Published online Sep 28, 2023. doi: 10.4329/wjr.v15.i9.256
Peer-review started: July 27, 2023
First decision: September 4, 2023
Revised: September 16, 2023
Accepted: September 22, 2023
Article in press: September 22, 2023
Published online: September 28, 2023
Processing time: 61 Days and 16.5 Hours
Among five types of pulmonary hypertension, chronic thromboembolic pulmo
Core Tip: Chronic thromboembolic pulmonary hypertension is an under-diagnosed disorder with high mortality if not diagnosed on time, however it can be fully cured with efficient diagnostic tools. Pulmonary perfusion magnetic resonance imaging (MRI) provides a non-invasive, reliable, radiation free and safer diagnostic test potentially replacing the standard techniques. Cardiopulmonary MRI also provides a comprehensive cardiopulmonary assessment in one single visit resulting in patients’ convenience and better utilization of healthcare resources and time.
- Citation: Lacharie M, Villa A, Milidonis X, Hasaneen H, Chiribiri A, Benedetti G. Role of pulmonary perfusion magnetic resonance imaging for the diagnosis of pulmonary hypertension: A review. World J Radiol 2023; 15(9): 256-273
- URL: https://www.wjgnet.com/1949-8470/full/v15/i9/256.htm
- DOI: https://dx.doi.org/10.4329/wjr.v15.i9.256
Pulmonary perfusion is crucial for the assessment of lung function, since proper pulmonary blood flow (PBF) and pulmonary ventilation are prerequisites for effective gas exchange. Pulmonary perfusion can be assessed by measuring mean pulmonary artery pressure (mPAP), pulmonary venous resistance (PVR), and pulmonary artery wedge pressure (PAWP) during right heart catheterization (RHC).
This normal pulmonary perfusion and ventilation pattern can be altered by several physiological and pathological conditions, including vascular (as in cases of chronic pulmonary thromboembolic disease - CTED) and parenchymal alterations (as in cases of lung fibrosis)[1]. These situations can lead to pulmonary hypertension (PH), PH is defined as mPAP of > 20 mmHg and PVR > 2.0 Woods Units[2].
PH is not a single entity, but rather a spectrum of disorders and can be due to multifactorial underlying pathophy
CTEPH is caused by the chronic occlusion of pulmonary arteries due to thromboembolic material with subsequent increased mPAP and PVR, potentially leading to right heart failure and death if left untreated[5,6]. Risk factors for pulmonary thromboembolic vessel occlusion are age over 70, female gender, pulmonary embolism at first venous thromboembolic event, deep vein thrombosis, chronic obstructive pulmonary disease, heart failure and atrial fibrillation[7]. This article will discuss contemporary diagnostic methods to diagnose PH, what methods have been researched so far in medical literature and their diagnostic accuracy. It further explores advantages and limitations of current methods routinely used in clinical practice. It also discusses newly emerging techniques and future horizons of research in PH diagnosis.
Currently, the differential diagnosis of PH involves a multimodality approach including RHC, echocardiography, nuclear medicine based planer ventilation/perfusion scintigraphy scan (V/Q), single photon emission computed tomography (SPECT) scintigraphy and more recently a hybrid approach of combining SPECT and conventional computed tomography (SPECT-CT) scan[8]. All nuclear medicine techniques involve intravenous injection of radionuclide which emits gamma rays after reaching the region of interest in the body. These gamma rays are detected by gamma cameras providing functional assessment of lungs.
Planer scintigraphy has been largely replaced by SPECT because in SPECT gamma cameras rotate over a 360-degree arc around the patient providing three dimensional images which are crucial to detect small segmental perfusion defects.
Recently, hybrid SPECT-CT has also been used for clinical practice. It involves two different types of scans at the same time, a nuclear medicine scan involving radionuclide aerosol of technetium-99m diethylenetriaminepentaacetic acid delivered through a non-breathing mask which reaches to the distal tracheobronchial tree to reflect regional ventilation. Second part of study is an intravenous injection of TC-99m macro aggregates which gets lodged in pulmonary precapillary arterioles to provide pulmonary perfusion assessment via gamma camera[9,10]. At the same time, conventional CT scanner built in with the SPECT scintigraphy machine provides the anatomical details. Consequently, hybrid SPECT-CT can be considered superior to planar scintigraphy and SPECT alone.
Additionally, computed tomography pulmonary angiography (CTPA) has also been demonstrated to accurately detect pulmonary embolism, however it lacks the ability of dynamic pulmonary perfusion to detect perfusion defects at segmental and sub segmental pulmonary level. CTPA involves intravenous injection of iodine-based contrast injection and imaging of pulmonary arteries for the detection of contrast filling defect denoting pulmonary embolism.
Sensitivity and specificity of CTPA for pulmonary embolism varies from 83% to 100% and with recent advancements in SPECT the potential for routinely used modalities for CTEPH detection has been validated for pulmonary perfusion defect detection[11,12]. This is made possible by the high image resolution offered by multi slice CT scanning, allowing an accurate assessment of the location and severity of thromboembolic material up to the sub-segmental level, providing crucial information for the assessment of patients with CTEPH considered suitable for pulmonary endarterectomy (PEA)[13]. Recently, dual energy computed tomography (CT) has further improved the assessment of patients with PH, thanks to the assessment of regional pulmonary perfusion with iodine perfusion maps[14].
Visual assessment of SPECT scintigraphy, SPECT-CT scintigraphy and CTPA images by expert radiologists is the routine clinical practice to identify partially or completely occluded pulmonary vessels and to assess lung perfusion.
Routine clinical tests for PH diagnosis are limited by radiation exposure causing carcinogenic stochastic effects (long-term cancer-causing effects after radiation exposure) and rarely deterministic effects (acute high dose exposure if radiation dose exceeds 100 mGy leading to skin erythema, organ damage/failure)[15]. However, these effects are extremely rare due to sophisticated equipment and specialised radiation dose lowering parameters.
The concern is exacerbated for women of child-bearing age, particularly considering the high female-to-male ratio (4.8:1) of incidence and prevalence of PH worldwide and harmful effects on foetal organogenesis[16,17]. Moreover, periodical follow-up to assess the disease progression is vital for PH management, imposing further radiation exposure.
The average effective radiation dose of V/Q scan has been estimated as 1.29 mSv, breast absorbed dose 0.37 mGy, uterus-absorbed dose 0.46 mGy and foetal absorbed doses of 0.40 mGy[18]. SPECT scintigraphy uses radionuclide doses of 25 to 30 MBq for ventilation and 100-120 MBq is usually required for a successful perfusion study[9,19].
Another limitation of current techniques like CTPA is ionising radiation exposure. The effective radiation dose of CTPA has been estimated to be 21 mSv and breast absorbed dose from 44 mGy to as high as 70 mGy to radiosensitive breast tissue[18,20]. Potential carcinogenic ionising radiation exposure and the need for frequent follow-up assessments by conventional tests for PH patients are a few limitations of current imaging tests for pulmonary embolism-based PH diagnosis[21].
Furthermore, as PH can inevitably affect cardiac functions, a single test including the assessment of left and right ventricular function and myocardial tissue characterisation would be desirable for a comprehensive assessment.
In a relevant study, researchers validated that cardiac magnetic resonance imaging (MRI) derived pulmonary artery pressure ejection fraction, RV stroke volume, cardiac output, ventricular mass index and pulmonary blood flow in non-treated lobes correlated with pulmonary blood flow changes in treated lobes post balloon pulmonary angioplasty P < 0.05. Furthermore, utility of pulmonary artery pulsatility and pulmonary artery flow measurement by cardiac MRI as an early marker of pulmonary hypertension has also been verified in another prospective study[22]. Therefore, these studies emphasize the significance of a comprehensive, non-invasive and ionising radiation free cardiopulmonary assessment using cardiac magnetic resonance and pulmonary MRI in a single setting.
Finally, in clinical practice these imaging tests are assessed visually only, which is prone to an observer-dependent evaluation, and the availability of quantitative analysis techniques could provide additional value.
Unfortunately, CTEPH retains high mortality, and the mean life expectancy has been reported as less than three years if left untreated[3,23]. Currently, CTEPH can be treated with PEA, which consists of the surgical resection of the thromboembolic material. Before PEA, the three-year mortality of CTEPH was over 50%[24]. It has been demonstrated that PEA can lead to significant improvements in hemodynamic and exercise capacity[23]. Delayed treatment however is still associated with poor prognosis, therefore early detection of CTEPH is critical[25].
A prospective European study of 679 patients with CTEPH, of which 386 underwent PEA, reported an in-hospital mortality rate as low as 4.7% and 1-year mortality of 7%, while the exercise capacity and pulmonary vascular resistance were significantly improved[26]. In another study, the mortality rate in 1500 consecutive CTEPH patients post PEA was investigated between 1999 and 2010. Outcomes were compared between historical cohorts and more recent patients, who received surgical treatment for distal disease affecting smaller pulmonary vessels. A significant reduction of mortality rate from 5.2% to 2.2% was observed, despite the more distal disease, reflecting a continuous improvement in surgical techniques and highlighting the need for more sensitive tests capable of identifying distal disease[27].
Most of CTEPH patients in these studies stated remarkable relief from symptoms after PEA and a return to near normal hemodynamic values[26-28]. These promising results of surgical treatment support the quest for new diagnostic methods capable of early differential diagnosis and further developments of surgical techniques for targeted intervention. Moreover, improved diagnostic tools are necessary for differentiating CTEPH from the other four types of PH because treatment pathways vary accordingly. Currently, this differentiation is based only on imaging tests that employ ionising radiations.
Recently, pulmonary perfusion MRI has been emerged as a powerful tool for the assessment of pulmonary hypertension, and particularly for the differential diagnosis of CTEPH[29]. Perfusion MRI can be performed using dynamic contrast imaging or without contrast using arterial spin labelling[30].
The aim of this review article is to provide an overview of the published studies on lung perfusion MRI, highlighting the limitations of standard tests and the role of perfusion MRI as a diagnostic tool for PH. Moreover, the review focuses on the methodology, advantages, challenges, and future directions for pulmonary perfusion MRI and its potential contribution to overall healthcare policies.
