Peer-review started: September 19, 2022
First decision: October 19, 2022
Revised: November 2, 2022
Accepted: December 27, 2022
Article in press: December 27, 2022
Published online: January 28, 2023
Processing time: 119 Days and 11 Hours
The liver has a complex vascular anatomy with a unique dual blood supply. Clinical conditions of the liver vary widely and include disorders originating in the vascular and biliary systems as well as the parenchyma. In most vascular disorders, the effects on the liver are generally subclinical because of its abundant blood supply. However, early diagnosis of such vascular diseases can signi
Core Tip: The liver has a complex vascular anatomy. Imaging findings may not be readily apparent in vascular disorders of the liver. Computed tomography angiography is an excellent imaging modality for visualizing the vascular anatomy of patients for treatment planning. This review article focuses on the vascular anatomy of the liver and imaging findings in some acute hepatic vascular diseases.
- Citation: Firat A, Abbasoglu TT, Karcaaltincaba M, Balaban YH. Clinical anatomy of hepatic vessels by computed tomography angiography: A minireview. World J Radiol 2023; 15(1): 1-9
- URL: https://www.wjgnet.com/1949-8470/full/v15/i1/1.htm
- DOI: https://dx.doi.org/10.4329/wjr.v15.i1.1
The liver performs a variety of metabolic processes for homeostasis, nutrition, and immune defence. For example, it is important for the removal and breakdown of toxic or potentially toxic substances from the blood; the regulation of blood glucose and lipids; the storage of certain vitamins, iron, and other micronutrients; the synthesis of proteins and clotting factors; the metabolism of amino acids; and the production of bile. It is involved in a variety of other biochemical reactions. Since most of these processes are exothermic, a significant portion of the body’s thermal energy production, especially at rest, is provided by the liver. Acute clinical conditions of the liver vary widely and include disorders originating in the vascular and biliary systems as well as the parenchyma. The liver has a complex vascular anatomy with a unique dual blood perfusion. The portal vein provides 70% of the hepatic blood flow, while the hepatic artery provides the remaining 30%[1]. In most vascular disorders, the effects on the liver are generally subclinical due to this rich blood supply. However, some acute diseases of these vessels may be accompanied by abdominal pain. Early diagnosis of such vascular disease can significantly reduce patient morbidity and mortality. Computed tomography angiography (CTA) is now the standard procedure for surgical planning of living liver donors in whom most of the right lobe of liver with associated vessels and ducts, is removed for transplantation to the recipient. CTA visualises both the vascular and parenchymal anatomy of the liver. In this technique, the contrast agent is administered directly into the splanchnic bed by injection into the superior mesenteric artery. CT images acquired after an appropriate delay provide hepatic and portal phase images of the liver. Rapid image acquisition of vessels with thin sections is a critical feature of an optimal CTA protocol that prevents artefacts due to motion and respiration. Two-phase liver protocols include both an arterial and portal venous phase. CTA is an excellent imaging modality for visualising the patient’s vascular anatomy for surgical planning of liver transplantation. Because imaging findings of vascular disorders may not be readily recognized, diagnosis can be difficult. In this review article, we focus on the vascular anatomy of the liver and the imaging findings in common acute diseases of the hepatic vasculature[2].
The liver begins to develop from the anterior bulge of the endoderm of the foregut. From the cephalic part of this diverticular structure, splanchnic mesodermal cells migrate into the septum transversum. These cells, which concentrate around the vitelline veins, differentiate into hepatic cells as single-layered plaques. Kupffer cells also differentiate from the endothelial cells of the vitelline plexus. Biliary capillaries and cholangioles also develop from the mesenchyme between the hepatocellular plates. The vitelline veins form a plexus in the liver, which then unites with the umbilical veins and opens into the sinus venosus of the heart. Later, some portions of the vitelline veins undergo atresia and become the portal vein, which unites with the splenic vein and the superior mesenteric veins. The proximal portions of the vitelline vein join the right and left hepatic veins (RHV and LHV, respectively) between the sinus venosus and the hepatic plexus. The entire right umbilical vein and the proximal portion of the left umbilical vein, which is connected to the hepatic sinusoids, atrophy. Over time, venous blood from the placenta flows into the right vitelline vein in the liver and then directly to the heart via the ductus venosus, a large venous trunk that separates from the hepatic sinusoids. Meanwhile, half of the blood from the umbilical vein flows through the liver and the other half through the ductus venosus. The umbilical vein settles in the falciform ligament as the ligamentum teres, and as the liver continues to grow in the abdomen, it maintains contact with the septum transversum under the diaphragm[3].
