low-frequency vibrations to quantitatively measure tissue elasticity and stiffness. In comparison to other quantitative MR techniques, it has the potential to overcome the common limitation of all techniques i.e., inter-observer variability. In a prospective study by Li et al[122] involving 73 patients, stiffness and fluidity quantification using tomoelastography improved the diagnostic yield of multiparametric MRI in detecting PCa in both PZ and TZ. Pre-operative MRE-based PCa stiffness measurement can even help in predicting the degree of lymph node metastasis, with sensitivity and specificity as high as 100% and 86.5%, respectively[123].
MRE is a non-invasive method that uses low-frequency vibrations to quantitatively measure tissue elasticity and stiffness. In comparison to other quantitative MR techniques, it has the potential to overcome the common limitation of all techniques i.e., inter-observer variability. In a prospective study by Li et al[122] involving 73 patients, stiffness and fluidity quantification using tomoelastography improved the diagnostic yield of multiparametric MRI in detecting PCa in both PZ and TZ. Pre-operative MRE-based PCa stiffness measurement can even help in predicting the degree of lymph node metastasis, with sensitivity and specificity as high as 100% and 86.5%, respectively[123].
Quantitative imaging has many advantages over conventional qualitative assessment. A few parameters have also been shown to correlate with the GS and can help in deciding disease prognosis and clinical management. Many such MRI based quantitative imaging metrics have been discussed thoroughly in this article. Few of these are already part of PIRADS, while many are under study and can be incorporated into clinical practice in the future after validation by rigorous multicentre studies. One major hurdle in achieving the quantification is the fact that numerical values obtained tend to differ from center to center because of many factors including differences in scanners, individual imaging protocols, and techniques, etc. The applications of features like TV and size, LCC, ADC values need a standardized cut off values to achieve their full clinical potential. An ideal metric would be one that is easy to calculate, and less prone to errors because of differences in scanners, individual imaging protocols, and techniques. A quantitative imaging marker could improve PCa detection by minimizing inter-radiologist subjectivity, thereby reducing overdiagnosis. This would help avoid unnecessary biopsies and decrease the overtreatment of slow-growing PCa.
| 1. | Bray F, Laversanne M, Sung H, Ferlay J, Siegel RL, Soerjomataram I, Jemal A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2024;74:229-263. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5690] [Cited by in RCA: 12953] [Article Influence: 6476.5] [Reference Citation Analysis (8)] |
| 2. | Reddy RR, Jagannathan NR. Potential of nuclear magnetic resonance metabolomics in the study of prostate cancer. Indian J Urol. 2022;38:99-109. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 3. | Fernandes MC, Yildirim O, Woo S, Vargas HA, Hricak H. The role of MRI in prostate cancer: current and future directions. MAGMA. 2022;35:503-521. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 41] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 4. | Rebello RJ, Oing C, Knudsen KE, Loeb S, Johnson DC, Reiter RE, Gillessen S, Van der Kwast T, Bristow RG. Prostate cancer. Nat Rev Dis Primers. 2021;7:9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 741] [Article Influence: 148.2] [Reference Citation Analysis (0)] |
| 5. | Lucarelli NM, Villanova I, Maggialetti N, Greco S, Tarantino F, Russo R, Trabucco SMR, Stabile Ianora AA, Scardapane A. Quantitative ADC: An Additional Tool in the Evaluation of Prostate Cancer? J Pers Med. 2023;13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 6. | Turkbey B, Rosenkrantz AB, Haider MA, Padhani AR, Villeirs G, Macura KJ, Tempany CM, Choyke PL, Cornud F, Margolis DJ, Thoeny HC, Verma S, Barentsz J, Weinreb JC. Prostate Imaging Reporting and Data System Version 2.1: 2019 Update of Prostate Imaging Reporting and Data System Version 2. Eur Urol. 2019;76:340-351. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 711] [Cited by in RCA: 1750] [Article Influence: 250.0] [Reference Citation Analysis (0)] |
| 7. | Qayyum A. Diffusion-weighted imaging in the abdomen and pelvis: concepts and applications. Radiographics. 2009;29:1797-1810. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 248] [Cited by in RCA: 265] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 8. | Thoeny HC, De Keyzer F. Extracranial applications of diffusion-weighted magnetic resonance imaging. Eur Radiol. 2007;17:1385-1393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 204] [Article Influence: 10.7] [Reference Citation Analysis (0)] |