A comprehensive literature search was performed using, PubMed, EMBASE and Medline databases. To develop the literature search question, a PICO framework was adopted: Population; PH patients, Intervention; MRI, Comparison; SPECT/CTPA, Outcome; diagnostic accuracy. Original prospective and retrospective studies were included in the literature search with or without direct comparison of pulmonary perfusion MRI to gold standard tests for the diagnosis of PH[31-55] in Table 1. Tables 2-4 provide the PICO framework, eligibility criteria for included studies and facet analysis with search strategy respectively. Figure 1 demonstrates the PRSMA flow chart for literature search.
| Ref. | Study design | Patients (n) | Demographics | Aims | Methodology | Analysis | Results (95%CI) |
| Amundsen et al[31], 1997 | Prospective, qualitative | 7 | 7 patients with suspected PE | To evaluate the feasibility of perfusion MRI for detection of perfusion defects distal to suspected pulmonary embolism compared to V/Q | Rapid acquisition of two sets of dynamic images in coronal and trans axial plane | Qualitative analysis (MRI Vs V/Q) | Perfusion MRI correctly identified 16/18 lung segments with perfusion defects |
| Amundsen et al[32], 2002 | Prospective, qualitative | 42 | 20 suspected PE, 11 Pneumonias, 11 COPD | To compare perfusion MRI and V/Q for the perfusion defects detection | Rapid acquisition of two sets of dynamic images in coronal and trans axial plane with an inversion recovery gradient MRI sequence | Qualitative analysis (MRI Vs V/Q) | For PE: Intra-modality kappa = 0.77, Inter-observer kappa = 0.92 |
| Ohno et al[33], 2004 | Prospective, qualitative | 40 | Controls=15, (Mean age 42 yr), PH patients=25, (Mean age 61 yr) | To assess regional differences in quantitative pulmonary perfusion parameters using MRI | Three dimensional ultrafast DCE-MRI was performed and PBF, PBV & MTT measured by signal intensity time course curve | MATLAB, For PBF, MTT, PBV, Mean, SD, ANOVA, Fisher’s PLSD test | PBF, PBV & MTT showed significant differences between normal volunteers and patients with PH (P < 0.05) |
| Nikolaou et al[34], 2005 | Prospective, qualitative | 29 | 16 females (mean age 54 ± 17 yr), 13 males (Mean age 57 ± 15 yr) | Pulmonary hypertension & CTEPH differentiation by perfusion MRI and pulmonary angiography | Turbo fast low angle shot gradient echo MRI sequence was performed by using generalized auto calibrating partially parallel technique or GRAPPA | Student t test for significance, ROC using SPSS software | ROC: MRA = 0.85, MRI = 0.82, MRA, MRI combined0.90 |
| Kluge et al[35], 2005 | Prospective, qualitative | 31 | 15 females, 18 males, (Mean age 59.4 yr) with acute PE | To compare the feasibility of perfusion MRI with CT for follow up examination in acute PE | Contrast enhanced 3-dimensional fast low angle shot or FLASH sequence was used for perfusion MRI and time to peak and peak enhancement was measured | T test for paired samples using SPSS | Follow up examination using MRI were feasible compared to CT for all patients |
| Kluge et al[36], 2006 | Prospective, qualitative | 41 | 41 patients with suspected PE | To assess the agreement of perfusion MRI with SPECT for identifying perfusion defects | Contrast enhanced 3-dimensional fast low angle shot or FLASH sequence was used for perfusion MRI | Not given | MRI and SPECT agreement kappa Lobar = 0.98, Segmental = 0.98, Subsegmental = 0.69 |
| Ohno et al[37], 2007 | Prospective, qualitative | 28 | Controls=14, (Mean age 34 yr), PH patients=14, (Mean age 41 yr) | To measure diagnostic potential of DCE-MRI for pulmonary hypertension | Three dimensional ultrafast DCE-MRI was performed and PBF, PBV & MTT measured by signal intensity time course curve | MATLAB, For PBF, MTT, PBV, MathWorks, Mean, SD, T test | Difference for study groups: PBF: P < 0.0001, PBV: P < 0.0001, MTT: P < 0.0001 |
| Ley et al[38], 2007 | Prospective, qualitative | 25 | Controls=5, PH patients=20 | To measure diagnostic potential of DCE-MRI for pulmonary hypertension | Contrast enhanced 3-dimensional fast low angle shot or FLASH sequence was used for perfusion MRI | Quantitative analysis of PBF, PBV and MTT, Mann-Whitney U-test | PBF, PBV & MTT showed significant differences between normal volunteers and patients with PH (P < 0.05) |
| Ohno et al[39], 2008 | Prospective, qualitative | 27 | Controls = 9, 18 gender and age matched CTD patients | To measure diagnostic potential of DCE-MRI for PAH | PBF, MTT and PBV measured by DCE-MRI and correlated by %DL(CO) measured by pulmonary function test and mPAP, sPAP measured by doppler echo | MATLAB, MathWorks, Mean, SD, T test, Correlation test | PBF, MTT, PBV correlated positively with %DL(CO) & sPAP (P < 0.05), PBF& PBV correlated positively with mPAP& moderately with PVR (P < 0.05) |
| Ohno et al[40], 2010 | Prospective, qualitative | 50 | 50 PE patients with acute pulmonary thromboembolism (APTE) | To measure diagnostic potential of DCE-MRI for acute pulmonary thromboembolism (APTE) | PBF, PBV, MTT & APTE index measured by DCE-MRI using 3-dimensional spoiled gradient sequence, MPAP, PVR measured by RHC. RV/LV diameter ratio, APTE index measured by CT & MRA | ROC curve, Logistic regression | PBF and MTT significantly lower for APTE segments to non-APTE segments (P < 0.05), APTE indexes from all modalities proved significant predictors for differentiating APTE patients |
| Stein et al[41], 2010 | Prospective, qualitative | 371 | 371 adults with diagnosed or excluded pulmonary embolism- (PIOPED III) | To assess performance of MRA and venography for pulmonary embolism detection | MRA was compared with CTPA, V/Q scan, venous ultrasonography, D-dimer assay, and clinical assessment, Qualitative assessment by expert reader only | Chi-square test ANOVA | Technically adequate images for MRA: SE: 78%, SP: 99% |
| Kang et al[42], 2011 | Prospective, qualitative | 35 | 35 PAH patients (Mean age 44 yr) | To assess if Cardiac MRI based pulmonary artery distensibility index correlates with RHC estimates for PAH | Pulmonary artery distensibility indices were derived from transverse view MRI and compared with PVR using RHC | Correlation | Non-invasive MRI based pulmonary artery distensibility index correlates with RHC based estimates P < 0.001 |
| Ohno et al[43], 2012 | Prospective, qualitative | 24 | Response group=13, Non-response group=11, 12 females & 12 males mean age 68 yr ± 8.6 | CTPA, MRA & DCE-MRI comparison for treatment response in inoperable CTEPH patients | PBF, PBV, MTT measured by DCE-MRI using 3-dimensional spoiled gradient sequence, RV/LV diameter ratio and embolic burden measured by CTPA & MRA | Mean of student T test, Correlation, ROC curve analysis, McNemar’s test | DCE-MRI SP = 90%, AC = 95%, CTPA SP = 36%, AC = 70%, MRA SP = 54%, AC = 79% |
| Ley et al[44], 2013 | Prospective, qualitative | 20 PAH or CTEPH patients | Controls 10, Training group 10 | To evaluate if training improves pulmonary perfusion in PH as assessed by MR perfusion imaging | Training group received in hospital exercise training while control group received conventional rehabilitation. 6 min walk test, PBF, PBV, MTT & peak flow velocity measured by MR perfusion were assessed for both groups from baseline to 3 wk | Mann-Whitney-Wilcoxon test, Spearman correlation coefficient | Training group had significantly improved 6-min walk test, MR flow and MR perfusion |
| Rajaram et al[45], 2013 | Prospective, qualitative | 132 | 78 CTEPH patients | To compare the diagnostic accuracy of perfusion MRI for CTEPH Vs. CTPA and V/Q | Pulmonary perfusion MRI using time resolved 3-Dimensional spoiled gradient and pulmonary MRA were compared with CTPA and V/Q | Not given | SE, SP in %, MRI: 97, 92, V/Q: 96, 90, CTPA: 94, 98 |
| Revel et al[46], 2013 | Prospective, qualitative | 274 | 274 suspected PE patients | To evaluate unenhanced, enhanced perfusion and MR angiography for PE detection | Unenhanced steady state free precession or SSFP, fast spoiled gradient echo for perfusion MRI and MR angiography were compared with CTPA | Chi-squared Kappa statistics | Kappa agreement MRA = 0.77, Perfusion MRI = 0.51, Unenhanced MRI = 0.62 |
| Sugimoto et al[47], 2013 | Prospective, qualitative | 34 | 34 congenital heart disease patients | To assess if velocity encoded cine imaging can measure pulmonary artery pressure in children with congenital heart disease | Pulmonary blood flow (QP), systemic blood flow (QS), acceleration time, ejection time, peak velocity, and maximal change in flow rate during ejection (MCFR) were measured by velocity encoded MRI and RHC | Velocity encoded MRI correlated strongly with RHC for QS, right to left QP ratio and QP/QS. Suggesting usefulness of MRI for pulmonary artery pressure measurement | |
| Schoenfeld et al[48], 2015 | Prospective, qualitative | 64 | 64 ruled out or confirmed PE patients | To compare perfusion weighted Fourier decomposition or PW-FD to DCE-MRI for PE detection | Time resolved angiography with stochastic trajectories or TWIST for DCE-MRI was used and compared with PW-FD | Qualitative only, Kappa statistics | For PW-FD per patient basis, SE = 100%, SP = 95%, PPV = 98%, NPV = 98%, Intraobserver k = 0.96, Interobserver k = 0.96 |
