Published online Aug 28, 2023. doi: 10.4329/wjr.v15.i8.241
Peer-review started: March 28, 2023
First decision: June 1, 2023
Revised: June 15, 2023
Accepted: July 27, 2023
Article in press: July 27, 2023
Published online: August 28, 2023
Processing time: 148 Days and 13.3 Hours
Diagnosis of prosthetic vascular graft infection with [(18)F]fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) allows for early detection of functional changes associated with infection, based on increased glucose utilization by activated macrophages and granulocytes. Aseptic vascular grafts, like all foreign bodies, can stimulate an inflammatory response, which can present as increased activity on 18F-FDG PET/CT. Conse
To investigate the visual, semiquantitative, and temporal characteristics of aseptic vascular grafts in patients status post EVAR.
In this observational retrospective cohort study, patients with history of EVAR who underwent 18F-FDG PET/CT for indications other than infection were identified retrospectively. All patients were asymptomatic for graft infection - no abdominal pain, fever of unknown origin, sepsis, or leukocytosis - at the time of imaging and for ≥ 2 mo after each PET/CT. Imaging studies such as CT for each patient were also reviewed, and any patients with suspected or confirmed vascular graft infection were excluded. One hundred two scans performed on 43 patients (34 males; 9 females; age = 77 ± 8 years at the time of the final PET/CT) were retrospectively reviewed. All 43 patients had an abdominal aortic (AA) vascular graft, 40 patients had a right iliac (RI) limb graft, and 41 patients had a left iliac (LI) limb graft. Twenty-two patients had 1 PET/CT and 21 patients had from 2 to 9 PET/CTs. Grafts were imaged between 2 mo to 168 mo (about 14 years) post placement. Eight grafts were imaged within 6 mo of placement, including three that were imaged within three months of placement. The mean interval between graft placement and PET/CT for all 102 scans was 51 ± 39 mo. PET/CT data was reconstructed with region-of-interest analysis of proximal, mid and distal portions of the grafts and background ascending aorta. Maximum standardized uptake value (SUVmax) was recorded for each region. SUVmax-to-background uptake ratios (URs) were calculated. Visual assessment was performed using a 2-pattern grading scale: Diffuse (homogeneous uptake less than liver uptake) and focal (one or more areas of focal uptake in any part of the graft). Statistical analysis was performed.
In total, there were 306 AA grafts, 285 LI grafts, 282 RI grafts, and 306 ascending aorta background SUVmax measurements. For all 102 scans, mean SUVmax values for AA grafts were 2.8-3.0 along proximal, mid, and distal segments. Mean SUVmax values for LI grafts and RI grafts were 2.7-2.8. Mean SUVmax values for background were 2.5 ± 0.5. Mean URs were 1.1-1.2. Visual analysis of the scans reflected results of quantitative analysis. On visual inspection, 98% revealed diffuse, homogeneous 18F-FDG uptake less than liver. Graft URs and visual pattern categories were significantly associated for AA graft URs (F-ratio = 21.5, P < 0.001), LI graft URs (F-ratio = 20.4, P < 0.001), and RI graft URs (F-ratio = 30.4, P < 0.001). Thus, visual patterns of 18F-FDG uptake corresponded statistically significantly to semiquantitative URs. The age of grafts showing focal patterns was greater than grafts showing diffuse patterns, 87 ± 89 vs 50 ± 37 mo, respectively (P = 0.02). URs were significantly associated with graft age for AA grafts (r = 0.19, P = 0.001). URs were also significantly associated with graft age for LI grafts (r = 0.25, P < 0.0001), and RI grafts (r = 0.31, P < 0.001). Quartiles of similar numbers of graft (n = 25-27) grouped by graft age indicated that URs were significantly higher for 4th quartile vs 2nd quartile URs (F-ratio = 19.5, P < 0.001). When evaluating URs, graft SUVmax values within 10%-20% of the ascending aorta SUVmax is evident in aseptic grafts, except for grafts in the oldest quartiles. In this study, grafts in the oldest quartiles (> 7 years post EVAR) showed SUVmax up to 30% higher than the ascending aorta SUVmax.
Characteristics of an aseptic vascular stent graft in the aorta and iliac vessels on 18F-FDG PET/CT include graft SUVmax values within 10%-20% of the ascending aorta background SUVmax. The SUVmax of older aseptic grafts can be as much as 30% above background. The visual uptake pattern of diffuse, homogeneous uptake less than liver was seen in 98% of aseptic vascular grafts, making this pattern particularly reassuring for clinicians.
Core Tip: In patients post endovascular aortic repair who undergo [(18)F]fluorodeoxyglucose positron emission tomography/computed tomography, aseptic vascular grafts show maximum standardized uptake value (SUVmax) within 10%-20% of background ascending aorta SUVmax values. Older aseptic vascular grafts can show up to 30% higher uptake vs background compared with younger aseptic vascular grafts. The visual uptake pattern of diffuse, homogeneous uptake less than liver was seen in 98% of aseptic vascular grafts, making this pattern particularly reassuring for clinicians.
