Published online Jul 28, 2023. doi: 10.4329/wjr.v15.i7.234
Peer-review started: April 3, 2023
First decision: May 19, 2023
Revised: June 2, 2023
Accepted: June 26, 2023
Article in press: June 26, 2023
Published online: July 28, 2023
Processing time: 111 Days and 23.3 Hours
Rhinocerebral mucormycosis (RCM) is a rare, fatal, invasive fungal infection infecting mainly patients with immunocompromised conditions, such as diabetes mellitus, hematologic malignancies, and organ transplantations. Coronavirus disease 2019 (COVID-19) disease in these patients further weakens the immune system due to several factors, including hypoxia, corticosteroid usage (further increasing hyperglycemic status), mechanical ventilation, increased serum ferritin levels, endothelitis due to free radicals, and glucose receptor protein upregulation. Timely diagnosis, judicious treatment decisions, and diabetes control with proper treatment guidelines in patients with coexisting COVID-19 disease can reduce complication rates and improve survival.
A 75-year-old male patient with diabetes and hypertension diagnosed with COVID-19 presented to the emergency department. Laboratory examinations revealed elevated blood glucose levels, as well as ketone bodies in the urine. He was treated with oxygen and steroids, as well as insulin to correct blood glucose levels. He complained of a headache 10 d later, and imaging demonstrated mucosal thickening in bilateral sphenoidal, ethmoidal, and maxillary sinuses with hyperdense foci in the right maxillary sinus but without central nervous system involvement. Surgical debridement was performed, and a histopathological study revealed fungi hyphae. Systemic antifungals (amphotericin b and posaconazole) were administered. Subsequently, on 15th day he developed right lower limb wea
Prompt early diagnosis, judicious treatment decisions, and diabetes control with proper treatment guidelines are necessary in patients with COVID-19 associated invasive RCM to reduce complication rates and improve patient survival.
Core Tip: Coronavirus disease 2019 associated invasive rhinocerebral mucormycosis is potentially life threatening in patients with uncontrolled diabetes mellitus and diabetic ketoacidosis. Early diagnosis and imaging the disease progression with proper treatment guidelines are essential for reducing the morbidity and mortality in these patients.
- Citation: Narra R, Rayapati S. Invasive rhinocerebral mucormycosis: Imaging the temporal evolution of disease in post COVID-19 case with diabetes: A case report. World J Radiol 2023; 15(7): 234-240
- URL: https://www.wjgnet.com/1949-8470/full/v15/i7/234.htm
- DOI: https://dx.doi.org/10.4329/wjr.v15.i7.234
Mucormycosis is a rare opportunistic and potentially lethal infection caused by members of the family mucoraceae, order mucorales, and class zygomycetes[1]. It is caused by fungi, which may be found in decaying food, soil, or other organic matter, such as animal excreta.
Rhinocerebral mucormycosis (RCM) is the most common type, accounting for 30%-50% of cases. Its extension to the orbit and brain is usual, making it a potentially life-threatening disease. Immunocompromised patients are especially susceptible, and timely diagnosis and judicious intervention are of utmost importance for the successful management and prevention of intracranial extension. Patients with coronavirus disease 2019 (COVID-19) with uncontrolled diabetes and diabetic ketoacidosis on corticosteroid treatment are potentially susceptible to invasive RCM and need aggressive treat
A 75-year-old male patient diagnosed with positive reverse transcription polymerase chain reaction for COVID-19 infection was admitted to the emergency department.
A 75-year-old male patient diagnosed with positive reverse transcription polymerase chain reaction for COVID-19 infection was admitted to the emergency department with fever, sore throat and generalized weakness for 3 d.
On 10th day he complained of frontal headache. On 15th day he complained of persistent frontal headache and left orbital pain, and a dragging sensation in the right foot. After 1 mo he progressed into altered sensorium and seizures and was admitted to emergency department.
He is known diabetic and hypertensive for 10 years.
No significant personal and family history.
On examination at admission temperature was 102.5 0F, pulse rate: 124/min, blood pressure (BP): 150/100 mmHg.