| Ingrisch et al[49], 2016 | Prospective, qualitative | 18 | 8 acute PE, 10 controls | DCE-MRI evaluation for acute PE detection compared with CTPA | Qualitative assessment of presence and absence of perfusion defects using DCE-MRI using TWIST sequence | Cohen’s kappa, Fisher’s exact test | SE: 87-93%, SP: 90-95%, PPV: 87-93%, NPV: 90-95%, Inter-reader agreement: k = 0.77, Intra-modality agreement: P < 0.001 |
| Johns et al[50], 2017 | Prospective, qualitative | 74 | 20 male, 26 female, Mean age 62 ± 14 yr | DCE-MRI, SPECT & CTPA comparison for CTEPH diagnosis | Qualitative comparison of presence/absence of perfusion defects on DCE-MRI using fast spoiled gradient echo, perfusion, SPECT and CTPA | 2*2 predictive table, Kappa (k) for inter-observer agreement | SE: 100%, SP: 81%, PPV: 90%, NPV: 100%, Inter-observer agreement for DCE-MRI: k = 0.88, SPECT: (k = 0.80) |
| Voskrebenzev et al[51], 2018 | Prospective, qualitative | 5 | 2 controls, 1 CTEPH patient 1 CF patient, 1 obstructive pulmonary disease patient | To assess the feasibility of phase resolved functional lung MRI (PREFUL) for quantitative reginal ventilation and perfusion | Time to peak, V/Q maps and fractional ventilation flow volume were calculated using PREFUL MRI | Full Cardiac and respiratory cycle were sorted using PREFUL | Post endarterectomy, CTEPH patient showed increased perfusion time to peak in visual agreement with DCE-MRI |
| Agoston-Coldea et al[52], 2018 | Prospective, qualitative | 30 | 30 consecutive patients with COPD and suspected secondary pulmonary hypertension | To evaluate ability of CMR right ventricular parameters and pulmonary artery stiffness to identify pulmonary hypertension | Clinical examination. 6-min walk test, echocardiography, RHC and cardiac functions and late gadolinium CMR imaging with phase contrast flow imaging of pulmonary artery. Followed up for a mean period of 16 mo | ROC curve analysis, Kolmogorov-Smirnov test, ANOVA test. Fischer’s exact test | Pulse wave velocity: SE = 93.5%, SP = 92.8% |
| Schoenfeld et al[53], 2019 | Prospective, qualitative | 29 | 20 CTEPH patients | Cardiopulmonary evaluation of treatment response after BPA in CTEPH patients | PBF and first pass bolus kinetic parameters and biventricular mass and functions were evaluated using MRI | Paired two sides Wilcoxon rank sum test, Spearman p correlation, Multiple linear regression | Post BPA, PBF changes in treated lobes were significantly higher than non-treated lobes P < 0.05, MRI derived pulmonary artery pressure ejection fraction, RV stroke volume, CO, ventricular mass index & PBF in non-treated lobes correlated with PBF changes in treated lobes P < 0.05 |
| Ray et al[54], 2019 | Prospective, qualitative | 51 | 20 mild PH, 31 moderate to severe PAH | Utility of pulmonary artery pulsatility by cardiac MRI as an early marker of pulmonary hypertension | Standards steady state free precession or cine SSFP for pulmonary artery pulsatility and phased contrast MRI imaging for pulmonary flow assessment | Wilcoxon rank sum test, Roc analysis | Pulmonary artery pulsatility declined from normal (53%), mild (22%) and moderate to severe PAH (17%) |
| Alsady et al[55], 2021 | Prospective, qualitative | 20 | 20 CTEPH patients | To compare DCE-MRI and computed tomography for lung perfusion defects before and after pulmonary endarterectomy | Lobe based analysis of perfusion defects using DCE-MRI and PBF and PBV measurement, comparison with dual energy computed tomography | Pearson product-moment correlation, Paired t test using MATLAB | Correlation between CT and MRI based perfusion defects (r > 0.78; P < 0.001) |
| Torres et al[56], 2022 | Prospective, qualitative | 41 | 20 IPF patients | DCE-MRI for the evaluation of lung perfusion in IPF | PBF CV, FVC% predicted %DL(CO) and LCI% were evaluated using DCE-MRI | Regression analysis, Spearman rank correlation | DCE-MRI identified regional perfusion defects between controls and IPF (P < 0.05). Correlation observed between PBF CV and %DL(CO) (r = 0.48, P < 0.001) |
| PICO | |
| Population | Pulmonary hypertension patients |
| Intervention | Cardio-pulmonary MRI, DCE-MRI and/or MRA and/or PREFUL Imaging |
| Comparator | Computed tomography pulmonary angiography (CTPA) and/or ventilation perfusion (V/Q) scan |
| Outcome | Diagnostic accuracy |
| Time frame | 1997-2022 |
| Study type | Original retrospective or prospective studies and randomised controlled trials only |
| Inclusion criteria | Exclusion criteria | Rationale |
| All publication to date | N/A | To avoid missing any relevant studies |
| English articles | Articles not translated into English | Difficult comprehension |
| Known or suspected PH patients | Other pulmonary conditions | Pulmonary hypertension is the focus of the study |
| Original research Prospective/Retrospective only | Reviews, meta-analyses, and case reports | Complicates the results Irrelevant study designs |
| Papers discussing diagnostic accuracy of MRI, CTPA and V/Q scan | Papers discussing diagnostic accuracy of gas exchange and other techniques | Irrelevant for current study focus |
| Electronic database | Search string |
| PubMed | (“Pulmonary hypertension” OR “Pulmonary arterial hypertension” OR “Chronic Thromboembolic Pulmonary Hypertension” OR “Left heart pulmonary hypertension” OR “Lung disease pulmonary hypertension” OR “Pulmonary veno-occulusive disease”) AND (“Magnetic Resonance Imaging” OR “Pulmonary perfusion Magnetic Resonance Imaging” OR “Cardiac Magnetic Resonance Imaging” OR “Magnetic resonance angiography” OR “Phase resolved functional ” OR “Dynamic Contrast Enhanced-Magnetic Resonance Imaging” OR “3Dimensional Dynamic Contrast Enhanced-Magnetic Resonance Imaging” AND (“Computed Tomography pulmonary angiography” OR Ventilation/perfusion scan”) AND (“Specificity” OR “Sensitivity” OR “Diagnostic accuracy” OR “Positive predictive value” OR “Area under the curve” OR “screening accuracy”) |
| EMBASE | (“Pulmonary hypertension” OR “Pulmonary arterial hypertension” OR “Chronic Thromboembolic Pulmonary Hypertension” OR “Left heart pulmonary hypertension” OR “Lung disease pulmonary hypertension” OR “Pulmonary veno-occulusive disease”) AND (“Magnetic Resonance Imaging” OR “Pulmonary perfusion Magnetic Resonance Imaging” OR “Cardiac Magnetic Resonance Imaging” OR “Magnetic resonance angiography” OR “Phase resolved functional ” OR “Dynamic Contrast Enhanced-Magnetic Resonance Imaging” OR “3Dimensional Dynamic Contrast Enhanced-Magnetic Resonance Imaging” AND (“Computed Tomography pulmonary angiography” OR Ventilation/perfusion scan”) AND (“Specificity” OR “Sensitivity” OR “Diagnostic accuracy” OR “Positive predictive value” OR “Area under the curve” OR “screening accuracy”) |
| Medline | (“Pulmonary hypertension” OR “Pulmonary arterial hypertension” OR “Chronic Thromboembolic Pulmonary Hypertension” OR “Left heart pulmonary hypertension” OR “Lung disease pulmonary hypertension” OR “Pulmonary veno-occulusive disease”) AND (“Magnetic Resonance Imaging” OR “Pulmonary perfusion Magnetic Resonance Imaging” OR “Cardiac Magnetic Resonance Imaging” OR “Magnetic resonance angiography” OR “Phase resolved functional ” OR “Dynamic Contrast Enhanced-Magnetic Resonance Imaging” OR “3Dimensional Dynamic Contrast Enhanced-Magnetic Resonance Imaging” AND (“Computed Tomography pulmonary angiography” OR Ventilation/perfusion scan”) AND (“Specificity” OR “Sensitivity” OR “Diagnostic accuracy” OR “Positive predictive value” OR “Area under the curve” OR “screening accuracy”) |
Note that the acronym “Dynamic contract enhanced MRI (DCE-MRI)” was frequently used in the selected studies instead of pulmonary perfusion MRI.
MRI uses the interaction between a magnetic field, hydrogen ions in the body and radiofrequency pulses to generate diagnostic images in multiple planes with high spatial resolution and without the need for ionising radiation. An MRI sequence involves repeated application of radiofrequency pulses to the magnetized hydrogen ions in the body and measuring the MRI signals while the hydrogen ions are demagnetized over time (echo time, TE)[56]. Because of the inherent difference in magnetic susceptibility between lung parenchyma and air, MRI of lungs is prone to wider frequency distribution and phase dispersion, leading to suboptimal noisy images[57]. To mitigate this susceptibility effect, recent advancements in lung MRI are ultra-short echo and zero echo time acquisitions, allowing accelerated scan time whilst preserving optimal image quality[58,59].
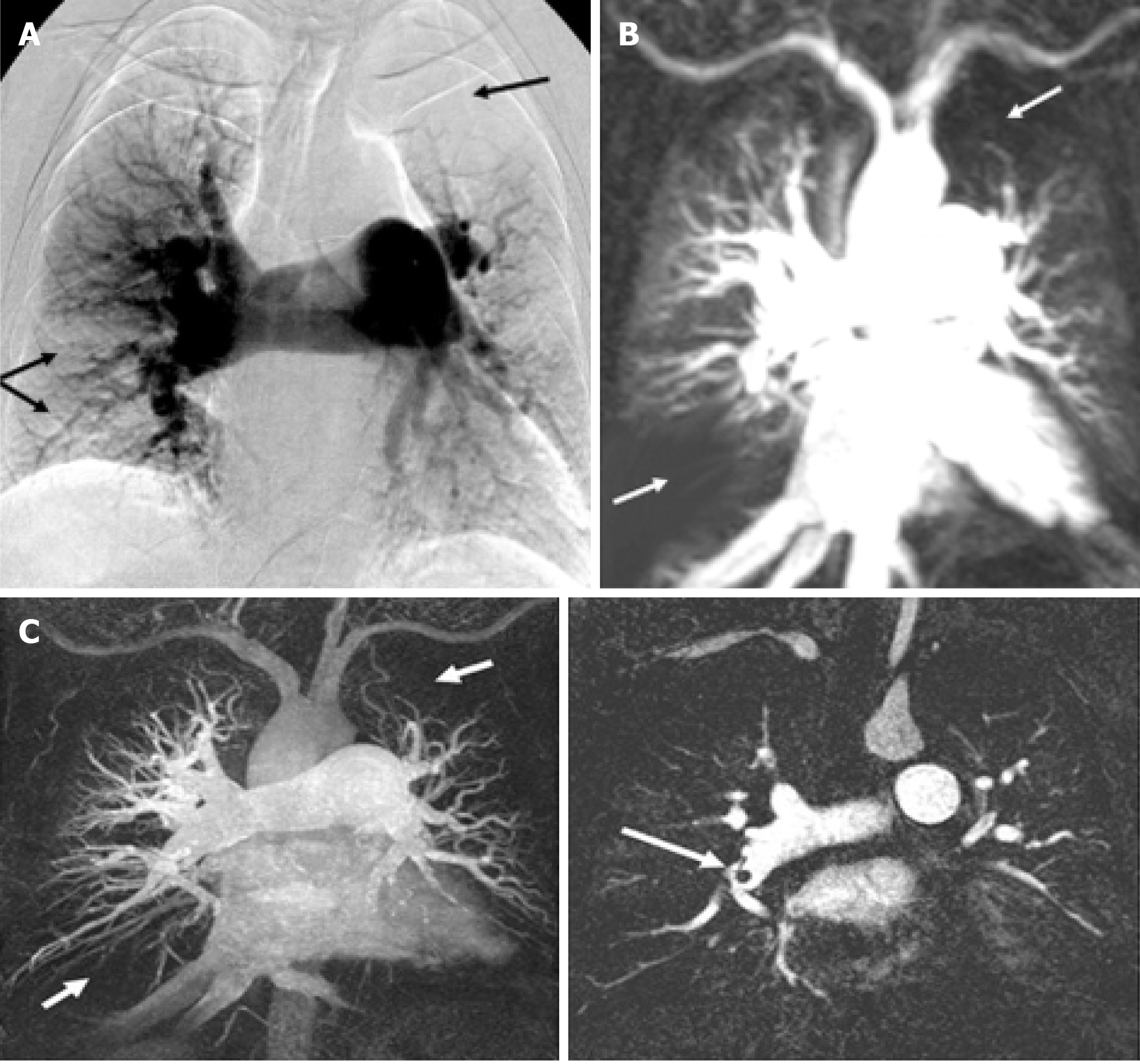
For fast pulmonary vasculature imaging, gradient pulse sequences with short radiofrequency repetition time and short time to receive the signal are typically employed, which allows acquisition of dynamic images of the lungs in real time as contrast agent flows through the pulmonary vasculature[60] (Figure 2[34]).
A commonly used gradient echo pulse sequence for perfusion MRI is 3-dimensional fast low angle shot (FLASH) MRI acquiring dynamic perfusion images of lungs[61]. The dynamic visualisation of the first pass of the contrast agent across the pulmonary circulation is a crucial requirement for the assessment of lung perfusion[61,62].
Several studies have used 3D FLASH perfusion MRI for the detection of chronic thromboembolic disease[33,34,37,63]. However, 3D FLASH perfusion MRI is limited by reduced image resolution and anatomical coverage[64,65]. This is due to the under sampling of the MRI k-space in FLASH MRI imaging. This under sampling of data leads to fast imaging at the expense of image resolution and anatomical coverage.