- Citation: Bennett P, Tomas MB, Koch CF, Nichols KJ, Palestro CJ. Appearance of aseptic vascular grafts after endovascular aortic repair on [(18)F]fluorodeoxyglucose positron emission tomography/computed tomography. World J Radiol 2023; 15(8): 241-249
- URL: https://www.wjgnet.com/1949-8470/full/v15/i8/241.htm
- DOI: https://dx.doi.org/10.4329/wjr.v15.i8.241
Diagnosis of prosthetic vascular graft infection with [(18)F]fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT) allows for detection of early functional changes associated with infection, based on increased glucose utilization by activated macrophages and granulocytes. 18F-FDG PET/CT can be an important diagnostic adjunct to CT, which depends on anatomic changes, such as perigraft air, fluid, soft tissue, fistula, and abscess for diagnosis of infection. However, sterile vascular grafts, like all foreign bodies, can stimulate an aseptic inflammatory response that presents as increased activity on 18F-FDG PET/CT. Consequently, distinguishing aseptic inflammation from vascular graft infection can be difficult, and standardized interpretation criteria for differentiating between these two conditions have not been universally adopted[1].
Currently, medical literature supports diagnostic sensitivity and specificity of 18F-FDG PET/CT in diagnosing vascular graft infection of 89%-98% and 59%-81%, respectively[2,3]. Note that the lower specificity raises the possibility of false-positive interpretations of 18F-FDG uptake on PET/CT. This has important clinical consequences, including unnecessary long-term antibiotic therapy, invasive procedures, and potential for graft explantation which carries an 18%-30% mortality rate due to complications[1,4,5]. As the negative predictive value of 18F-FDG PET/CT for excluding vascular graft infection is high (about 93%), the expected physiological patterns of 18F-FDG uptake in uninfected vascular grafts should be identified to avoid false-positive interpretation[4,6].
However, evidence on the appearance of aseptic vascular grafts over time on 18F-FDG PET/CT is sparse, maximum standardized uptake value (SUVmax) cutoff values for aseptic grafts have not been clearly defined, and visual pattern analysis is often suggested to distinguish aseptic from infected vascular grafts[2,7-10]. In the case of endovascular aneurysm repair (EVAR), a minimally invasive procedure involving the transfemoral insertion of an endoprosthetic stent graft, the normal postoperative appearance of these grafts on 18F-FDG PET/CT can vary over time, potentially con
In this observational retrospective cohort study, patients with history of EVAR who underwent 18F-FDG PET/CT for indications other than infection were identified retrospectively. All patients were asymptomatic for graft infection - no abdominal pain, fever of unknown origin, sepsis, or leukocytosis - at the time of imaging and for ≥ 2 mo after each PET/CT. Imaging studies such as CT for each patient were also reviewed, and any patients with suspected or confirmed vascular graft infection were excluded. One hundred two scans performed on 43 patients (34 males; 9 females; age = 77 ± 8 years at the time of the final PET/CT) were retrospectively reviewed. All 43 patients had an abdominal aortic (AA) vascular graft, 40 patients had a right iliac (RI) limb graft, and 41 patients had a left iliac (LI) limb graft. Twenty-two patients had 1 PET/CT and 21 patients had from 2 to 9 PET/CTs. Grafts were imaged between 2 mo to 168 mo (about 14 years) post placement. Eight grafts were imaged within 6 mo of placement, including three that were imaged within three months of placement. The mean interval between graft placement and PET/CT for all 102 scans was 51 ± 39 mo. Types of graft material were obtained from the patients’ medical records, when available (n = 19). The Institutional Review Board approved this retrospective study and the requirement to obtain informed consent was waived. All data were handled in compliance with the Health Insurance Portability and Accountability Act of 1996.
Data were acquired on 4 PET/CT systems: 2 Siemens Biograph mCT 64 (Munich, Germany) and 2 GE D710 (GE Healthcare, Chicago, IL, United States) systems. Data were reconstructed using manufacturer recommended 18F-FDG PET/CT reconstruction parameters on associated workstations at which data were acquired.
All reconstructed data were reviewed on a single GE AW workstation (GE Healthcare, Chicago, IL, United States). One nuclear medicine physician (MBT) analyzed all PET/CT images and obtained semiquantitative SUVmax using manually drawn region of interest (ROI) analysis. For each graft, a square ROI was drawn encompassing the width of the graft, cross-referenced on CT and confirmed on fused PET/CT images (Figure 1). ROIs were drawn around the proximal, mid, and distal portions of the AA graft, and SUVmax was recorded for each region. A similar ROI was used to measure SUVmax in the ascending aorta as the background (BKG) reference. SUVmax was also measured at proximal, mid, and distal portions of the RI and LI grafts when present. ROIs for each of the 3 locations along the grafts were placed equidistant.
