Published online Apr 28, 2023. doi: 10.4329/wjr.v15.i4.89
Peer-review started: November 18, 2022
First decision: February 15, 2023
Revised: March 3, 2023
Accepted: March 30, 2023
Article in press: March 30, 2023
Published online: April 28, 2023
Processing time: 158 Days and 23.6 Hours
Radiomics is a hot topic in the research on customized oncology treatment, efficacy evaluation, and tumor prognosis prediction. To achieve the goal of mining the heterogeneity information within the tumor tissue, the image features concealed within the tumoral images are turned into quantifiable data features. This article primarily describes the research progress of radiomics and clinical-radiomics combined model in the prediction of efficacy, the choice of treatment modality, and survival in transarterial chemoembolization (TACE) and TACE combination therapy for hepatocellular carcinoma.
Core Tip: Hepatic cancer is a highly varied primary liver malignancy, and distinct tumor stages necessitate different techniques to guarantee appropriate therapeutic efficacy. Radiomics is a potential method, which can predict the benefits of patients with different treatment methods and different tumor stages through the obtained potential information of clinical medical imaging that is difficult to identify with the naked eye, and predict the outcome of patients, thereby assisting precision medical decision-making. In this paper, we propose and discuss the possible use of radiomics in the selection of treatment modalities and efficacy prediction for liver cancer.
- Citation: Chen TY, Yang ZG, Li Y, Li MQ. Radiomic advances in the transarterial chemoembolization related therapy for hepatocellular carcinoma. World J Radiol 2023; 15(4): 89-97
- URL: https://www.wjgnet.com/1949-8470/full/v15/i4/89.htm
- DOI: https://dx.doi.org/10.4329/wjr.v15.i4.89
Approximately 90% of all liver tumors are primary hepatocellular carcinomas (HCC), which are the most frequent primary liver tumors[1]. Among the most prevalent malignant tumors worldwide, HCC is the sixth most prevalent. HCC ranks third in the mortality rate of malignant tumors, which has a significant impact on people's quality of life and physical well-being[2]. According to statistics from the World Health Organization 2018, there are 840000 new instances of HCC each year, of which 780000 cases die[3].
The occurrence and progression of HCC is a complex process with multi-factor participation and multi-step interconnection. The majority of patients would lose their opportunity for radical treatment, such as surgical resection and liver transplantation, due to its insidious onset, atypical symptoms and signs, low early diagnosis rate, rapid disease progression, tumor multicentric origin, abundant blood supply, easy to form cancer thrombus, cause metastasis, highly heterogeneous tumor, and other malignant biological behaviors. The selection of HCC treatments is also constrained by characteristics other than the tumor itself, including the degree of cirrhosis, liver function reserve, and portal hypertension. The effectiveness of a single surgical procedure has demonstrated a "ceiling impact" in light of the HCC's biological nature, which is very aggressive.
With the continuous development of interventional oncology therapeutics, interventional therapy for HCC has developed from straightforward transhepatic arterial therapy to a diversified, combined treatment mode, covering early-, intermediate- and advanced-stage HCC. In the field of minimally invasive treatment of HCC, transarterial chemoembolization (TACE) is the most commonly used technique, which uses lipiodol and/or drug-eluting beads. In process of TACE, cytotoxic drugs like doxorubicin or cisplatin are injected while being emulsified in the radiopaque oil compound lipiodol. After that, the embolic agents, like a gelatin sponge, are given. Lipiodol promotes embolization of the tumor microcirculation and delivers cytotoxic drugs directly to the tumor[4]. Nevertheless, there is no acceptable approach to accurately assess the efficiency of interventional therapy due to the significant individual discrepancies presently.
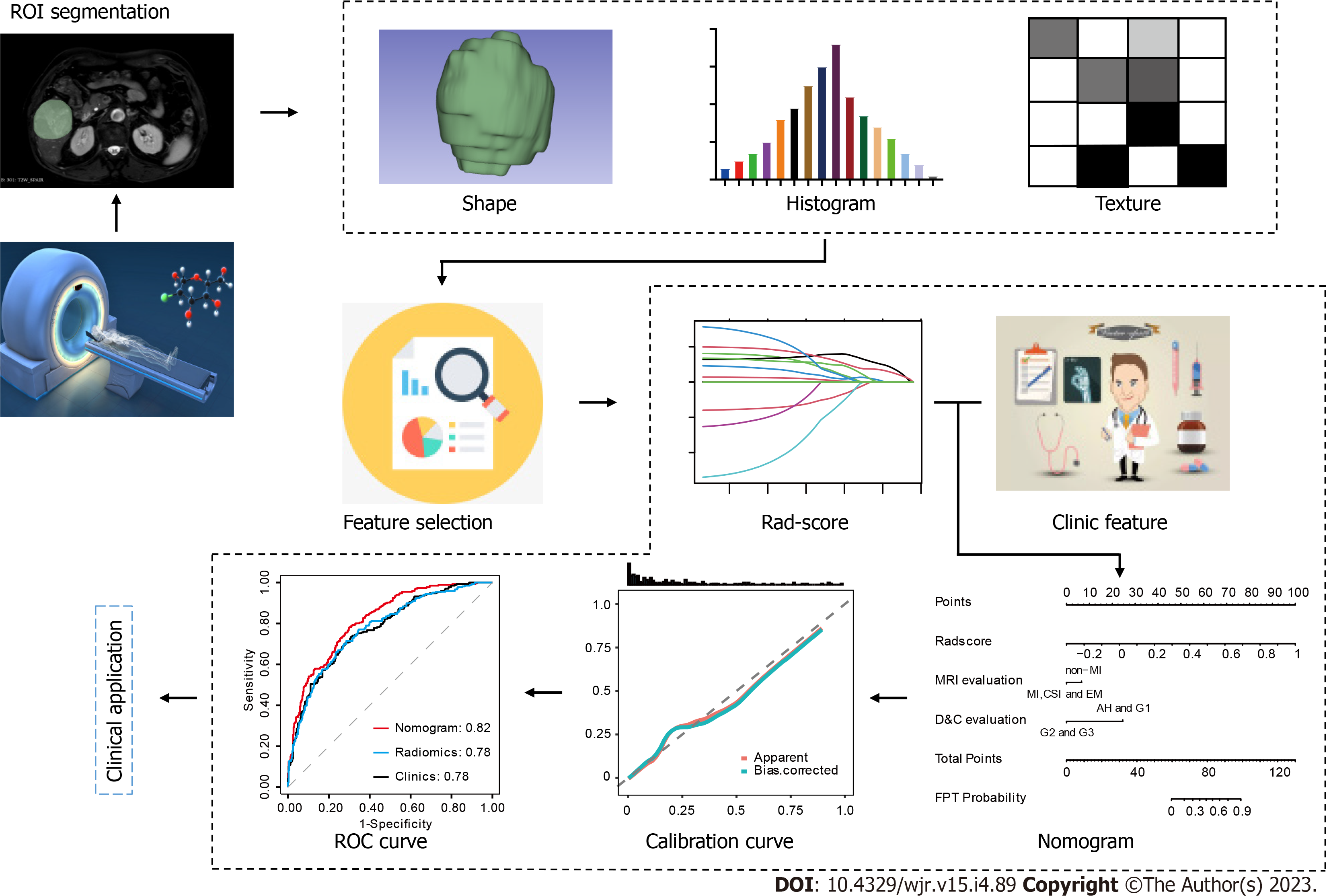