On 10th day temperature was 101.5 0F, pulse rate: 88/min, BP: 150/90 mmHg. On 15th day temperature was 99 0F, pulse rate: 108/min, BP: 130/90 mmHg, neurological examination revealed reduced power in the right lower limb (2/5) and left lateral rectus palsy. On 30th day temperature was 102 0F, pulse rate: 115/min, BP: 160/100 mmHg, glasgow coma scale was poor (8/15).
On admission: Elevated C-reactive protein (188 mg/dL), serum ferritin (513 ng/mL), blood glucose (400 mg/dL), and glycosylated hemoglobin (16%) levels and the presence of ketone bodies (+++) in the urine. Other parameters were within the normal limits: Hemoglobin of 11 gm%; red blood cells of 5.2 × 106/μL; total leukocyte count of 9000/cumm; differential leukocyte count, including neutrophils of 75%, lymphocytes of 22%, eosinophils of 02%, and macrophages of 01%; platelets of 2.4 Lakhs/cumm; serum potassium of 3.8 mmol/L; serum sodium of 138 mmol/L; blood urea of 12 mg/dL; and serum creatinine of 0.8 mg/dL.
On 10th day C-reactive protein (106 mg/dL), serum ferritin (498 ng/mL), blood glucose (260 mg/dL), and the presence of ketone bodies (+) in the urine.
On 15th day C-reactive protein (108 mg/dL), serum ferritin (450 ng/mL), blood glucose (250 mg/dL), and the presence of ketone bodies (+) in the urine.
After 1 mo C-reactive protein (198 mg/dL), serum ferritin (415 ng/mL), blood glucose (450 mg/dL), and the presence of ketone bodies (+++) in the urine.
Rest of the routine laboratory investigations were within normal limits.
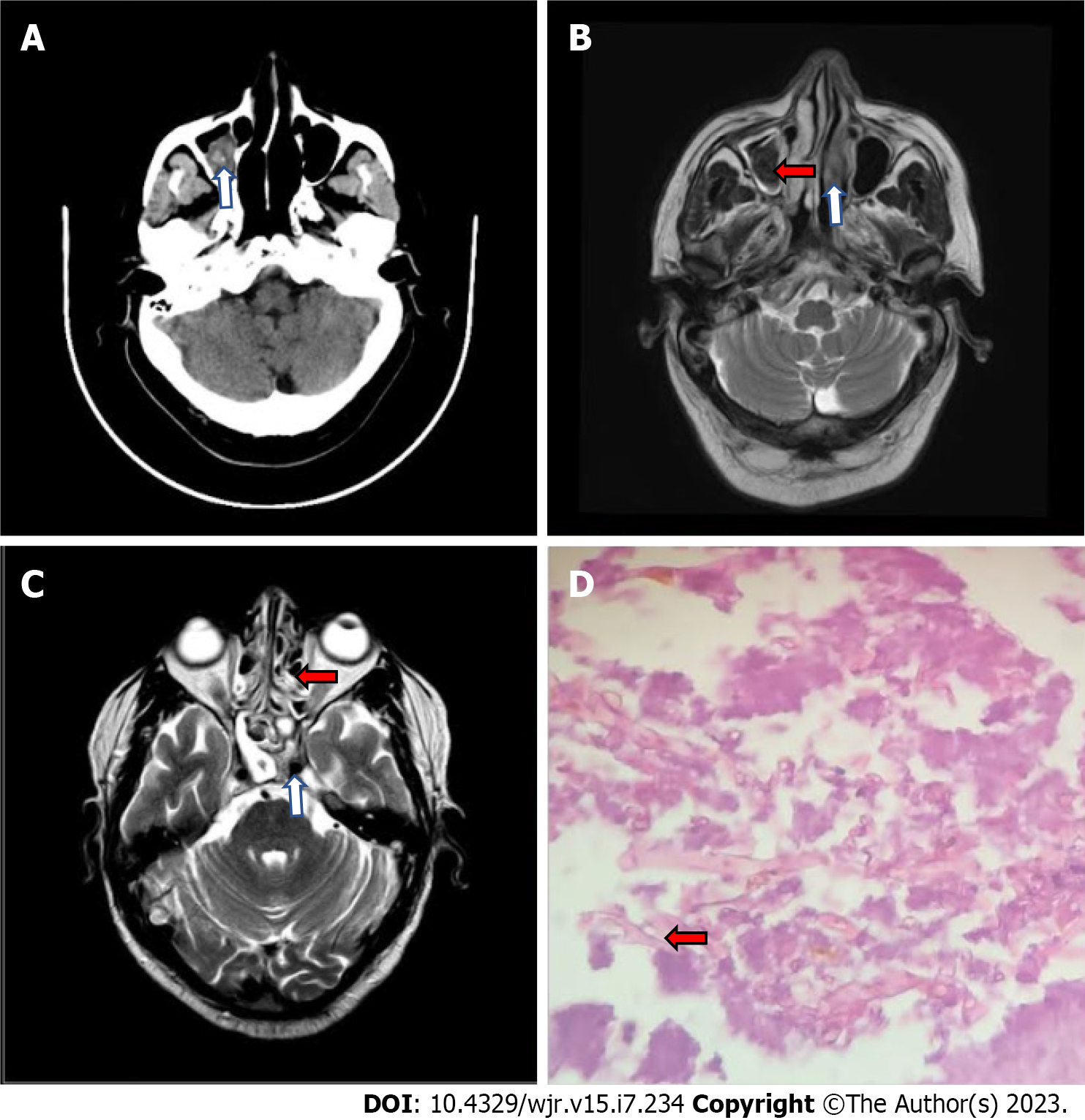
