Published online May 28, 2023. doi: 10.4329/wjr.v15.i5.146
Peer-review started: November 26, 2022
First decision: March 15, 2023
Revised: April 4, 2023
Accepted: April 24, 2023
Article in press: April 24, 2023
Published online: May 28, 2023
Processing time: 178 Days and 2 Hours
Although lung volumes are usually normal in individuals with chronic thromboembolic pulmonary hypertension (CTEPH), approximately 20%-29% of patients exhibit a restrictive pattern on pulmonary function testing.
To quantify longitudinal changes in lung volume and cardiac cross-sectional area (CSA) in patients with CTEPH.
In a retrospective cohort study of patients seen in our hospital between January 2012 and December 2019, we evaluated 15 patients with CTEPH who had chest computed tomography (CT) performed at baseline and after at least 6 mo of therapy. We matched the CTEPH cohort with 45 control patients by age, sex, and observation period. CT-based lung volumes and maximum cardiac CSAs were measured and compared using the Wilcoxon signed-rank test and the Mann-Whitney u test.
Total, right lung, and right lower lobe volumes were significantly reduced in the CTEPH cohort at follow-up vs baseline (total, P = 0.004; right lung, P = 0.003; right lower lobe; P = 0.01). In the CTEPH group, the reduction in lung volume and cardiac CSA was significantly greater than the corresponding changes in the control group (total, P = 0.01; right lung, P = 0.007; right lower lobe, P = 0.01; CSA, P = 0.0002). There was a negative correlation between lung volume change and cardiac CSA change in the control group but not in the CTEPH cohort.
After at least 6 mo of treatment, CT showed an unexpected loss of total lung volume in patients with CTEPH that may reflect continued parenchymal remodeling.
Core Tip: The total lung volume, right lower lobe volume, and cardiac cross-sectional area were reduced after at least 6 mo of follow-up after treatment in patients with chronic thromboembolic pulmonary hypertension (CTEPH). This finding suggests that structural lung changes have occurred in CTEPH, possibly from continued infarction with secondary volume loss from fibrosis or bronchoconstriction. The loss of lung volume may prove to be an important clinical consideration in CTEPH treatment because pulmonary function may continue to deteriorate despite improved right heart function in patients with CTEPH.
- Citation: Tsuchiya N, Xu YY, Ito J, Yamashiro T, Ikemiyagi H, Mummy D, Schiebler ML, Yonemoto K, Murayama S, Nishie A. Chronic thromboembolic pulmonary hypertension is associated with a loss of total lung volume on computed tomography. World J Radiol 2023; 15(5): 146-156
- URL: https://www.wjgnet.com/1949-8470/full/v15/i5/146.htm
- DOI: https://dx.doi.org/10.4329/wjr.v15.i5.146
Chronic thromboembolic pulmonary hypertension (CTEPH) is characterized by the presence of residual organized thrombi and vascular remodeling, leading to progressive pulmonary hypertension and right ventricular failure[1]. Surgical pulmonary endarterectomy (PEA) is a treatment for surgically accessible CTEPH. However, it has been reported that 17%-25% of patients undergoing PEA have residual or recurrent pulmonary hypertension[2]. In addition, approximately 30% of patients are not eligible for PEA because of the distal location of their pulmonary thromboemboli[3]; balloon pulmonary angioplasty (BPA) or medical therapy are the preferred treatment strategies for such patients[1,4,5].
Spirometry results and lung volumes are usually normal in patients with CTEPH, but approximately 20%-29% of patients exhibit a restrictive pattern on pulmonary function testing[6-8]. This reduction in lung volume is thought to be caused by parenchymal scarring from pulmonary infarction and not by displacement from proximal vessel hypertrophy and dilatation[7].
The nature of the progression of this observed reduction in lung volume in patients with CTEPH has not previously been described[3,9]. In our practice, we sometimes encounter patients who are receiving BPA or medical treatment for CTEPH but are gradually losing lung volume on chest computed tomography (CT); these patients require long-term follow-up. Based on this experience, we hypo
The purpose of this study was to investigate longitudinal changes in CT lung volume and to evaluate the relationship between lung volume and heart size in patients with CTEPH.
Our institutional review board approved this retrospective cohort study and waived the requirement for patient informed consent.
