Published online Jun 28, 2023. doi: 10.4329/wjr.v15.i6.182
Peer-review started: March 1, 2023
First decision: April 13, 2023
Revised: April 27, 2023
Accepted: May 30, 2023
Article in press: May 30, 2023
Published online: June 28, 2023
Processing time: 117 Days and 3 Hours
The course and variations of thyroid arteries must be understood by surgeons to prevent bleeding during operative procedures of the thyroid gland. There is limited scientific literature regarding the radiological anatomy of thyroid arteries in this geographical area, the Garhwal region of Sub-Himalayan belt, which is considered to be the endemic belt of goiter. Computed tomography angiography provides a three-dimensional orientation of the vascular and surgical anatomy of the entire cervical region.
To estimate the proportion of variation in origin of thyroid arteries using Computed Tomography Angiography.
Using Computed Tomography Angiography, the presence and origin of the superior thyroid artery, inferior thyroid artery, and thyroid ima artery were observed and assessed.
Out of total 210 subjects, superior thyroid artery was seen to be emerging from external carotid artery in 77.1% cases. The artery was found to be originating at the level of bifurcation of common carotid artery in 14.3% cases, whereas in 8.6% cases, it emerged as a direct branch of the common carotid artery. Similarly, the inferior thyroid artery was observed to be emerging from thyrocervical trunk, subclavian artery and vertebral artery in 95.7% cases, 3.3% and 1% cases, respectively. Thyroid ima artery was also reported in a subject, arising from the brachiocephalic trunk.
To avoid vascular injuries, excessive and uncontrollable bleeding, intra-operative difficulties, and post-operative issues, it is imperative for surgeons to be aware of the course and variations of thyroid arteries
Core Tip: To avoid vascular injuries, excessive and uncontrollable bleeding, intra-operative difficulties, and post-operative issues, it is imperative for surgeons to be aware of the course and variations of thyroid arteries. The current study will assist the surgeons in understanding the variation in thyroid arteries among people of Garhwal and west Uttar Pradesh region in a non-invasive manner using computed tomography angiography.
- Citation: Bhardwaj Y, Singh B, Bhadoria P, Malhotra R, Tarafdar S, Bisht K. Computed tomography angiographic study of surgical anatomy of thyroid arteries: Clinical implications in neck dissection. World J Radiol 2023; 15(6): 182-190
- URL: https://www.wjgnet.com/1949-8470/full/v15/i6/182.htm
- DOI: https://dx.doi.org/10.4329/wjr.v15.i6.182
The thyroid gland is an endocrine gland, which is essential for the normal metabolism and growth of an individual. Conditions affecting the thyroid gland range from simple goiter to malignant thyroid nodule and thyroid carcinoma. Thyroidectomy or hemithyroidectomy is occasionally indicated to remove a malignant thyroid tumor. The neck is also explored for surgical procedures, such as lobectomy, selective embolization of the thyroid, emergency cricothyroidotomy, radical neck dissection, diagnostic and therapeutic catheterization, reconstruction of the aneurysm, and carotid endarterectomy[1]. Bleeding during the operative procedure of the thyroid gland may compress the trachea causing difficulty in breathing[2].
Thyroid is a richly vascularized gland, which is supplied by superior thyroid artery (STA), inferior thyroid artery (ITA) and thyroid ima artery (TIA). The superior thyroid artery usually arises from external carotid artery and runs in close proximity to external branch of superior laryngeal nerve, before entering the fascia of thyroid and giving anterior and posterior branch.
Inferior thyroid artery usually commences from thyrocervical trunk[3]. Occasionally there is presence of a single minor artery, thyroid ima artery in 10% of the individuals[4].
The course and variations of the thyroid arteries must be understood by surgeons to prevent bleeding during the operative procedure of the thyroid gland.
There is limited scientific literature regarding the radiological anatomy of thyroid arteries in this geographical area, the Garhwal region of Sub-Himalayan belt, which is considered to be the endemic belt of goiter[5]. Computed tomography (CT) angiography provides a three-dimensional orientation of the vascular and surgical anatomy of the entire cervical region. For vascular mapping, radiography is a non-invasive multiplanar imaging method[6]. The present study investigated the origin and variation of thyroid arteries with the help of CT angiography.