The liver has a spongy structure consisting of irregular single-row cell strands and spaces called lacunae between them. The diameter of the lacunae is larger than that of the hepatocytes. The capillaries within these lacunae are called sinusoids, which are lined with a basement membrane and are permeable to macromolecules. Kupffer cells, which are part of the reticuloendothelial system, are located around the endothelium of the sinusoids. The hexagonal cells of the cell strands are called hepatocytes. They form the critical cell layer between the sinusoids and the bile canaliculi. They have a unique polarity, with the basement membrane facing the sinusoidal endothelium, while one or more apical poles may contribute to multiple bile canaliculi[2]. Between the endothelium of the sinusoids and the hepatocytes is a potential gap called the space of Disse. The fluid accumulated in this space is drained by the lymphatic vessels and blood capillaries. Since hepatocytes are polygonal in shape and have many facets, two or three facets are in contact with the space of Disse, while the remaining facets are in contact with the neighbouring liver cells. Bile canaliculi run between adjacent hepatocytes. Hepatocyte polarity is essential in explaining many functions of the liver. Hepatocyte strands and sinusoids run past each other in a branched and interlocking fashion, which increases metabolic exchange between the blood and biliary systems. The combination of the bile duct, hepatic artery, and hepatic portal vein is termed a portal triad. As they enter the liver, each divides into segmental branches surrounded by a perivascular fibrous capsule. The hepatic lobule is a hexagonal unit consisting of a concentric arrangement of a central vein in the middle and surrounding portal triads located at the vertices of each hexagon and hepatocyte strands, although the boundaries are not very sharp. Each portal triad is surrounded by a hepatocyte plate, which is traversed by the bile canaliculi and blood vessels. Between the portal triad and the hepatocyte strand is a gap called the space of Mall (periportal area). The hepatic lobule is perfused by the branches of the hepatic artery and portal vein at the periphery and drained by the central vein in the middle of the lobule. Blood flows centrifugally from the portal triad. Bile produced by the hepatocytes also flows centrifugally to the periphery. There is blood flow from the portal vein and hepatic artery to the hepatic vein depending on the blood pressure gradient. Each central vein opens through a sublobular vein, which eventually forms the right and LHVs, and opens into the inferior vena cava. Since there is no such blood pressure gradient in the embryo, the lobular cell arrangement is not observed, and this arrangement develops after birth. In this case, the lobule structure may change with blood pressure and blood flow[3].
Another functional concept is the liver acinus: A diamond-shaped area supplied by the terminal branches of the hepatic artery and portal vein. This small unit is important in describing functional and pathologic changes in many clinical conditions. Its short axis runs along the borders of the hepatic lobules and its imaginary long axis is located between the two central veins. The hepatocytes are located in three elliptical zones around the short axis[3-5]. The areas near the center of the acini receive more oxygenated blood because they are near the afferent vessels and are referred to as zone 1 (periportal zone). Zone 2 (intermediate zone) is the intermediate region that is more peripheral to the acini, and zone 3 is the region closest to the central vein at the end of the acini. Zone 3 is most affected by ischemic damage. The lobule and acinus formation systems, which complement each other, can be related to the gross anatomy that integrates functional modulation[6,7].
In the right and left lobes of the liver, arteries, portal veins, and bile ducts run parallel to each other. These vessels and ducts branch to disperse into segments and show many individual variations, and no anastomosis was observed between the intraparenchymal branches.
The bile duct, hepatic artery, portal vein, lymphatics, and nerves open horizontally into the porta hepatis, which is located in the middle part of the visceral surface of the liver.
CT examinations were performed with a 16-MDCT scanner (Somatom Sensation 16, Siemens, Germany). All patients received 100 mL of iodinated contrast material (Ultravist 300/100 mg/mL; Bayer Schering Pharma, Berlin, Germany) at a flow rate of 4 mL/s using a power injector. CT images were obtained 70 s after the injection of contrast for the portal venous phase, 15-20 s for the early arterial phase, and 30-40 s for the late arterial phase. Technical parameters were detector collimation, 1.5 mm; pitch, 1.5; gantry rotation time: 0.5 s. Axial images were reconstructed at a thickness of 2 mm and 5 mm and transferred to Picture Archiving and Communication System.