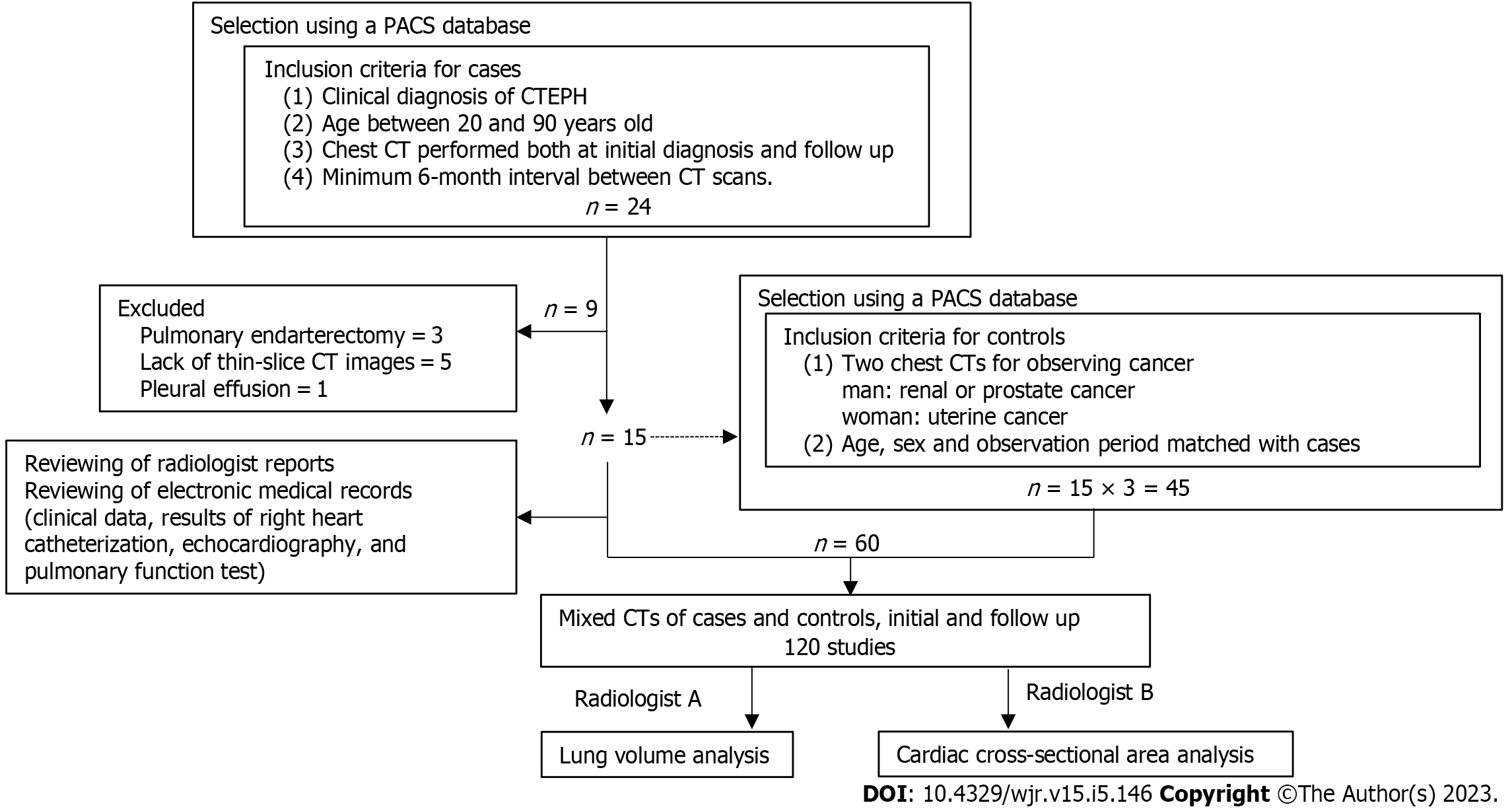
We conducted a retrospective review of patients seen at our institution between January 2012 and December 2019. Patients were initially identified using a picture archiving and communications system database, and patient selection was further refined by reviewing the electronic medical records. The inclusion criteria were a clinical diagnosis of CTEPH, age between 20 years and 90 years, chest CT performed at initial diagnosis and at the time of follow-up, and a minimum 6-mo interval between CT imaging sessions (Figure 1). We excluded patients who underwent PEA, patients for whom there was a lack of thin-slice CT images (< 2 mm) or poor image quality (e.g., motion artifact from a missed breath hold), and patients with other conditions that might affect lung volume (e.g., pleural effusion, underlying chronic pulmonary disease such as emphysema, interstitial lung disease, or old tuberculosis, and a history of thoracic surgery). The inclusion criterion for individuals in the control group, which was matched by age, sex, and observation period, was the presence of two chest CTs performed to observe cancer status (renal or prostate cancer for men; uterine cancer for women). Individuals were excluded from the control group if there was a lack of thin-slice CT images, poor image quality, or any abnormal findings on chest CT.
The diagnosis of CTEPH was determined by cardiologists based on a detailed medical history, physical examination, chest radiography, chest CT, echocardiography, lung ventilation–perfusion scintigraphy, right heart catheterization (RHC), and angiographic demonstration of multiple stenoses and obstruction of bilateral pulmonary arteries[10]. Radiologist reports were reviewed to determine which pulmonary arteries were affected by chronic thrombosis. Clinical data, including age, height, weight, and treatment details (e.g., anticoagulant therapy, BPA), were extracted from the medical records. We also noted the results of RHC (mean pulmonary arterial pressure, cardiac output, cardiac index) and echocardiography (tricuspid regurgitation pressure gradient, left ventricular end-diastolic diameter, left ventricular end-systolic diameter) at the examination closest in time to the chest CT.
Chest CT, with or without contrast, was performed as part of routine clinical practice. Two scanners were used: The Light Speed VCT 64-row CT (GE HealthCare, Milwaukee, WI, United States) and the Aquilion ONE 320-row CT (Canon Medical Systems, Odawara, Japan). Imaging was performed during a supine breath-hold at full inspiration. Instructions for full inspiration were given using an automatic voice system to keep the degree of inspiration constant. The imaging parameters were: Voltage, 120 kVp; current, automatic exposure control; collimation, 0.5 (Canon) or 0.625 mm (GE); rotation time, 0.5 sec; matrix, 512 × 512; and slice thicknesses, 1 mm (Canon) or 1.25 mm (GE).