| 9. | Kumar V, Jagannathan NR, Kumar R, Das SC, Jindal L, Thulkar S, Gupta SD, Dwivedi SN, Roell S, Hemal AK, Gupta NP. Correlation between metabolite ratios and ADC values of prostate in men with increased PSA level. Magn Reson Imaging. 2006;24:541-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 52] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 10. | Kumar V, Jagannathan NR, Kumar R, Thulkar S, Gupta SD, Dwivedi SN, Hemal AK, Gupta NP. Apparent diffusion coefficient of the prostate in men prior to biopsy: determination of a cut-off value to predict malignancy of the peripheral zone. NMR Biomed. 2007;20:505-511. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 49] [Cited by in RCA: 46] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 11. | Rosenkrantz AB, Parikh N, Kierans AS, Kong MX, Babb JS, Taneja SS, Ream JM. Prostate Cancer Detection Using Computed Very High b-value Diffusion-weighted Imaging: How High Should We Go? Acad Radiol. 2016;23:704-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 12. | Rosenkrantz AB, Hindman N, Lim RP, Das K, Babb JS, Mussi TC, Taneja SS. Diffusion-weighted imaging of the prostate: Comparison of b1000 and b2000 image sets for index lesion detection. J Magn Reson Imaging. 2013;38:694-700. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 77] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 13. | Ueno Y, Kitajima K, Sugimura K, Kawakami F, Miyake H, Obara M, Takahashi S. Ultra-high b-value diffusion-weighted MRI for the detection of prostate cancer with 3-T MRI. J Magn Reson Imaging. 2013;38:154-160. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 50] [Cited by in RCA: 56] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 14. | Wang X, Qian Y, Liu B, Cao L, Fan Y, Zhang JJ, Yu Y. High-b-value diffusion-weighted MRI for the detection of prostate cancer at 3 T. Clin Radiol. 2014;69:1165-1170. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 22] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 15. | Wetter A, Nensa F, Lipponer C, Guberina N, Olbricht T, Schenck M, Schlosser TW, Gratz M, Lauenstein TC. High and ultra-high b-value diffusion-weighted imaging in prostate cancer: a quantitative analysis. Acta Radiol. 2015;56:1009-1015. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 27] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 16. | Metens T, Miranda D, Absil J, Matos C. What is the optimal b value in diffusion-weighted MR imaging to depict prostate cancer at 3T? Eur Radiol. 2012;22:703-709. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 96] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 17. | Agarwal HK, Mertan FV, Sankineni S, Bernardo M, Senegas J, Keupp J, Daar D, Merino M, Wood BJ, Pinto PA, Choyke PL, Turkbey B. Optimal high b-value for diffusion weighted MRI in diagnosing high risk prostate cancers in the peripheral zone. J Magn Reson Imaging. 2017;45:125-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 18. | Ohgiya Y, Suyama J, Seino N, Hashizume T, Kawahara M, Sai S, Saiki M, Munechika J, Hirose M, Gokan T. Diagnostic accuracy of ultra-high-b-value 3.0-T diffusion-weighted MR imaging for detection of prostate cancer. Clin Imaging. 2012;36:526-531. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 38] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 19. | Rosenkrantz AB, Chandarana H, Hindman N, Deng FM, Babb JS, Taneja SS, Geppert C. Computed diffusion-weighted imaging of the prostate at 3 T: impact on image quality and tumour detection. Eur Radiol. 2013;23:3170-3177. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 63] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 20. | Kwon MR, Kim CK, Kim JH. PI-RADS version 2: evaluation of diffusion-weighted imaging interpretation between b = 1000 and b = 1500 s mm(-)(2). Br J Radiol. 2017;90:20170438. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 7] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 21. | Rosenkrantz AB, Padhani AR, Chenevert TL, Koh DM, De Keyzer F, Taouli B, Le Bihan D. Body diffusion kurtosis imaging: Basic principles, applications, and considerations for clinical practice. J Magn Reson Imaging. 2015;42:1190-1202. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 258] [Cited by in RCA: 302] [Article Influence: 27.5] [Reference Citation Analysis (0)] |
| 22. | Weinreb JC, Barentsz JO, Choyke PL, Cornud F, Haider MA, Macura KJ, Margolis D, Schnall MD, Shtern F, Tempany CM, Thoeny HC, Verma S. PI-RADS Prostate Imaging - Reporting and Data System: 2015, Version 2. Eur Urol. 2016;69:16-40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1765] [Cited by in RCA: 2349] [Article Influence: 213.5] [Reference Citation Analysis (0)] |
| 23. | Verma S, Rajesh A, Morales H, Lemen L, Bills G, Delworth M, Gaitonde K, Ying J, Samartunga R, Lamba M. Assessment of aggressiveness of prostate cancer: correlation of apparent diffusion coefficient with histologic grade after radical prostatectomy. AJR Am J Roentgenol. 2011;196:374-381. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 174] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 24. | Woodfield CA, Tung GA, Grand DJ, Pezzullo JA, Machan JT, Renzulli JF 2nd. Diffusion-weighted MRI of peripheral zone prostate cancer: comparison of tumor apparent diffusion coefficient with Gleason score and percentage of tumor on core biopsy. AJR Am J Roentgenol. 2010;194:W316-W322. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 158] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 25. | Vargas HA, Akin O, Franiel T, Mazaheri Y, Zheng J, Moskowitz C, Udo K, Eastham J, Hricak H. Diffusion-weighted endorectal MR imaging at 3 T for prostate cancer: tumor detection and assessment of aggressiveness. Radiology. 2011;259:775-784. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 328] [Cited by in RCA: 338] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 26. | Donati OF, Mazaheri Y, Afaq A, Vargas HA, Zheng J, Moskowitz CS, Hricak H, Akin O. Prostate cancer aggressiveness: assessment with whole-lesion histogram analysis of the apparent diffusion coefficient. Radiology. 