Ideally, a combination of high temporal resolution (fast imaging) – capable to resolve the dynamic first-pass of the contrast agent, full coverage of the lung fields, and high spatial resolution to capture small pulmonary defects would be required for the optimal differential diagnosis of CTED.
Recently, simultaneous multi-slice (SMS) balanced steady state free precession (b-SSFP) imaging has emerged to solve this problem, allowing better anatomical coverage, increased image resolution and accelerated scan time enabling acquisition of multiple slices simultaneously[65,66]. Therefore, it potentially leads to better visualisation of small pulmonary perfusion defects which can be easily missed in case of sub optimal image quality.
SMS b-SSFP has been recently validated for cardiac perfusion imaging where it doubled spatial coverage by acquiring 6 slices at the same time it takes for conventional sequences to acquire 3 slices, while preserving in-plane resolution[65]. The use of SMS b-SSFP for pulmonary perfusion imaging has not been extensively researched, yet further research is warranted considering its promising results for increased anatomical coverage with high image resolution and shorter scan time for cardiac perfusion.
Additionally, pulmonary perfusion MRI involves antero-posterior coronal images covering both lungs and image acquisition on inspiration to minimize respiratory artefacts. To assess perfusion defects, a gadolinium-based MRI contrast injection is used, which offers hyper-intense or hypo-intense signals depending on the amount of blood perfusion in the region of interest over time (dynamic imaging). Recently, free breathing lung MRI was also validated for image quality and reproducibility, showing promise for increased patient comfort during scanning of PH and patients with other lung diseases causing poor breath holding[67,68].
Pulmonary perfusion MRI images can be assessed qualitatively and quantitatively.
Current clinical practice is to visually assess the lung perfusion MRI images during the passage of contrast agent through the pulmonary circulation. Dynamic pulmonary perfusion MRI leads to the visualisation of pulmonary arteries’ contrast distribution: a pulmonary segment with normal perfusion shows a good and homogeneous perfusion, while the lung territory supplied by a pulmonary artery with partial or complete occlusion due to thromboembolic material will show reduced and delayed perfusion. Impaired lung perfusion can be visually assessed as a wedge-shaped perfusion defect on the pulmonary perfusion images.
However, this observer based qualitative assessment is prone to inter- and intra-observer variations leading to subjective interpretations[69,70].
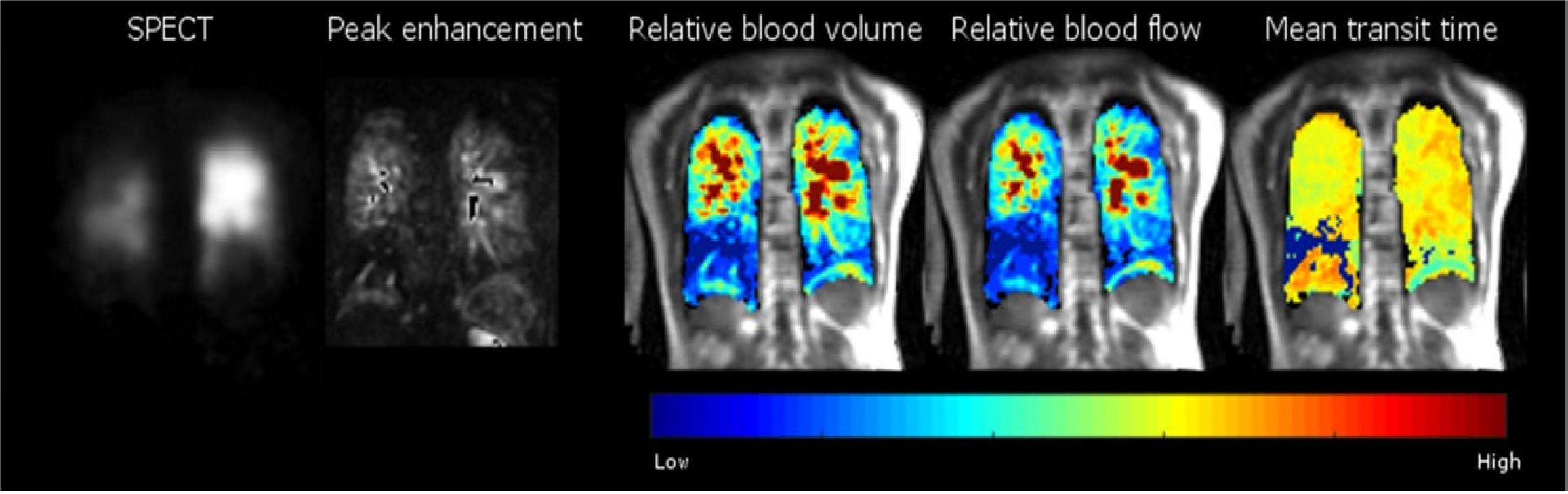
To mitigate subjective bias, pulmonary perfusion MRI also allows quantification of perfusion parameters: Pulmonary blood flow, pulmonary blood volume and mean transient time of blood flow (Figures 3 and 4[33,50]) which requires tracing of the contrast agent passing through the tissue of interest and an arterial input function (AIF) curve corresponding to the signal intensity in the blood pool over time[66].
Perfusion quantification is based on the principles of the indicator dilution theory, relating the AIF and tissue enhancement curves via the process of deconvolution[71-73], and is often used for the measurement of myocardial blood flow[74]. Pulmonary blood volume (PBV) and mean transit time (MTT) can be subsequently estimated according to the central volume theorem[33,73]. Quantification is, however, significantly impacted by the nonlinear relationship of the measured signal with contrast agent concentration leading to errors in quantitative metrics.
Non-linearity is caused by spatial signal variations due to variations in sensitivity profiles of MRI surface coils, contrast bolus related T2* decay and subsequent signal loss[66]. To mitigate this effect, a dual-contrast bolus approach can be used, where a dilute (low concentration) bolus is injected for AIF measurement and a neat (high concentration) bolus is subsequently injected for signal intensity measurement in the lung tissue[75]. Researchers reported that the dual-bolus approach is feasible for pulmonary perfusion MRI, reducing the inherent nonlinearity between contrast concentration and signal intensity[76].
Recently, the dual-sequence approach was introduced to avoid the complexity of injector set up and issues of non-linearity for cardiac perfusion MRI[77]. The dual-sequence approach is more practical in busy clinical settings, as it involves injection of a single contrast bolus. The sequence acquires a low resolution slice by FLASH readout for sampling the AIF, followed by acquisition of high resolution short-axis slices using SSFP or FLASH readout[78]. This dual-sequence technique has been validated for cardiac perfusion imaging for its linearity for signal conversion to contrast bolus concentration[66].
Clinically, MRI perfusion quantification permits user-independence quantification of PBF, PBV and MTT at the global, lobar, and segmental pulmonary levels, minimising inter- and intra-observer qualitative bias[69]. Quantification of perfusion parameters allow narrowing down the differential diagnosis of lesions associated with increased or decreased PBF or PBV, (as in the case of chronic thromboembolic partial or complete obstruction of pulmonary vasculature[79].
Evaluation of pulmonary thromboembolic disease by lung perfusion MRI is a relatively new imaging technique, and its diagnostic potential has been reported comparable with the routinely performed tests of CTPA and SPECT scintigraphy[43,50,63]. The diagnostic accuracy of pulmonary perfusion has been tested qualitatively and quantitatively.
Qualitative assessment: An early feasibility study looking at the diagnostic accuracy of perfusion MRI for detecting lung perfusion defects in patients with pulmonary embolism revealed a good inter-modality agreement with V/Q scan by correctly identifying 16 out of 18 perfusion defects[31].
The diagnostic accuracy of pulmonary perfusion MRI has been demonstrated comparable to V/Q scan measured by the Kappa statistic (k) for inter-observer and inter-modality agreement (inter-observer agreement: k = 0.63 and inter-modality agreement: k = 0.79) in another prospective qualitative study assessing the treatment response of inoperable CTEPH patients[34].
A prospective qualitative study on 78 CTEPH patients assessing the diagnostic accuracy of pulmonary perfusion MRI concluded a sensitivity of 97% and specificity of 92% compared to V/Q and CTPA[45]. Another qualitative comparison for evaluating the diagnostic potential of pulmonary perfusion MRI for the detection of pulmonary embolism reported a sensitivity of 87%-93% and a specificity of 90%-95% with an inter-observer agreement of k = 0.77[49]. Similarly, another study prospectively comparing the diagnostic potential of perfusion MRI, perfusion SPECT and CTPA revealed sensitivity 100% and specificity 81% with inter-observer agreement of k=0.88 for perfusion MRI compared to inter-observer agreement of k = 0.80 (P < 0.0001) for SPECT scintigraphy[50].
Quantitative assessment: Prospective quantitative studies affirm the diagnostic potential of pulmonary perfusion MRI by measuring the perfusion parameters of PBF, PBV and MTT for normal, CTEPH and PH of other aetiologies leading to early differential diagnosis and better disease management[37,39,40,43].
Pulmonary perfusion parameters of PBF, PBV and MTT for healthy volunteers and PH patients were quantitatively assessed in a prospective study. Pulmonary perfusion parameters were significantly lower for PH patients accurately differentiating the two study groups[33]. Furthermore, pulmonary perfusion MRI diagnostic accuracy was confirmed in another relevant study where significant results were reported for all three pulmonary perfusion MRI parameters correctly differentiating the two study groups and aiding the differential diagnosis of primary pulmonary hypertension[37].
Additionally, the capability of pulmonary perfusion MRI for the evaluation of disease severity and progression of PAH was compared with pulmonary function test and Doppler echocardiography. Pulmonary perfusion MRI was positively correlated with diffusing capacity of carbon monoxide %DL(CO) measured by pulmonary function test. In the same study, pulmonary perfusion MRI parameters of PBF, PBV and MTT were also positively correlated with mean pulmonary artery pressure and systolic pulmonary artery pressure measured by Doppler echocardiography[39]. This significant correlation implies promising diagnostic potential of pulmonary perfusion MRI for detecting PAH when compared to routine tests.
In another quantitative prospective study, specificity and accuracy of pulmonary perfusion MRI were compared with MDCT and magnetic resonance angiography (MRA) for PH diagnosis. Specificity and accuracy were significantly higher for pulmonary perfusion MRI when right ventricle/Left ventricle diameter ratio and acute pulmonary thromboembolic indices were compared for perfusion MRI, MDCT and MRA (P < 0.05). This implies comparable diagnostic capability for pulmonary perfusion MRI to routine tests for PH diagnosis. In this study, PBF was reported as a more accurate perfusion MRI parameter than PBV and MTT[40].
Moreover, researchers compared the accuracy of treatment response in inoperable CTEPH patients using pulmonary perfusion MRI, CTPA and MRA. This comparative prospective study found higher diagnostic accuracy (95%) and specificity (90%) for pulmonary perfusion MRI compared to CTPA to differentiate treatment responders and non-responders[43]. A recent quantitative study assessed the regional PBF pre and post PEA using pulmonary perfusion MRI and compared it with exercise capacity post PEA for CTEPH patient. They concluded a positive correlation between perfusion MRI for identifying improvement in PBF in the lower lungs and exercise capacity after successful surgery[80].