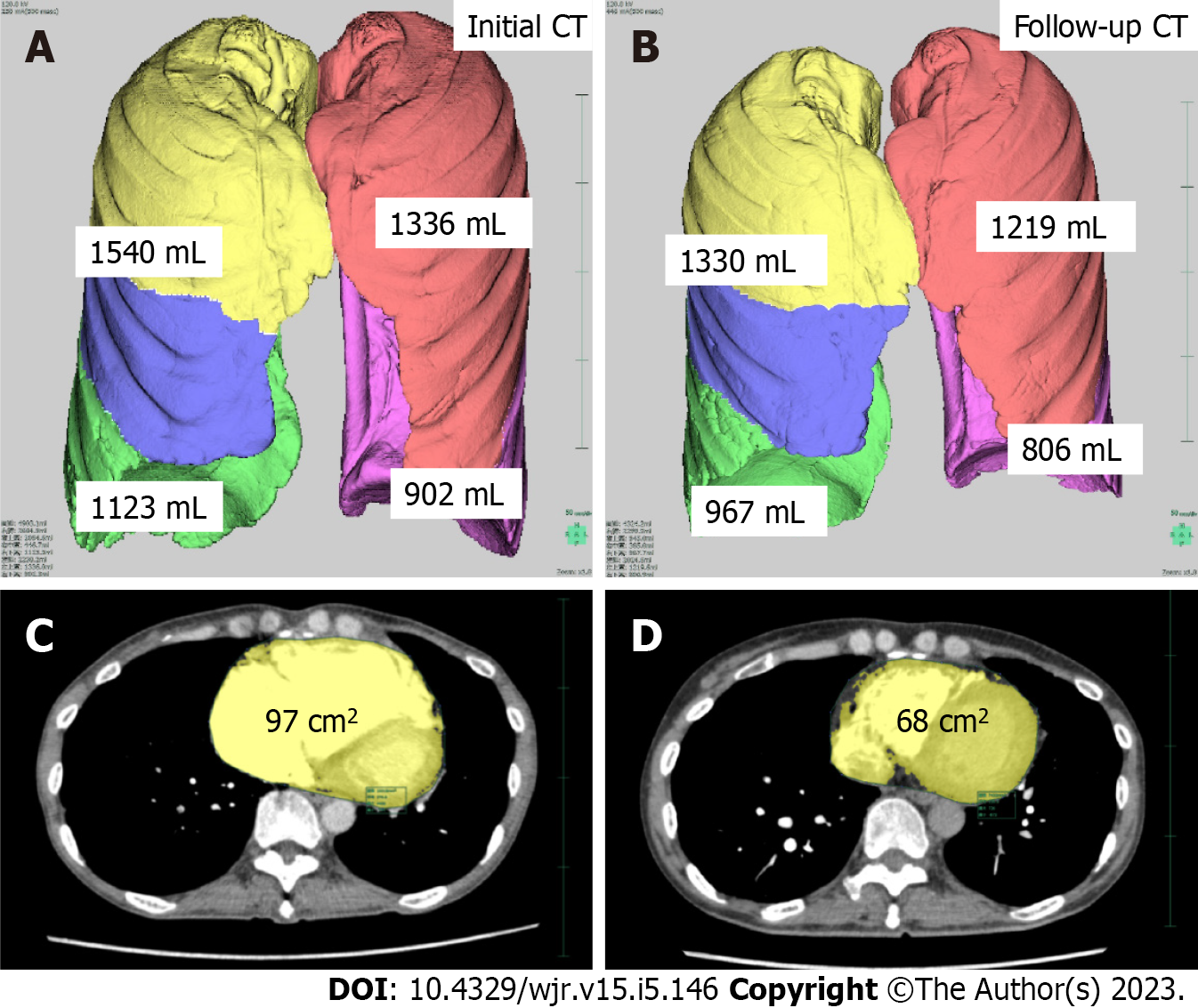
Lung volumes were automatically segmented and calculated using a commercially available workstation (SYNAPSE VINCENT, version 4.1; Fujifilm Healthcare Corporation, Tokyo, Japan). We used images with a soft tissue reconstruction algorithm [Standard (GE) or FC14 (Cannon)] for analysis. Lung volumes were assessed based on lobe anatomy: Right upper and middle lobe; right lower lobe; left upper lobe; and left lower lobe. The right upper and middle lobes were assessed together because of the frequent presence of incomplete lobulation.
We calculated the amount of change in lung volume between the initial CT and follow-up CT. The slice with the maximal cardiac cross-sectional area (CSA) in cm2 was measured semiautomatically using a commercially available workstation (SYNAPSE VINCENT, version 4.1). Based on a previously published method[11], the following process was used for each patient. A hounsfield unit threshold was set to exclude the pericardial fat pad (0-300 hounsfield unit). All images that contained the heart were then identified, and the maximum cardiac CSA was determined. Finally, the boundary of the heart was traced, and the maximum cardiac CSA recorded. In addition, we calculated the size change (difference in area) in cardiac CSA between the initial and follow-up CT images. Lung volume and cardiac CSA were analyzed separately by different radiologists (NT and YX) who were blinded to the patients’ clinical information (Figure 1).
The sample size for this study was determined by the following method[12]: Average lung volume, 2000 mL; standard deviation, 400 mL; effect size, 400 mL (20% of average); patient to control ratio, 1:3; α, 0.05; and power, 0.8.
Statistical analyses were performed using JMP 11 (SAS Institute Japan, Tokyo, Japan). Continuous variables were calculated as the mean and 95% confidence interval. The Wilcoxon signed-rank test was used to compare parameters between the initial and follow-up CT for both groups. The Mann-Whitney U test was used to compare parameters between patients with CTEPH and those in the control group. The Spearman correlation coefficient was used to determine the correlation between lung volume and cardiac CSA. A P value of less than 0.05 was considered statistically significant.