The proposed study was done in the Department of Anatomy in collaboration with the Department of Radiodiagnosis over a period of 18 months. A total of 210 samples were included as per inclusion and exclusion criteria. Patients above 18 years who underwent CT angiography were included. Patients who had undergone previous common carotid occlusions or known vascular abnormalities (carotid hypoplasia, plaque in the carotid bifurcation, aneurysms) were excluded. Following the approval of Institutional Ethics Committee, with ethical clearance number AIIMS/IEC/21/323, CT angiographic data from 64-slice CT scanner and 128-slice CT scanner were collected from the Department of Radiodiagnosis. Reconstruction of the three-dimensional views of the images were done using RadiAnt DICOM Viewer (RadiAnt ©), a software installed in the MSI laptop PC. The images, thus obtained, were observed and evaluated for variations. The images were analyzed as per the general anatomical course and branches of vessels and variations, if present, were noted. The statistical analysis was done using the chi square test and the Stuart–Maxwell test.
The sample size for the proposed study was 208, considering our primary objective, which was to study variation in origin of superior thyroid artery. Previous studies have shown that the percentage of variation in the superior thyroid artery ranged from 6.5%–18.5%. We selected 6.5% to calculate our sample size with 10% (65) of relative procedure and finite population correction factor as 216 with 95% of confidence level and alpha error as 5%. The calculated sample size was 208. We used Open Epi, Version 3, open source calculator for the calculation of same.
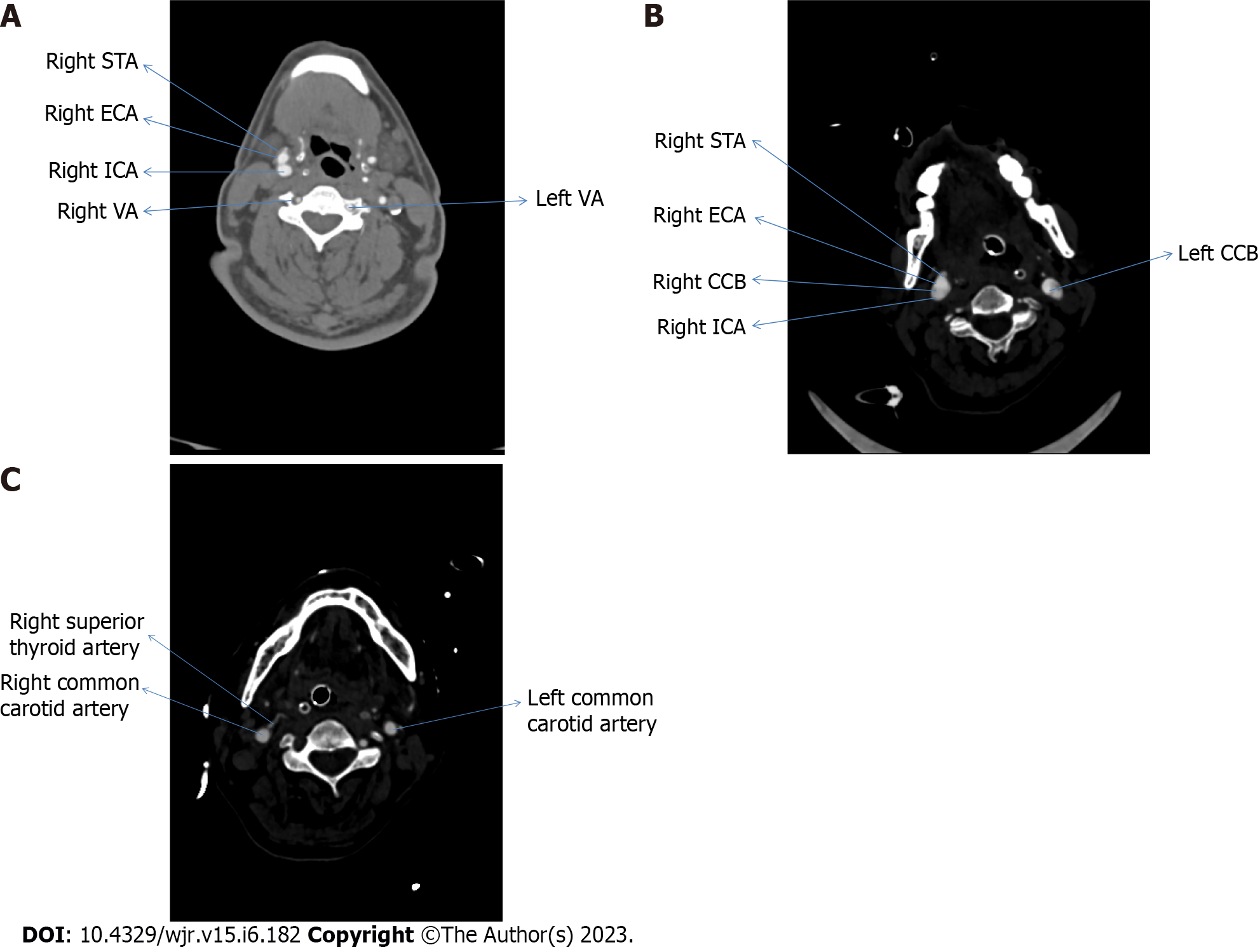
We collected 210 samples; however, the calculated sample size for the present study was 208. Out of total 210 participants, 162 (77.1%) participants had the superior thyroid artery originating from the external carotid artery. Participants with superior thyroid artery originating from level of bifurcation of common carotid artery were 30 (14.3%), whereas participants who had origin of superior thyroid artery from common carotid artery were 18 (8.6%). Figure 1 represents the CT angiographic image of various origin of superior thyroid artery in the axial plane.