The portal vein is formed by the union of the superior mesenteric and splenic veins. It begins at the level of the second lumbar vertebra, where it lies behind the neck of the pancreas. It has an average length of 6.50 cm and a diameter of 1.09 cm and rises behind the first part of the duodenum. After entering the right margin of the lesser omentum anterior to the epiploic foramen, it reaches the right end of the porta hepatis and divides into right and left hepatic branches that accompany the corresponding branches of the hepatic artery, lymphatic vessels, and hepatic nerve plexus. It is located behind the common bile duct and hepatic artery in the lesser omentum. The superior gastroduodenal vein empties into the portal vein from the lower right, into the left gastric vein from the lower right, and into the right gastric vein from the right. The cholecystic vein drains into the right hepatic branch of the portal vein. The left portal vein receives only the obliterated umbilical vein. The right branch supplies segments V, VI, VII, and VIII. Segments I, II, III, and IV receives the left obliterated umbilical vein via the ligamentum teres. It eventually opens into intraparenchymal venules that arise at right angles from a distributive vein and are less than 0.3 mm in diameter. Blood in the branches of the portal vein and hepatic artery flows into the sinusoidal microcirculation. Blood within the sinusoids flows through the radially arranged plates and empties into the central vein[3,6,7].
Some anatomic variations in the portal venous system lead to contraindications for certain surgeries such as transplantation or increase the risk of postoperative complications. Shunt operations for portal hypertension have increased interest in portal vein anatomy and studies have shown that the portal vein has fewer variations than the hepatic artery. Portal venous variants are frequent and account approximately 20%-35% of the population[8]: (1) The most common variant is the so-called ‘‘portal vein trifurcation’’ in which the main portal vein divides into three branches: The left portal vein, the right anterior portal vein, and the right posterior portal vein; (2) the second most common variant is a right posterior portal vein arising as the first branch of the portal vein; (3) in 10% of cases, no vein opens into the main trunk of the portal vein; (4) the left gastric vein joins the main trunk of the portal vein to the left side of the junction of the superior mesenteric vein and the splenic vein; (5) in 24% of cases, the left gastric vein joins the splenic vein; (6) the inferior mesenteric vein may drain from the junction of the splenic vein and the superior mesenteric vein into the portal vein; and (7) the inferior mesenteric vein may join with the superior mesenteric vein.
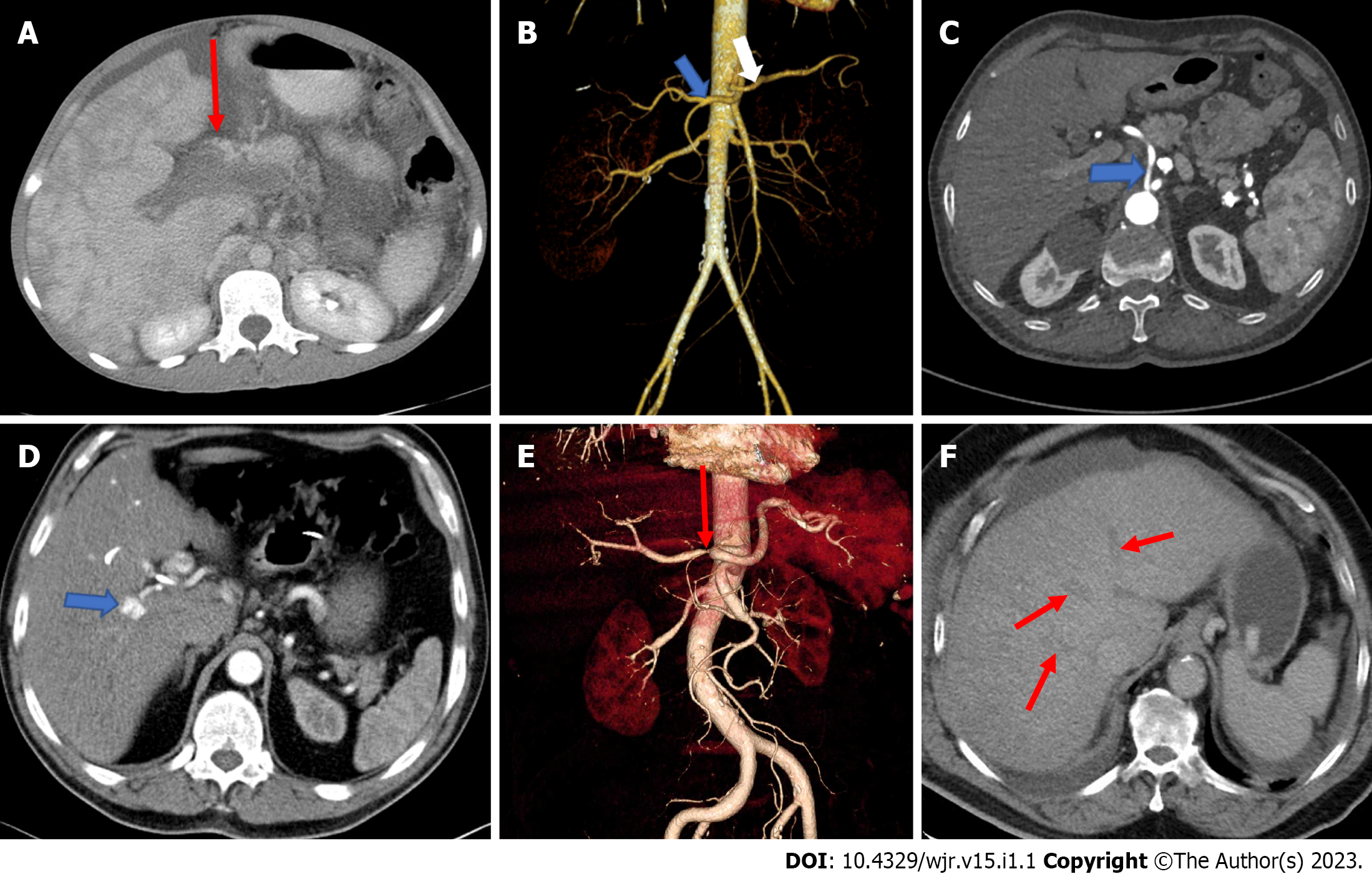
The anatomy of the portal vein is best visualised in the coronal plane of CT angiography. Acute portal vein thrombosis is sudden blood clot formation in the portal vein lumen[9]. It can occur in cirrhosis, pylephlebitis, hematologic disorders, pancreatitis, diverticulitis of the colon, appendicitis, malignancies, and surgical procedures[10]. CT is commonly used for diagnosis and usually shows the absence/ presence of enhancement within the thrombus, which is critical for characterising the thrombus (Figure 1A). Acute thrombosis is usually hyperdense on pre-contrast CT images, whereas chronic thrombosis can lead to calcifications in the portal vein and abnormal liver perfusion areas can be detected in the liver parenchyma, especially in the arterial phase[11,12]. A decrease in portal vein flow may be caused by portal vein or hepatic vein thrombosis, compression by focal masses such as abscesses, long-standing biliary obstruction, or parenchymal trauma. Pylephlebitis is defined as infectious