Radiomics is a technique that combines high-throughput extraction of advanced quantitative features to objectively and quantitatively define tumor behaviors. It was first proposed in 2012 by Dutch researcher Lambin[5]. Radiomics extract a huge number of features from medical pictures in high throughput and turn imaging data into a high-resolution mining data space by automatic or semi-automatic analysis methods[5,6]. It is possible to comprehensively, non-invasively, and quantitatively monitor the spatial and temporal heterogeneity of tumors. Radiomics can be divided into below parts: (1) Image acquisition; (2) Region of interest (ROI) segmentation; (3) Feature Extraction; (4) Feature Selection; (5) Model establishment and evaluation; and (6) Clinical application[5,6]. Radiomics has no restrictions on the sorts of medical pictures used for sample collection, and the objects of analysis can include ultrasound, X-ray, CT, magnetic resonance imaging (MRI), positron emission tomography, and other medical images. The ROI can be altered depending on the clinical situation, whether it is normal tissue, the full tumor area, or a metastatic lesion. Generally, both manual and automatic segmentation of the ROI is used in radiomics. However, the segmentation effect of these automatic segmentation techniques is unstable. Currently, manual ROI segmentation by skilled imaging specialists is the approach most frequently used in medical imaging toolkits (such as ITK-SNAP segmentation software). Nevertheless, manual segmentation takes a lot of time, costs a lot of money, and is susceptible to subjective factors despite having a high accuracy rate. Extraction of high-throughput features for quantitative analysis of the important ROI attributes is the first stage in radiomics. The following three categories generally correspond to the extracted features: (1) Shape feature: Reflecting the information of tumor morphology, size, and regularity; (2) Histogram features: The most basic statistical descriptor is based on a global histogram of grayscale values, which includes the grayscale mean, maximum, minimum, variance, and percentile. These features are also known as first-order features because they are based on single-pixel or monomer element analysis; and (3) Texture feature: characteristics can measure details that are hard for the eye to easily see, such as tissue distribution inside the tumor or textural patterns. Such high-dimensional abstract features like the Baud sign may play varied roles to capture clinical information that is not readily perceptible by the vision for clinical problems that are difficult to convey by simple visual features of tumor images. Many hundred to tens of thousands of features may be obtained by feature extraction for feature screening, but not all of them are connected to the clinical issues that need to be resolved. On the other hand, because there are so few samples and so many characteristics in practice, it is simple to cause the overfitting phenomena in later models. Hence, choosing the extracted features is a crucial step in the study of radiomics. The commonly used feature selection methods include filtering, packaging, and embedding. The last step is model construction and model evaluation. To establish the key features of the above feature screening based on clinical research questions, or to further combine features other than imaging omics (such as clinical signs, pathology, and genetic test data) to establish predictive models (Figure 1). According to previous studies[5-8], radiomics properties are directly associated with tumor aggressiveness and heterogeneity indices at the cellular level. Radiomics is believed to be able to forecast clinical endpoints including survival and therapeutic response. Second, radiomics data are mineable, which means that, in sufficiently big data groups, they may be utilized to find previously unidentified indicators and patterns of illness evolution, progression, and treatment response.
The aim of this review was to summarize the predictive value of radiomics in TACE alone and combination therapy for HCC and its guiding significance for the formulation of treatment regimens.