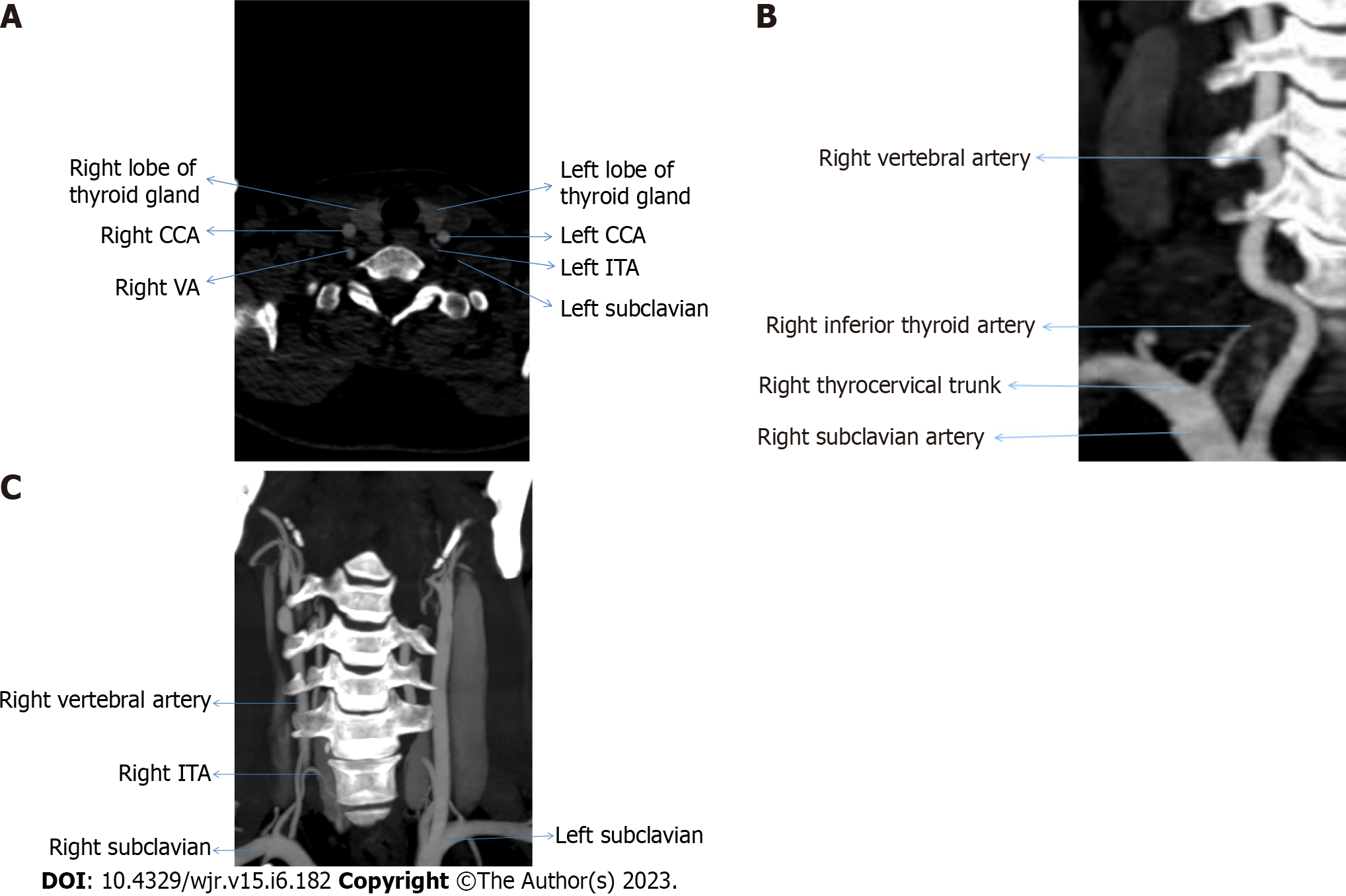
Participants who had origin of inferior thyroid artery from thyrocervical trunk, subclavian artery, and vertebral artery were 201 (95.7%), 7 (3.3%), and 2 (1.0%), respectively. Figure 2 represent the origin of inferior thyroid artery from left & right subclavian arteries and thyrocervical trunk respectively.