thrombosis of either the portal vein or its intrahepatic branches or both and may lead to abscess formation in the parenchyma[13]. Diverticulitis of the colon is the most common cause of septic thrombophlebitis of the mesenteric and portal venous systems[14]. The standard treatment for acute portal vein thrombosis is anticoagulation. Portal vein aneurysms are rare and account for 3% of all venous aneuryms[15]. Most of these aneurysms are acquired, as a significant number of portal vein aneurysms are discovered in patients with underlying hepatocellular disease and portal hypertension.
The proper hepatic artery supplies the liver. It is a branch of the common hepatic artery separated from the coeliac trunk. The coeliac trunk is an anterior branch of the abdominal aorta and passes through the hiatus aorticus. It runs anteriorly and transversely over the pancreas and after a short course divides into the left gastric, splenic, and common hepatic branches. The common hepatic artery runs anteriorly and enters the lesser omentum from the right margin. Here it is located to the left of the common bile duct and anterior to the portal vein. On its way up, it branches into the gastroduodenal artery, the supraduodenal artery, and the right gastric artery. Simultaneously, it continues to ascend as the proper hepatic artery. When it reaches the porta hepatis of the liver, it branches into the right (RHA) and left (LHA) hepatic arteries. Normally, the RHA passes behind the common hepatic duct and branches into the cholecystic artery. In the portal triad, the branches of the hepatic artery run parallel to the portal vein. The arterioles distribute in the lobular parenchyma and they form a peribiliary plexus around the biliary capillaries.
The Michels’ classification of the hepatic arterial anatomy with the approximate frequency of occurrence of each type of variant in the general population is shown in Table 1[16].
| Type | Frequency (%) | Description |
| I | 55.0 | RHA, MHA, and LHA arise from common hepatic artery |
| II | 10.0 | RHA, MHA, and LHA arise from common hepatic artery; replaced LHA arises from left gastric artery |
| IV | 1.0 | Replaced RHA and LHA |
| V | 8.0 | RHA, MHA, and LHA arise from common hepatic artery; accessory LHA arises from left gastric artery |
| VI | 7.0 | RHA, MHA, and LHA arise from common hepatic artery; accessory RHA |
| VII | 1.0 | Accessory RHA and LHA |
| VIII | 4.0 | Replaced RHA and accessory LHA or replaced LHA and accessory RHA |
| IX | 4.5 | Entire hepatic trunk arises from superior mesenteric artery |
| X | 0.5 | Entire hepatic trunk arises from left gastric artery |
Classical branching of the hepatic artery is observed in around 76% of people[17]. Thus, a significant proportion of patients have a variant arterial anatomy (Figure 1B and C). Hepatic artery variations do not cause absolute contraindications for transplantation. However, being informed about these variations prior to the transplantation is a factor that would simplify the surgery, decrease the rate of contraindications, and improve the chance of technical success[16,17]: (1) The common hepatic artery, as a branch of the superior mesenteric artery, passes through or behind the pancreas and courses upward; (2) the common hepatic artery branches more proximal to the right and left hepatic artery before reaching the porta hepatis; (3) the right hepatic artery arises from the superior mesenteric artery, and the left hepatic artery arises from the coeliac trunk; (4) presence of accessory right hepatic artery originating from the superior mesenteric artery; (5) presence of accessory left hepatic artery originating from the left gastric artery; (6) presence of accessory left gastric artery coming out of the right gastric artery; and (7) the right hepatic artery crosses the common bile duct anteriorly.