We identified 27 individuals with CTEPH who were seen during the study period. Of these, 24 had two chest CTs with an interval greater than 6 mo. We excluded 3 patients who underwent PEA, 5 patients who lacked thin-slice CT images, and 1 patient with a pleural effusion. The size of the CTEPH population was thus fixed at 15 (4 males and 11 females) due to the retrospective nature of the study. The mean patient age was 48 years (range: 40-83 years). The size of the control group was set at 45 individuals (3 times the size of the CTEPH group). All individuals with CTEPH received anticoagulant therapy, and 14 patients underwent BPA. Patient characteristics are summarized in Table 1. The results of echocardiography and RHC performed before and after therapy are shown in Table 2.
| Characteristic | CTEPH, n = 15 | Control, n = 45 | P value | |
| Age in yr, mean (95%CI) | 48 (40, 83) | 48 (40, 83) | - | |
| Height in cm, mean (95%CI) | 159 (151, 162) | 159 (154, 163) | 0.5 | |
| Weight in kg, mean (95%CI) | 58 (49, 75) | 61 (54, 72) | 0.9 | |
| Interval between initial and follow-up CT in d, mean (95%CI) | 547 (273, 846) | 531 (294, 815) | 0.9 | |
| Results of RHC and echocardiography | Initial, n = 15 | Follow-up, n = 15 | P value | |
| Pulmonary arterial pressure in mmHg, mean (95%CI) | 47 (38, 59) | 34 (23, 44) | - | 0.001b |
| Cardiac output in L/min, mean (95%CI) | 3.4 (2.8, 4.3) | 4.6 (3.6, 6.1) | - | 0.01a |
| Cardiac index in L/min/m2, mean (95%CI) | 2.2 (1.6, 2.7) | 2.7 (2.4, 3.6) | - | 0.01a |
| Tricuspid regurgitation pressure gradient in mmHg, mean (95%CI) | 66 (51, 77) | 42 (23, 61) | - | 0.01a |
| Left ventricular end-diastolic diameter in mm, mean (95%CI) | 38 (37, 42) | 44 (41, 47) | - | 0.02a |
| Left ventricular end-systolic diameter in mm, mean (95%CI) | 25 (22, 28) | 27 (24, 31) | - | 0.5 |
| Results of spirometry | Initial, n = 10 | Follow-up, n = 10 | ||
| Vital capacity % predicted | 83 (70, 102) | 87 (76, 99) | - | 0.2 |
| Forced vital capacity % predicted | 76 (71, 97) | 83 (74, 97) | - | 0.3 |
| Forced expiratory volume-one second % predicted | 74 (63, 959 | 83 (69, 96) | - | 0.5 |
| CT measurement | CTEPH, n = 15 | Control, n = 45 | ||||
| Initial | Follow-up | P value | Initial | Follow-up | P value | |
| Total lung volume in mL, mean (95%CI) | 3238 (3016, 4494) | 3360 (2687, 4043) | 0.004b | 3669 (3063, 4167) | 3657 (2993, 4385) | 0.9 |
| Right lung volume in mL, mean (95%CI) | 1879 (1700, 2619) | 1817 (1522, 2299) | 0.003b | 1939 (1635, 2268) | 1990 (1614, 2347) | 0.9 |
| Right upper + middle lobe volume in mL, mean (95%CI) | 977 (913, 1103) | 973 (900, 1148) | 0.2 | 1028 (868, 1169) | 1010 (865, 1212) | 1.0 |
| Right lower lobe lung volume in mL, mean (95%CI) | 978 (737, 1123) | 815 (611, 1111) | 0.01a | 931 (707, 1112) | 911 (733, 1148) | 0.8 |
| Left lung volume in mL, mean (95%CI) | 1396 (1316, 1875) | 1455 (1216, 1744) | 0.1 | 1692 (1376, 1940) | 1659 (1403, 2023) | 0.7 |
| Left upper lobe lung volume in mL, mean (95%CI) | 836 (752, 925) | 802 (689, 1000) | 0.4 | 867 (747, 1042) | 869 (741, 1089) | 0.9 |
| Left lower lobe lung volume in mL, mean (95%CI) | 636 (555, 902) | 652 (476, 806) | 0.05 | 826 (628, 939) | 749 (622, 999) | 0.6 |
| Heart cross-sectional area in cm2, mean (95%CI) | 97 (86, 112) | 83 (69, 100) | 0.001b | 75 (65, 85) | 74 (64, 85) | 0.7 |
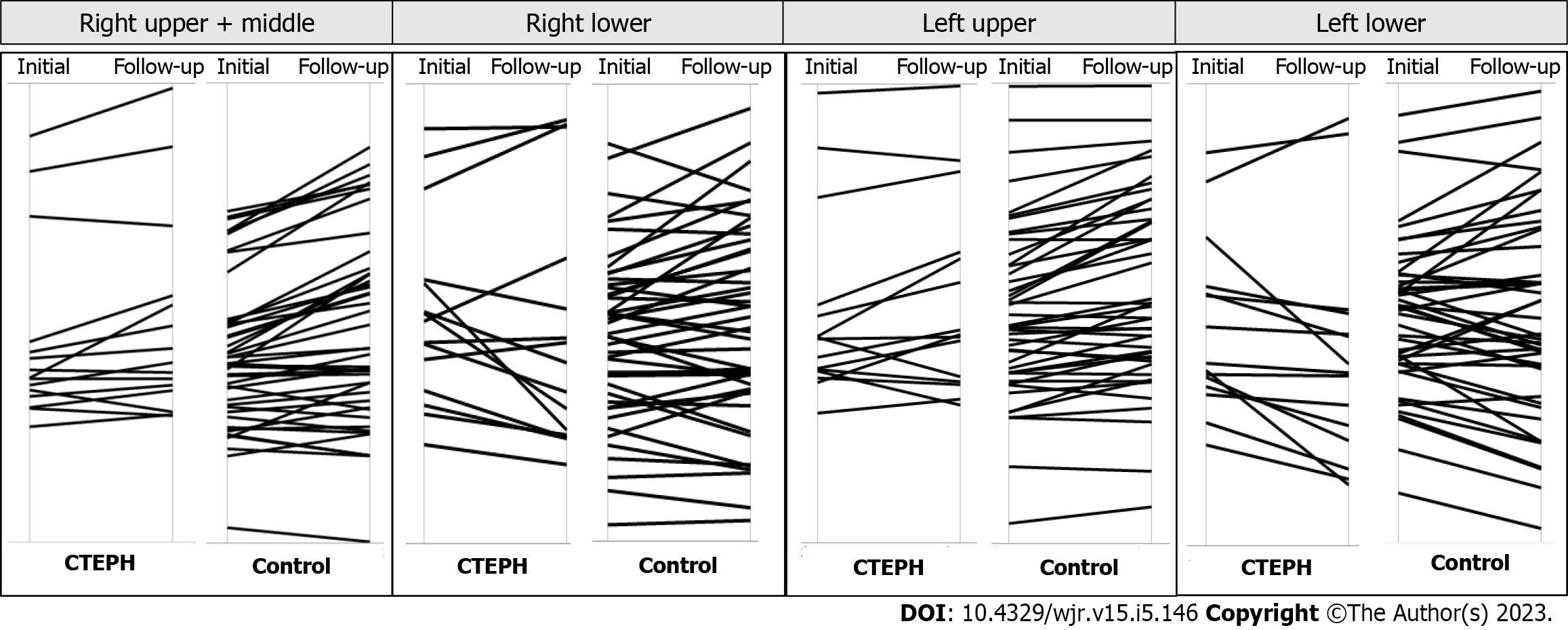
The initial and follow-up measurements for lung volume are shown in Table 2. The total lung volume (P = 0.004), right lung volume (P = 0.003), and the right lower lobe volume (P = 0.01) at total lung capacity (TLC) were significantly reduced in patients with CTEPH at follow-up (Figures 2 and 3), but there were no significant longitudinal changes in lung volumes in the control group. The cardiac CSA was significantly reduced in the CTEPH cohort at follow-up (P = 0.001), but this change was not observed in the control group.