On 10th day post admission computed tomography (CT) and magnetic resonance imaging (MRI) demonstrated mucosal thickening in the bilateral ethmoidal, sphenoidal and maxillary sinuses with hyperdensity (CT) and hypointensity (MRI) in the right maxillary sinus suggestive of fungal sinusitis (Figure 1A-C). The patient underwent functional endoscopic sinus surgery under general anesthesia, and a biopsy was performed for histopathological examination which revealed fungus of mucorales species (Figure 1D).
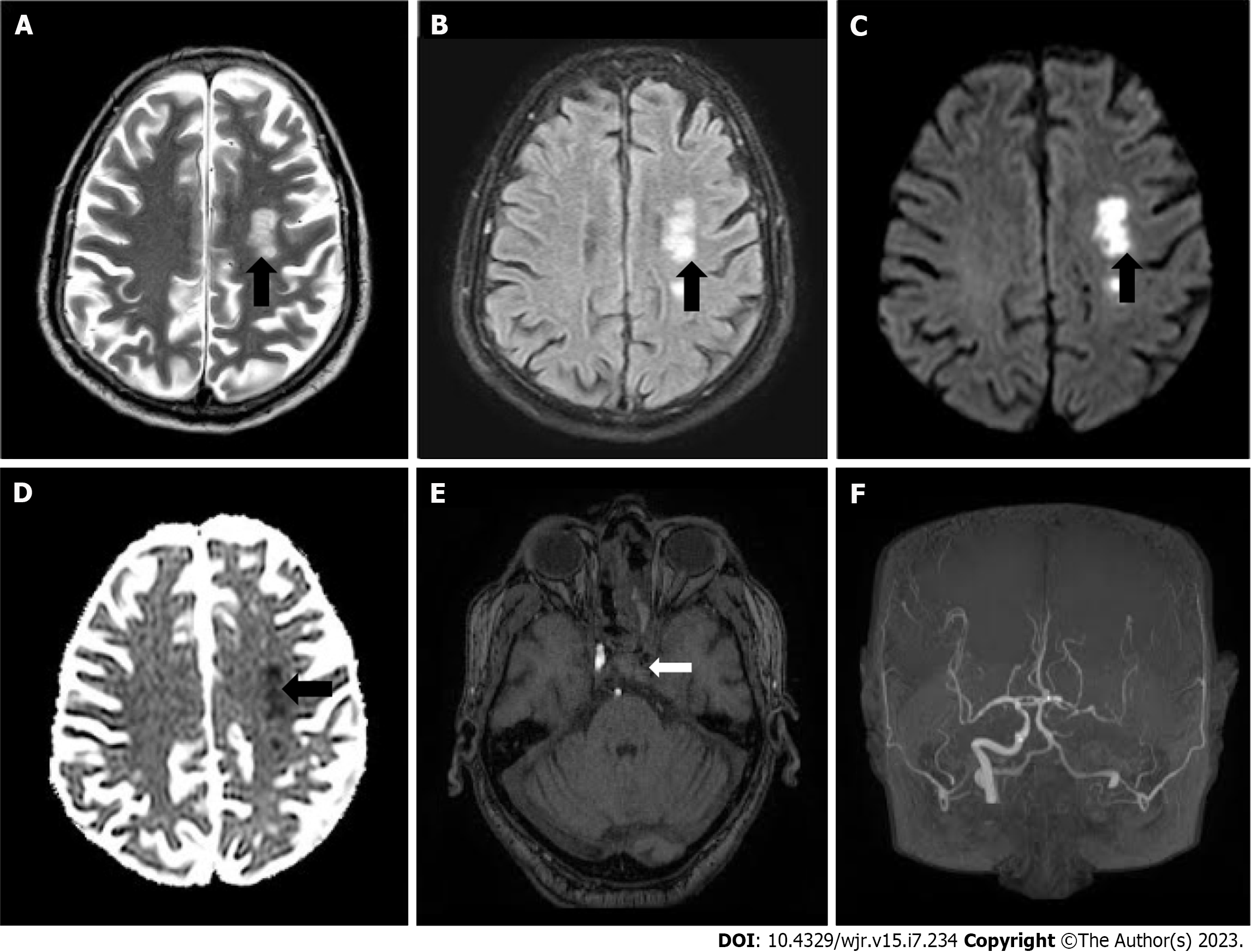
On 15th day post admission MRI scan of the brain, paranasal sinuses and the orbits with IV contrast was advised which demonstrated acute infarcts in the watershed territories of left anterior cerebral artery, middle cerebral artery and posterior cerebral arteries (Figure 2A-D) with filling defects in the left cavernous sinus suggestive of