Analyses were performed for SUVmax values to avoid underrepresentation of 18F-FDG uptake that could result from sampling tissue outside of graft tissues. To avoid the possibility of different PET/CT systems or software generating SUVmax values that were different from one another, the uptake ratio (UR) of SUVmax was calculated for each graft location using the formula: UR = SUVmax graft/SUVmax BKG. The URs were analyzed to minimize effects of using different PET/CT systems and image reconstruction algorithms.
The same nuclear medicine physician who placed ROIs for semiquantitative analysis also classified uptake according to two visual patterns for aseptic grafts: Diffuse and focal. Diffuse was defined as mild, homogeneous uptake less than liver. Focal was defined as one or more areas of focal uptake in any part of the graft. Reference for visual analysis was 18F-FDG uptake in the liver.
Analyses were performed using commercially available software (“MedCalc” Statistical Software version 20.110; MedCalc Software Ltd, Ostend, Belgium; https://www.medcalc.org; 2022). Values were reported as mean ± SD. The Kolmogorov-Smirnov method assessed whether continuous variables were normally distributed and provided means and distribution percentiles. ANOVA with Bonferroni correction compared SUVmax and URs grouped by age of grafts, ROI locations, and graft material. Significance of differences between mean values were assessed by the unpaired student’s t-test for normally distributed variables and by the Mann-Whitney test for non-normally distributed variables. Significance of changes over time was determined by linear regression of URs vs graft age. Linear regression of URs vs the time difference from the first through the last scan was performed for each patient with more than 1 PET/CT. Also, a separate subgroup analysis of patients with 3 or more scans was performed similarly with URs compared with the time difference from the first through the last scan of each patient. The Tukey test was applied to URs to detect outliers. For all tests, P < 0.05 was defined as statistically significant, or as adjusted by Bonferroni corrections for comparisons among multiple categories.
In total, there were 306 AA grafts, 285 LI grafts, 282 RI grafts, and 306 BKG SUVmax measurements. For all 102 scans, mean SUVmax values for AA grafts were 2.8-3.0 along proximal, mid, and distal segments (Table 1). Mean SUVmax values for LI grafts and RI grafts were 2.7-2.8. Mean SUVmax values for BKG were 2.5 ± 0.5 (Table 2). Mean URs were 1.1-1.2 (Tables 1 and 2).
| Location | SUVmax | Uptake ratio |
| Proximal aortic graft | 2.8 ± 0.8 | 1.1 ± 0.3 |
| Mid aortic graft | 2.9 ± 0.8 | 1.2 ± 0.3 |
| Distal aortic graft | 3.0 ± 0.9 | 1.2 ± 0.3 |
| Background | 2.5 ± 0.5 | - |
| Location | SUVmax | Uptake ratio |
| Aortic graft | 2.9 ± 0.8 | 1.2 ± 0.3 |
| Left Iliac graft | 2.7 ± 0.8 | 1.1 ± 0.3 |
| Right Iliac graft | 2.8 ± 0.8 | 1.2 ± 0.4 |
| Background | 2.5 ± 0.5 | - |
Of the 43 patients, graft material was identifiable for 10 patients who had polyethylene terephthalate (PT) grafts and 9 patients who had polytetrafluoroethylene (PTFE) grafts. There were 87 SUVmax measurements of PT grafts and 78 SUVmax measurements of PTFE grafts. ANOVA indicated a modest difference (F-ratio = 5.1, P = 0.03) of AA graft URs between PT and PTFE graft materials (1.2 ± 0.3 vs 1.1 ± 0.2, P = 0.03).