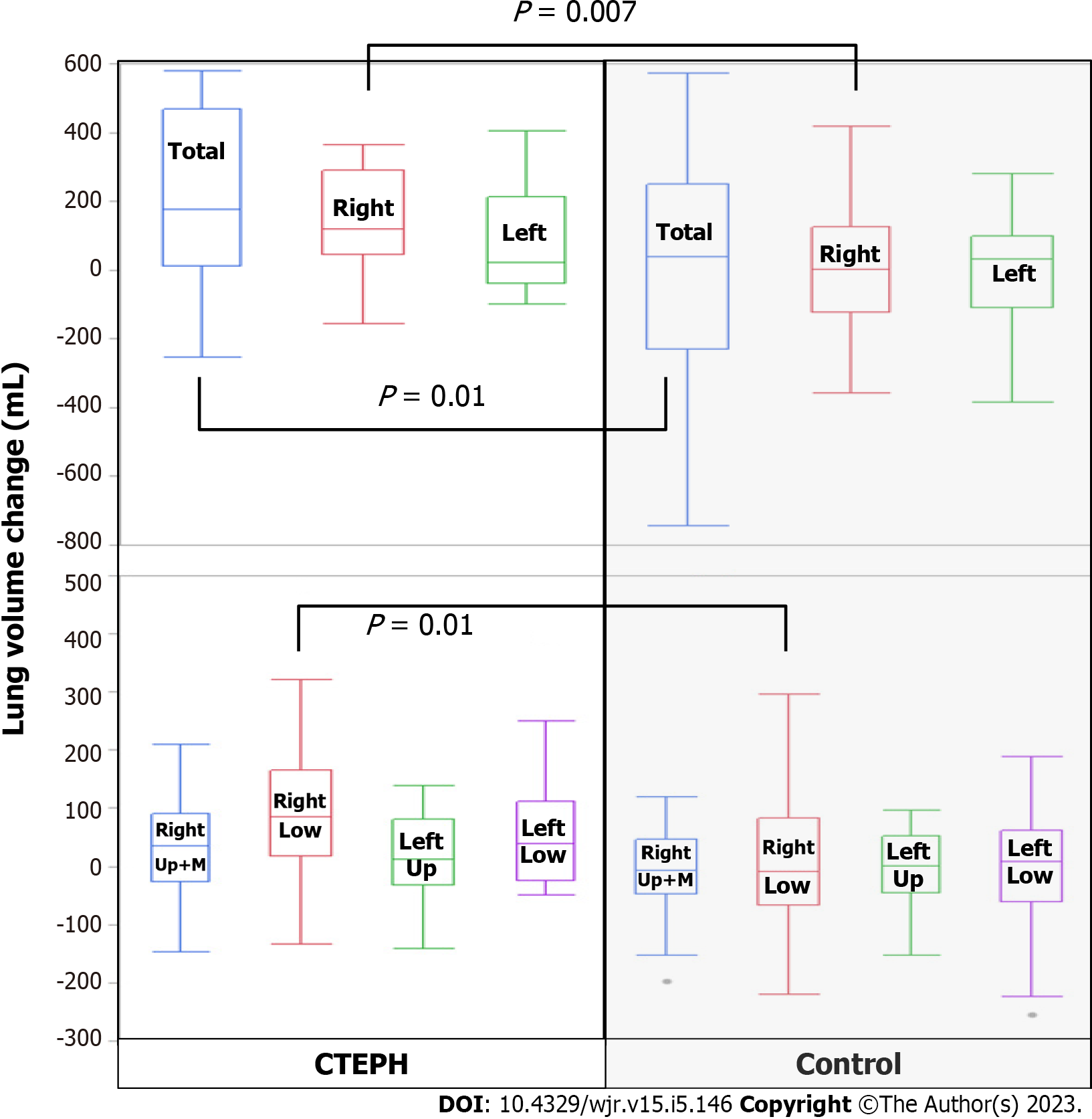
The lung volumes and cardiac CSA measurements for the CTEPH cohort and the control group are shown in Table 3. There was no significant difference in lung volumes between groups, but the cardiac CSA was larger in the CTEPH group than in the control group at both initial and follow-up assessments (P < 0.0001). In the CTEPH cohort, the reduction in total lung volume (P = 0.01) and in right lung volume (P = 0.007) and right lower lobe volume (P = 0.01) was significantly larger than that seen in the control group (Figure 4). The reduction in cardiac CSA in the CTEPH cohort was significantly greater than in the control group (P = 0.0002).