Kuang et al[9] retrospectively collected MRI images and clinical data of 153 patients from 3 hospitals, and divided the subjects into 1 internal training group (n = 113) and 1 external validation group (n = 40) to establish MRI radiomics nomograms that can forecast a patient's short-term response to TACE for an HCC with a diameter under 5 cm. By examining pictures [enhanced computed tomography (CT)/magnetic resonance (MR) scans] of all patients 3/4 mo after their initial TACE treatment, the response of HCC patients to TACE was assessed using the modified Response Evaluation Criteria in Solid Tumors (mRECIST). All cases were split into two groups based on the evaluation results: those who responded well (complete response, CR, and partial response, PR), and those who did not (stable disease, SD, and progressive disease, PD). Images from dynamic enhanced MRI (DCE-MRI) arterial phase and T2-weighted imaging (T2WI) were used to establish radiomics model, clinical model, and radiomics nomogram (by combining the radiomics and clinical model). Radiomics selected the image features using the minimum absolute contraction and selection operator (LASSO) regression and the maximum correlation-minimum redundancy (mRMR) algorithm and then selected 11 of the best subsets of features based on the arterial phase to calculate the radscore. The clinical model based on T2WI was ultimately developed by combining platelet count (PLT), pseudocapsule, boundary, and peritumoral augmentation, and well-response was suggested by a normal PLT value (> 100 × 109/L), pseudocapsule, and obvious border on the T2WI sequence. Another clinical model based on DCE-MRI arterial phase (AP) was developed by combining Child-Pugh class, border, and peritumoral augmentation. Low ChildPugh class (class A), clear border, and no peritumoral enhancement on AP all point to well-response. In contrast with radiomics and clinical models alone, the results showed that the nomogram had superior predictive potential for postoperative response to TACE. Clinics models and Radiomics were obtained which could predict the postoperative response of TACE, and the maximum area under the curve (AUC) value was 0.76, and 0.78 respectively. When combing Clinics models with Radiomics models, predictive power peaked at 0.84. The T2WI-Nomogram and AP-Nomogram were the models with the highest clinical decision effectiveness, according to a comparison of each model's clinical decision effectiveness. Furthermore, T2WI-based and DCE-MRI-based nomograms had approximately comparable predictive abilities. It is demonstrated that the T2WI sequence may effectively represent the heterogeneity of lesions and that adopting a T2WI sequence can result in the formulation of a stable prediction model, eliminate the need for enhanced scanning, lower the side effects of unnecessary contrast injections and medical costs. Patients included in this study were under MRI scanning from different manufacturers (GE and Siemens) with various field strengths (1.5T and 3.0T) and with various sequence parameters. It was discovered through analyzing and processing data that various machines and magnetic field strengths had a mild influence on the radiomics findings. This result indicated that the radiomics has a high degree of repeatability and stability.
TACE offers a considerable survival advantage over supportive therapy for patients with stage B HCC who are initially diagnosed with Barcelona clinical liver cancer (BCLC). These patients had significant survival heterogeneity following TACE despite getting comparable therapies. Some pre-existing prognostic models have been put forth to forecast clinical outcomes following TACE, including Six-and-twelve score[10], Four-and-seven score[11], Hepatoma arterial-embolization prognostic (HAP score)[12], modified hepatoma arterial embolization prognostic (mHAP score)[13], modified hepatoma arterial embolization II (mHAP-II score)[14], modified hepatoma arterial embolization III (mHAP-III score)[15] and albumin-bilirubin(ALBI) grade[16] for arterial embolization of liver cancer. Using minimum absolute contraction and selection operator approaches, the author of this study creates radiomic signatures for survival (rad signatures). The most predictive 6 radiomic features were ultimately chosen. The remaining 4 features were generated from tumor variants of interest (VOI) imaging in the portal vein phase, leaving 2 of the 6 features based on tumor VOI and peritumoral VOI imaging in the arterial phase. Univariate Cox regression analyses were used to ascertain prognostic clinical factors. With multivariate Cox regression analyses, a combined radiomics-clinic (CRC) model was developed using the Rad-signature and clinical factors with a potential association with overall survival (OS). And then contrasted with a prognostic model that had previously been published. Researchers[17] conducted a retrospective multicenter analysis, and the outcomes revealed that the CRC model which was finally established with tumor number (< 4 vs ≥ 4) and the Rad-signature performed better than the other previously mentioned seven established prognostic models, with Harrell’s concordance-index (C-index) of 0.73 (95%CI: 0.68–0.79) and 0.70 (95%CI: 0.62–0.82) in the training and testing cohort, respectively. Among the seven models tested, the six-and-12 score and four-and-seven criteria performed better than the other models, with C-indices of 0.64 (95%CI: 0.58–0.70) and 0.65 (95%CI: 0.55–0.75) in the testing cohort, respectively. The authors created customized risk scores by linearly combining the radscore and the number of tumors (< 4 vs ≥ 4), weighted by their respective coefficients in the multivariate Cox regression model, in order to aid clinical practice. Patients were separated into two groups based on their median risk scores for the training cohort (0.0214): stratum 1, with a risk score of < -0.0214, and stratum 2, with a risk score of > -0.0214. In the training cohort, stratum 1 patients had a considerably longer median survival (31.3 mo) than stratum 2 patients (12.5 mo), with a hazard ratio of 3.63 (95%CI: 2.36-5.60, log-rank test P < 0.0001). Applying the same cutoff to the testing cohort the hazard ratio was 2.43 (95%CI: 1.91-4.98, P = 0.0014), and the median survival for the two groups was 30.9 and 17.0 mo, respectively. The CRC model showed improved survival predictive performance, and researchers believe that the CT radiomics signature represents an independent biomarker of survival in patients with HCC undergoing TACE.
Sun et al[18] used five distinct MR machines with various field strengths for traditional T1WI, T2WI, and T2 grease pressing as well as diffusion-weighted imaging (DWI) (b = 0 and b = 500) scanning. A total of 84 BCLC B HCC patients were enrolled, of whom 67 were enrolled in the training group and 17 in the test group. The mRECIST was used to assign patients into the disease progression (PD) group and nonprogressive disease (N-PD) group if they progressed 6 mo following TACE treatment. In the test group, the calculated AUC of the DWI feature-based model for TACE efficacy prediction is 0.79 (b = 0) and 0.73 (b = 500), followed by T2WI (0.729) and ADC (0.71). In comparison to any single MRI-based radiomics feature, the multiparametric MRI-based radiomics features' AUC rises to 0.80. While clinical data including age, α-fetoprotein (AFP), and tumor size were not statistically significant, a multivariate logistic analysis could identify the independent prognostic indicators of the radiomics signature of PD.