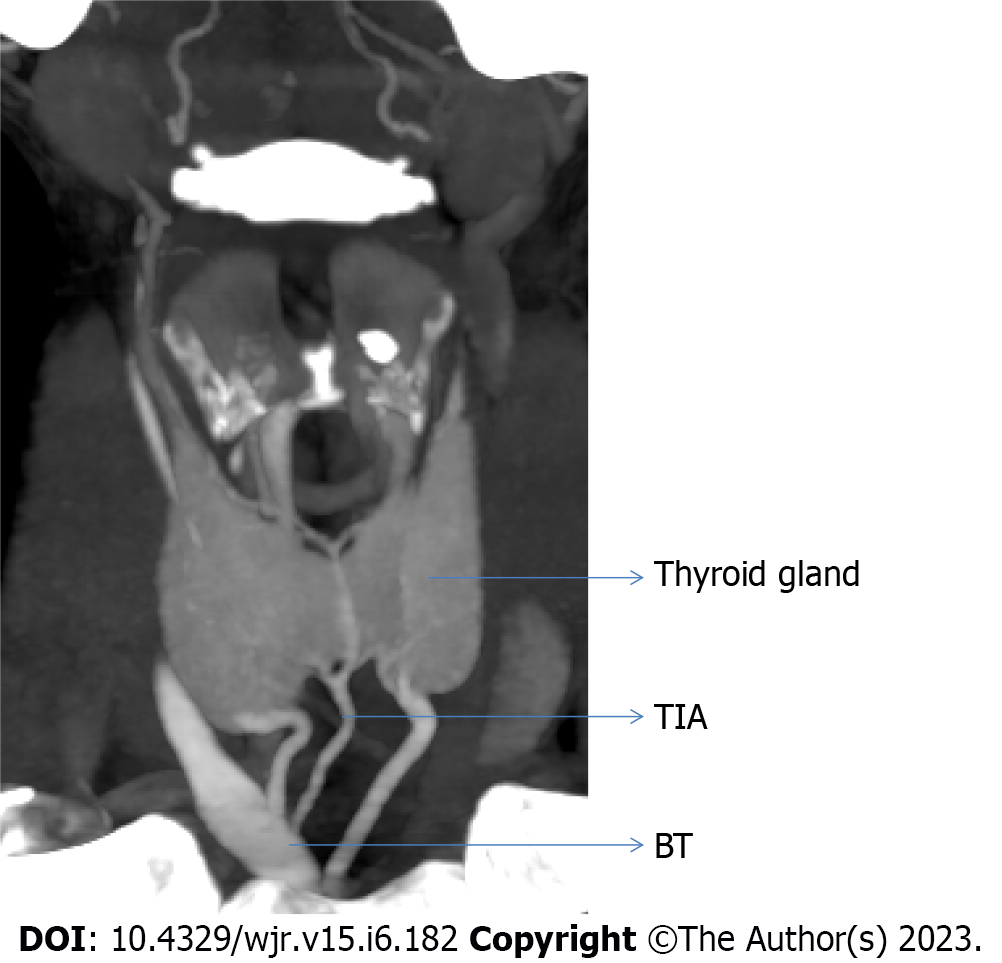
Out of total 210 cases, we observed one case that had origin of thyroid ima artery from brachiocephalic trunk (Figure 3).