This literature search concludes the approving potential of pulmonary perfusion MRI for the differential diagnosis of PH. However, most of these comparative studies were single centre with small sample size, therefore further large-scale multicentre research is recommended.
Hyperpolarised gases like 3He, 129Xe and Oxygen-enhanced 1H have also gained popularity for assessing pulmonary ventilation and perfusion in lung diseases. A retrospective study on 15 patients was conducted to assess regional lung ventilation as part of pre-operative planning of lobectomy, pneumonectomy or lung volume reduction surgery. This study reported good agreement between 3He MRI and scintigraphy ventilation imaging to diagnose ventilation defects[81]. Another case study of CTEPH patient demonstrated the use of 3He ventilation scan combined with dynamic contrast enhanced perfusion MRI to assess the treatment response of pre and post PEA[82].
129Xe is of particular interest for CTEPH diagnosis because 129Xe has an ability to dissolve in pulmonary tissues. After inhalation, 129Xe is easily soluble in lung tissues and blood leading to frequency shift which can be quantitatively measured by MR spectroscopy[83]. 129Xe has been tested to demonstrate a reduced uptake of red blood cells in CTEPH and pulmonary vascular disease patients indicating perfusion defects[84,85]. Another study on 10 PAH patients, 129Xe MRI showed reduced RBC amplitude oscillations on MRI spectroscopy implying its potential to diagnose pulmonary vascular diseases from other lung disorders[86]. These initial results reflect the potential of combining hyperpolarised gas MRI imaging with cardiopulmonary MRI to comprehensively assess the structural and functional ventilation and perfusion pulmonary disorders.
However, hyperpolarised inhaled contrast agent pulmonary MRI is still an emerging modality and further large-scale research is needed to strengthen its diagnostic potential to conventional perfusion scintigraphy.
Ionising radiation free diagnostic test: Screening and follow-up scans to monitor the disease progression is a crucial part of management strategy for patients with PH. In a prospective longitudinal study in a cohort of 675 emergency department patients researchers concluded that at least one third of patients undergoing CTPA were called for a follow up scan within 5 years, with one fifth of patients being women younger than 40 years old[21]. In addition, it is estimated that 5% to 11% of CTPA scans for the diagnosis of CTEPH are repeated due to artefacts and technical reasons, which further exacerbate the risk of excessive radiation dose in another study[87]. This unnecessary ionising radiation exposure can be reduced by using pulmonary perfusion MRI at screening and diagnostic stages of CTEPH leading to better patient healthcare strategy.
An all-inclusive test: Besides lung perfusion imaging, the cardiac assessment is also important in patients with PH. Cardiac involvement in PH is an inevitable step in the disease progression and PH due to left heart disease is the most common type[88,89]. It is reported that more than half of heart failure patients suffer from PH[90]. The pressure increase in the pulmonary arteries leads to an increase in the RV pressure and cardiac remodelling, correlated with increased mortality. RV end-systolic volume index has been reported as an independent predictor of PAH prognosis in a study using 288 derivation cohort and 288 validation PAH cohort[91].
Moreover, another relevant study validated that cardiac MRI based pulmonary artery distensibility index correlates positively with the right heart catheterization estimates for PAH, suggesting non-invasive cardiac MRI a valuable tool for PAH diagnosis[42].
Additionally, cardiac magnetic resonance imaging has also been used to assess pulmonary artery pressure measurements in children with congenital heart disease. Researchers found velocity encoded flow cardiac MRI images strongly correlated with right heart catheterization for systemic blood flow (QS), right to left pulmonary blood flow (QP) ratio and QP/QS ratio[47]. This suggests usefulness of cardiac MRI for pulmonary artery pressure measurement by using the standard SSFP cine imaging and phase contrast flow imaging.
Pulmonary perfusion can be combined with cardiac MRI, including cardiac cine images for structural and functional assessment of heart chambers, blood flow assessment by phase contrast imaging, MRA, post contrast late gadolinium enhancement imaging for myocardial fibrosis/scarring and four-dimensional phase contrast pulmonary artery flow scan. A comprehensive cardio-pulmonary assessment can be achieved in a single examination during the same visit, leading to better use of health resources and limiting the patients’ stress[83].
Quantification mitigates observer bias: MRI pulmonary perfusion offers quantification of PBF, PBV and MTT with improved diagnostic accuracy. In a study assessing the diagnostic accuracy of qualitative and quantitative cardiac MR perfusion in coronary artery disease, the level of training was reported as a major determinant for the accuracy of qualitative assessment (the diagnostic accuracy of level-3 operators was 83.6%, level-2 operators 65.7% and level-1 operators 55.7%, P < 0.001), while the diagnostic accuracy of quantitative assessment was 86.3% (significantly higher than level-2 and level-1 operators), highlighting the usefulness of operator-independent quantification techniques[70].
Moreover, higher consistency and reproducibility results were achieved using quantification techniques, and an increased sensitivity (from 77% to 83%) was reported when using quantitative measures for the evaluation of myocardial perfusion[92]. Researchers also studied MRI signal abnormalities in the brain and reported higher reproducibility and accuracy of quantification techniques compared to qualitative analysis by expert radiologists[93]. The quantification of MRI perfusion parameters mitigates this observer bias, which is not achievable with current tests to diagnose PH.
CT scan uses iodine-containing contrast media which can unfortunately cause serious complications, such as contrast induced nephropathy. Gadolinium-based contrast agents are considered safer than iodinated contrast media, thus they can be safely used also in patients with impaired kidney function[94]. CT contrast also involves a huge dose and contrast related discomfort has also been reported. Additionally, the iodine-based contrast can potentially worsen the thyroid function, on the other hand gadolinium based MRI contrast agents have been reported safer for pregnant patients and patients with compromised renal functions[95]. Some other contraindications for CT contrast injection include multiple myeloma and metformin.
An MRI scanner is a long, tunnel-like enclosed chamber where patients need to lie down still for a long time (minimum 40 min). Patients are further fastened by the radiofrequency coil placed on the chest. The MRI scanner also produces loud banging noise (110 decibels at its loudest)[96]. Lying down still for a long time in such an enclosed chamber and tolerating the loud noise can be challenging for claustrophobic patients or patients with hearing problems. Magnet related safety for medical devices’ including cardiac pacemakers, aneurysmal clips, intra orbital metallic injury and some other incompatible metallic implants are some other limitations of MRI scanning.
In comparison to computed tomography, MRI is prone to breathing artefacts and it inherently retains low image resolution for moving organs like heart and lungs. Scan time is another limitation for MRI, longer scan time leads to patient discomfort. To mitigate, advanced motion correction techniques of parallel imaging, patient training for breath hold, respiratory/ electrocardiogram gating for cardiac MRI and saturation band to supress signal from moving chest wall and heart motion have been adapted[97].
Moreover, recently researchers have successfully validated free breathing, real time MRI imaging with short scan time and high image resolution for cardiac perfusion imaging[98,99]. More research is warranted to apply this technique for pulmonary perfusion
Pulmonary perfusion quantification is technically challenging. It needs a skilled operator and availability of a specific software package for quantification. Furthermore, it is prone to errors when images have respiratory and motion artefacts. Lack of integration with other clinical findings while quantification is another limitation.
The Fleischner Society’s position paper recommends MRI for clinical use for cystic fibrosis, lung cancer staging, lung nodule characterisation and pulmonary hypertension. The position paper endorses further investigating the role of perfusion MRI for the diagnosis of chronic obstructive pulmonary disease and pulmonary parenchymal abnormalities in future[57]. However, MRI imaging for lungs is still limited in clinical and research fields implying the need for further clinically implementable research in this field[57,100]. An ongoing prospective, multicentre, comparative phase III clinical trial (Change-MRI) is currently being carried out to compare the diagnostic accuracy of perfusion MRI and SPECT scintigraphy for CTEPH diagnosis[101]. Pulmonary perfusion MRI has clinical and technical aspects to be considered for future research on this topic.
Clinically, combining cardiac magnetic resonance with pulmonary perfusion imaging can help differentiate lung disorders of multiple aetiologies. 4-dimentional flow MRI imaging to assess pulmonary artery pressure has been demonstrated to aid CTEPH diagnosis[53,102,103]. Moreover, as SMS b-SSFP MRI sequence has been validated for better anatomical coverage and improved resolution for cardiac imaging, its use can be expanded for pulmonary perfusion imaging and specifically thromboembolic vascular lung disorders. Additionally, sophisticated software to quantitatively analyse perfusion parameters can further improve diagnostic accuracy in future[57].
Quantitative analysis of perfusion MRI parameters of PBF, PBV and MTT can be performed manually or automatically. Manual quantification can be time consuming and laborious. To improve efficiency, researchers established the validity of automated quantitative analysis for myocardial perfusion MRI and found it more efficient and reproducible than manual analysis[104-106].
Similarly, the automatic analysis of lung MRI has also been researched using artificial intelligence (AI) and deep learning algorithms for automatic lung segmentation of 20 cystic fibrosis patients. This study found automatic lung segmentation comparable with manual segmentation and capable of accurate estimation of lung volume[107]. Researchers also used AI techniques to automatically quantify the neonatal lung structure and compared it with manual segmentation by expert operators. This study found that AI-supported automatic segmentation of lung MRI was accurate and comparable to expert-level accuracy[108]. Therefore, for efficiency of time and consistency of diagnostic parameters, further research on the accuracy of automatic lung segmentation and quantification of perfusion parameters is recommended.
Currently, the dual-bolus approach is preferred in routine clinical practice for perfusion MRI imaging, but the dual-sequence approach to account for signal intensity nonlinearity and operator-friendly set up of injector pump in busy clinical settings is also recommended for future research on this topic[66].