These variations are also important in terms of collaterals that may occur after the ligation or occlusion of an artery. In transplantation, the mapping and perfect knowledge of hepatic vascularisation is essential for the surgeon.
The vascular anatomy of the liver influences surgical interventions to minimize surgical morbidity. It is possible to diagnose hepatic arterial disorders (thrombosis, aneurysm, and dissection and pseudoaneurysm formation) preoperatively and after liver transplantation by CTA. Coronal oblique slices are ideal for evaluating hepatic arterial anatomy. If the portal venous supply of the liver is intact, occlusion or stenosis of hepatic arterial flow does not generally result in parenchymal infarction (Figure 1E). CTA is the most commonly utilized modality to demonstrate localization and severity of the abrupt vessel. A wedge-shaped low-attenuation area in the liver periphery can be observed when hepatic infarctions are caused by hepatic artery flow disturbances. Central liver infarcts are characterized by a round-shaped lesion instead of wedge-shaped[18]. Vascular complications following liver transplantation include hepatic artery thrombosis occurring in 2%-5% of cases and stenosis occurring in 5%-11%, which may lead to irreversible infarctions and loss of the liver graft[19,20]. Hepatic artery dissection may occur spontaneously and/or following liver transplants. Hypertension and preexisting vascular disease prior to surgery may be related with spontaneous dissection[21]. Hepatic artery aneurysms represent 20% of all visceral aneurysms. They usually have an extrahepatic location at the level of common hepatic or right hepatic arteries and common etiologies are trauma, tumors, infection, and iatrogenic reasons[22]. CT is an excellent diagnostic tool in which aneurysm appears as a fusiform dilatation of the vessel lumen, as well-defined focal enhancing lesions that may simulate hypervascular tumours[18]. Hepatic artery pseudoaneurysms (Figure 1D) differ from true aneurysms with the appearance of more irregular peripheral margins[22]. Hepatic arterio-portal shunts (HAPS) occur due to fistulization of blood flow from the hepatic artery to the portal vein. HAPS are frequently asymptomatic. However, systemic complications may arise including the development of portal hypertension, as well as accelerated metastasis in patients with malignant tumors. HAPS is divided into three types according to the anatomic location of the shunt within the liver: (1) Central HAPS: When the shunt is located in the porta hepatis, such central HAPS is manifested as earlier enhancement and arterial-phase opacification of the central portal veins during CT scanning; (2) Peripheral HAPS: When the shunt is located in peripheral liver parenchyma. Given the smaller hepatic vascular size in this region, early contrast into the portal veins generally does not occur. Shunts in this region of the liver usually manifest as transient hepatic enhancement differences; and (3) mixed HAPS: Alternatively, HAPS may demonstrate both central and peripheral findings[22]. Abnormal vessels that arise from the hepatic arteries promptly opacify the liver, thereby giving rise to the characteristic blush of an arteriovenous shunt.