According to mRECIST 1.1, Bai et al[19] aim to evaluate the effectiveness of radiomics features based on preoperative contrast-enhanced computed tomography (CECT) in predicting response to transarterial chemotherapy (TACE), categorized 111 patients with intermediate HCC who received CE-CT in the arterial phase and venous phase before and following TACE into objective response group (n = 38) and non-response group (n = 73) groups. Radiomic feature extraction from CECT pictures. The best monophasic radiomic features of AP and VP in the training set were discovered using two feature ranking methods and three classifiers. In parallel, decision level fusion and feature level fusion were used to combine the pictures of the two CECT phases and establish the multiphase radiomic properties. For the AP signature, the combination of minimum redundancy maximum relevance (MRMR), and support vector machine (SVM) showed the best performance (AUC = 0.814). For VP signatures, the best performance was obtained by MRMR and LASSO (AUC = 0.861). For the performance of multiphase radiomic signatures, DLF signatures had the highest AUC value of 0.883 among all radiomic signatures by using the features selected by MRMR and SVM classifiers. Eventually, a nomogram was constructed by combining two common features (tumor size and tumor number) and radiomics signature, using multivariate logistic regression, and its prediction ability was assessed by AUC on the test data. To determine the accuracy, sensitivity, and specificity values, the cut-off point closest to the upper left corner of the training receiver operating characteristic (ROC) was used. Scores below or above the cutoff were considered to be either objective-response or non-response. It was discovered that multiphase radiomics features (AUC = 0.883) outperformed the best single-phase radiomics signature (AUC = 0.861) in predicting response to TACE treatment. The nomogram in the test dataset and training dataset showed better performance than any radiomics signatures. The authors conclude that the radiomic model will be helpful in assessing the therapeutic advantages of TACE treatment.
Kong et al[20] enrolled 99 patients with intermediate and advanced HCC (BCLC B and C) treated with TACE. Among them, 69 cases were enrolled in the training group and 30 cases in the test group. MRI was performed before TACE, and efficacy was evaluated according to mRECIST criteria 3 mo after TACE. The patients were divided into response groups (CR and PR) and non-response groups (PD and SD). There were significant differences in AFP values, Child-Pugh scores, and BCLC staging between the two groups. Before TACE, 396 radiomics characteristics were extracted from T2WI, and 6 features were chosen using minimal absolute contraction and selection operator (LASSO) regression. These features are variance, inverse difference moment angle90 offset7, long run emphasis angle90 offset7, short run emphasis all direction offset 4 SD, short run emphasis angle 135 offset1, and high intensity large area emphasis. They are combined to provide radscore to create response prediction models. In the training and validation cohorts, the AUCs of the ROC curve based on Radscore were 0.812 and 0.866, respectively. While the prediction performance of the radiomics model was greatly enhanced when the Child-Pugh class, BCLC stage, and AFP level were added. The AUC of the training group was 0.861 (95%CI: 0.774–0.949) with specificity and sensitivity as high as 0.811 and 0.844, respectively. The AUC of the validation group was 0.884 (95%CI: 0.764–1.000), and the specificity and sensitivity were 0.75 and 1.00, respectively. Based on the above conclusion, the authors believe that the quantitative nomogram model based on radscore and clinical predictors can accurately predict the response to TACE in intermediate and advanced HCC and can also be used as an auxiliary diagnosis for clinical prognosis.
Retrospectively, 122 HCC (BCLC A and B) patients who had MRI scanning prior to initial TACE (response = 63; non-response = 59) were recruited by Ying Zhao et al[21] and randomly divided into training group (n = 85) and validation group (n = 37). The manual segmentation of arteries, veins, and delayed periods on CE-MRI yielded a total of 2367 radiomics characteristics. Of all radiomics features, the three-phase radiomics model performed better in the training group, with an AUC of 0.838. The AUC of radiomics nomogram combining three-phase radiomics score and clinical-radiological risk factors (total bilirubin, tumor shape, and tumor encapsulation) were 0.878 and 0.833, respectively, which showed good calibration and prediction with better predictive power. However, other studies[22,23] have demonstrated that while total bilirubin can predict survival in HCC patients treated with TACE, it cannot be utilized as a clinical risk factor to evaluate the effectiveness of treatment.
Almost all the guidelines advocate transitioning to systemic medication or combination therapy when a patient's response to TACE is poor. To tackle this problem, the concept of "TACE refractoriness" is put forth[24,25]. According to the Japanese Liver Association[26], vascular invasion, distant metastases, persistently increased tumor markers or an ongoing partial response to at least two TACEs are indicative of intractable resolution of TACE. New vascular invasion or distant metastases can operate as stand-in markers for refractory TACE during TACE therapy, according to a Korean study[27]. It's critical to anticipate TACE refractoriness while considering a potentially better treatment. The ability to predict TACE refractoriness before surgery has been demonstrated by some researchers utilizing the radiomics nomogram approach on arterial CT scans of 80 HCC patients who did not have extrahepatic metastases or macrovascular involvement[28]. The authors believe that the radscore, which consists of the Gray-Level Zone Length Matrix (GLZLM)-Long-Zone Low Gray-Level Emphasis and GLZLM-Gray-Level Non-Uniformity, T-stage, log AFP, and bilobar distribution is significantly related to TACE refractoriness. Multivariate logistic regression model reveals that these factors, such as radscore+T-stage (AUC = 0.95); radscore+bilobar distribution (AUC = 0.91); and radscore+logAFP (AUC = 0.91), perform well in predicting TACE refractoriness. Additionally, according to Niu et al[29], the radiomics nomogram of CT can be used to forecast the pretreatment prediction of TACE refractoriness and offer better guidance for choosing the next course of TACE treatment.