cavernous sinus thrombosis. In addition magnetic resonance angiogram demonstrated complete occlusion of left internal carotid artery (ICA) (Figure 2E and F).
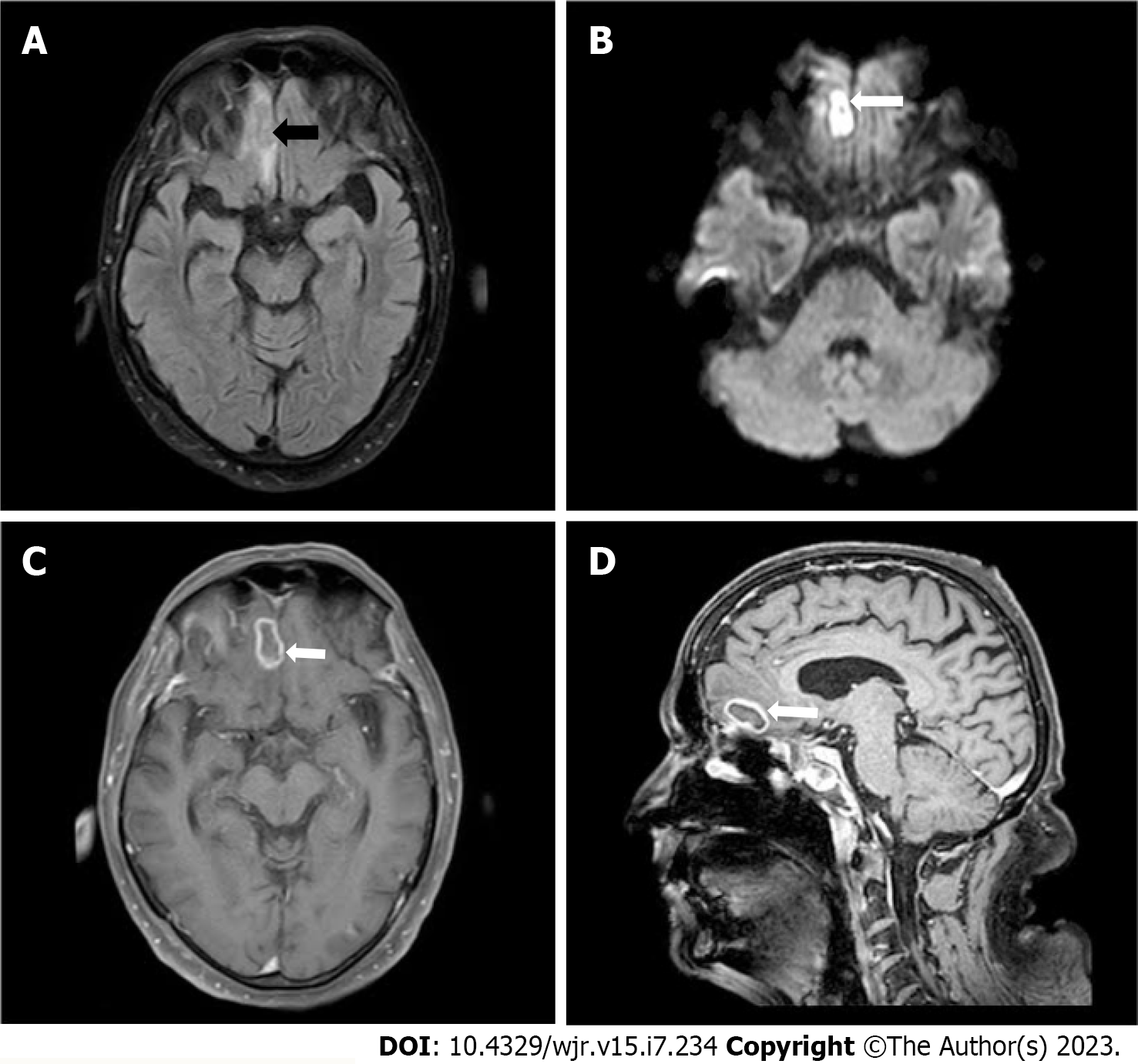
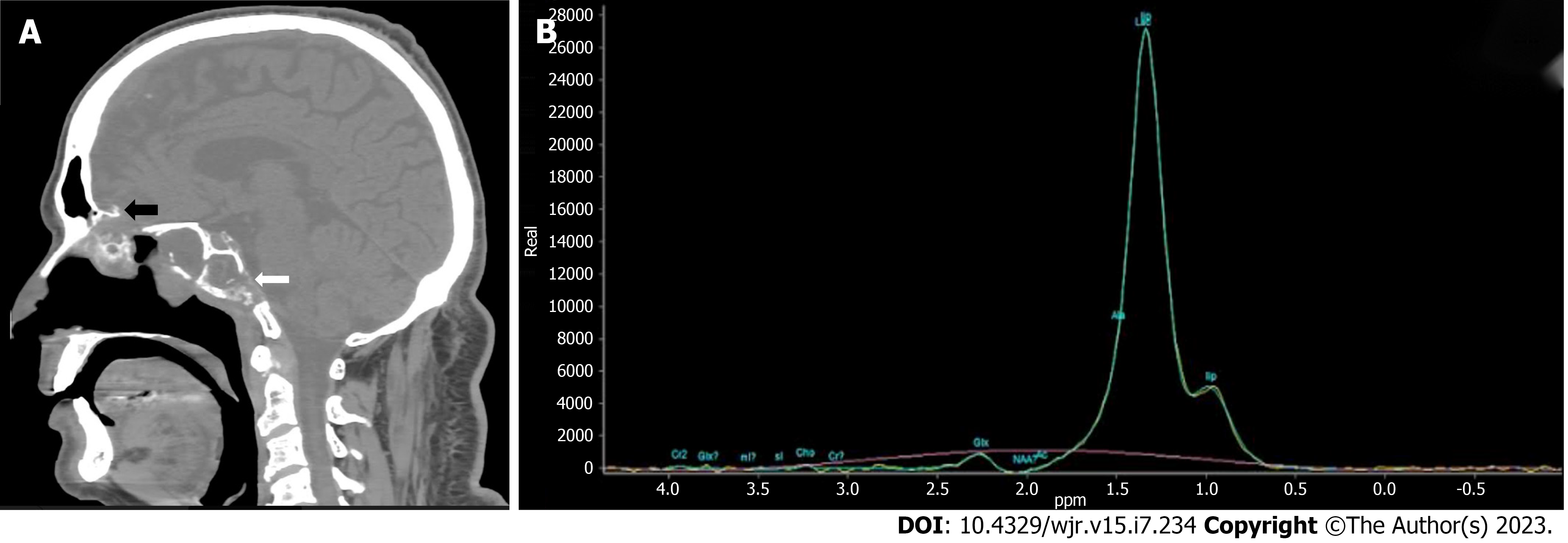
After 1 mo MRI of brain with IV contrast demonstrated an hyperintense lesion on T2 Weighted and fluid attenuated inversion recovery sequence with surrounding edema and peripheral rim enhancement of approximately 21 mm × 12 mm in the right basi-frontal lobe with restriction on diffusion weighted imaging and reduced apparent diffusion coefficient suggestive of fungal abscess (Figure 3). CT and MRI of brain demonstrated destruction of cribriform plate, clivus, sphenoid sinus and magnetic resonance spectroscopy demonstrated elevated lactate peak within the lesion (Figure 4).