The liver is the largest lymph-producing organ, accounting for 25%-50% of lymph passing through the thoracic duct[23]. The hepatic lymphatic system transport lymph through lymphatic vessels to draining lymph nodes to remove waste products and immune cells. Sinusoids are covered by fenestrated liver sinusoidal endothelial cells (LSECs). Hepatic lymphatic fluid originates mainly from plasma components filtered through the fenestrae of LSECs and flows into the space of Disse. In this tight space between the hepatocytes and the sinusoid, fluid and solid materials pass through. The space of Disse cannot be observed by biopsy and is mainly occupied by reticular arcuate fibers. Especially during passive hepatic congestion, anoxia, or in some toxic conditions resulting from hepatic edema, we observe a dilatation of this perisinusoidal space filled with protein-rich fluid. These spaces have contact with the periportal spaces (space of Mall) between the stroma of the portal tract and the outermost hepatocytes. The lymphatic fluid inside the perisinusoidal and periportal spaces drains into the lymphatics in the portal triad. A rich lymphatic network is also shown around the bile ducts, reaching under the epithelium. There may also be very thin lymphatic vessels accompanying arterioles in the parenchyma. In addition, under the Glisson capsule, there is a subperitoneal lymph drainage communicating with both the lymphatics in the gallbladder bed and the parenchymal lymph vessels. The lymphatic vessels in the portal triad take different routes: Most of them first travel to the hepatic lymph nodes and drain into the coeliac lymph nodes and the lymph nodes around the cisterna chyli; some reach the thoracic duct directly from the porta hepatis; some drain into the lymph nodes around the inferior vena cava. The subcapsular lymphatics drain to the lymph nodes around the inferior vena cava and the diaphragmatic hiatus and then reach the thoracic duct directly. Lymphatics coming from the left side of the posterior surface of the liver drain into the paracardial group of the left gastric lymph nodes, and those coming from the right side directly into the coeliac lymph nodes. Although the lymph from the gallbladder and extrahepatic bile ducts mostly drains into the hepatic lymph nodes, a few vessels from the common hepatic duct go to the pyloric lymph nodes. Normally, hepatic lymph vessels do not anastomose with duodenal and pancreatic lymph vessels. A significant increase in the number of lymphatic vessels (lymphangiogenesis) has been reported in various pathological conditions of the liver, including cirrhosis, viral hepatitis, lymphedema, cholestasis, portal hypertension, biliary cholangitis, and hepatocellular carcinoma[23-29].
Most of the venous blood of the liver is drained by the three main hepatic veins (RHV, LHV, and intermediate hepatic vein), which open into the suprahepatic part of the superior vena cava. Numerous small hepatic veins open into the intrahepatic portion (at the sulcus for the inferior vena cava) of the inferior vena cava. The RHV drains liver segments V-VII, the middle hepatic vein (MHV) drains segments IV, V, VIII, and the LHV drains segments II and III[3]. Impairment of the hepatic venous outflow can be catastrophic. Patients may experience rapid onset jaundice and severe ascites. The increased intrahepatic pressure, caused by the inability of the massive blood flow into the liver, causes severe centrilobular congestion and subsequent parenchymal necrosis. This clinical condition of hepatic venous outflow obstruction is called the Budd-Chiari syndrome (BCS) (Figure 1F)[18]. This definition excludes the outflow impairment that results from heart failure or pericardial diseases. The definition also excludes sinusoidal obstruction syndrome. The compromised hepatic venous flow results in dramatically increased sinusoidal pressure that reverses hepatic drainage into the portal venous system. In approximately 75% of the cases, a hematologic abnormality or an underlying thrombotic diathesis can be identified[30,31]. CT clearly shows the obstructed hepatic veins and associating parenchymal changes[32]. The absence of contrast enhancement in the hepatic vein is the pathognomonic imaging findings on CT[12]. In the acute phase, the liver becomes enlarged with smooth contours and appears heterogeneously edematous with parenchymal perfusion abnormalities with the decreased flow in the liver periphery and enhanced flow in the central liver parenchyma in the hepatic arterial phase. Patchy and decreased contrast enhancement in the liver periphery, in the arterial phase, results from edema and congestion in the peripheral regions due to flow stasis in portal vein branches and the liver sinusoids[12,19,33]. This finding is related to elevated post-sinusoidal pressure[33]. A heterogeneous and mottled mosaic pattern of parenchymal enhancement can also be encountered in congestive hepatopathy. Congestion is due to passive stasis of blood within the liver parenchyma secondary to impaired hepatic venous outflow due to decompensated cardiac disease[18,31]. Visualization of patency of hepatic veins and inferior vena cava differentiates congestive hepatopathy from BCS. A mosaic pattern of enhancement with linear and curvilinear areas of poor enhancement is seen in the portal venous phase images, described as nutmeg liver. A “flip-flop” pattern may occur in the portal venous phase as the central part of the liver demonstrates low attenuation due to contrast wash-out and gradual increase of the peripheral attenuation. The caudate lobe may demonstrate better venous enhancement due to its unique venous drainage pattern[32]. Associated features of cardiac dysfunction aid diagnosis[18].
The vascular variants that are of surgical importance include variants of the hepatic vein also: (1) An accessory RHV occurs in 52.5% of patients; (2) two accessory hepatic veins in 12%; (3) an accessory vein draining the caudate lobe in 12%; (4) an accessory inferior RHV; and (5) early branching of the vein that drains the right superoanterior segment (segment VIII) into the MHV.
Significant hepatic venous variants affecting both donors and recipients are seen in approximately 30% of patients, thus presenting a problem for the surgeon, and may result in a modification of the hepatectomy plane[34].