The overall difference in the origin of superior thyroid artery was statistically significant (Stuart–Maxwell test: χ2 = 11.632, P = 0.003) (Table 1).
| Origin of superior thyroid artery | Category | Chi-squared test | |||
| Right | Left | Total | χ2 | P value | |
| Bifurcation of common carotid artery | 7 (6.7) | 23 (21.9) | 30 (14.3) | 10.756 | 0.005 |
| Common carotid artery | 8 (7.6) | 10 (9.5) | 18 (8.6) | ||
| External carotid artery | 90 (85.7) | 72 (68.6) | 162 (77.1) | ||
| Total | 105 (100.0) | 105 (100.0) | 210 (100.0) | ||
The overall difference in the origin of inferior thyroid artery was not statistically significant (Stuart–Maxwell test: χ2 = 5.000, P = 0.082) (Table 2).
| Origin of inferior thyroid artery | Category | Fisher's exact test | |||
| Right | Left | Total | χ2 | P value | |
| Subclavian artery | 1 (1.0) | 6 (5.7) | 7 (3.3) | 3.696 | 0.119 |
| Thyrocervical trunk | 103 (98.1) | 98 (93.3) | 201 (95.7) | ||
| Vertebral artery | 1 (1.0) | 1 (1.0) | 2 (1.0) | ||
| Total | 105 (100.0) | 105 (100.0) | 210 (100.0) | ||
The following variables were significantly associated (P < 0.05) with the variable 'Gender':
The association between “gender” and the “origin of STA” was investigated using the χ2 test (Table 3).
| Origin of STA | Gender | Chi-squared test | |||
| Male | Female | Total | χ2 | P value | |
| External carotid Artery | 89 (79.5) | 73 (74.5) | 162 (77.1) | 1.409 | 0.494 |
| Bifurcation of CCA | 13 (11.6) | 17 (17.3) | 30 (14.3) | ||
| CCA | 10 (8.9) | 8 (8.2) | 18 (8.6) | ||
| Total | 112 (100.0) | 98 (100.0) | 210 (100.0) | ||
Regarding the distribution of the origin of the STA, there was no discernible difference between the various groups (χ2 = 1.409, P = 0.494).
Cramer’s V value for the strength of association between the two variables is 0.08, which is considered to have little or no association in this study.
The distribution of the origin of ITA did not significantly differ across the different groups (χ2 = 2.059, P = 0.545) (Table 4).
| Origin of ITA | Gender | Fisher’s exact test | |||
| Male | Female | Total | χ2 | P value | |
| Thyrocervical Trunk | 107 (95.5) | 94 (95.9) | 201 (95.7) | 2.059 | 0.545 |
| SA | 3 (2.7) | 4 (4.1) | 7 (3.3) | ||
| VA | 2 (1.8) | 0 (0.0) | 2 (1.0) | ||
| Total | 112 (100.0) | 98 (100.0) | 210 (100.0) | ||
The connection of the two variables for Cramer’s V strength was 0.1, which is considered to be little or no association in this study.
The STA is considered to be a branch of external carotid artery (ECA) in normal anatomy. The STA can also originate from common carotid artery or bifurcation of common carotid artery, according to studies that have investigated the variability in the origin site of the superior thyroid artery from the carotid arteries. The inferior thyroid artery is generally considered a branch of the thyrocervical trunk, while it can also originate from subclavian artery, vertebral artery and common carotid artery according to previous research[7]. Although thyroid ima artery is considered to originate from brachiocephalic trunk, in some cases it originates directly from arch of aorta[8].
In the present study, we have observed that the STA originated from ECA, common carotid artery (CCA) and bifurcation of common carotid artery (CCB) in 77%, 14.3% and 8.6% of the cases, respectively. However, in the case of ITA the variation in origin was seen only in 3.3% of cases from subclavian artery (SA) and in 1% case from vertebral artery (VA) with varying level of origin of thyrocervical trunk (TCT).