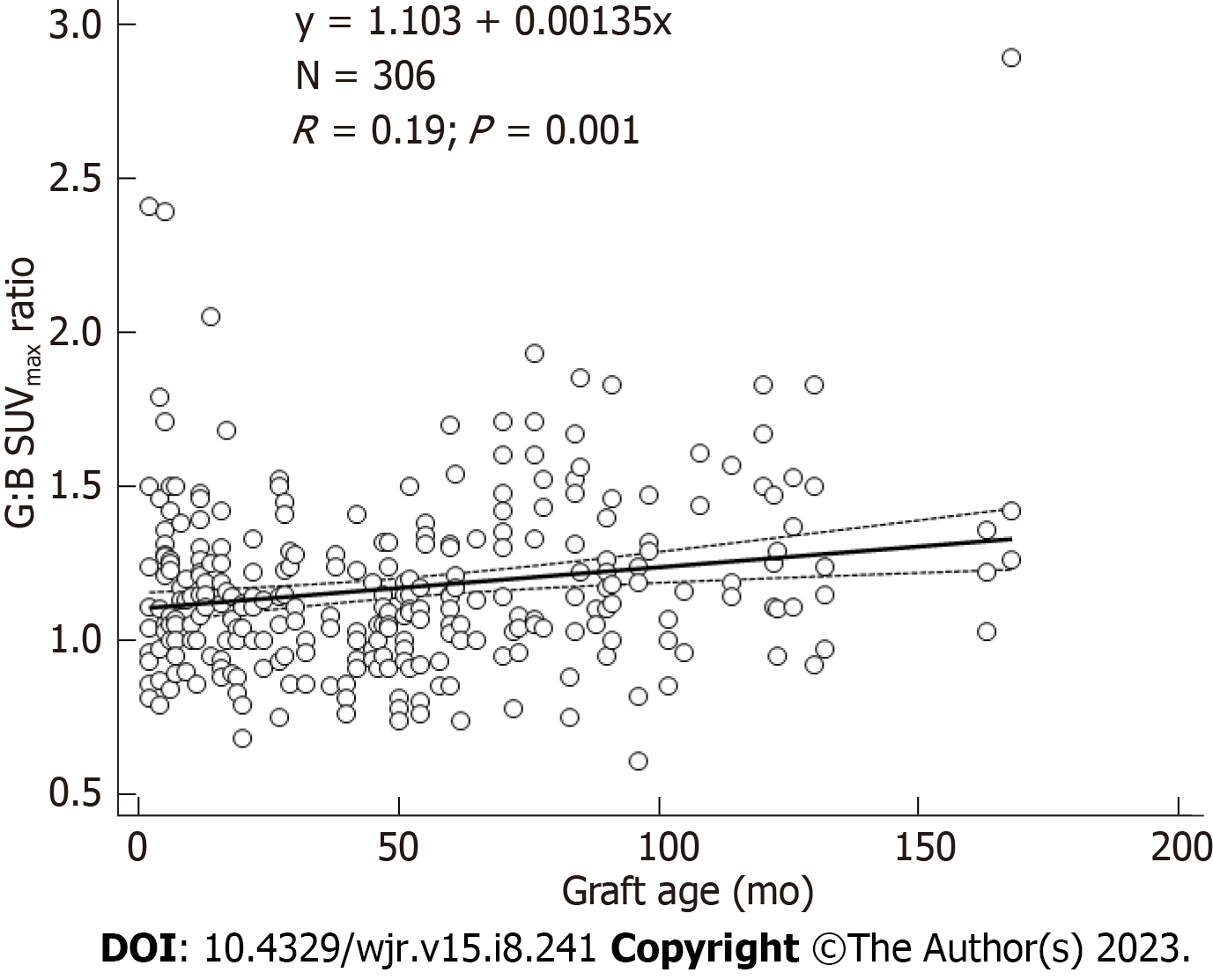
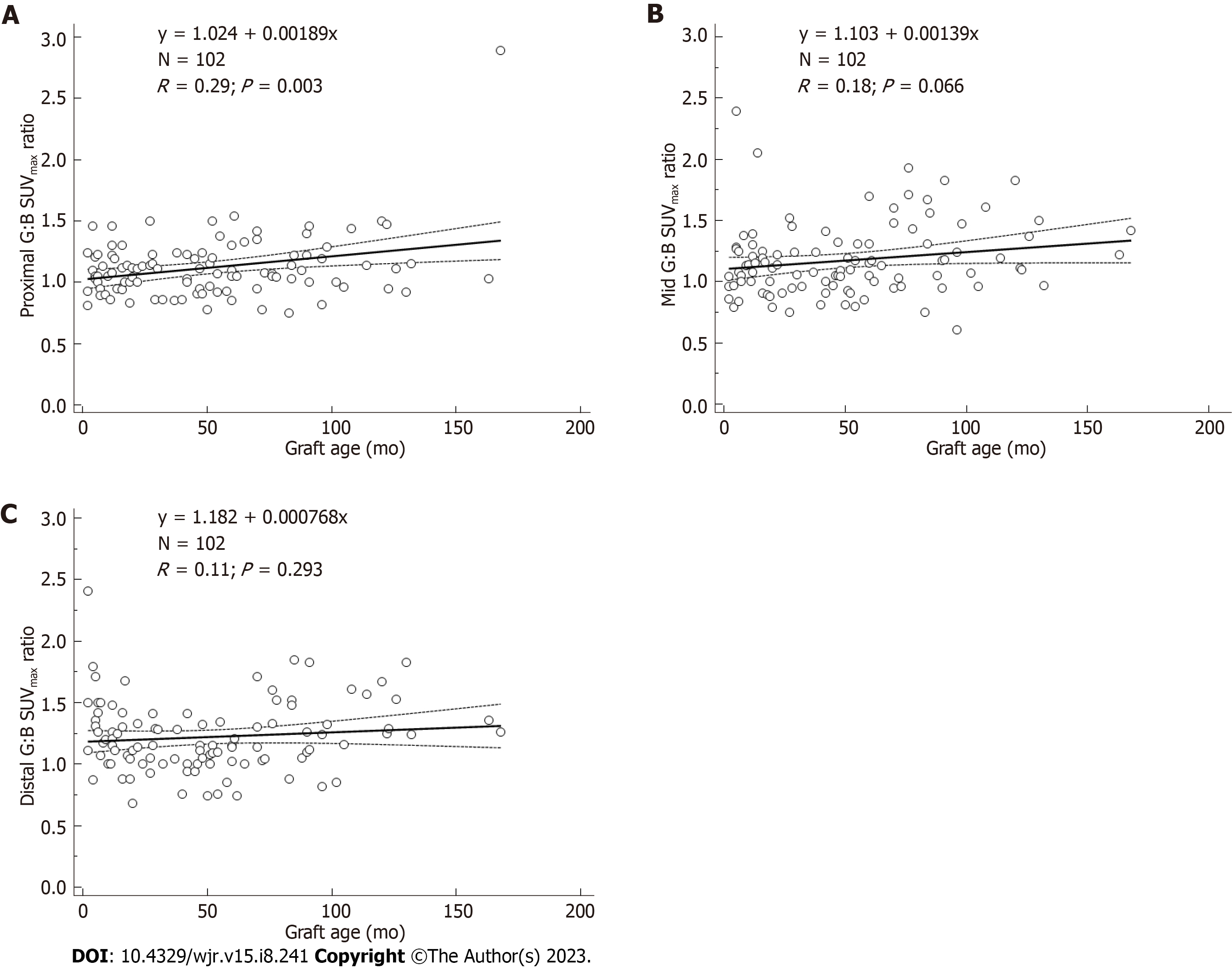
URs were significantly associated with graft age for AA grafts (r = 0.19, P = 0.001) (Figure 2). URs were also significantly associated with graft age for LI grafts (r = 0.25, P < 0.0001), and RI grafts (r = 0.31, P < 0.001). Quartiles of similar numbers of graft (n = 25-27) grouped by graft age indicated that URs were significantly higher for 4th quartile vs 2nd quartile URs (F-ratio = 19.5, P < 0.001) (Table 3). URs were similar for patients for whom graft placement was < 3 mo vs those with older grafts and were likewise similar for patients for whom graft placement was < 6 mo vs those with older grafts (F-ratio < 2.0, P > 0.05). While correlation of URs versus graft age was significant for all AA grafts (Figure 2), when analyzed separately by location, strongest correlation vs AA graft age was for proximal ROIs, less strong for mid ROIs, and not significant for distal ROIs (Figure 3). The highest UR value (2.89) corresponded to the patch region of the graft in one patient.
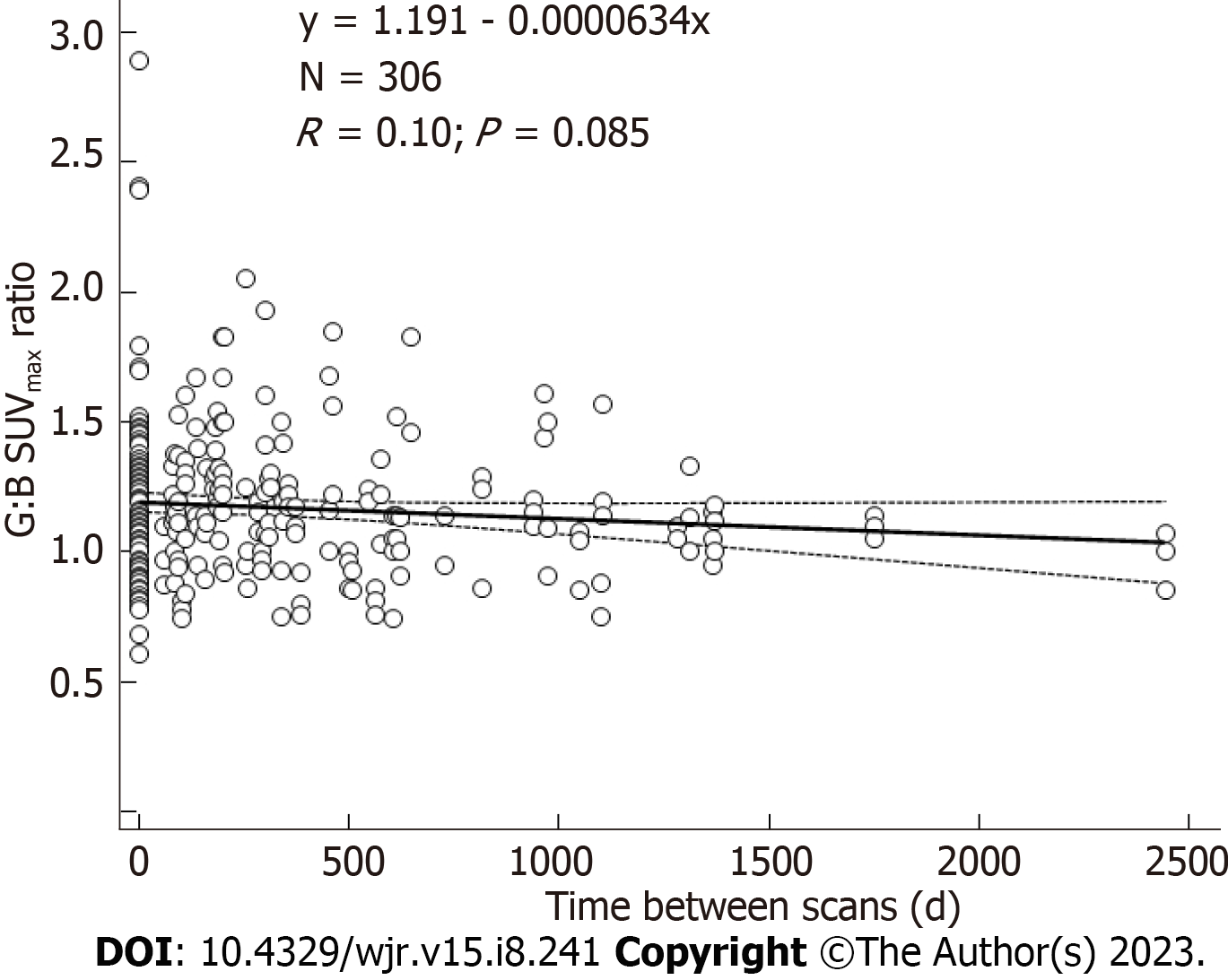
A total of 80 18F-FDG PET/CTs were performed on the 21 patients with repeat scans: 5 patients had 2; 8 patients had 3; 3 patients had 4; 3 patients had 6; 1 patient had 7; and 1 patient had 9 scans. Correlations of URs over time from the first through the last scan were not significant (r = 0.10, P = 0.09) (Figure 4). There were 210 URs evaluated for the subgroup of patients with 3 or more scans. For this subgroup, URs were not correlated with time from the first through the last scan (r = 0.12, P = 0.07).