CTA may slightly be favourable in hepatic vascular disorders due to being time saving in critically ill patients with distended abdomen and pain. For a complete mapping of the hepatic vasculature, CT scan is preferable to other imaging modalities. Correct diagnosis and proper intervention are crucial for appropriate clinical management. CTA is an effective, high-resolution, noninvasive imaging technique that demonstrates the presence of vascular pathology, with a direct impact on treatment decisions including patient selection for surgical management. Also, the objective of vascular imaging in patients with liver neoplasms is to provide a vascular map for understanding the relationship of the tumor to adjacent vessels, which is needed for tumor resection that includes a peripheral tumor-free margin. Multiplanar reformation and 3D reconstruction are very helpful in demonstrating the relationship of liver tumors to the hepatic veins and inferior vena cava. Awareness of the imaging findings of hepatic vascularization is crucial for the correct diagnosis and optimization of the treatment to avoid complications.
| 1. | Colagrande S, Centi N, La Villa G, Villari N. Transient hepatic attenuation differences. AJR Am J Roentgenol. 2004;183:459-464. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 2. | Price M, Patino M, Sahani D. Computed Tomography Angiography of the Hepatic, Pancreatic, and Splenic Circulation. Radiol Clin North Am. 2016;54:55-70. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 3. | Standring S. Gray’s Anatomy The Anatomical Basis of Clinical Practice. 42nd ed. London: Churchill Livingstone Elsevier, 2021: 327-329, 1205-1217. |
| 4. | Gissen P, Arias IM. Structural and functional hepatocyte polarity and liver disease. J Hepatol. 2015;63:1023-1037. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 206] [Cited by in RCA: 227] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 5. | Sun P, Zhang G, Su X, Jin C, Yu B, Yu X, Lv Z, Ma H, Zhang M, Wei W, Li W. Maintenance of Primary Hepatocyte Functions In Vitro by Inhibiting Mechanical Tension-Induced YAP Activation. Cell Rep. 2019;29:3212-3222.e4. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 50] [Article Influence: 8.3] [Reference Citation Analysis (0)] |
| 6. | Erwin K, Hans-Dieter K. Hepatology: Textbook and Atlas. 3rd ed. Heidelberg: Springer Medizin Verlag, 2008: 26-28. |
| 7. | William KO, Patrick CN. Netter's Essential Histology. 2nd ed. Philadelphia: Elsevier Sauders, 2013: 314-316. |
| 8. | Schmidt S, Demartines N, Soler L, Schnyder P, Denys A. Portal vein normal anatomy and variants: implication for liver surgery and portal vein embolization. Semin Intervent Radiol. 2008;25:86-91. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 31] [Cited by in RCA: 29] [Article Influence: 1.9] [Reference Citation Analysis (0)] |
| 9. | DeLeve LD, Valla DC, Garcia-Tsao G; American Association for the Study Liver Diseases. Vascular disorders of the liver. Hepatology. 2009;49:1729-1764. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 739] [Cited by in RCA: 670] [Article Influence: 39.4] [Reference Citation Analysis (0)] |
| 10. | Chawla Y, Duseja A, Dhiman RK. Review article: the modern management of portal vein thrombosis. Aliment Pharmacol Ther. 2009;30:881-894. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 101] [Cited by in RCA: 103] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 11. | Onur MR, Karaosmanoglu AD, Akca O, Ocal O, Akpinar E, Karcaaltincaba M. Imaging features of non-traumatic vascular liver emergencies. Jpn J Radiol. 2017;35:215-224. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1] [Reference Citation Analysis (0)] |
| 12. | Gallego C, Velasco M, Marcuello P, Tejedor D, De Campo L, Friera A. Congenital and acquired anomalies of the portal venous system. Radiographics. 2002;22:141-159. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 178] [Article Influence: 7.4] [Reference Citation Analysis (0)] |
| 13. | Bazan F, Busto M. IMAGES IN CLINICAL MEDICINE. Pylephlebitis as a Complication of Diverticulitis. N Engl J Med. 2015;373:2270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 14. | Kanellopoulou T, Alexopoulou A, Theodossiades G, Koskinas J, Archimandritis AJ. Pylephlebitis: an overview of non-cirrhotic cases and factors related to outcome. Scand J Infect Dis. 2010;42:804-811. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 108] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 15. | Turner KC, Bohannon WT, Atkins MD. Portal vein aneurysm: a rare occurrence. J Vasc Nurs. 2011;29:135-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 9] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 16. | Michels NA. Blood supply and anatomy of the upper abdominal organs with a descriptive atlas. Philadelphia: Lippincott, 1955: 64-69. |
| 17. | Favelier S, Germain T, Genson PY, Cercueil JP, Denys A, Krausé D, Guiu B. Anatomy of liver arteries for interventional radiology. Diagn Interv Imaging. 2015;96:537-546. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 32] [Cited by in RCA: 39] [Article Influence: 3.3] [Reference Citation Analysis (0)] |