Sreedharan et al[7] in 2018 did a cadaveric study depending on the frequency of origin of STA coming from ECA, CCB, or CCA. With an incidence of 88.33%, the STA originated from ECA in 53 of the 60 hemi-neck specimens. A total of 5 out of 60 hemi-necks showed that STA appeared at the CCB in 8.33% of cases. The STA developed from the CCA in 2 of the 60 patients, with an incidence of 3.33%. In the present study the overall difference in the origin of STA was statistically significant (Stuart–Maxwell test: χ2 = 11.632, P = 0.003) on right and left sides, which is shown in Table 1.
Anagnostopoulou et al[9] in 2014 conducted a study on 68 formalin-embalmed adult cadavers regarding superior thyroid artery only and categorized their result in three major groups. Type A consisted of cases in which the STA originates from ECA, Type B includes cases in which STA originates from CCA and Type C constitutes cases in which STA originates from the level of CCB. In 36.8% (25/68) of the patients, Type A was found on the right side, and in 42.6% (29/68) of the cases, on the left. In 32.4% (22/68) and 38.2% (26/68) of the individuals, type B was seen on the right and left side, respectively. In 19.1% (13/68) and 30.9% (21/68) of cases, Type C variation was noted on the right and left sides. In our study, superior thyroid artery originated from ECA in majority of cases followed by level of CCB and then CCA, on left side with 72 (68.6%), 23 (21.9%) and 10 (9.5%) cases, respectively and on the right side the order of frequency of origin of superior thyroid artery is from ECA, CCA and then CCB contrary to study done by Anagnostopoulou et al[9].
Shankar et al[10] in 2017 did a cadaveric study on 80 superior thyroid arteries with 40 right and 40 Left arteries, and found that it originated from the external carotid artery in 43 (53.75%), from CCB in 12 (15%), and the CCA in 25 (31.265%) cases[6]. The results of this study are similar to that of the present study as in the present study STA arose from ECA in 90 (85.7%) and 72 (68.6%) cases, on the right and left sides respectively, as the most common site of origin on both sides.
Esen et al[11] in 2017 did an angiographic study on 640 patients in which on the right (64.5%) and left (39.7%) sides, the STA was frequently originated from the ECA. In their study, there were 608 (95%) and 578 (90.3%) right and left ITAs emerging from the thyrocervical trunk, respectively. A single root from the SA gave rise to 18 (2.8%) right and 13 (2.0%) left ITAs. The VA was the origin of four left ITAs. In 15 patients (2.3%), the TIA was identified. A total of 12 of these were brachiocephalic arteries, 2 were right CCAs, and 1 was an aortic arch[7]. In present study the right and left STA arises from ECA in 90 (85.7%) and 72 (68.6%) cases respectively, whereas in the remaining 30 (14.3%) and 18 (8.6%) cases the STA originates from the level of CCB and main trunk of CCA respectively. However, ITA arise from TCT in 103 (98.1%) cases on right and 98 (93.3%) cases on left and among remaining cases 7 (3.3%) arise from SA and 2 (1.0%) arise from VA. We found TIA in 1 case originating from BT.
Tsegay et al[12] performed a cadaveric study in 2019 and observed that, the ECA was identified as the most frequent location of STA origin. The TCT gave rise to the ITA in each case, while in the present study the result for STA corresponds with the given study as in majority of cases the STA arises from ECA on both the sides, whereas in the case of ITA present study does not correspond with given study as in present study the ITA takes origin from thyrocervical trunk, SA, and VA in 201 (95.7%), 7 (3.3%) and 2 (1.0%) cases, respectively.
Gupta et al[13] performed an angiographic study in 2020, on 15 Indian patients indigenously from New Delhi region who were examined with a total of 25 selective STA angiograms. ECA was the primary source of STA on both sides. Ten patients (71.5%) had right STAs with ECA origins, while eight (72.5%) had left STAs with ECA origins. The bifurcation of the CCA accounted for 3 (21%) right STA and 2 (18.5%) left STA, making it the second most frequent site of STA genesis. CCA (right STA) and internal carotid artery (left STA) were the least frequent sites of origin, each accounting for one instance. In the present study, we considered a sample size of 210 patients from population of Uttarakhand, India. Gupta el al[13] in their study used DSA technique and also noted the branching patterns of STA only but in our study we observed the CT angiographic data and evaluated STA, ITA as well as TIA.
Dhindsa et al[14] in 2014 found during a routine cadaveric dissection on male cadaver that STA on left side was arising from CCA, while on right it was originating from ECA only[10]. In the present study, we evaluated the data of 112 male patients and found that in 89 (79.5%) cases the STA arose from ECA, in 13 (11.6%) cases STA originated from level of CCB and in 10 (8.9%) cases the STA originated from CCA main trunk.