PH is multifactorial and requires timely multimodality differential diagnosis for better disease prognosis. Pulmonary perfusion MRI can be considered a reliable and safe diagnostic imaging tool with comparable diagnostic accuracy to reference gold standards of SPECT, CTPA and V/Q imaging. It offers cardiopulmonary assessment when combined with CMR at the same visit without any radiation exposure making it a comprehensive test for screening, differential diagnosis and follow up scans for PH management. Furthermore, quantification of pulmonary perfusion parameters could improve the reliability and accuracy of diagnostic testing and help mitigate subjective interpretation of imaging findings which is not possible by qualitative assessment alone offered by standard tests.
| 1. | Verhoeff K, Mitchell JR. Cardiopulmonary physiology: why the heart and lungs are inextricably linked. Adv Physiol Educ. 2017;41:348-353. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 40] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 2. | Maron BA. Revised Definition of Pulmonary Hypertension and Approach to Management: A Clinical Primer. J Am Heart Assoc. 2023;12:e029024. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 109] [Article Influence: 36.3] [Reference Citation Analysis (0)] |
| 3. | Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M; ESC Scientific Document Group. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J. 2016;37:67-119. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3636] [Cited by in RCA: 3916] [Article Influence: 356.0] [Reference Citation Analysis (0)] |
| 4. | Kiely DG, Levin D, Hassoun P, Ivy DD, Jone PN, Bwika J, Kawut SM, Lordan J, Lungu A, Mazurek J, Moledina S, Olschewski H, Peacock A, Puri GD, Rahaghi F, Schafer M, Schiebler M, Screaton N, Tawhai M, Van Beek EJ, Vonk-Noordegraaf A, Vanderpool RR, Wort J, Zhao L, Wild J, Vogel-Claussen J, Swift AJ. EXPRESS: Statement on imaging and pulmonary hypertension from the Pulmonary Vascular Research Institute (PVRI). Pulm Circ. 2019;9:2045894019841990. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 88] [Cited by in RCA: 107] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 5. | Lan NSH, Massam BD, Kulkarni SS, Lang CC. Pulmonary Arterial Hypertension: Pathophysiology and Treatment. Diseases. 2018;6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 74] [Cited by in RCA: 119] [Article Influence: 14.9] [Reference Citation Analysis (0)] |
| 6. | Yaghi S, Novikov A, Trandafirescu T. Clinical update on pulmonary hypertension. J Investig Med. 2020;68:821-827. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 39] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Martinez C, Wallenhorst C, Teal S, Cohen AT, Peacock AJ. Incidence and risk factors of chronic thromboembolic pulmonary hypertension following venous thromboembolism, a population-based cohort study in England. Pulm Circ. 2018;8:2045894018791358. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 51] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 8. | Ohno Y, Koyama H, Lee HY, Miura S, Yoshikawa T, Sugimura K. Contrast-enhanced CT- and MRI-based perfusion assessment for pulmonary diseases: basics and clinical applications. Diagn Interv Radiol. 2016;22:407-421. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 25] [Article Influence: 2.8] [Reference Citation Analysis (1)] |
| 9. | Mortensen J, Gutte H. SPECT/CT and pulmonary embolism. Eur J Nucl Med Mol Imaging. 2014;41 Suppl 1:S81-S90. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 30] [Cited by in RCA: 39] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 10. | Musch G, Layfield JD, Harris RS, Melo MF, Winkler T, Callahan RJ, Fischman AJ, Venegas JG. Topographical distribution of pulmonary perfusion and ventilation, assessed by PET in supine and prone humans. J Appl Physiol (1985). 2002;93:1841-1851. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 169] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 11. | Doğan H, de Roos A, Geleijins J, Huisman MV, Kroft LJ. The role of computed tomography in the diagnosis of acute and chronic pulmonary embolism. Diagn Interv Radiol. 2015;21:307-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 78] [Article Influence: 7.8] [Reference Citation Analysis (0)] |
| 12. | Soler X, Hoh CK, Test VJ, Kerr KM, Marsh JJ, Morris TA. Single photon emission computed tomography in chronic thromboembolic pulmonary hypertension. Respirology. 2011;16:131-137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 44] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 13. | Grosse A, Grosse C, Lang I. Evaluation of the CT imaging findings in patients newly diagnosed with chronic thromboembolic pulmonary hypertension. PLoS One. 2018;13:e0201468. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 35] [Article Influence: 4.4] [Reference Citation Analysis (0)] |
| 14. | Remy-Jardin M, Faivre JB, Pontana F, Molinari F, Tacelli N, Remy J. Thoracic applications of dual energy. Semin Respir Crit Care Med. 2014;35:64-73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 15. | Havránková R. Biological effects of ionizing radiation. Cas Lek Cesk. 2020;159:258-260. [PubMed] |
| 16. | Levine DJ. Pulmonary arterial hypertension: updates in epidemiology and evaluation of patients. Am J Manag Care. 2021;27:S35-S41. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 48] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 17. | Lin EC. Radiation risk from medical imaging. Mayo Clin Proc. 2010;85:1142-1146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 306] [Cited by in RCA: 355] [Article Influence: 22.2] [Reference Citation Analysis (0)] |
| 18. | Astani SA, Davis LC, Harkness BA, Supanich MP, Dalal I. Detection of pulmonary embolism during pregnancy: comparing radiation doses of CTPA and pulmonary scintigraphy. Nucl Med Commun. 2014;35:704-711. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 43] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 19. | Bajc M, Neilly B, Miniati M, Mortensen J, Jonson B. Methodology for ventilation/perfusion SPECT. Semin Nucl Med. 2010;40:415-425. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 20. | Schembri GP, Miller AE, Smart R. Radiation dosimetry and safety issues in the investigation of pulmonary embolism. Semin Nucl Med. 2010;40:442-454. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 68] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 21. | Kline JA, Courtney DM, Beam DM, King MC, Steuerwald M. Incidence and predictors of repeated computed tomographic pulmonary angiography in emergency department patients. Ann Emerg Med. 2009;54:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 67] [Cited by in RCA: 69] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 22. | Ray JC, Burger C, Mergo P, Safford R, Blackshear J, Austin C, Fairweather D, Heckman MG, Zeiger T, Dubin M, Shapiro B. Pulmonary arterial stiffness assessed by cardiovascular magnetic resonance imaging is a predictor of mild pulmonary arterial hypertension. Int J Cardiovasc Imaging. 2019;35:1881-1892. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 28] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 23. | Olsson KM, Meyer B, Hinrichs J, Vogel-Claussen J, Hoeper MM, Cebotari S. Chronic thromboembolic pulmonary hypertension. Dtsch Arztebl Int. 2014;111:856-862. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 13] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 24. | Riedel M, Stanek V, Widimsky J, Prerovsky I. Longterm follow-up of patients with pulmonary thromboembolism. Late prognosis and evolution of hemodynamic and respiratory data. Chest. 1982;81:151-158. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 562] [Cited by in RCA: 553] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 25. | Gopalan D, Delcroix M, Held M. Diagnosis of chronic thromboembolic pulmonary hypertension. Eur Respir Rev. 2017;26. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 127] [Article Influence: 14.1] [Reference Citation Analysis (0)] |
| 26. | Mayer E, Jenkins D, Lindner J, D'Armini A, Kloek J, Meyns B, Ilkjaer LB, Klepetko W, Delcroix M, Lang I, Pepke-Zaba J, Simonneau G, Dartevelle P. Surgical management and outcome of patients with chronic thromboembolic pulmonary hypertension: results from an international prospective registry. J Thorac Cardiovasc Surg. 2011;141:702-710. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 441] [Cited by in RCA: 519] [Article Influence: 34.6] [Reference Citation Analysis (0)] |
| 27. | Madani MM, Auger WR, Pretorius V, Sakakibara N, Kerr KM, Kim NH, Fedullo PF, Jamieson SW. Pulmonary endarterectomy: recent changes in a single institution's experience of more than 2,700 patients. Ann Thorac Surg. 2012;94:97-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 368] [Cited by in RCA: 421] [Article Influence: 30.1] [Reference Citation Analysis (0)] |
| 28. | Jenkins D, Mayer E, Screaton N, Madani M. State-of-the-art chronic thromboembolic pulmonary hypertension diagnosis and management. Eur Respir Rev. 2012;21:32-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 96] [Article Influence: 6.9] [Reference Citation Analysis (0)] |
| 29. | Hopkins SR, Wielpütz MO, Kauczor HU. Imaging lung perfusion. J Appl Physiol (1985). 2012;113:328-339. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 80] [Article Influence: 5.7] [Reference Citation Analysis (0)] |
| 30. | Aziz M, Krishnam M, Madhuranthakam AJ, Rajiah P. Update on MR imaging of the pulmonary vasculature. Int J Cardiovasc Imaging. 2019;35:1483-1497. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 31. | Amundsen T, Kvaerness J, Jones RA, Waage A, Bjermer L, Nilsen G, Haraldseth O. Pulmonary embolism: detection with MR perfusion imaging of lung--a feasibility study. Radiology. 1997;203:181-185. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 74] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 32. | Amundsen T, Torheim G, Kvistad KA, Waage A, Bjermer L, Nordlid KK, Johnsen H, Asberg A, Haraldseth O. Perfusion abnormalities in pulmonary embolism studied with perfusion MRI and ventilation-perfusion scintigraphy: an intra-modality and inter-modality agreement study. J Magn Reson Imaging. 2002;15:386-394. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 48] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 33. | Ohno Y, Hatabu H, Murase K, Higashino T, Kawamitsu H, Watanabe H, Takenaka D, Fujii M, Sugimura K. Quantitative assessment of regional pulmonary perfusion in the entire lung using three-dimensional ultrafast dynamic contrast-enhanced magnetic resonance imaging: Preliminary experience in 40 subjects. J Magn Reson Imaging. 2004;20:353-365. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 153] [Cited by in RCA: 150] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 34. | Nikolaou K, Schoenberg SO, Attenberger U, Scheidler J, Dietrich O, Kuehn B, Rosa F, Huber A, Leuchte H, Baumgartner R, Behr J, Reiser MF. Pulmonary arterial hypertension: diagnosis with fast perfusion MR imaging and high-spatial-resolution MR angiography--preliminary experience. Radiology. 2005;236:694-703. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 105] [Cited by in RCA: 74] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 35. | Kluge A, Gerriets T, Lange U, Bachman G. MRI for short-term follow-up of acute pulmonary embolism. Assessment of thrombus appearance and pulmonary perfusion: a feasibility study. Eur Radiol. 2005;15:1969-1977. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 30] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 36. | Kluge A, Gerriets T, Stolz E, Dill T, Mueller KD, Mueller C, Bachmann G. Pulmonary perfusion in acute pulmonary embolism: agreement of MRI and SPECT for lobar, segmental and subsegmental perfusion defects. Acta Radiol. 2006;47:933-940. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 37. | Ohno Y, Hatabu H, Murase K, Higashino T, Nogami M, Yoshikawa T, Sugimura K. Primary pulmonary hypertension: 3D dynamic perfusion MRI for quantitative analysis of regional pulmonary perfusion. AJR Am J Roentgenol. 2007;188:48-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 38. | Ley S, Mereles D, Risse F, Grünig E, Ley-Zaporozhan J, Tecer Z, Puderbach M, Fink C, Kauczor HU. Quantitative 3D pulmonary MR-perfusion in patients with pulmonary arterial hypertension: correlation with invasive pressure measurements. Eur J Radiol. 2007;61:251-255. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 39. | Ohno Y, Koyama H, Nogami M, Takenaka D, Matsumoto S, Onishi Y, Matsumoto K, Murase K, Sugimura K. Dynamic perfusion MRI: capability for evaluation of disease severity and progression of pulmonary arterial hypertension in patients with connective tissue disease. J Magn Reson Imaging. 2008;28:887-899. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 37] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 40. | Ohno Y, Koyama H, Matsumoto K, Onishi Y, Nogami M, Takenaka D, Yoshikawa T, Matsumoto S, Sugimura K. Dynamic MR perfusion imaging: capability for quantitative assessment of disease extent and prediction of outcome for patients with acute pulmonary thromboembolism. J Magn Reson Imaging. 2010;31:1081-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 34] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 41. | Stein PD, Chenevert TL, Fowler SE, Goodman LR, Gottschalk A, Hales CA, Hull RD, Jablonski KA, Leeper KV Jr, Naidich DP, Sak DJ, Sostman HD, Tapson VF, Weg JG, Woodard PK; PIOPED III (Prospective Investigation of Pulmonary Embolism Diagnosis III) Investigators. Gadolinium-enhanced magnetic resonance angiography for pulmonary embolism: a multicenter prospective study (PIOPED III). Ann Intern Med. 2010;152:434-443. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 316] [Cited by in RCA: 255] [Article Influence: 15.9] [Reference Citation Analysis (0)] |