A multicenter retrospective analysis included 140 patients with TACE HCC (BCLC A+B+C)[30]. In order to predict tumor response, response-related radscores were created using T2WI and DCE-MRI, respectively. The T2WI outperformed 4 MRI sequences with an AUC of 0.75. The T2WI radscore, BCLC stage, and albumin-bilirubin grade were combined to generate a nomogram using the logistic regression model. The nomogram yielded AUCs of 0.81 and 0.78 for the train and test groups for predicting tumor response. Progression-free survival (PFS) and OS in survival analyses showed substantial differences between responders and non-responders of TACE treatment. Albumin bilirubin grading, satellite nodes, and BCLC staging are independent predictors of OS. PFS and OS were independently predicted by albumin bilirubin grading and BCLC staging. According to the Cox model, PFS and OS's consistency indices were 0.70 and 0.73, respectively.
Another study extracted radiomics features from intratumoral and perineoplastic dilatation (1, 3, and 5 mm, respectively) from 184 patients with HCC treated with TACE. The radiomics model was constructed using LASSO[31], which was used to investigate the relationship between preoperatively CE-MRI and recurrence-free survival (RFS) in HCC patients regarding the RFS of HCC patients after TACE. Seven models of radiomics (APETV, PVPETV, PVPB1, PVPB3, and PVPB5, in which the TV stands for the total tumor volume and B represents millimeters beyond the tumor boundary), clinical-radiology parameters, and a combination model (nomogram) were generated. The results showed that sex, AFP, BCLC stage, IM (defined as a tumor with a budding portion at its periphery in both the AP and PVP), and APE (which was interpreted as the contrast enhanced shadow around the tumor in the AP, and isointensity compared with the background liver parenchyma in the PVP and delayed phase) were independent risk factors related to RFS after TACE. The performance of the PVPB3 model was 0.714 (C-index), which was equivalent to that of the portal stage intratumor model (C-index, 0.727, P = 0.409). The nomogram, which had a C-index of 0.802 with the best performance, incorporated five independent risk factors from PVPETV characteristics and clinical-radiological parameters. It was more useful for assessing RFS in HCC patients following TACE treatment. The combined model's cutoff relative to the median (1.74) allowed for a precise division of these individuals into high- and low-risk groupings, according to the Kaplan-Meier analysis. A study[29] based on radiomics features by CE-CT in the arterial phase had also revealed that radscore, BCLC stage, irregular tumor margin, largest tumor size, and tumor number were independent influencing factors for OS. CT-based radiomics nomogram could classify patients into high-risk (> 3.5) and low-risk (≤ 3.5) groups with significantly different prognoses between the two groups (OS: 12.3 mo vs 23.6 mo, P < 0.001). Another study came to the same conclusion that the combined model (nomogram) included five radiomics features (surface area-to-volume ratio, kurtosis, median, gray-level co-occurrence matrix contrast, and size zone variability) and three clinical factors (Child-Pugh score, α-fetoprotein level, and tumor size) was better in predicting survival than either the clinical scoring model or the radscore model (hazard ratio 19.88; P < 0.001).
TACE and RFA combination therapy have synergistic cytotoxic effects for intermediate and advanced HCC, compared to TACE alone, which can better control local tumor recurrence and lengthen survival[4]. TACE lessens the "heat sink" that blood flow creates during radiofrequency ablation. It aggravates the edema of tissues, and clearly displays the outlines of lesions induced by iodine oil deposition on CT, making it easier to determine the RFA range. These elements may increase the severity of coagulation necrosis and, as a result, improve OS prominently[32]. However, there are also significant differences in the efficacy of TACE combined with RFA. Some scholars[33] combined radiomics and clinical factors to construct a predictive nomogram model, which can be used as a new strategy for predicting advanced HCC PFS in the treatment of TACE+RFA.
Lenvatinib is just as efficient as sorafenib as a multikinase inhibitor with antiangiogenic effects in the first-line treatment of advanced HCC[34]. Combination therapy with TACE and lenvatinib has been shown to be effective in recent studies[35,36]. According to the findings, combination therapy outperformed lenvatinib monotherapy and TACE by a wide margin. Luo et al[37] studied 61 HCC patients and extracted radiomics characteristics from T1WI, T1WI arterial phase, T1WI portal phase, T1WI delay phase, T2WI, DWI (b = 800), and ADC maps. The results showed that the number of tumors [risk ratio, HR = 4.64, 95%CI: 1.03-20.88, P = 0.045] and arterial phase intensity HR = 0.24, (95%CI: 0.09-0.64) with a sensitivity of 0.99, specificity of 0.95, and accuracy of 0.71. The combined model significantly increased the clinical model's performance in predicting disease progression.
We discussed the use of radiomics in predicting TACE treatment in HCC, which quantifies the heterogeneity of HCC on medical images. These radiomics features can be further used as predictors of clinical outcomes to determine the efficacy of TACE, as well as provide individualized guidance for patient treatment options. However, there are also some difficulties need to be further resolved. First, it might be challenging by using a computer to pinpoint the tumor boundary automatically, and hand outlining may vulnerable to significant subjective variation. Second, considering radiomics is still in its infancy and different researchers employ different imaging tools and image algorithms, it is impossible for other researchers to evaluate and put the results to clinical use[38]. Last but not the least, it is challenging to create a worldwide uniform paradigm for radiomics in HCC research because the underlying etiology of the disease varies between different regions[39].