Visual analysis of the scans reflected results of quantitative analysis (Table 4). On visual inspection, 98% revealed diffuse, homogeneous 18F-FDG uptake less than liver. Graft URs and visual pattern categories were significantly associated for AA graft URs (F-ratio = 21.5, P < 0.001), LI graft URs (F-ratio = 20.4, P < 0.001), and RI graft URs (F-ratio = 30.4, P < 0.001). Thus, visual patterns of 18F-FDG uptake corresponded statistically significantly to semiquantitative URs. The age of grafts showing focal patterns was greater than grafts showing diffuse patterns, 87 ± 89 vs 50 ± 37 mo, res
| Visual uptake pattern | n | Uptake ratio |
| Diffuse | 300 | 1.1 ± 0.3 |
| Focal | 6 | 1.8 ± 0.7a |
Visual uptake patterns were similar for different graft materials, when known, in that similar percentages of PT grafts and PTFE grafts were scored with focal visual patterns (2% vs 1%, P = 0.63) (Table 5), and similar to the 2% (6/306) of focal visual patterns for all grafts (Table 4).
The Tukey test showed there were 3 outlier cases for 3 different patients among the 306 graft URs. Even after excluding these 3 cases, there was significant association with URs and graft age for AA graft URs (r = 0.19, P = 0.001) (Figure 2). Similarly, there was significant association with URs and graft age for LI graft URs (r = 0.25, P < 0.001) and RI graft URs (r = 0.31, P < 0.001). Thus, no results were altered by excluding the 3 outliers.