| 42. | Kang KW, Chang HJ, Kim YJ, Choi BW, Lee HS, Yang WI, Shim CY, Ha J, Chung N. Cardiac magnetic resonance imaging-derived pulmonary artery distensibility index correlates with pulmonary artery stiffness and predicts functional capacity in patients with pulmonary arterial hypertension. Circ J. 2011;75:2244-2251. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 42] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 43. | Ohno Y, Koyama H, Yoshikawa T, Nishio M, Matsumoto S, Matsumoto K, Aoyama N, Nogami M, Murase K, Sugimura K. Contrast-enhanced multidetector-row computed tomography vs. Time-resolved magnetic resonance angiography vs. contrast-enhanced perfusion MRI: assessment of treatment response by patients with inoperable chronic thromboembolic pulmonary hypertension. J Magn Reson Imaging. 2012;36:612-623. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 26] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 44. | Ley S, Fink C, Risse F, Ehlken N, Fischer C, Ley-Zaporozhan J, Kauczor HU, Klose H, Gruenig E. Magnetic resonance imaging to assess the effect of exercise training on pulmonary perfusion and blood flow in patients with pulmonary hypertension. Eur Radiol. 2013;23:324-331. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 45. | Rajaram S, Swift AJ, Telfer A, Hurdman J, Marshall H, Lorenz E, Capener D, Davies C, Hill C, Elliot C, Condliffe R, Wild JM, Kiely DG. 3D contrast-enhanced lung perfusion MRI is an effective screening tool for chronic thromboembolic pulmonary hypertension: results from the ASPIRE Registry. Thorax. 2013;68:677-678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 108] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 46. | Revel MP, Sanchez O, Lefort C, Meyer G, Couchon S, Hernigou A, Niarra R, Chatellier G, Frija G. Diagnostic accuracy of unenhanced, contrast-enhanced perfusion and angiographic MRI sequences for pulmonary embolism diagnosis: results of independent sequence readings. Eur Radiol. 2013;23:2374-2382. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 27] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 47. | Sugimoto M, Kajino H, Kajihama A, Nakau K, Murakami N, Azuma H. Assessment of pulmonary arterial pressure by velocity-encoded cine magnetic resonance imaging in children with congenital heart disease. Circ J. 2013;77:3015-3022. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 48. | Schönfeld C, Cebotari S, Voskrebenzev A, Gutberlet M, Hinrichs J, Renne J, Hoeper MM, Olsson KM, Welte T, Wacker F, Vogel-Claussen J. Performance of perfusion-weighted Fourier decomposition MRI for detection of chronic pulmonary emboli. J Magn Reson Imaging. 2015;42:72-79. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 34] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 49. | Ingrisch M, Maxien D, Meinel FG, Reiser MF, Nikolaou K, Dietrich O. Detection of pulmonary embolism with free-breathing dynamic contrast-enhanced MRI. J Magn Reson Imaging. 2016;43:887-893. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 50. | Johns CS, Swift AJ, Rajaram S, Hughes PJC, Capener DJ, Kiely DG, Wild JM. Lung perfusion: MRI vs. SPECT for screening in suspected chronic thromboembolic pulmonary hypertension. J Magn Reson Imaging. 2017;46:1693-1697. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 45] [Cited by in RCA: 71] [Article Influence: 7.9] [Reference Citation Analysis (0)] |
| 51. | Voskrebenzev A, Gutberlet M, Klimeš F, Kaireit TF, Schönfeld C, Rotärmel A, Wacker F, Vogel-Claussen J. Feasibility of quantitative regional ventilation and perfusion mapping with phase-resolved functional lung (PREFUL) MRI in healthy volunteers and COPD, CTEPH, and CF patients. Magn Reson Med. 2018;79:2306-2314. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 166] [Article Influence: 18.4] [Reference Citation Analysis (0)] |
| 52. | Agoston-Coldea L, Lupu S, Mocan T. Pulmonary Artery Stiffness by Cardiac Magnetic Resonance Imaging Predicts Major Adverse Cardiovascular Events in patients with Chronic Obstructive Pulmonary Disease. Sci Rep. 2018;8:14447. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 23] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 53. | Schoenfeld C, Hinrichs JB, Olsson KM, Kuettner MA, Renne J, Kaireit T, Czerner C, Wacker F, Hoeper MM, Meyer BC, Vogel-Claussen J. Cardio-pulmonary MRI for detection of treatment response after a single BPA treatment session in CTEPH patients. Eur Radiol. 2019;29:1693-1702. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 54. | Moher Alsady T, Kaireit TF, Behrendt L, Winther HB, Olsson KM, Wacker F, Hoeper MM, Cebotari S, Vogel-Claussen J. Comparison of dual-energy computer tomography and dynamic contrast-enhanced MRI for evaluating lung perfusion defects in chronic thromboembolic pulmonary hypertension. PLoS One. 2021;16:e0251740. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 55. | Torres LA, Lee KE, Barton GP, Hahn AD, Sandbo N, Schiebler ML, Fain SB. Dynamic contrast enhanced MRI for the evaluation of lung perfusion in idiopathic pulmonary fibrosis. Eur Respir J. 2022;60. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 15] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 56. | Yousaf T, Dervenoulas G, Politis M. Advances in MRI Methodology. Int Rev Neurobiol. 2018;141:31-76. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 57. | Hatabu H, Ohno Y, Gefter WB, Parraga G, Madore B, Lee KS, Altes TA, Lynch DA, Mayo JR, Seo JB, Wild JM, van Beek EJR, Schiebler ML, Kauczor HU; Fleischner Society. Expanding Applications of Pulmonary MRI in the Clinical Evaluation of Lung Disorders: Fleischner Society Position Paper. Radiology. 2020;297:286-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 136] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 58. | Alsop DC, Hatabu H, Bonnet M, Listerud J, Gefter W. Multi-slice, breathhold imaging of the lung with submillisecond echo times. Magn Reson Med. 1995;33:678-682. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 85] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 59. | Mayo JR, MacKay A, Müller NL. MR imaging of the lungs: value of short TE spin-echo pulse sequences. AJR Am J Roentgenol. 1992;159:951-956. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 80] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 60. | Gordon Y, Partovi S, Müller-Eschner M, Amarteifio E, Bäuerle T, Weber MA, Kauczor HU, Rengier F. Dynamic contrast-enhanced magnetic resonance imaging: fundamentals and application to the evaluation of the peripheral perfusion. Cardiovasc Diagn Ther. 2014;4:147-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 56] [Reference Citation Analysis (0)] |
| 61. | Patel SH, Batchala PP, Schallert K, Patrie JT, Abbas SO, Ornan DA, Mukherjee S, Huerta T, Mugler JP 3rd. 3D fast low-angle shot (FLASH) technique for 3T contrast-enhanced brain MRI in the inpatient and emergency setting: comparison with 3D magnetization-prepared rapid gradient echo (MPRAGE) technique. Neuroradiology. 2021;63:897-904. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 62. | Cuenod CA, Balvay D. Perfusion and vascular permeability: basic concepts and measurement in DCE-CT and DCE-MRI. Diagn Interv Imaging. 2013;94:1187-1204. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 160] [Cited by in RCA: 232] [Article Influence: 17.8] [Reference Citation Analysis (0)] |
| 63. | Rajaram S, Swift AJ, Capener D, Telfer A, Davies C, Hill C, Condliffe R, Elliot C, Hurdman J, Kiely DG, Wild JM. Diagnostic accuracy of contrast-enhanced MR angiography and unenhanced proton MR imaging compared with CT pulmonary angiography in chronic thromboembolic pulmonary hypertension. Eur Radiol. 2012;22:310-317. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 57] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 64. | Deshmane A, Gulani V, Griswold MA, Seiberlich N. Parallel MR imaging. J Magn Reson Imaging. 2012;36:55-72. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 280] [Cited by in RCA: 399] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 65. | Nazir MS, Neji R, Speier P, Reid F, Stäb D, Schmidt M, Forman C, Razavi R, Plein S, Ismail TF, Chiribiri A, Roujol S. Simultaneous multi slice (SMS) balanced steady state free precession first-pass myocardial perfusion cardiovascular magnetic resonance with iterative reconstruction at 1.5 T. J Cardiovasc Magn Reson. 2018;20:84. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 32] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 66. | Kellman P, Hansen MS, Nielles-Vallespin S, Nickander J, Themudo R, Ugander M, Xue H. Myocardial perfusion cardiovascular magnetic resonance: optimized dual sequence and reconstruction for quantification. J Cardiovasc Magn Reson. 2017;19:43. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 179] [Cited by in RCA: 242] [Article Influence: 26.9] [Reference Citation Analysis (0)] |
| 67. | Ingrisch M, Maxien D, Schwab F, Reiser MF, Nikolaou K, Dietrich O. Assessment of pulmonary perfusion with breath-hold and free-breathing dynamic contrast-enhanced magnetic resonance imaging: quantification and reproducibility. Invest Radiol. 2014;49:382-389. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 23] [Cited by in RCA: 24] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 68. | Maxien D, Ingrisch M, Meinel FG, Reiser M, Dietrich O, Nikolaou K. Quantification of pulmonary perfusion with free-breathing dynamic contrast-enhanced MRI--a pilot study in healthy volunteers. Rofo. 2013;185:1175-1181. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 6] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 69. | Borhani AA, Hosseinzadeh K. Quantitative Versus Qualitative Methods in Evaluation of T2 Signal Intensity to Improve Accuracy in Diagnosis of Pheochromocytoma. AJR Am J Roentgenol. 2015;205:302-310. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 70. | Villa ADM, Corsinovi L, Ntalas I, Milidonis X, Scannell C, Di Giovine G, Child N, Ferreira C, Nazir MS, Karady J, Eshja E, De Francesco V, Bettencourt N, Schuster A, Ismail TF, Razavi R, Chiribiri A. Importance of operator training and rest perfusion on the diagnostic accuracy of stress perfusion cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2018;20:74. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 46] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 71. | Jerosch-Herold M. Quantification of myocardial perfusion by cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2010;12:57. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 155] [Cited by in RCA: 164] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 72. | Jerosch-Herold M, Wilke N, Stillman AE. Magnetic resonance quantification of the myocardial perfusion reserve with a Fermi function model for constrained deconvolution. Med Phys. 1998;25:73-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 319] [Cited by in RCA: 320] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 73. | Zierler K. Indicator dilution methods for measuring blood flow, volume, and other properties of biological systems: a brief history and memoir. Ann Biomed Eng. 2000;28:836-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 106] [Cited by in RCA: 87] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 74. | Calamante F. Arterial input function in perfusion MRI: a comprehensive review. Prog Nucl Magn Reson Spectrosc. 2013;74:1-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 142] [Cited by in RCA: 156] [Article Influence: 12.0] [Reference Citation Analysis (0)] |