Radiomics can forecast the efficacy of TACE and TACE combined therapy regimens before the treatment conducting. Tailored counsel for patient treatment based on radiomics and increased comprehensive prediction abilities would be achieved when combined with clinical data.
| 1. | Dy GW, Gore JL, Forouzanfar MH, Naghavi M, Fitzmaurice C. Global Burden of Urologic Cancers, 1990-2013. Eur Urol. 2017;71:437-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 178] [Cited by in RCA: 266] [Article Influence: 29.6] [Reference Citation Analysis (0)] |
| 2. | Yang JD, Hainaut P, Gores GJ, Amadou A, Plymoth A, Roberts LR. A global view of hepatocellular carcinoma: trends, risk, prevention and management. Nat Rev Gastroenterol Hepatol. 2019;16:589-604. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2184] [Cited by in RCA: 3128] [Article Influence: 446.9] [Reference Citation Analysis (17)] |
| 3. | Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394-424. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53206] [Cited by in RCA: 56700] [Article Influence: 7087.5] [Reference Citation Analysis (135)] |
| 4. | Chang Y, Jeong SW, Young Jang J, Jae Kim Y. Recent Updates of Transarterial Chemoembolilzation in Hepatocellular Carcinoma. Int J Mol Sci. 2020;21. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 109] [Cited by in RCA: 247] [Article Influence: 41.2] [Reference Citation Analysis (0)] |
| 5. | Lambin P, Rios-Velazquez E, Leijenaar R, Carvalho S, van Stiphout RG, Granton P, Zegers CM, Gillies R, Boellard R, Dekker A, Aerts HJ. Radiomics: extracting more information from medical images using advanced feature analysis. Eur J Cancer. 2012;48:441-446. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2415] [Cited by in RCA: 4201] [Article Influence: 300.1] [Reference Citation Analysis (4)] |
| 6. | Kumar V, Gu Y, Basu S, Berglund A, Eschrich SA, Schabath MB, Forster K, Aerts HJ, Dekker A, Fenstermacher D, Goldgof DB, Hall LO, Lambin P, Balagurunathan Y, Gatenby RA, Gillies RJ. Radiomics: the process and the challenges. Magn Reson Imaging. 2012;30:1234-1248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1505] [Cited by in RCA: 1630] [Article Influence: 116.4] [Reference Citation Analysis (0)] |
| 7. | Choi ER, Lee HY, Jeong JY, Choi YL, Kim J, Bae J, Lee KS, Shim YM. Quantitative image variables reflect the intratumoral pathologic heterogeneity of lung adenocarcinoma. Oncotarget. 2016;7:67302-67313. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 71] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 8. | Sala E, Mema E, Himoto Y, Veeraraghavan H, Brenton JD, Snyder A, Weigelt B, Vargas HA. Unravelling tumour heterogeneity using next-generation imaging: radiomics, radiogenomics, and habitat imaging. Clin Radiol. 2017;72:3-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 218] [Cited by in RCA: 269] [Article Influence: 29.9] [Reference Citation Analysis (0)] |
| 9. | Kuang Y, Li R, Jia P, Ye W, Zhou R, Zhu R, Wang J, Lin S, Pang P, Ji W. MRI-Based Radiomics: Nomograms predicting the short-term response after transcatheter arterial chemoembolization (TACE) in hepatocellular carcinoma patients with diameter less than 5 cm. Abdom Radiol (NY). 2021;46:3772-3789. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 38] [Article Influence: 7.6] [Reference Citation Analysis (0)] |
| 10. | Wang Q, Xia D, Bai W, Wang E, Sun J, Huang M, Mu W, Yin G, Li H, Zhao H, Li J, Zhang C, Zhu X, Wu J, Gong W, Li Z, Lin Z, Pan X, Shi H, Shao G, Liu J, Yang S, Zheng Y, Xu J, Song J, Wang W, Wang Z, Zhang Y, Ding R, Zhang H, Yu H, Zheng L, Gu W, You N, Wang G, Zhang S, Feng L, Liu L, Zhang P, Li X, Chen J, Xu T, Zhou W, Zeng H, Huang W, Jiang W, Zhang W, Shao W, Li L, Niu J, Yuan J, Lv Y, Li K, Yin Z, Xia J, Fan D, Han G; China HCC-TACE Study Group. Development of a prognostic score for recommended TACE candidates with hepatocellular carcinoma: A multicentre observational study. J Hepatol. 2019;70:893-903. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 97] [Cited by in RCA: 188] [Article Influence: 26.9] [Reference Citation Analysis (6)] |
| 11. | Yamakado K, Miyayama S, Hirota S, Mizunuma K, Nakamura K, Inaba Y, Maeda H, Matsuo K, Nishida N, Aramaki T, Anai H, Koura S, Oikawa S, Watanabe K, Yasumoto T, Furuichi K, Yamaguchi M. Subgrouping of intermediate-stage (BCLC stage B) hepatocellular carcinoma based on tumor number and size and Child-Pugh grade correlated with prognosis after transarterial chemoembolization. Jpn J Radiol. 2014;32:260-265. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 59] [Cited by in RCA: 76] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 12. | Kadalayil L, Benini R, Pallan L, O'Beirne J, Marelli L, Yu D, Hackshaw A, Fox R, Johnson P, Burroughs AK, Palmer DH, Meyer T. A simple prognostic scoring system for patients receiving transarterial embolisation for hepatocellular cancer. Ann Oncol. 2013;24:2565-2570. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 207] [Cited by in RCA: 295] [Article Influence: 22.7] [Reference Citation Analysis (0)] |
| 13. | Pinato DJ, Arizumi T, Allara E, Jang JW, Smirne C, Kim YW, Kudo M, Pirisi M, Sharma R. Validation of the hepatoma arterial embolization prognostic score in European and Asian populations and proposed modification. Clin Gastroenterol Hepatol. 2015;13:1204-8.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 58] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 14. | Park Y, Kim SU, Kim BK, Park JY, Kim DY, Ahn SH, Park YE, Park JH, Lee YI, Yun HR, Han KH. Addition of tumor multiplicity improves the prognostic performance of the hepatoma arterial-embolization prognostic score. Liver Int. 2016;36:100-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 85] [Cited by in RCA: 81] [Article Influence: 8.1] [Reference Citation Analysis (0)] |