In this study, the 18F-FDG PET/CT appearance of aseptic vascular grafts was delineated on 43 patients post EVAR without clinical signs and symptoms of vascular graft infection who underwent 18F-FDG PET/CT for oncologic indications. Visual, semiquantitative SUVmax and graft-to-background UR analysis was performed for 306 AA grafts, 285 LI grafts and 282 RI grafts. To our knowledge, this is the largest analysis of aseptic vascular graft appearance on 18F-FDG PET/CT to date.
All patients with aseptic aortic and iliac grafts showed graft SUVmax values of 3 or below. This is supported by a study by Tsuda et al[13] showing SUVmax below 4.5 in uninfected grafts, which was not dependent on time after surgery or whether the graft was placed in an open or endovascular fashion. Other studies have reported SUVmax values greater than 3.8-4.5 as significant for infection, which is supported by this study showing lower SUVmax values in aseptic grafts[14,15].
As SUVmax values can vary based on differences in PET/CT scanners, reconstruction algorithms and quality control efforts, we chose to include graft-to-background URs in our analyses. When evaluating URs, graft SUVmax values within 10%-20% of the ascending aorta SUVmax is evident in aseptic grafts, except for grafts in the oldest quartiles. In this study, grafts in the oldest quartile (> 7 years post EVAR) showed SUVmax up to 30% higher than the ascending aorta SUVmax.