Post COVID-19 associated invasive RCM.
On admission: Patient was given mechanical ventilation and oxygen support, anti-pyretics, azithromycin, remedesivir (200 mg loading dose) followed by 100 mg daily for 5 d, and intravenous dexamethasone 10 mg intravenous for 10 d. The patient was administered insulin 0.1 U/kg as an intravenous bolus dose followed by infusion of 0.1 U/kg/h for a targeted glucose level of 200 mg/dL.
On 10th day: The patient was advised and started with amphotericin b injection at 3 mg/kg/day for 14 d and oral posaconazole at 300 mg twice daily for 6 wk. The renal profile was checked every 3 d for nephrotoxicity from amphotericin b. Poor patient tolerance was noted and steroids discontinued.
On 15th day: The patient was administered aspirin 150 mg, clopitab 75 mg, and atorvastatin 40 mg. After stabilization and rehabilitation patient was counselled for diabetes control and discharged. He was maintained on human mixtard insulin thrice daily.
After 1mo: The patient was treated with antiepileptics, and insulin and anti-fungal treatment was continued with supportive treatment.
After stabilization there was very slow recovery of the patient with residual neurological deficits and increased morbidity.
RCM is a rare invasive infection caused by fungi of the class phycomycetes commonly involving the nasal and sinus mucosae of immunocompromised patients and spreads rapidly to the orbit and brain. Diabetes mellitus (especially with ketoacidosis) and hematologic malignancies with neutropenia are the principal predisposing factors. High blood glucose levels and ketoacidosis in diabetes enhance fungal growth. The altered number or function of neutrophils increases the risk of infection because they are responsible for defense against fungi[3]. COVID-19 disease in these patients further weakens the immune system due to several factors, including hypoxia, corticosteroid usage (further increasing hyperglycemic status), mechanical ventilation, increased serum ferritin levels, endothelitis due to free radicals, and glucose receptor protein upregulation, increased iron reduces the function of gamma interferon which prevents phagocytic function on fungus[4]. Other identified risk factors are steroid therapy, organ transplantation, chemotherapy, and chronic kidney disease[5-7]. Extensive angioinvasion is considered the main cause of vascular thrombosis and tissue necrosis. Vascular involvement is a more common cause of increased morbidity and mortality, resulting in ICA thrombosis causing brain ischemic infarcts and infiltrating the cavernous sinus and orbital apex, causing facial cellulitis and vision loss. Histopathological examination of the thrombus demonstrated the fungus in some cases of ICA thrombosis following thrombectomy[8]. Mucormycosis may spread intracranially from the paranasal sinuses along the cribriform plate into the anterior cranial fossa, leading to a cerebral abscess.
Brain and paranasal sinus MRI provides a better evaluation of intracranial and soft tissue involvement, skull base invasion, perineural spread, and vascular obstruction. MRI contrast study demonstrates orbital soft tissue invasion, skull base infiltration, perineural spread, intracranial complications, and vascular obstruction, involving the ICA[9,10].
At present, antifungal therapy and aggressive surgical debridement are used in active mucormycosis treatment. The overall mortality of patients with mucormycosis remains high and approaches 40% in patients with diabetes with invasive mucormycosis despite antifungal therapy and surgical debridement. Prophylactic treatment with antiplatelet drugs, including aspirin, clopitab, and statins, should also be initiated in aggressive cases where complications, such as ICA occlusion, thrombosis, and angioinvasion may develop.