| Parameter | CTEPH, n = 15 | Control, n = 45 | P value |
| Total lung volume change in mL, mean (95%CI) | 176 (13, 468) | 39 (-228, 252) | 0.01a |
| Right lung volume change in mL, mean (95%CI) | 120 (47, 292) | 4 (-121, 126) | 0.007b |
| Right upper + middle lobe lung volume change in mL, mean (95%CI) | 35 (-26, 91) | -7 (-46, 46) | 0.9 |
| Right lower lobe lung volume change in mL, mean (95%CI) | 85 (19, 167) | -9 (-66, 83) | 0.01a |
| Left lung volume change in mL, mean (95%CI) | 23 (-37, 214) | 33 (-109, 99) | 0.1 |
| Left upper lobe lung volume change in mL, mean (95%CI) | 13 (-31, 81) | 1 (-44, 53) | 0.4 |
| Left lower lobe lung volume change in mL, mean (95%CI) | 40 (-23, 112) | 9 (-59, 63) | 0.1 |
| Cardiac cross-sectional area change in cm2, mean (95%CI) | 19 (4, 25) | -0.3 (-2, 3) | 0.0002c |
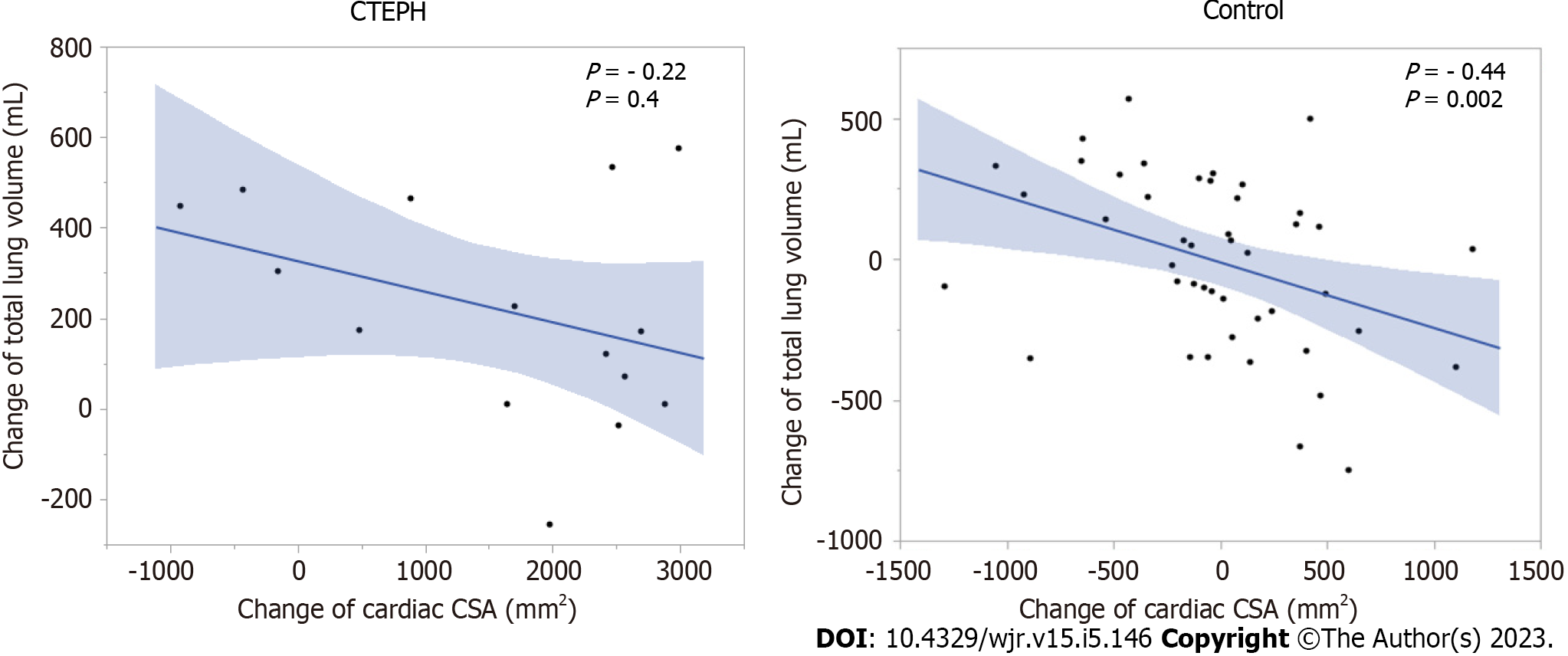
There were no significant correlations between lung volume and cardiac CSA in the CTEPH cohort or the control group at either initial or follow-up CT. In the CTEPH group, there were no significant correlations between lung volume change and the change in cardiac CSA. In the control group, the change in cardiac CSA was negatively correlated with total lung volume change (ρ = -0.44; P = 0.002), right lung volume change (ρ = -0.34; P = 0.01), right upper and middle lobe volume change (ρ = -0.27; P = 0.06), right lower lobe volume change (ρ = -0.34; P = 0.02), left lung volume change (ρ = -0.50; P = 0.0004), left upper lobe volume change (ρ = -0.40; P = 0.0005), and left lower lobe volume change (ρ = -0.52; P = 0.0002) (Figure 5).
This pilot study showed that the lung volume in patients with CTEPH who are treated medically decreases over 6 mo of follow-up, despite a reduction in heart size during that same period. These results suggest that a reduction in lung volume may continue in patients with CTEPH who have not undergone PEA, and that this reduction may occur independently of right heart enlargement quantified using cardiac CSA.