| 18. | Virmani V, Ramanathan S, Virmani VS, Kielar A, Sheikh A, Ryan J. Non-neoplastic hepatic vascular diseases: spectrum of CT and MRI appearances. Clin Radiol. 2014;69:538-548. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 19. | Khalaf H. Vascular complications after deceased and living donor liver transplantation: a single-center experience. Transplant Proc. 2010;42:865-870. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 87] [Article Influence: 5.4] [Reference Citation Analysis (1)] |
| 20. | Hwang JY, Kim KW, Lee SJ, Kim SY, Lee JS, Kim HJ, Lee J, Song GW, Lee SG. The computed tomographic angiography finding of hepatic artery dissection after living donor liver transplantation; what is the clinical significance? Clin Imaging. 2016;40:130-136. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 0.9] [Reference Citation Analysis (0)] |
| 21. | Müller MF, Kim D. Spontaneous dissection of the hepatic artery. Abdom Imaging. 1995;20:462-465. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 24] [Cited by in RCA: 21] [Article Influence: 0.7] [Reference Citation Analysis (0)] |
| 22. | O'Driscoll D, Olliff SP, Olliff JF. Hepatic artery aneurysm. Br J Radiol. 1999;72:1018-1025. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 98] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 23. | Tanaka M, Iwakiri Y. The Hepatic Lymphatic Vascular System: Structure, Function, Markers, and Lymphangiogenesis. Cell Mol Gastroenterol Hepatol. 2016;2:733-749. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 61] [Cited by in RCA: 114] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 24. | Wang Q, Koniaris LG, Milgrom DP, Patel A, Hu M, Cui E, Deng Y, Akisik F. CT and MRI imaging and interpretation of hepatic arterioportal shunts. Transl Gastroenterol Hepatol. 2019;4:34. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 16] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 25. | Chung C, Iwakiri Y. The lymphatic vascular system in liver diseases: its role in ascites formation. Clin Mol Hepatol. 2013;19:99-104. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 72] [Article Influence: 5.5] [Reference Citation Analysis (1)] |
| 26. | Thelen A, Jonas S, Benckert C, Weichert W, Schott E, Bötcher C, Dietz E, Wiedenmann B, Neuhaus P, Scholz A. Tumor-associated lymphangiogenesis correlates with prognosis after resection of human hepatocellular carcinoma. Ann Surg Oncol. 2009;16:1222-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 27] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 27. | Oikawa H, Masuda T, Sato S, Yashima A, Suzuki K, Satodate R. Changes in lymph vessels and portal veins in the portal tract of patients with idiopathic portal hypertension: a morphometric study. Hepatology. 1998;27:1607-1610. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 33] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 28. | Yamauchi Y, Michitaka K, Onji M. Morphometric analysis of lymphatic and blood vessels in human chronic viral liver diseases. Am J Pathol. 1998;153:1131-1137. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 43] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 29. | Soyer P, Heath D, Bluemke DA, Choti MA, Kuhlman JE, Reichle R, Fishman EK. Three-dimensional helical CT of intrahepatic venous structures: comparison of three rendering techniques. J Comput Assist Tomogr. 1996;20:122-127. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 60] [Cited by in RCA: 49] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 30. | Cura M, Haskal Z, Lopera J. Diagnostic and interventional radiology for Budd-Chiari syndrome. Radiographics. 2009;29:669-681. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 90] [Cited by in RCA: 66] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 31. | Valla DC, Cazals-Hatem D. Vascular liver diseases on the clinical side: definitions and diagnosis, new concepts. Virchows Arch. 2018;473:3-13. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 18] [Cited by in RCA: 30] [Article Influence: 3.8] [Reference Citation Analysis (0)] |
| 32. | Karaosmanoglu AD, Uysal A, Akata D, Ozmen MN, Karcaaltincaba M. Role of imaging in visceral vascular emergencies. Insights Imaging. 2020;11:112. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 11] [Article Influence: 1.8] [Reference Citation Analysis (0)] |
| 33. | Torabi M, Hosseinzadeh K, Federle MP. CT of nonneoplastic hepatic vascular and perfusion disorders. Radiographics. 2008;28:1967-1982. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 79] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 34. | Sahani D, Mehta A, Blake M, Prasad S, Harris G, Saini S. Preoperative hepatic vascular evaluation with CT and MR angiography: implications for surgery. Radiographics. 2004;24:1367-1380. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 140] [Cited by in RCA: 129] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Anatomy and morphology
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Rasti S, Iran; Wu SZ, China S-Editor: Chen YL L-Editor: Wang TQ P-Editor: Chen YL