2014;271:143-152. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 239] [Cited by in RCA: 251] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 27. | Tamada T, Prabhu V, Li J, Babb JS, Taneja SS, Rosenkrantz AB. Assessment of prostate cancer aggressiveness using apparent diffusion coefficient values: impact of patient race and age. Abdom Radiol (NY). 2017;42:1744-1751. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 28. | Litjens GJ, Hambrock T, Hulsbergen-van de Kaa C, Barentsz JO, Huisman HJ. Interpatient variation in normal peripheral zone apparent diffusion coefficient: effect on the prediction of prostate cancer aggressiveness. Radiology. 2012;265:260-266. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 51] [Cited by in RCA: 64] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 29. | Chatterjee A, Gallan AJ, He D, Fan X, Mustafi D, Yousuf A, Antic T, Karczmar GS, Oto A. Revisiting quantitative multi-parametric MRI of benign prostatic hyperplasia and its differentiation from transition zone cancer. Abdom Radiol (NY). 2019;44:2233-2243. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 31] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 30. | Kitzing YX, Prando A, Varol C, Karczmar GS, Maclean F, Oto A. Benign Conditions That Mimic Prostate Carcinoma: MR Imaging Features with Histopathologic Correlation. Radiographics. 2016;36:162-175. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 129] [Article Influence: 11.7] [Reference Citation Analysis (0)] |
| 31. | Woo S, Kim SY, Cho JY, Kim SH. Preoperative Evaluation of Prostate Cancer Aggressiveness: Using ADC and ADC Ratio in Determining Gleason Score. AJR Am J Roentgenol. 2016;207:114-120. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 32. | Rosenkrantz AB, Kopec M, Kong X, Melamed J, Dakwar G, Babb JS, Taouli B. Prostate cancer vs. post-biopsy hemorrhage: diagnosis with T2- and diffusion-weighted imaging. J Magn Reson Imaging. 2010;31:1387-1394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 73] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 33. | Bajgiran AM, Mirak SA, Sung K, Sisk AE, Reiter RE, Raman SS. Apparent Diffusion Coefficient (ADC) Ratio Versus Conventional ADC for Detecting Clinically Significant Prostate Cancer With 3-T MRI. AJR Am J Roentgenol. 2019;213:W134-W142. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 34. | Giganti F, Pecoraro M, Fierro D, Campa R, Del Giudice F, Punwani S, Kirkham A, Allen C, Emberton M, Catalano C, Moore CM, Panebianco V. DWI and PRECISE criteria in men on active surveillance for prostate cancer: A multicentre preliminary experience of different ADC calculations. Magn Reson Imaging. 2020;67:50-58. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 35. | Fennessy FM, Maier SE. Quantitative diffusion MRI in prostate cancer: Image quality, what we can measure and how it improves clinical assessment. Eur J Radiol. 2023;167:111066. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 36. | Jyoti R, Jain TP, Haxhimolla H, Liddell H, Barrett SE. Correlation of apparent diffusion coefficient ratio on 3.0 T MRI with prostate cancer Gleason score. Eur J Radiol Open. 2018;5:58-63. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 21] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 37. | Barrett T, Priest AN, Lawrence EM, Goldman DA, Warren AY, Gnanapragasam VJ, Sala E, Gallagher FA. Ratio of Tumor to Normal Prostate Tissue Apparent Diffusion Coefficient as a Method for Quantifying DWI of the Prostate. AJR Am J Roentgenol. 2015;205:W585-W593. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 38. | De Cobelli F, Ravelli S, Esposito A, Giganti F, Gallina A, Montorsi F, Del Maschio A. Apparent diffusion coefficient value and ratio as noninvasive potential biomarkers to predict prostate cancer grading: comparison with prostate biopsy and radical prostatectomy specimen. AJR Am J Roentgenol. 2015;204:550-557. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 70] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 39. | Rozenberg R, Thornhill RE, Flood TA, Hakim SW, Lim C, Schieda N. Whole-Tumor Quantitative Apparent Diffusion Coefficient Histogram and Texture Analysis to Predict Gleason Score Upgrading in Intermediate-Risk 3 + 4 = 7 Prostate Cancer. AJR Am J Roentgenol. 2016;206:775-782. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 71] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 40. | Wu X, Reinikainen P, Vanhanen A, Kapanen M, Vierikko T, Ryymin P, Hyödynmaa S, Kellokumpu-Lehtinen PL. Correlation between apparent diffusion coefficient value on diffusion-weighted MR imaging and Gleason score in prostate cancer. Diagn Interv Imaging. 2017;98:63-71. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 49] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 41. | Zhang Z, Xu H, Xue Y, Li J, Ye Q. Risk Stratification of Prostate Cancer Using the Combination of Histogram Analysis of Apparent Diffusion Coefficient Across Tumor Diffusion Volume and Clinical Information: A Pilot Study. J Magn Reson Imaging. 2019;49:556-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 42. | Surov A, Meyer HJ, Wienke A. Correlations between Apparent Diffusion Coefficient and Gleason Score in Prostate Cancer: A Systematic Review. Eur Urol Oncol. 