Vázquez et al[15] performed a cadaveric study and indicated that there are four different types of origin of STA. In type 1 STA originated from the level of CCB in 102 (49%) of cases. Type 2 was when STA originated from CCA in 55 (27%) of cases. There is a statistically significant difference between the frequency of type 2 origins on the left and right. On the other hand, there was no statistically significant distinction between male and female Type 2 origins. In Type 3, the STA arose from ECA in 48 (23%) cases. In our study we did not categorize the types, but we found that STA arose 120 from CCA, level of CCB and ECA in 18 (8.6%), 30 (14.3%), and 162 (77.1%) cases, respectively and in our study the overall difference in the origin of STA was statistically significant (Stuart–Maxwell test: χ2 = 11.632, P = 0.003) on right and left sides which is shown in Table 1.
In the present study the measurement of the thyroid arteries was not done.
In our study, 77% of the cases had ECA as the source of STA and in more than 22% of cases variation were seen in the origin of STA, from CCA and CCB. Variation in the level of bifurcation of CCA was also seen, which greatly changes the course of STA. The present study reveals the variation in origin of ITA is 4%, while its course varied with varying level of origin of TCT. We found that thyroid ima artery is not frequently present, but was present in only one case and can lead to severe hemorrhagic condition if remains unnoticed.
An individual's normal growth and metabolism depend on the thyroid gland, an endocrine organ. Simple goiter, thyroid cancer, and malignant thyroid nodules are just a few of the conditions that can affect the thyroid gland. Sometimes it is necessary to perform a thyroidectomy or hemithyroidectomy in order to remove a malignant thyroid tumor. Additionally, the neck is examined for surgical treatments such as lobectomy, carotid endarterectomy, emergency cricothyroidotomy, radical neck dissection, diagnostic and therapeutic catheterization, and selective thyroid embolization. Breathing difficulties may result from tracheal compression brought on by bleeding during the thyroid gland surgery.
To avoid complications during thyroid surgeries, surgeons must be aware of the path and variations of the thyroid arteries. The Garhwal region of the Sub-Himalayan belt, which is thought to be the endemic zone of goitre, has little scientific literature on the radiological anatomy of thyroid arteries. A three-dimensional orientation of the vascular and surgical anatomy of the entire cervical region is provided by computed tomography angiography.
Radiography is a non-invasive multiplanar imaging technique for vascular mapping. The objective of the current research was to examine the origin and variation of thyroid arteries, with the aid of Computed Tomography angiography, Surgeons must be familiar with the course and variations of the thyroid arteries to prevent difficulties during thyroid operations.
Patients with known vascular anomalies (carotid hypoplasia, plaque in the carotid bifurcation, aneurysms) or past common carotid occlusions were excluded. Following the Institutional Ethics Committee's consent, the Department of Radiodiagnosis obtained computed tomography (CT) angiographic data from 64-slice and 128-slice CT scanners. RadiAnt DICOM Viewer (RadiAnt), a programme installed on the MSI laptop PC, was used to reconstruct the three-dimensional views of the photos. The Stuart-Maxwell test and the chi square test were employed in the statistical study.
Out of 210 individuals, 77.1% of the time the superior thyroid artery was observed to be arising from the external carotid artery. In 14.3% of cases, the artery was shown to originate at the point where the common carotid artery splits, whereas in 8.6% of cases, it was discovered to be a direct branch of the common carotid artery. Similar to this, in 95.7%, 3.3%, and 1% of cases, respectively, the inferior thyroid artery was seen to be arising from the thyrocervical trunk, subclavian artery, and vertebral artery. Another person was found to have a thyroid ima artery that emerged from the brachiocephalic trunk.
In our study, superior thyroid artery (STA) originated from external carotid artery in 77% of instances, and the common carotid artery (CCA) and common carotid bifurcation were the sources of STA in more than 22% of cases. Variation in the degree of CCA bifurcation was also observed, which significantly alters the path of STA. The current study shows that there is a 4% variance in inferior thyroid artery origin, whereas its course varies depending on the level of thyrocervical trunk origin. We discovered that the thyroid ima artery is only occasionally present in a small number of cases—it was only present in one case—and that if it goes unnoticed, it can cause serious hemorrhagic disease.