The highest difference in URs was evident in PT grafts compared to PTFE grafts, although this modest difference is likely not clinically significant (1.2 ± 0.3 vs 1.1 ± 0.2, P = 0.03). When vascular grafts are encountered in the PET/CT clinic, two measurements of the ascending aorta and the graft can help to confirm a clinically noninfected appearance.
Visual analysis of vascular grafts in these patients was useful to detect a diffuse, homogeneous pattern of 18F-FDG uptake less than liver uptake, with results comparable to semiquantitative SUVmax and UR analysis. This suggests that visual comparison to the liver during image evaluation can be used to confirm a noninfected graft. The uptake pattern of 18F-FDG in aseptic vascular grafts was usually diffuse (300/306 = 98%), making this pattern particularly reassuring for clinicians.
When considering graft age, our data show a tendency for older grafts to exhibit higher 18F-FDG uptake. Those in the oldest quartile of the study (mean age 107 ± 24 mo) had mean URs of 1.3-1.4. Grafts in the lower 3 graft-age quartiles had mean URs closer to 1.1. Therefore, clinicians should consider the possibility of graft SUVmax being as much as 30% above ascending aorta background for old vascular grafts, particularly in proximal graft regions.
Limitations of this study include its retrospective nature, with chart review analysis the only means available to confirm absence of vascular graft infection in these patients. In addition, not all patients had contrast-enhanced CT for correlation with presence or absence of findings of vascular graft infection on anatomic imaging. Information regarding graft material composition was not available on all patients, potentially limiting analysis based on graft material. Another limitation is that a sole reader evaluated all data points on the PET/CT scans, including SUVmax and visual analysis. Therefore, interobserver variability in interpretation was not analyzed. Finally, our study did not include analysis of 18F-FDG PET/CT in patients with suspected or confirmed vascular graft infections, to compare with findings in aseptic vascular grafts in a similar patient population.
For 18F-FDG PET/CT interpreters, the visual, semiquantitative, and temporal characteristics of aseptic vascular stent grafts in patients’ status post EVAR can be useful in interpreting PET/CT, whether stent grafts are encountered as incidental findings on oncologic scans or on scans performed for suspected vascular graft infection. Our findings reinforce prior research in determining the characteristics of aseptic vascular grafts in a large cohort of grafts analyzed over time.
On [(18)F]fluorodeoxyglucose positron emission tomography/computed tomography (18F-FDG PET/CT), the inflammatory response caused by endoprosthetic stent grafts after endovascular aneurysm repair (EVAR) can show increased 18F-FDG uptake. However, the visual, semiquantitative, and temporal characteristics of uninfected, or aseptic, endovascular aneurysm grafts has not been fully elucidated.
Characterization of aseptic vascular stent grafts on 18F-FDG PET/CT is important to distinguish the normal inflammatory response to graft material vs vascular graft infection.
The purpose of this study was to characterize aseptic vascular stent grafts over time.
In this observational retrospective cohort study, patients with EVAR who underwent 18F-FDG PET/CT for routine oncologic indications were included. Any patients with suspected or confirmed vascular stent graft infection were excluded. Visual and semiquantitative region of interest (ROI) analysis with maximum standardized uptake value (SUVmax) and graft-to-background ascending aorta uptake ratios (URs) of the grafts were obtained. We compared visual analysis and semiquantitative values, grouped by age of grafts, ROI locations, and graft materials.
Characteristics of an aseptic vascular stent graft on 18F-FDG PET/CT include graft SUVmax values within 10%-20% of the ascending aorta background SUVmax. The SUVmax of older aseptic grafts can be as much as 30% above background. The visual uptake pattern of diffuse, homogeneous uptake less than liver was seen in 98% of aseptic vascular stent grafts.
Aseptic vascular stent grafts post endovascular repair show mildly increased 18F-FDG uptake, with mean graft-to-background URs of 1.1-1.2. Diffuse homogeneous 18F-FDG uptake less than liver in vascular stent grafts is particularly reassuring as a sign of an uninfected graft.