2020;3:489-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 42] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 43. | Priester A, Natarajan S, Khoshnoodi P, Margolis DJ, Raman SS, Reiter RE, Huang J, Grundfest W, Marks LS. Magnetic Resonance Imaging Underestimation of Prostate Cancer Geometry: Use of Patient Specific Molds to Correlate Images with Whole Mount Pathology. J Urol. 2017;197:320-326. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 198] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 44. | Stamey TA, McNeal JE, Freiha FS, Redwine E. Morphometric and clinical studies on 68 consecutive radical prostatectomies. J Urol. 1988;139:1235-1241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 366] [Cited by in RCA: 340] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 45. | Knoedler JJ, Karnes RJ, Thompson RH, Rangel LJ, Bergstralh EJ, Boorjian SA. The association of tumor volume with mortality following radical prostatectomy. Prostate Cancer Prostatic Dis. 2014;17:144-148. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 40] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 46. | Egevad L, Norberg M, Mattson S, Norlén BJ, Busch C. Estimation of prostate cancer volume by multiple core biopsies before radical prostatectomy. Urology. 1998;52:653-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 32] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 47. | Muller BG, Fütterer JJ, Gupta RT, Katz A, Kirkham A, Kurhanewicz J, Moul JW, Pinto PA, Rastinehad AR, Robertson C, de la Rosette J, Sanchez-Salas R, Jones JS, Ukimura O, Verma S, Wijkstra H, Marberger M. The role of magnetic resonance imaging (MRI) in focal therapy for prostate cancer: recommendations from a consensus panel. BJU Int. 2014;113:218-227. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 71] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 48. | Moore CM, Giganti F, Albertsen P, Allen C, Bangma C, Briganti A, Carroll P, Haider M, Kasivisvanathan V, Kirkham A, Klotz L, Ouzzane A, Padhani AR, Panebianco V, Pinto P, Puech P, Rannikko A, Renard-Penna R, Touijer K, Turkbey B, van Poppel H, Valdagni R, Walz J, Schoots I. Reporting Magnetic Resonance Imaging in Men on Active Surveillance for Prostate Cancer: The PRECISE Recommendations-A Report of a European School of Oncology Task Force. Eur Urol. 2017;71:648-655. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 198] [Article Influence: 22.0] [Reference Citation Analysis (0)] |
| 49. | Gholami N, Haghparast A, Alipourfard I, Nazari M. Prostate cancer in omics era. Cancer Cell Int. 2022;22:274. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 8] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 50. | Mazzucchelli R, Scarpelli M, Cheng L, Lopez-Beltran A, Galosi AB, Kirkali Z, Montironi R. Pathology of prostate cancer and focal therapy ('male lumpectomy'). Anticancer Res. 2009;29:5155-5161. [PubMed] |
| 51. | Lindner U, Lawrentschuk N, Trachtenberg J. Image guidance for focal therapy of prostate cancer. World J Urol. 2010;28:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 22] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 52. | McNeal JE, Villers AA, Redwine EA, Freiha FS, Stamey TA. Capsular penetration in prostate cancer. Significance for natural history and treatment. Am J Surg Pathol. 1990;14:240-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 198] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 53. | Villers AA, McNeal JE, Redwine EA, Freiha FS, Stamey TA. Pathogenesis and biological significance of seminal vesicle invasion in prostatic adenocarcinoma. J Urol. 1990;143:1183-1187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 142] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 54. | McNeal JE, Villers AA, Redwine EA, Freiha FS, Stamey TA. Histologic differentiation, cancer volume, and pelvic lymph node metastasis in adenocarcinoma of the prostate. Cancer. 1990;66:1225-1233. [PubMed] [DOI] [Full Text] |
| 55. | Bostwick DG, Graham SD Jr, Napalkov P, Abrahamsson PA, di Sant'agnese PA, Algaba F, Hoisaeter PA, Lee F, Littrup P, Mostofi FK. Staging of early prostate cancer: a proposed tumor volume-based prognostic index. Urology. 1993;41:403-411. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 118] [Cited by in RCA: 103] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 56. | Hambrock T, Somford DM, Huisman HJ, van Oort IM, Witjes JA, Hulsbergen-van de Kaa CA, Scheenen T, Barentsz JO. Relationship between apparent diffusion coefficients at 3.0-T MR imaging and Gleason grade in peripheral zone prostate cancer. Radiology. 2011;259:453-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 453] [Cited by in RCA: 477] [Article Influence: 31.8] [Reference Citation Analysis (0)] |
| 57. | Mazaheri Y, Hricak H, Fine SW, Akin O, Shukla-Dave A, Ishill NM, Moskowitz CS, Grater JE, Reuter VE, Zakian KL, Touijer KA, Koutcher JA. Prostate tumor volume measurement with combined T2-weighted imaging and diffusion-weighted MR: correlation with pathologic tumor volume. Radiology. 2009;252:449-457. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 167] [Cited by in RCA: 160] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 58. | Singh D, Das CJ, Kumar V, Singh A, Mehndiratta A. Quantification of prostate tumour diameter and volume from MR images using 3D ellipsoid model and its impact on PI-RADS v2.1 assessment. Sci Rep. 2022;12:21501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 2] [Reference Citation Analysis (0)] |