The Garhwal region of the Sub-Himalayan belt, which is thought to be the endemic zone of goitre, has little scientific literature on the radiological anatomy of thyroid arteries. A three-dimensional orientation of the vascular and surgical anatomy of the entire cervical region is provided by computed tomography angiography. The objective of the current research was to examine the origin and variation of thyroid arteries, with the aid of Computed Tomography angiography, Surgeons must be familiar with the course and variations of the thyroid arteries to prevent difficulties during thyroid operations. Additional research in this area using even better methods can further add to the existing knowledge on thyroid arteries.
We would like to thank the attendants and fellows from the Radiology department of AIIMS Rishikesh as well as the laboratory staff, Mr. Devender, for their continued support and assistance during data collection.
Provenance and peer review: Unsolicited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anatomy and morphology
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Taydas O, Turkey; Yang L, China S-Editor: Liu JH L-Editor: A P-Editor: Zhao S
| 1. | Kaplan E, Angelos P, Applewhite M, Mercier F, Grogan RH. Chapter 21. Surgery of the Thyroid. In: Feingold KR, Anawalt B, Blackman MR, Boyce A, Chrousos G, Corpas E, et al, editors. South. Dartmouth (MA). 2000;. [DOI] [Full Text] |
| 2. | Lemke J, Schreiber MN, Henne-Bruns D, Cammerer G, Hillenbrand A. Thyroid gland hemorrhage after blunt neck trauma: case report and review of the literature. BMC Surg. 2017;17:115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 13] [Cited by in RCA: 21] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 4. | Gray’s Anatomy, 39th Edition: The Anatomical Basis of Clinical Practice. Vol. American Journal of Neuroradiology. 2005;2703-2704. [RCA] [DOI] [Full Text] [Reference Citation Analysis (0)] |
| 5. | Sareen N, Kapil U, Nambiar V, Pandey RM, Khenduja P. Iodine nutritional status in Uttarakhand State, India. Indian J Endocrinol Metab. 2016;20:171-176. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 6. | Kumamaru KK, Hoppel BE, Mather RT, Rybicki FJ. CT angiography: current technology and clinical use. Radiol Clin North Am. 2010;48:213-235, vii. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 72] [Article Influence: 4.5] [Reference Citation Analysis (0)] |
| 7. | Sreedharan R, Krishna L, Shetty A. Origin of superior thyroid artery: under the surgeon's knife. J Vasc Bras. 2018;17:290-295. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 12] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 8. | Jayaraju RM, Mohiyuddin A, Merchant S, Raj S, Sasidharan B. Thyroidea ima artery: a report of two cases. Int J Head Neck Surg. 2015;5:89-90. [DOI] [Full Text] |
| 9. | Anagnostopoulou S, Mavridis I. Emerging patterns of the human superior thyroid artery and review of its clinical anatomy. Surg Radiol Anat. 2014;36:33-38. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 14] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 10. | Shankar VV, N K, Shetty S. A cross-sectional study of superior thyroid artery in human cadavers. Int J Anat Res. 2017;5:4751-4755. [DOI] [Full Text] |
| 11. | Esen K, Ozgur A, Balci Y, Tok S, Kara E. Variations in the origins of the thyroid arteries on CT angiography. Jpn J Radiol. 2018;36:96-102. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
| 12. | Tsegay AT, Berhe T, Amdeslase F, Hayelom H. Variations on arterial supply of thyroid gland and its clinical significance in selected universities of North Ethiopia. Int J Anat Res. 2019;7:6830-6834. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 2] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 13. | Gupta P, Bhalla AS, Thulkar S, Kumar A, Mohanti BK, Thakar A, Sharma A. Variations in superior thyroid artery: A selective angiographic study. Indian J Radiol Imaging. 2014;24:66-71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 18] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Dhindsa GS, Sodhi S. Variation in the origin of superior thyroid artery. J Evol Med Dent Sci. 2014;3:5969-5972. [DOI] [Full Text] |
| 15. | Vázquez T, Cobiella R, Maranillo E, Valderrama FJ, McHanwell S, Parkin I, Sañudo JR. Anatomical variations of the superior thyroid and superior laryngeal arteries. Head Neck. 2009;31:1078-1085. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 50] [Article Influence: 2.9] [Reference Citation Analysis (0)] |