Our case report explains the disease progression from infection initially involving the paranasal sinuses to subsequent brain involvement. Mucosal thickening of the bilateral sphenoidal, ethmoidal, and maxillary sinuses was initially noted on imaging in the present report, with no central nervous system involvement with a normal ICA and without any bony involvement. However, ICA thrombosis causing ischemic infarcts was noted on subsequent imaging, probably due to poor patient compliance to antifungal drugs causing increased fungal growth. The patient was stabilized and treated with aspirin 150 mg, clopitab 75 mg, and atorvastatin 40 mg and rehabilitation. However, the patient was readmitted to the hospital with seizures and altered consciousness after a few days, and subsequent imaging revealed the spread of infection through the destruction of the cribriform plate and clivus to the brain causing a cerebral abscess. This was due to poor diabetes control and diabetic ketoacidosis after patient discharge, which caused the intracranial spread of the disease.
Invasive RCM is a rare but fatal fungal infection that primarily affects immunocompromised patients. Early diagnosis and prompt management with aggressive surgical intervention and antifungal therapy are crucial for improving patient outcomes. This case report highlights the importance of close monitoring and imaging in patients with post-COVID-19 treated with oxygen therapy and corticosteroids, as well as diabetes, and ensuring optimal glycemic control to prevent rapid disease progression and intracranial spread. Regular monitoring of serum ferritin levels, lymphocyte count and further strengthening the immune system with regular follow-up imaging is essential to monitor disease progression and identify potential complications.
Dr Rama Krishna Gorantla for his expert advice and guidance.
| 1. | Kim JG, Park HJ, Park JH, Baek J, Kim HJ, Cha IH, Nam W. Importance of immediate surgical intervention and antifungal treatment for rhinocerebral mucormycosis: a case report. J Korean Assoc Oral Maxillofac Surg. 2013;39:246-250. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 10] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 2. | Mallis A, Mastronikolis SN, Naxakis SS, Papadas AT. Rhinocerebral mucormycosis: an update. Eur Rev Med Pharmacol Sci. 2010;14:987-992. [PubMed] |
| 3. | Dilek A, Ozaras R, Ozkaya S, Sunbul M, Sen EI, Leblebicioglu H. COVID-19-associated mucormycosis: Case report and systematic review. Travel Med Infect Dis. 2021;44:102148. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 62] [Article Influence: 12.4] [Reference Citation Analysis (0)] |
| 4. | Aggarwal SK, Kaur U, Talda D, Pandey A, Jaiswal S, Kanakan A, Singh A, Chakrabarti SS. Case Report: Rhino-orbital Mucormycosis Related to COVID-19: A Case Series Exploring Risk Factors. Am J Trop Med Hyg. 2021;106:566-570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2] [Cited by in RCA: 5] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 5. | Spellberg B, Edwards J Jr, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clin Microbiol Rev. 2005;18:556-569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 980] [Cited by in RCA: 929] [Article Influence: 44.2] [Reference Citation Analysis (0)] |
| 6. | Bhansali A, Bhadada S, Sharma A, Suresh V, Gupta A, Singh P, Chakarbarti A, Dash RJ. Presentation and outcome of rhino-orbital-cerebral mucormycosis in patients with diabetes. Postgrad Med J. 2004;80:670-674. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 137] [Cited by in RCA: 158] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 7. | Onyango JF, Kayima JK, Owen WO. Rhinocerebral mucormycosis: case report. East Afr Med J. 2002;79:390-393. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 14] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 8. | Kashyap S, Bernstein J, Ghanchi H, Bowen I, Cortez V. Diagnosis of Rhinocerebral Mucormycosis by Treatment of Cavernous Right Internal Carotid Artery Occlusion With Mechanical Thrombectomy: Special Case Presentation and Literature Review. Front Neurol. 2019;10:264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 9. | McDevitt GR Jr, Brantley MJ, Cawthon MA. Rhinocerebral mucormycosis: a case report with magnetic resonance imaging findings. Clin Imaging. 1989;13:317-320. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 19] [Cited by in RCA: 18] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 10. | Parsi K, Itgampalli RK, Vittal R, Kumar A. Perineural spread of rhino-orbitocerebral mucormycosis caused by Apophysomyces elegans. Ann Indian Acad Neurol. 2013;16:414-417. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 23] [Cited by in RCA: 31] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Mayotte
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Jain M, India; Yu YB, China S-Editor: Qu XL L-Editor: A P-Editor: Zhao S