Although decreased TLC has been previously observed in patients with CTEPH[7], this report analyzed regional contributions to reduction in lung volume using CT measurements, showing that the right lung and right lower lung have significantly reduced volumes. The reason for this may be related to continued lung remodeling and loss of parenchyma from continuing pulmonary infarction. Reports of pulmonary embolism show a striking lower lobe predominance, with an embolus rate in the right lower lobe twice that of the left lower lobe[13]. Thus, it is plausible that the right lower lung is a major contributor to lung volume reduction because of its status as a predominant region of pulmonary infarction.
A retrospective study by Morris et al[7] found that 22% of the 51 patients with CTEPH who were candidates for PEA had restrictive lung disease on pulmonary function testing, defined as a TLC less than 80% of predicted. They also demonstrated that the reduction in lung volume was correlated to the degree of parenchymal scarring occurring after pulmonary infarction. Pulmonary infarction is more common with peripheral pulmonary emboli than with central pulmonary emboli due to the relatively lower contribution of bronchial arterial supply to the peripheral lung[13,14].
The decline in heart size seen with CTEPH is most likely related to the improvement in right ventricular function and decreased right ventricular end-diastolic and end-systolic volumes with medical therapy or BPA. Echocardiography and RHC show these improvements at follow-up. Cardiac CSA, measured in the transverse plane, is affected by the phase of ventilation. Inhalation stretches the heart in the vertical plane and reduces cardiac CSA, whereas expiration lifts the diaphragm and pushes the heart upward, increasing cardiac CSA[11]. The control group in this study had a significant negative correlation between heart size change, as measured by CSA, and lung volume change. However, the correlation between these changes disappeared in the CTEPH cohort. We initially speculated that cardiomegaly was a factor in lung volume decline in the CTEPH group. In addition to the physiologic increase in cardiac CSA caused by poor inspiration, the increase in the right ventricle and right atrium volume caused by exacerbation of right heart failure would compress the lung parenchyma, leading to a decrease in lung volume. However, we found the opposite to be true: lung volumes decreased in the CTEPH group despite a reduction in heart size. In other words, a reduction in heart size that is associated with improved heart function can occur simultaneously with a loss of lung volume in CTEPH. An additional explanation of this negative correlation between heart size and lung volume is that in normal individuals more vigorous inspiration when measuring TLC reduces venous filling of the right atrium. In patients with CTEPH, there should be less of an effect because these individuals already have volume overload in the right heart.
There have been remarkable recent advances in treatment for inoperable CTEPH, and survival has significantly improved in the population treated with medication or BPA[2]. However, whether quality of life improves after medical therapy has been controversial, and there have been no reports of changes in quality of life following BPA[15-19]. While TLC reportedly increases following BPA for CTEPH[20], this is only a short-term outcome. Further study is needed to clarify whether this temporary increase in lung volume and subsequent regression may influence quality of life in patients with CTEPH.
There are several limitations to this study. First, this is a retrospective single-center study with a small number of patients, and spirometry data are lacking. Second, height is an important factor influencing lung volume, but this was not matched between patients in the CTEPH and control groups; however, mean height was not significantly different between the groups. Therefore, this effect is likely to be negligible. Third, there were multiple hardware vendors for the CT scanners used in this project. Finally, the absolute inspiration volume for CT was not measured with spirometry.
The total lung volume, right lower lobe volume, and the cardiac CSA were reduced after at least 6 mo of follow-up after treatment in patients with CTEPH. This finding suggests that structural changes in the lung have occurred, possibly from continued infarction with secondary volume loss from fibrosis or bronchoconstriction. The results of this study suggest that pulmonary function may continue to deteriorate in patients with CTEPH despite improvements in right heart function and decreased right ventricular volumes from lowered pulmonary arterial pressure after treatment. This loss of lung volume may prove to be an important clinical consideration in CTEPH treatment.
In chronic thromboembolic pulmonary hypertension (CTEPH), about 20% of patients have lung restriction due to parenchymal scarring. We sometimes follow CTEPH patients who gradually lose lung volume. There is no report describing the temporal change in lung volume of CTEPH patients.
The loss of lung volume may be an important clinical consideration in CTEPH treatment.
The purpose of this study was to assess the temporal lung volume changes in CTEPH.
Included in the study were patients with CTEPH who underwent two thoracic computed tomography (CT) examinations with a between-test interval that was greater than 6 mo. We also assessed controls matched by age, sex, and observation period. The lung volume was measured on the left and right sides by thin-slice CT scanning. Lung volume was automatically measured by lung analysis software. We analyzed the lung volume changes between the initial CT and follow-up CT in patients and controls by the Wilcoxon signed-rank test.
The total and right lung volumes were significantly reduced from the initial CT to the follow-up CT in the patients with CTEPH. In CTEPH patients, there was no significant change in the left lung volume. In controls, there were no significant changes in lung volume.
In patients with CTEPH, the lung volume was reduced temporally. The right lung was more affected than the left lung by the lung volume reduction.
Further study is needed to clarify whether this temporary increase in lung volume and subsequent regression may influence quality of life in patients with CTEPH.