| 15. | Campani C, Vitale A, Dragoni G, Arena U, Laffi G, Cillo U, Giannini EG, Tovoli F, Rapaccini GL, Di Marco M, Caturelli E, Zoli M, Sacco R, Cabibbo G, Mega A, Guarino M, Gasbarrini A, Svegliati-Baroni G, Foschi FG, Biasini E, Masotto A, Nardone G, Raimondo G, Azzaroli F, Vidili G, Brunetto MR, Farinati F, Trevisani F, Marra F. Time-Varying mHAP-III Is the Most Accurate Predictor of Survival in Patients with Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization. Liver Cancer. 2021;10:126-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 16. | Hiraoka A, Kumada T, Kudo M, Hirooka M, Tsuji K, Itobayashi E, Kariyama K, Ishikawa T, Tajiri K, Ochi H, Tada T, Toyoda H, Nouso K, Joko K, Kawasaki H, Hiasa Y, Michitaka K; Real-Life Practice Experts for HCC (RELPEC) Study Group and HCC 48 Group (hepatocellular carcinoma experts from 48 clinics). Albumin-Bilirubin (ALBI) Grade as Part of the Evidence-Based Clinical Practice Guideline for HCC of the Japan Society of Hepatology: A Comparison with the Liver Damage and Child-Pugh Classifications. Liver Cancer. 2017;6:204-215. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 158] [Cited by in RCA: 176] [Article Influence: 19.6] [Reference Citation Analysis (2)] |
| 17. | Meng XP, Wang YC, Ju S, Lu CQ, Zhong BY, Ni CF, Zhang Q, Yu Q, Xu J, Ji J, Zhang XM, Tang TY, Yang G, Zhao Z. Radiomics Analysis on Multiphase Contrast-Enhanced CT: A Survival Prediction Tool in Patients With Hepatocellular Carcinoma Undergoing Transarterial Chemoembolization. Front Oncol. 2020;10:1196. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 39] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 18. | Sun Y, Bai H, Xia W, Wang D, Zhou B, Zhao X, Yang G, Xu L, Zhang W, Liu P, Xu J, Meng S, Liu R, Gao X. Predicting the Outcome of Transcatheter Arterial Embolization Therapy for Unresectable Hepatocellular Carcinoma Based on Radiomics of Preoperative Multiparameter MRI. J Magn Reson Imaging. 2020;52:1083-1090. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 51] [Article Influence: 8.5] [Reference Citation Analysis (1)] |
| 19. | Bai H, Meng S, Xiong C, Liu Z, Shi W, Ren Q, Xia W, Zhao X, Jian J, Song Y, Ni C, Gao X, Li Z. Preoperative CECT-based Radiomic Signature for Predicting the Response of Transarterial Chemoembolization (TACE) Therapy in Hepatocellular Carcinoma. Cardiovasc Intervent Radiol. 2022;45:1524-1533. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 13] [Reference Citation Analysis (0)] |
| 20. | Kong C, Zhao Z, Chen W, Lv X, Shu G, Ye M, Song J, Ying X, Weng Q, Weng W, Fang S, Chen M, Tu J, Ji J. Prediction of tumor response via a pretreatment MRI radiomics-based nomogram in HCC treated with TACE. Eur Radiol. 2021;31:7500-7511. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 103] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 21. | Zhao Y, Wang N, Wu J, Zhang Q, Lin T, Yao Y, Chen Z, Wang M, Sheng L, Liu J, Song Q, Wang F, An X, Guo Y, Li X, Wu T, Liu AL. Radiomics Analysis Based on Contrast-Enhanced MRI for Prediction of Therapeutic Response to Transarterial Chemoembolization in Hepatocellular Carcinoma. Front Oncol. 2021;11:582788. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 30] [Article Influence: 6.0] [Reference Citation Analysis (0)] |
| 22. | Abajian A, Murali N, Savic LJ, Laage-Gaupp FM, Nezami N, Duncan JS, Schlachter T, Lin M, Geschwind JF, Chapiro J. Predicting Treatment Response to Intra-arterial Therapies for Hepatocellular Carcinoma with the Use of Supervised Machine Learning-An Artificial Intelligence Concept. J Vasc Interv Radiol. 2018;29:850-857.e1. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 132] [Article Influence: 16.5] [Reference Citation Analysis (0)] |
| 23. | Zhang H, He X, Yu J, Song W, Liu X, Liu Y, Zhou J, Guo D. Preoperative MRI features and clinical laboratory indicators for predicting the early therapeutic response of hepatocellular carcinoma to transcatheter arterial chemoembolization combined with High-intensity focused ultrasound treatment. Br J Radiol. 2019;92:20190073. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 5] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Raoul JL, Sangro B, Forner A, Mazzaferro V, Piscaglia F, Bolondi L, Lencioni R. Evolving strategies for the management of intermediate-stage hepatocellular carcinoma: available evidence and expert opinion on the use of transarterial chemoembolization. Cancer Treat Rev. 2011;37:212-220. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 365] [Cited by in RCA: 413] [Article Influence: 25.8] [Reference Citation Analysis (0)] |
| 25. | Yamashita T, Kaneko S. Treatment strategies for hepatocellular carcinoma in Japan. Hepatol Res. 2013;43:44-50. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 14] [Cited by in RCA: 17] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 26. | Kudo M, Izumi N, Kokudo N, Matsui O, Sakamoto M, Nakashima O, Kojiro M, Makuuchi M; HCC Expert Panel of Japan Society of Hepatology. Management of hepatocellular carcinoma in Japan: Consensus-Based Clinical Practice Guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339-364. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 642] [Cited by in RCA: 677] [Article Influence: 45.1] [Reference Citation Analysis (0)] |