| 59. | Lovegrove CE, Matanhelia M, Randeva J, Eldred-Evans D, Tam H, Miah S, Winkler M, Ahmed HU, Shah TT. Prostate imaging features that indicate benign or malignant pathology on biopsy. Transl Androl Urol. 2018;7:S420-S435. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 19] [Cited by in RCA: 29] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 60. | Barentsz JO, Weinreb JC, Verma S, Thoeny HC, Tempany CM, Shtern F, Padhani AR, Margolis D, Macura KJ, Haider MA, Cornud F, Choyke PL. Synopsis of the PI-RADS v2 Guidelines for Multiparametric Prostate Magnetic Resonance Imaging and Recommendations for Use. Eur Urol. 2016;69:41-49. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 372] [Cited by in RCA: 435] [Article Influence: 39.5] [Reference Citation Analysis (0)] |
| 61. | Moosavi B, Flood TA, Al-Dandan O, Breau RH, Cagiannos I, Morash C, Malone SC, Schieda N. Multiparametric MRI of the anterior prostate gland: clinical-radiological-histopathological correlation. Clin Radiol. 2016;71:405-417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 18] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 62. | Shaish H, Taneja SS, Rosenkrantz AB. Prostate MR Imaging: An Update. Radiol Clin North Am. 2017;55:303-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 63. | Li Q, Lu H, Choi J, Gage K, Feuerlein S, Pow-Sang JM, Gillies R, Balagurunathan Y. Radiological semantics discriminate clinically significant grade prostate cancer. Cancer Imaging. 2019;19:81. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 64. | Abreu-Gomez J, Wu M, McInnes MDF, Thornhill RE, Flood TA, Schieda N. Shape Analysis of Peripheral Zone Observations on Prostate DWI: Correlation to Histopathology Outcomes After Radical Prostatectomy. AJR Am J Roentgenol. 2020;214:1239-1247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 16] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 65. | Krishna S, Schieda N, McInnes MD, Flood TA, Thornhill RE. Diagnosis of transition zone prostate cancer using T2-weighted (T2W) MRI: comparison of subjective features and quantitative shape analysis. Eur Radiol. 2019;29:1133-1143. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 22] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 66. | Cuocolo R, Stanzione A, Ponsiglione A, Romeo V, Verde F, Creta M, La Rocca R, Longo N, Pace L, Imbriaco M. Clinically significant prostate cancer detection on MRI: A radiomic shape features study. Eur J Radiol. 2019;116:144-149. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 71] [Article Influence: 10.1] [Reference Citation Analysis (0)] |
| 67. | Baco E, Rud E, Vlatkovic L, Svindland A, Eggesbø HB, Hung AJ, Matsugasumi T, Bernhard JC, Gill IS, Ukimura O. Predictive value of magnetic resonance imaging determined tumor contact length for extracapsular extension of prostate cancer. J Urol. 2015;193:466-472. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 84] [Cited by in RCA: 99] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 68. | Wibmer A, Vargas HA, Donahue TF, Zheng J, Moskowitz C, Eastham J, Sala E, Hricak H. Diagnosis of Extracapsular Extension of Prostate Cancer on Prostate MRI: Impact of Second-Opinion Readings by Subspecialized Genitourinary Oncologic Radiologists. AJR Am J Roentgenol. 2015;205:W73-W78. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 61] [Cited by in RCA: 80] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 69. | de Rooij M, Hamoen EH, Witjes JA, Barentsz JO, Rovers MM. Accuracy of Magnetic Resonance Imaging for Local Staging of Prostate Cancer: A Diagnostic Meta-analysis. Eur Urol. 2016;70:233-245. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 337] [Cited by in RCA: 488] [Article Influence: 48.8] [Reference Citation Analysis (0)] |
| 70. | Fütterer JJ, Engelbrecht MR, Jager GJ, Hartman RP, King BF, Hulsbergen-Van de Kaa CA, Witjes JA, Barentsz JO. Prostate cancer: comparison of local staging accuracy of pelvic phased-array coil alone versus integrated endorectal-pelvic phased-array coils. Local staging accuracy of prostate cancer using endorectal coil MR imaging. Eur Radiol. 2007;17:1055-1065. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 122] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 71. | Onay A, Vural M, Armutlu A, Ozel Yıldız S, Kiremit MC, Esen T, Bakır B. Evaluation of the most optimal multiparametric magnetic resonance imaging sequence for determining pathological length of capsular contact. Eur J Radiol. 2019;112:192-199. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 17] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 72. | Rosenkrantz AB, Shanbhogue AK, Wang A, Kong MX, Babb JS, Taneja SS. Length of capsular contact for diagnosing extraprostatic extension on prostate MRI: Assessment at an optimal threshold. J Magn Reson Imaging. 2016;43:990-997. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 70] [Cited by in RCA: 91] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 73. | Woo S, Kim SY, Cho JY, Kim SH. Length of capsular contact on prostate MRI as a predictor of extracapsular extension: which is the most optimal sequence? Acta Radiol. 2017;58:489-497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 31] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 74. | Kim TH, Woo S, Han S, Suh CH, Ghafoor S, Hricak H, Vargas HA. The Diagnostic Performance of the Length of Tumor Capsular Contact on MRI for Detecting Prostate Cancer Extraprostatic Extension: A Systematic Review and Meta-Analysis. Korean J Radiol. 