| 75. | Ishida M, Schuster A, Morton G, Chiribiri A, Hussain S, Paul M, Merkle N, Steen H, Lossnitzer D, Schnackenburg B, Alfakih K, Plein S, Nagel E. Development of a universal dual-bolus injection scheme for the quantitative assessment of myocardial perfusion cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2011;13:28. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 94] [Cited by in RCA: 91] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 76. | Risse F, Semmler W, Kauczor HU, Fink C. Dual-bolus approach to quantitative measurement of pulmonary perfusion by contrast-enhanced MRI. J Magn Reson Imaging. 2006;24:1284-1290. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 42] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 77. | Broadbent DA, Biglands JD, Ripley DP, Higgins DM, Greenwood JP, Plein S, Buckley DL. Sensitivity of quantitative myocardial dynamic contrast-enhanced MRI to saturation pulse efficiency, noise and t1 measurement error: Comparison of nonlinearity correction methods. Magn Reson Med. 2016;75:1290-1300. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 15] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 78. | Gatehouse PD, Elkington AG, Ablitt NA, Yang GZ, Pennell DJ, Firmin DN. Accurate assessment of the arterial input function during high-dose myocardial perfusion cardiovascular magnetic resonance. J Magn Reson Imaging. 2004;20:39-45. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 145] [Cited by in RCA: 143] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 79. | Jahng GH, Li KL, Ostergaard L, Calamante F. Perfusion magnetic resonance imaging: a comprehensive update on principles and techniques. Korean J Radiol. 2014;15:554-577. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 118] [Cited by in RCA: 153] [Article Influence: 12.8] [Reference Citation Analysis (0)] |
| 80. | Schoenfeld C, Cebotari S, Hinrichs J, Renne J, Kaireit T, Olsson KM, Voskrebenzev A, Gutberlet M, Hoeper MM, Welte T, Haverich A, Wacker F, Vogel-Claussen J. MR Imaging-derived Regional Pulmonary Parenchymal Perfusion and Cardiac Function for Monitoring Patients with Chronic Thromboembolic Pulmonary Hypertension before and after Pulmonary Endarterectomy. Radiology. 2016;279:925-934. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 81. | Altes TA, Rehm PK, Harrell F, Salerno M, Daniel TM, De Lange EE. Ventilation imaging of the lung: comparison of hyperpolarized helium-3 MR imaging with Xe-133 scintigraphy. Acad Radiol. 2004;11:729-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 31] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 82. | Marshall H, Kiely DG, Parra-Robles J, Capener D, Deppe MH, van Beek EJ, Swift AJ, Rajaram S, Hurdman J, Condliffe R, Elliot CA, Wild JM. Magnetic resonance imaging of ventilation and perfusion changes in response to pulmonary endarterectomy in chronic thromboembolic pulmonary hypertension. Am J Respir Crit Care Med. 2014;190:e18-e19. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 16] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 83. | Saunders LC, Hughes PJC, Alabed S, Capener DJ, Marshall H, Vogel-Claussen J, van Beek EJR, Kiely DG, Swift AJ, Wild JM. Integrated Cardiopulmonary MRI Assessment of Pulmonary Hypertension. J Magn Reson Imaging. 2022;55:633-652. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 11] [Article Influence: 2.8] [Reference Citation Analysis (0)] |
| 84. | Wang Z, He M, Bier E, Rankine L, Schrank G, Rajagopal S, Huang YC, Kelsey C, Womack S, Mammarappallil J, Driehuys B. Hyperpolarized (129) Xe gas transfer MRI: the transition from 1.5T to 3T. Magn Reson Med. 2018;80:2374-2383. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 28] [Cited by in RCA: 36] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 85. | Dahhan T, Kaushik SS, He M, Mammarappallil JG, Tapson VF, McAdams HP, Sporn TA, Driehuys B, Rajagopal S. Abnormalities in hyperpolarized (129)Xe magnetic resonance imaging and spectroscopy in two patients with pulmonary vascular disease. Pulm Circ. 2016;6:126-131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 21] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 86. | Wang Z, Bier EA, Swaminathan A, Parikh K, Nouls J, He M, Mammarappallil JG, Luo S, Driehuys B, Rajagopal S. Diverse cardiopulmonary diseases are associated with distinct xenon magnetic resonance imaging signatures. Eur Respir J. 2019;54. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 68] [Article Influence: 9.7] [Reference Citation Analysis (0)] |
| 87. | U-King-Im JM, Freeman SJ, Boylan T, Cheow HK. Quality of CT pulmonary angiography for suspected pulmonary embolus in pregnancy. Eur Radiol. 2008;18:2709-2715. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 88. | Mehra P, Mehta V, Sukhija R, Sinha AK, Gupta M, Girish MP, Aronow WS. Pulmonary hypertension in left heart disease. Arch Med Sci. 2019;15:262-273. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 89. | Rosenkranz S, Howard LS, Gomberg-Maitland M, Hoeper MM. Systemic Consequences of Pulmonary Hypertension and Right-Sided Heart Failure. Circulation. 2020;141:678-693. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 209] [Article Influence: 34.8] [Reference Citation Analysis (0)] |
| 90. | Calderaro D, Alves Junior JL, Fernandes CJCDS, Souza R. Pulmonary Hypertension in General Cardiology Practice. Arq Bras Cardiol. 2019;113:419-428. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
| 91. | Swift AJ, Capener D, Johns C, Hamilton N, Rothman A, Elliot C, Condliffe R, Charalampopoulos A, Rajaram S, Lawrie A, Campbell MJ, Wild JM, Kiely DG. Magnetic Resonance Imaging in the Prognostic Evaluation of Patients with Pulmonary Arterial Hypertension. Am J Respir Crit Care Med. 2017;196:228-239. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 141] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 92. | Yun CH, Tsai JP, Tsai CT, Mok GS, Sun JY, Hung CL, Wu TH, Huang WT, Yang FS, Lee JJ, Cury RC, Fares A, Nshisso LD, Bezerra HG. Qualitative and semi-quantitative evaluation of myocardium perfusion with 3 T stress cardiac MRI. BMC Cardiovasc Disord. 2015;15:164. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 12] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 93. | Wei X, Warfield SK, Zou KH, Wu Y, Li X, Guimond A, Mugler JP 3rd, Benson RR, Wolfson L, Weiner HL, Guttmann CR. Quantitative analysis of MRI signal abnormalities of brain white matter with high reproducibility and accuracy. J Magn Reson Imaging. 2002;15:203-209. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 92] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 94. | Hasebroock KM, Serkova NJ. Toxicity of MRI and CT contrast agents. Expert Opin Drug Metab Toxicol. 2009;5:403-416. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 161] [Cited by in RCA: 167] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 95. | Webb JA, Thomsen HS, Morcos SK; Members of Contrast Media Safety Committee of European Society of Urogenital Radiology (ESUR). The use of iodinated and gadolinium contrast media during pregnancy and lactation. Eur Radiol. 2005;15:1234-1240. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 408] [Cited by in RCA: 343] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 96. | Salvi R, Sheppard A. Is Noise in the MR Imager a Significant Risk Factor for Hearing Loss? Radiology. 2018;286:609-610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 97. | Serai SD, Hu HH, Ahmad R, White S, Pednekar A, Anupindi SA, Lee EY. Newly Developed Methods for Reducing Motion Artifacts in Pediatric Abdominal MRI: Tips and Pearls. AJR Am J Roentgenol. 2020;214:1042-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 37] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 98. | Scannell CM, Correia T, Villa ADM, Schneider T, Lee J, Breeuwer M, Chiribiri A, Henningsson M. Feasibility of free-breathing quantitative myocardial perfusion using multi-echo Dixon magnetic resonance imaging. Sci Rep. 2020;10:12684. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 99. | McElroy S, Ferrazzi G, Nazir MS, Evans C, Ferreira J, Bosio F, Mughal N, Kunze KP, Neji R, Speier P, Stäb D, Ismail TF, Masci PG, Villa ADM, Razavi R, Chiribiri A, Roujol S. Simultaneous multislice steady-state free precession myocardial perfusion with full left ventricular coverage and high resolution at 1.5 T. Magn Reson Med. 2022;88:663-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1] [Cited by in RCA: 7] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 100. | Ley S, Ley-Zaporozhan J. Pulmonary perfusion imaging using MRI: clinical application. Insights Imaging. 2012;3:61-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 29] [Cited by in RCA: 31] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 101. | Lasch F, Karch A, Koch A, Derlin T, Voskrebenzev A, Alsady TM, Hoeper MM, Gall H, Roller F, Harth S, Steiner D, Krombach G, Ghofrani HA, Rengier F, Heußel CP, Grünig E, Beitzke D, Hacker M, Lang IM, Behr J, Bartenstein P, Dinkel J, Schmidt KH, Kreitner KF, Frauenfelder T, Ulrich S, Hamer OW, Pfeifer M, Johns CS, Kiely DG, Swift AJ, Wild J, Vogel-Claussen J. Comparison of MRI and VQ-SPECT as a Screening Test for Patients With Suspected CTEPH: CHANGE-MRI Study Design and Rationale. Front Cardiovasc Med. 2020;7:51. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 15] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 102. | Lechartier B, Chaouat A, Aubert JD, Schwitter J; Swiss Society for Pulmonary Hypertension (SSPH). Magnetic resonance imaging in pulmonary hypertension: an overview of current applications and future perspectives. Swiss Med Wkly. 2022;152:w30055. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 103. | Ota H, Sugimura K, Miura M, Shimokawa H. Four-dimensional flow magnetic resonance imaging visualizes drastic change in vortex flow in the main pulmonary artery after percutaneous transluminal pulmonary angioplasty in a patient with chronic thromboembolic pulmonary hypertension. Eur Heart J. 2015;36:1630. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 24] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 104. | Jacobs M, Benovoy M, Chang LC, Corcoran D, Berry C, Arai AE, Hsu LY. Automated Segmental Analysis of Fully Quantitative Myocardial Blood Flow Maps by First-Pass Perfusion Cardiovascular Magnetic Resonance. IEEE Access. 2021;9:52796-52811. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 16] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 105. | Scannell CM, Veta M, Villa ADM, Sammut EC, Lee J, Breeuwer M, Chiribiri A. Deep-Learning-Based Preprocessing for Quantitative Myocardial Perfusion MRI. J Magn Reson Imaging. 2020;51:1689-1696. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 57] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 106. | Xue H, Davies RH, Brown LAE, Knott KD, Kotecha T, Fontana M, Plein S, Moon JC, Kellman P. Automated Inline Analysis of Myocardial Perfusion MRI with Deep Learning. Radiol Artif Intell. 2020;2:e200009. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 36] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 107. | Weng AM, Heidenreich JF, Metz C, Veldhoen S, Bley TA, Wech T. Deep learning-based segmentation of the lung in MR-images acquired by a stack-of-spirals trajectory at ultra-short echo-times. BMC Med Imaging. 2021;21:79. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 8] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 108. | Mairhörmann B, Castelblanco A, Häfner F, Pfahler V, Haist L, Waibel D, Flemmer A, Ehrhardt H, Stoecklein S, Dietrich O, Foerster K, Hilgendorff A, Schubert B. Deep Learning-Based Magnetic Resonance Imaging Lung Segmentation and Volumetric Marker Extraction in Preterm Infants. 2021 Preprint. Available from: medRxiv 2021.08.06.21261648. [DOI] [Full Text] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author's Membership in Professional Societies: Health and Care Professionalcouncil, RA75559.
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu YJ, Taiwan; Xu L, China S-Editor: Liu JH L-Editor: A P-Editor: Zhao S