The authors are grateful to Ms. Chihiro Siroma for contribution to collection of the data.
| 1. | Robbins IM, Pugh ME, Hemnes AR. Update on chronic thromboembolic pulmonary hypertension. Trends Cardiovasc Med. 2017;27:29-37. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 26] [Cited by in RCA: 33] [Article Influence: 3.7] [Reference Citation Analysis (0)] |
| 2. | Miwa H, Tanabe N, Jujo T, Kato F, Anazawa R, Yamamoto K, Naito A, Kasai H, Nishimura R, Suda R, Sugiura T, Sakao S, Ishida K, Masuda M, Tatsumi K. Long-Term Outcome of Chronic Thromboembolic Pulmonary Hypertension at a Single Japanese Pulmonary Endarterectomy Center. Circ J. 2018;82:1428-1436. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 25] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 3. | Low AT, Medford AR, Millar AB, Tulloh RM. Lung function in pulmonary hypertension. Respir Med. 2015;109:1244-1249. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 4. | Moriarty JM, Khan SN, Kao SD, Saggar R. Balloon Pulmonary Angioplasty for Chronic Thromboembolic Pulmonary Hypertension. Cardiovasc Intervent Radiol. 2018;41:1826-1839. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 10] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 5. | Aoki T, Sugimura K, Tatebe S, Miura M, Yamamoto S, Yaoita N, Suzuki H, Sato H, Kozu K, Konno R, Miyata S, Nochioka K, Satoh K, Shimokawa H. Comprehensive evaluation of the effectiveness and safety of balloon pulmonary angioplasty for inoperable chronic thrombo-embolic pulmonary hypertension: long-term effects and procedure-related complications. Eur Heart J. 2017;38:3152-3159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 129] [Article Influence: 16.1] [Reference Citation Analysis (0)] |
| 6. | Horn M, Ries A, Neveu C, Moser K. Restrictive ventilatory pattern in precapillary pulmonary hypertension. Am Rev Respir Dis. 1983;128:163-165. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 33] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 7. | Morris TA, Auger WR, Ysrael MZ, Olson LK, Channick RN, Fedullo PF, Moser KM. Parenchymal scarring is associated with restrictive spirometric defects in patients with chronic thromboembolic pulmonary hypertension. Chest. 1996;110:399-403. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 43] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 8. | Fukushi K, Kataoka M, Shimura N, Inami T, Fukuda K, Yoshino H, Satoh T. Impaired Respiratory Function in Chronic Thromboembolic Pulmonary Hypertension: A Comparative Study with Healthy Control Subjects. Ann Am Thorac Soc. 2016;13:1183-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 7] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 9. | Kahn SR, Houweling AH, Granton J, Rudski L, Dennie C, Hirsch A. Long-term outcomes after pulmonary embolism: current knowledge and future research. Blood Coagul Fibrinolysis. 2014;25:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 43] [Cited by in RCA: 48] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 10. | Kim NH, Delcroix M, Jenkins DP, Channick R, Dartevelle P, Jansa P, Lang I, Madani MM, Ogino H, Pengo V, Mayer E. Chronic thromboembolic pulmonary hypertension. J Am Coll Cardiol. 2013;62:D92-D99. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 394] [Cited by in RCA: 426] [Article Influence: 32.8] [Reference Citation Analysis (0)] |
| 11. | Tomita H, Yamashiro T, Matsuoka S, Matsushita S, Kurihara Y, Nakajima Y. Changes in Cross-Sectional Area and Transverse Diameter of the Heart on Inspiratory and Expiratory Chest CT: Correlation with Changes in Lung Size and Influence on Cardiothoracic Ratio Measurement. PLoS One. 2015;10:e0131902. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 17] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 12. | Kohn MA, Senyak J. Sample Size Calculators. 2021 December 20 [cited 24 January 2020]. San Francisco: UCSF CTSI. [DOI] [Full Text] |
| 13. | He H, Stein MW, Zalta B, Haramati LB. Pulmonary infarction: spectrum of findings on multidetector helical CT. J Thorac Imaging. 2006;21:1-7. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 62] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 14. | Tsao MS, Schraufnagel D, Wang NS. Pathogenesis of pulmonary infarction. Am J Med. 1982;72:599-606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 65] [Cited by in RCA: 60] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 15. | Mathai SC, Ghofrani HA, Mayer E, Pepke-Zaba J, Nikkho S, Simonneau G. Quality of life in patients with chronic thromboembolic pulmonary hypertension. Eur Respir J. 2016;48:526-537. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 37] [Cited by in RCA: 52] [Article Influence: 5.2] [Reference Citation Analysis (0)] |
| 16. | Scholzel BE, Post MC, Thijs Plokker HW, Snijder RJ. Clinical worsening during long-term follow-up in inoperable chronic thromboembolic pulmonary hypertension. Lung. 2012;190:161-167. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 21] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 17. | Suntharalingam J, Treacy CM, Doughty NJ, Goldsmith K, Soon E, Toshner MR, Sheares KK, Hughes R, Morrell NW, Pepke-Zaba J. Long-term use of sildenafil in inoperable chronic thromboembolic pulmonary hypertension. Chest. 2008;134:229-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 212] [Cited by in RCA: 184] [Article Influence: 10.2] [Reference Citation Analysis (0)] |
| 18. | Jaïs X, D'Armini AM, Jansa P, Torbicki A, Delcroix M, Ghofrani HA, Hoeper MM, Lang IM, Mayer E, Pepke-Zaba J, Perchenet L, Morganti A, Simonneau G, Rubin LJ; Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension Study Group. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol. 2008;52:2127-2134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 446] [Cited by in RCA: 393] [Article Influence: 21.8] [Reference Citation Analysis (0)] |
| 19. | Halank M, Hoeper MM, Ghofrani HA, Meyer FJ, Stähler G, Behr J, Ewert R, Fletcher M, Colorado P, Nikkho S, Grimminger F. Riociguat for pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension: Results from a phase II long-term extension study. Respir Med. 2017;128:50-56. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 27] [Cited by in RCA: 32] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 20. | Takei M, Kataoka M, Kawakami T, Kuwahira I, Fukuda K. Respiratory function and oxygenation after balloon pulmonary angioplasty. Int J Cardiol. 2016;212:190-191. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 16] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Japan
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Chen Q, China; Sharma D, India S-Editor: Liu XF L-Editor: Filipodia P-Editor: Zhao S