2020;21:684-694. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 35] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 75. | Kongnyuy M, Sidana A, George AK, Muthigi A, Iyer A, Ho R, Chelluri R, Mertan F, Frye TP, Su D, Merino MJ, Choyke PL, Wood BJ, Pinto PA, Turkbey B. Tumor contact with prostate capsule on magnetic resonance imaging: A potential biomarker for staging and prognosis. Urol Oncol. 2017;35:30.e1-30.e8. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 76. | Granja MF, Pedraza CM, Flórez DC, Romero JA, Palau MA, Aguirre DA. Predicting extracapsular involvement in prostate cancer through the tumor contact length and the apparent diffusion coefficient. Radiologia. 2017;59:313-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 77. | Lawrence EM, Gallagher FA, Barrett T, Warren AY, Priest AN, Goldman DA, Sala E, Gnanapragasam VJ. Preoperative 3-T diffusion-weighted MRI for the qualitative and quantitative assessment of extracapsular extension in patients with intermediate- or high-risk prostate cancer. AJR Am J Roentgenol. 2014;203:W280-W286. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 78. | Tamada T, Sone T, Jo Y, Toshimitsu S, Yamashita T, Yamamoto A, Tanimoto D, Ito K. Apparent diffusion coefficient values in peripheral and transition zones of the prostate: comparison between normal and malignant prostatic tissues and correlation with histologic grade. J Magn Reson Imaging. 2008;28:720-726. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 207] [Cited by in RCA: 207] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 79. | Schlemmer HP, Merkle J, Grobholz R, Jaeger T, Michel MS, Werner A, Rabe J, van Kaick G. Can pre-operative contrast-enhanced dynamic MR imaging for prostate cancer predict microvessel density in prostatectomy specimens? Eur Radiol. 2004;14:309-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 116] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 80. | Kiessling F, Lichy M, Grobholz R, Heilmann M, Farhan N, Michel MS, Trojan L, Ederle J, Abel U, Kauczor HU, Semmler W, Delorme S. Simple models improve the discrimination of prostate cancers from the peripheral gland by T1-weighted dynamic MRI. Eur Radiol. 2004;14:1793-1801. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 81. | Franiel T, Lüdemann L, Rudolph B, Rehbein H, Stephan C, Taupitz M, Beyersdorff D. Prostate MR imaging: tissue characterization with pharmacokinetic volume and blood flow parameters and correlation with histologic parameters. Radiology. 2009;252:101-108. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 45] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 82. | Verma S, Turkbey B, Muradyan N, Rajesh A, Cornud F, Haider MA, Choyke PL, Harisinghani M. Overview of dynamic contrast-enhanced MRI in prostate cancer diagnosis and management. AJR Am J Roentgenol. 2012;198:1277-1288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 194] [Cited by in RCA: 222] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 83. | Vos EK, Litjens GJ, Kobus T, Hambrock T, Hulsbergen-van de Kaa CA, Barentsz JO, Huisman HJ, Scheenen TW. Assessment of prostate cancer aggressiveness using dynamic contrast-enhanced magnetic resonance imaging at 3 T. Eur Urol. 2013;64:448-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 134] [Cited by in RCA: 143] [Article Influence: 11.0] [Reference Citation Analysis (0)] |
| 84. | Rosida YR, Sukmaningtyas H, Imawati S, Prajoko YW, Sadhana U. Qualitative and quantitative parameters of dynamic contrast-enhanced (DCE) MRI as a diagnostic determinant of soft tissue tumor malignancy: a study from Indonesia. Egypt J Radiol Nucl Med. 2023;54:118. [DOI] [Full Text] |
| 85. | Li C, Chen M, Li S, Zhao X, Zhang C, Luo X, Zhou C. Detection of prostate cancer in peripheral zone: comparison of MR diffusion tensor imaging, quantitative dynamic contrast-enhanced MRI, and the two techniques combined at 3.0 T. Acta Radiol. 2014;55:239-247. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 26] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 86. | Hötker AM, Mazaheri Y, Aras Ö, Zheng J, Moskowitz CS, Gondo T, Matsumoto K, Hricak H, Akin O. Assessment of Prostate Cancer Aggressiveness by Use of the Combination of Quantitative DWI and Dynamic Contrast-Enhanced MRI. AJR Am J Roentgenol. 2016;206:756-763. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 87. | Turkbey B, Pinto PA, Mani H, Bernardo M, Pang Y, McKinney YL, Khurana K, Ravizzini GC, Albert PS, Merino MJ, Choyke PL. Prostate cancer: value of multiparametric MR imaging at 3 T for detection--histopathologic correlation. Radiology. 2010;255:89-99. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 392] [Cited by in RCA: 413] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 88. | Berman RM, Brown AM, Chang SD, Sankineni S, Kadakia M, Wood BJ, Pinto PA, Choyke PL, Turkbey B. DCE MRI of prostate cancer. Abdom Radiol (NY). 2016;41:844-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 63] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 89. | Oto A, Yang C, Kayhan A, Tretiakova M, Antic T, Schmid-Tannwald C, Eggener S, Karczmar GS, Stadler WM. Diffusion-weighted and dynamic contrast-enhanced MRI of prostate cancer: correlation of quantitative MR parameters with Gleason score and tumor angiogenesis. AJR Am J Roentgenol. 2011;197:1382-1390. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 198] [Cited by in RCA: 196] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 90. | Feng X, Chen X, Peng P, Zhou H, Hong Y, Zhu C, Lu L, Xie S, Zhang S, Long L. Values of multiparametric and biparametric MRI in diagnosing clinically significant prostate cancer: a multivariate analysis. BMC Urol. 2024;24:40. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 3] [Reference Citation Analysis (0)] |