This study reinforces prior research in characterizing aseptic vascular grafts on 18F-FDG PET/CT.
| 1. | Casali M, Lauri C, Altini C, Bertagna F, Cassarino G, Cistaro A, Erba AP, Ferrari C, Mainolfi CG, Palucci A, Prandini N, Baldari S, Bartoli F, Bartolomei M, D'Antonio A, Dondi F, Gandolfo P, Giordano A, Laudicella R, Massollo M, Nieri A, Piccardo A, Vendramin L, Muratore F, Lavelli V, Albano D, Burroni L, Cuocolo A, Evangelista L, Lazzeri E, Quartuccio N, Rossi B, Rubini G, Sollini M, Versari A, Signore A. State of the art of (18)F-FDG PET/CT application in inflammation and infection: a guide for image acquisition and interpretation. Clin Transl Imaging. 2021;9:299-339. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 112] [Cited by in RCA: 126] [Article Influence: 25.2] [Reference Citation Analysis (0)] |
| 2. | Arnon-Sheleg E, Keidar Z. Vascular Graft Infection Imaging. Semin Nucl Med. 2023;53:70-77. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 9] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 3. | Bowles H, Ambrosioni J, Mestres G, Hernández-Meneses M, Sánchez N, Llopis J, Yugueros X, Almela M, Moreno A, Riambau V, Fuster D, Miró JM; Hospital Clinic Endocarditis Study Group. Diagnostic yield of (18)F-FDG PET/CT in suspected diagnosis of vascular graft infection: A prospective cohort study. J Nucl Cardiol. 2020;27:294-302. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 2.5] [Reference Citation Analysis (0)] |
| 4. | Chrapko BE, Chrapko M, Nocuń A, Zubilewicz T, Stefaniak B, Mitura J, Wolski A, Terelecki P. Patterns of vascular graft infection in 18F-FDG PET/CT. Nucl Med Rev Cent East Eur. 2020;23:63-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 5. | Schaefers JF, Donas KP, Panuccio G, Kasprzak B, Heine B, Torsello GB, Osada N, Usai MV. Outcomes of Surgical Explantation of Infected Aortic Grafts After Endovascular and Open Abdominal Aneurysm Repair. Eur J Vasc Endovasc Surg. 2019;57:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 15] [Cited by in RCA: 25] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 6. | Sarrazin JF, Trottier M, Tessier M. How useful is 18F-FDG PET/CT in patients with suspected vascular graft infection? J Nucl Cardiol. 2020;27:303-304. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 7. | Jamar F, Buscombe J, Chiti A, Christian PE, Delbeke D, Donohoe KJ, Israel O, Martin-Comin J, Signore A. EANM/SNMMI guideline for 18F-FDG use in inflammation and infection. J Nucl Med. 2013;54:647-658. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 376] [Cited by in RCA: 426] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 8. | Reinders Folmer EI, Von Meijenfeldt GCI, Van der Laan MJ, Glaudemans AWJM, Slart RHJA, Saleem BR, Zeebregts CJ. Diagnostic Imaging in Vascular Graft Infection: A Systematic Review and Meta-Analysis. Eur J Vasc Endovasc Surg. 2018;56:719-729. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 83] [Article Influence: 10.4] [Reference Citation Analysis (0)] |
| 9. | Mahmoodi Z, Salarzaei M, Sheikh M. Prosthetic vascular graft infection: A systematic review and meta-analysis on diagnostic accuracy of 18FDG PET/CT. Gen Thorac Cardiovasc Surg. 2022;70:219-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 12] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 10. | Rojoa D, Kontopodis N, Antoniou SA, Ioannou CV, Antoniou GA. 18F-FDG PET in the Diagnosis of Vascular Prosthetic Graft Infection: A Diagnostic Test Accuracy Meta-Analysis. Eur J Vasc Endovasc Surg. 2019;57:292-301. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 48] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 11. | Keidar Z, Pirmisashvili N, Leiderman M, Nitecki S, Israel O. 18F-FDG uptake in noninfected prosthetic vascular grafts: incidence, patterns, and changes over time. J Nucl Med. 2014;55:392-395. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 12. | Liddy S, Mallia A, Collins CD, Killeen RP, Skehan S, Dodd JD, Subesinghe M, Murphy DJ. Vascular findings on FDG PET/CT. Br J Radiol. 2020;93:20200103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 7] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 13. | Tsuda K, Washiyama N, Takahashi D, Natsume K, Ohashi Y, Hirano M, Takeuchi Y, Shiiya N. 18-Fluorodeoxyglucose positron emission tomography in the diagnosis of prosthetic aortic graft infection: the difference between open and endovascular repair. Eur J Cardiothorac Surg. 2022;63. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 5] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 14. | Kim A, Koshevarova V, Shure A, Joseph S, Villanueva-Meyer J, Bhargava P. FDG PET/CT in abdominal aortic graft infection: A case report and literature review. Radiol Case Rep. 2023;18:27-30. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Reference Citation Analysis (0)] |
| 15. | Rahimi M, Adlouni M, Ahmed AI, Alnabelsi T, Chinnadurai P, Al-Mallah MH. Diagnostic Accuracy of FDG PET for the Identification of Vascular Graft Infection. Ann Vasc Surg. 2022;87:422-429. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Corresponding Author’s Membership in Professional Societies: Society of Nuclear Medicine and Molecular Imaging, 218327.
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Covantsev S, Russia; Long X, China S-Editor: Wang JJ L-Editor: A P-Editor: Wang JJ