| 27. | Kim HY, Park JW, Joo J, Jung SJ, An S, Woo SM, Kim HB, Koh YH, Lee WJ, Kim CM. Severity and timing of progression predict refractoriness to transarterial chemoembolization in hepatocellular carcinoma. J Gastroenterol Hepatol. 2012;27:1051-1056. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 56] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 28. | Sheen H, Kim JS, Lee JK, Choi SY, Baek SY, Kim JY. A radiomics nomogram for predicting transcatheter arterial chemoembolization refractoriness of hepatocellular carcinoma without extrahepatic metastasis or macrovascular invasion. Abdom Radiol (NY). 2021;46:2839-2849. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 21] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 29. | Niu XK, He XF. Development of a computed tomography-based radiomics nomogram for prediction of transarterial chemoembolization refractoriness in hepatocellular carcinoma. World J Gastroenterol. 2021;27:189-207. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 27] [Cited by in RCA: 37] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 30. | Liu QP, Yang KL, Xu X, Liu XS, Qu JR, Zhang YD. Radiomics analysis of pretreatment MRI in predicting tumor response and outcome in hepatocellular carcinoma with transarterial chemoembolization: a two-center collaborative study. Abdom Radiol (NY). 2022;47:651-663. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Cited by in RCA: 38] [Article Influence: 9.5] [Reference Citation Analysis (0)] |
| 31. | Song W, Yu X, Guo D, Liu H, Tang Z, Liu X, Zhou J, Zhang H, Liu Y. MRI-Based Radiomics: Associations With the Recurrence-Free Survival of Patients With Hepatocellular Carcinoma Treated With Conventional Transcatheter Arterial Chemoembolization. J Magn Reson Imaging. 2020;52:461-473. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 33] [Cited by in RCA: 72] [Article Influence: 10.3] [Reference Citation Analysis (0)] |
| 32. | Iezzi R, Pompili M, Posa A, Coppola G, Gasbarrini A, Bonomo L. Combined locoregional treatment of patients with hepatocellular carcinoma: State of the art. World J Gastroenterol. 2016;22:1935-1942. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 48] [Cited by in RCA: 58] [Article Influence: 5.8] [Reference Citation Analysis (0)] |
| 33. | Fang S, Lai L, Zhu J, Zheng L, Xu Y, Chen W, Wu F, Wu X, Chen M, Weng Q, Ji J, Zhao Z, Tu J. A Radiomics Signature-Based Nomogram to Predict the Progression-Free Survival of Patients With Hepatocellular Carcinoma After Transcatheter Arterial Chemoembolization Plus Radiofrequency Ablation. Front Mol Biosci. 2021;8:662366. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in RCA: 11] [Reference Citation Analysis (0)] |
| 34. | Kudo M, Finn RS, Qin S, Han KH, Ikeda K, Piscaglia F, Baron A, Park JW, Han G, Jassem J, Blanc JF, Vogel A, Komov D, Evans TRJ, Lopez C, Dutcus C, Guo M, Saito K, Kraljevic S, Tamai T, Ren M, Cheng AL. Lenvatinib vs sorafenib in first-line treatment of patients with unresectable hepatocellular carcinoma: a randomised phase 3 non-inferiority trial. Lancet. 2018;391:1163-1173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4432] [Cited by in RCA: 4122] [Article Influence: 515.3] [Reference Citation Analysis (5)] |
| 35. | Ando Y, Kawaoka T, Amioka K, Naruto K, Ogawa Y, Yoshikawa Y, Kikukawa C, Kosaka Y, Uchikawa S, Morio K, Fujino H, Nakahara T, Murakami E, Yamauchi M, Tsuge M, Hiramatsu A, Fukuhara T, Mori N, Takaki S, Tsuji K, Nonaka M, Hyogo H, Aisaka Y, Masaki K, Honda Y, Moriya T, Naeshiro N, Takahashi S, Imamura M, Chayama K, Aikata H. Efficacy and Safety of Lenvatinib-Transcatheter Arterial Chemoembolization Sequential Therapy for Patients with Intermediate-Stage Hepatocellular Carcinoma. Oncology. 2021;99:507-517. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 36. | Fu Z, Li X, Zhong J, Chen X, Cao K, Ding N, Liu L, Zhang X, Zhai J, Qu Z. Lenvatinib in combination with transarterial chemoembolization for treatment of unresectable hepatocellular carcinoma (uHCC): a retrospective controlled study. Hepatol Int. 2021;15:663-675. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 130] [Article Influence: 26.0] [Reference Citation Analysis (0)] |
| 37. | Luo J, Huang Z, Wang M, Li T, Huang J. Prognostic role of multiparameter MRI and radiomics in progression of advanced unresectable hepatocellular carcinoma following combined transcatheter arterial chemoembolization and lenvatinib therapy. BMC Gastroenterol. 2022;22:108. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 28] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 38. | Orlhac F, Frouin F, Nioche C, Ayache N, Buvat I. Validation of A Method to Compensate Multicenter Effects Affecting CT Radiomics. Radiology. 2019;291:53-59. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 157] [Cited by in RCA: 171] [Article Influence: 24.4] [Reference Citation Analysis (0)] |
| 39. | Kim DY, Ryu HJ, Choi JY, Park JY, Lee DY, Kim BK, Kim SU, Ahn SH, Chon CY, Han KH. Radiological response predicts survival following transarterial chemoembolisation in patients with unresectable hepatocellular carcinoma. Aliment Pharmacol Ther. 2012;35:1343-1350. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 78] [Cited by in RCA: 77] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Gastroenterology and hepatology
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Elsayed MOK, United Kingdom; Nakano H, Japan S-Editor: Liu JH L-Editor: A P-Editor: Zhao S