| 91. | Le Bihan D, Breton E, Lallemand D, Aubin ML, Vignaud J, Laval-Jeantet M. Separation of diffusion and perfusion in intravoxel incoherent motion MR imaging. Radiology. 1988;168:497-505. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2349] [Cited by in RCA: 2505] [Article Influence: 65.9] [Reference Citation Analysis (0)] |
| 92. | Le Bihan D. Intravoxel incoherent motion perfusion MR imaging: a wake-up call. Radiology. 2008;249:748-752. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 233] [Cited by in RCA: 249] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 93. | Tamura C, Shinmoto H, Soga S, Okamura T, Sato H, Okuaki T, Pang Y, Kosuda S, Kaji T. Diffusion kurtosis imaging study of prostate cancer: preliminary findings. J Magn Reson Imaging. 2014;40:723-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 94. | Jensen JH, Helpern JA. MRI quantification of non-Gaussian water diffusion by kurtosis analysis. NMR Biomed. 2010;23:698-710. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1060] [Cited by in RCA: 1001] [Article Influence: 62.6] [Reference Citation Analysis (0)] |
| 95. | Malagi AV, Das CJ, Khare K, Calamante F, Mehndiratta A. Effect of combination and number of b values in IVIM analysis with post-processing methodology: simulation and clinical study. MAGMA. 2019;32:519-527. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 14] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 96. | Malagi AV, Netaji A, Kumar V, Baidya Kayal E, Khare K, Das CJ, Calamante F, Mehndiratta A. IVIM-DKI for differentiation between prostate cancer and benign prostatic hyperplasia: comparison of 1.5 T vs. 3 T MRI. MAGMA. 2022;35:609-620. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 17] [Article Influence: 4.3] [Reference Citation Analysis (0)] |
| 97. | Bao J, Wang X, Hu C, Hou J, Dong F, Guo L. Differentiation of prostate cancer lesions in the Transition Zone by diffusion-weighted MRI. Eur J Radiol Open. 2017;4:123-128. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 24] [Cited by in RCA: 37] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 98. | Chang CB, Lin YC, Wong YC, Lin SN, Lin CY, Lin YH, Sheng TW, Huang CC, Yang LY, Wang LJ. IVIM Parameters on MRI Could Predict ISUP Risk Groups of Prostate Cancers on Radical Prostatectomy. Front Oncol. 2021;11:659014. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 99. | Liu Y, Wang X, Cui Y, Jiang Y, Yu L, Liu M, Zhang W, Shi K, Zhang J, Zhang C, Li C, Chen M. Comparative Study of Monoexponential, Intravoxel Incoherent Motion, Kurtosis, and IVIM-Kurtosis Models for the Diagnosis and Aggressiveness Assessment of Prostate Cancer. Front Oncol. 2020;10:1763. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 23] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 100. | Das CJ, Malagi AV, Sharma R, Mehndiratta A, Kumar V, Khan MA, Seth A, Kaushal S, Nayak B, Kumar R, Gupta AK. Intravoxel incoherent motion and diffusion kurtosis imaging and their machine-learning-based texture analysis for detection and assessment of prostate cancer severity at 3 T. NMR Biomed. 2024;37:e5144. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 101. | Kuru TH, Roethke MC, Stieltjes B, Maier-Hein K, Schlemmer HP, Hadaschik BA, Fenchel M. Intravoxel incoherent motion (IVIM) diffusion imaging in prostate cancer - what does it add? J Comput Assist Tomogr. 2014;38:558-564. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 102. | Zhang YD, Wang Q, Wu CJ, Wang XN, Zhang J, Liu H, Liu XS, Shi HB. The histogram analysis of diffusion-weighted intravoxel incoherent motion (IVIM) imaging for differentiating the gleason grade of prostate cancer. Eur Radiol. 2015;25:994-1004. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 104] [Cited by in RCA: 123] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 103. | Yang DM, Kim HC, Kim SW, Jahng GH, Won KY, Lim SJ, Oh JH. Prostate cancer: correlation of intravoxel incoherent motion MR parameters with Gleason score. Clin Imaging. 2016;40:445-450. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 22] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 104. | Shan Y, Chen X, Liu K, Zeng M, Zhou J. Prostate cancer aggressive prediction: preponderant diagnostic performances of intravoxel incoherent motion (IVIM) imaging and diffusion kurtosis imaging (DKI) beyond ADC at 3.0 T scanner with gleason score at final pathology. Abdom Radiol (NY). 2019;44:3441-3452. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 24] [Article Influence: 3.4] [Reference Citation Analysis (0)] |
| 105. | Panda A, Gulani V, Ponsky LE. Reading MRI of the Prostate: A Practical Guide. Cham: Springer, 2020. |
| 106. | Ma D, Gulani V, Seiberlich N, Liu K, Sunshine JL, Duerk JL, Griswold MA. Magnetic resonance fingerprinting. Nature. 2013;495:187-192. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 863] [Cited by in RCA: 1130] [Article Influence: 86.9] [Reference Citation Analysis (1)] |
| 107. | Jiang Y, Ma D, Seiberlich N, Gulani V, Griswold MA. MR fingerprinting using fast imaging with steady state precession (FISP) with spiral readout. Magn Reson Med. 2015;74:1621-1631. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 325] [Article Influence: 27.1] [Reference Citation Analysis (0)] |
| 108. | Gibbs P, Liney GP, Pickles MD, Zelhof B, Rodrigues G, Turnbull LW. Correlation of ADC and T2 measurements with cell density in prostate cancer at 3.0 Tesla. Invest Radiol. 2009;44:572-576. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 228] [Cited by in RCA: 236] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 109. | Simpkin CJ, Morgan VA, Giles SL, Riches SF, Parker C, deSouza NM. Relationship between T2 relaxation and apparent diffusion coefficient in malignant and non-malignant prostate regions and the effect of peripheral zone fractional volume. Br J Radiol. 2013;86:20120469. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 110. | Panda A, OʼConnor G, Lo WC, Jiang Y, Margevicius S, Schluchter M, Ponsky LE, Gulani V. Targeted Biopsy Validation of Peripheral Zone Prostate Cancer Characterization With Magnetic Resonance Fingerprinting and Diffusion Mapping. Invest Radiol. 2019;54:485-493. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 45] [Cited by in RCA: 54] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 111. | Panda A, Obmann VC, Lo WC, Margevicius S, Jiang Y, Schluchter M, Patel IJ, Nakamoto D, Badve C, Griswold MA, Jaeger I, Ponsky LE, Gulani V. MR Fingerprinting and ADC Mapping for Characterization of Lesions in the Transition Zone of the Prostate Gland. Radiology. 2019;292:685-694. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 112. | Yu AC, Badve C, Ponsky LE, Pahwa S, Dastmalchian S, Rogers M, Jiang Y, Margevicius S, Schluchter M, Tabayoyong W, Abouassaly R, McGivney D, Griswold MA, Gulani V. Development of a Combined MR Fingerprinting and Diffusion Examination for Prostate Cancer. Radiology. 2017;283:729-738. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 96] [Cited by in RCA: 130] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 113. | Davnall F, Yip CS, Ljungqvist G, Selmi M, Ng F, Sanghera B, Ganeshan B, Miles KA, Cook GJ, Goh V. Assessment of tumor heterogeneity: an emerging imaging tool for clinical practice? Insights Imaging. 2012;3:573-589. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 592] [Cited by in RCA: 713] [Article Influence: 50.9] [Reference Citation Analysis (0)] |
| 114. | Miles KA, Ganeshan B, Hayball MP. CT texture analysis using the filtration-histogram method: what do the measurements mean? Cancer Imaging. 2013;13:400-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 202] [Cited by in RCA: 251] [Article Influence: 19.3] [Reference Citation Analysis (0)] |
| 115. | Schieda N, Lim CS, Zabihollahy F, Abreu-Gomez J, Krishna S, Woo S, Melkus G, Ukwatta E, Turkbey B. Quantitative Prostate MRI. J Magn Reson Imaging. 2021;53:1632-1645. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 46] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 116. | Hameed M, Ganeshan B, Shur J, Mukherjee S, Afaq A, Batura D. The clinical utility of prostate cancer heterogeneity using texture analysis of multiparametric MRI. Int Urol Nephrol. 2019;51:817-824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 117. | Wibmer A, Hricak H, Gondo T, Matsumoto K, Veeraraghavan H, Fehr D, Zheng J, Goldman D, Moskowitz C, Fine SW, Reuter VE, Eastham J, Sala E, Vargas HA. Haralick texture analysis of prostate MRI: utility for differentiating non-cancerous prostate from prostate cancer and differentiating prostate cancers with different Gleason scores. Eur Radiol. 2015;25:2840-2850. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 300] [Article Influence: 27.3] [Reference Citation Analysis (0)] |
| 118. | Baek TW, Kim SH, Park SJ, Park EJ. Texture analysis on bi-parametric MRI for evaluation of aggressiveness in patients with prostate cancer. Abdom Radiol (NY). 2020;45:4214-4222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 119. | Krishna S, Lim CS, McInnes MDF, Flood TA, Shabana WM, Lim RS, Schieda N. Evaluation of MRI for diagnosis of extraprostatic extension in prostate cancer. J Magn Reson Imaging. 2018;47:176-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 41] [Cited by in RCA: 65] [Article Influence: 7.2] [Reference Citation Analysis (0)] |
| 120. | Xiong H, He X, Guo D. Value of MRI texture analysis for predicting high-grade prostate cancer. Clin Imaging. 2021;72:168-174. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 121. | Chu L, Si Y, Liu RB. [The Value of MRI Texture Analysis in Identifying Intraductal Carcinoma of the Prostate Gland]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2020;51:42-48. [RCA] [PubMed] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 122. | Li M, Guo J, Hu P, Jiang H, Chen J, Hu J, Asbach P, Sack I, Li W. Tomoelastography Based on Multifrequency MR Elastography for Prostate Cancer Detection: Comparison with Multiparametric MRI. Radiology. 2021;299:362-370. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 123. | Hu B, Deng Y, Chen J, Kuang S, Tang W, He B, Zhang L, Xiao Y, Chen J, Rossman P, Arani A, Yin Z, Glaser KJ, Yin M, Venkatesh SK, Ehman RL, Wang J. Evaluation of MR elastography for prediction of lymph node metastasis in prostate cancer. Abdom Radiol (NY). 2021;46:3387-3400. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/