Published online Jun 28, 2022. doi: 10.4329/wjr.v14.i6.151
Peer-review started: January 31, 2022
First decision: April 8, 2022
Revised: April 20, 2022
Accepted: June 13, 2022
Article in press: June 13, 2022
Published online: June 28, 2022
Processing time: 147 Days and 15.9 Hours
The use of artificial intelligence plays a crucial role in developing precision medicine in nuclear medicine. Artificial intelligence refers to a field of computer science aimed at imitating the performance of tasks typically requiring human intelligence. From machine learning to generative adversarial networks, artificial intelligence automized the workflow of medical imaging. In this mini-review, we encapsulate artificial intelligence models and their use in nuclear medicine imaging workflow.
Core Tip: Artificial intelligence is a distinguished tool for creating tailor-made medicine. Artificial intelligence (AI) consists of machine learning, deep learning, artificial neural networks, convolutional neural networks, and generative adversarial networks. These AI applications affect all phases of a routine medical imaging workflow in nuclear medicine: planning, image acquisition, and interpretation. The integration of AI into clinical workflow and protocols of medical imaging will provide the opportunity to decrease the error rate of physicians and eventually lead to improved patient management.
- Citation: Tamam MO, Tamam MC. Artificial intelligence technologies in nuclear medicine. World J Radiol 2022; 14(6): 151-154
- URL: https://www.wjgnet.com/1949-8470/full/v14/i6/151.htm
- DOI: https://dx.doi.org/10.4329/wjr.v14.i6.151
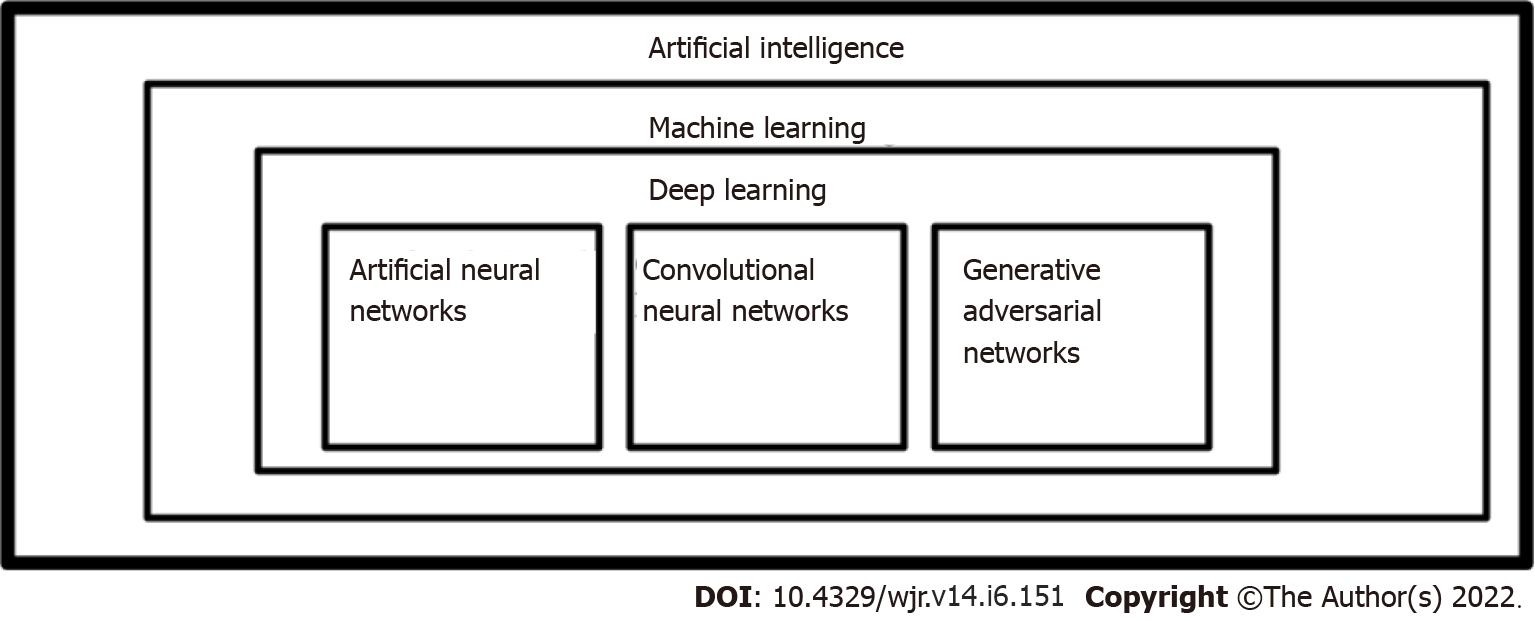
Personalized medicine (precision medicine) is a developing medical practice that develops tailor-made approaches for individual patients, leading to increased reliability and a significant impact on preventative, diagnostic, and therapeutic pathways[1]. Artificial intelligence (AI) integration plays a significant role in achieving precision medicine in nuclear medicine[2]. It refers to a field of computer science aimed at imitating the performance of tasks typically requiring human intelligence[3]. Advancements in AI have allowed for precision medicine models to be developed for individual patients (Figure 1, Table 1). The advancements in AI have been in the order of machine learning (ML), deep learning (DL), artificial neural networks (ANNs), convolutional neural networks (CNNs), and generative adversarial networks (GANs)[4,5].
| Machine learning (ML) |
| Deep learning (DL) |
| Artificial neural networks (ANNs) |
| Convolutional neural networks (CNNs) |
| Generative adversarial networks(GANs) |
Machine learning is not a singular algorithm, but a subset of AI. It processes a set of training data and constructs a model that carries the associations among the variables that are relevant to a particular outcome. It usually needs handcrafted features, requiring more human intervention, for data extraction and filtration[2]. There are many ML methods, some of which are supervised learning, unsupervised learning, semi-supervised learning, and reinforcement machine learning[5,6]. DL is a subset of ML, automating many parts of input extraction, enabling less human intervention. In contrast, ML requires more human intervention for data extraction and filtration[2,5,6].
Artificial Neural Networks are a subfield of DL. ANNs are connected nodes with weighted paths. Each node has parent nodes that influence it, an activation function, firing threshold, and an output value. ANNs are analogous to neurons and their intercommunication[4,5].
Convolutional Neural Networks are made up of convoluting series of pooling layers. CNNs apply a neural-network layer to a part of an image and systematically traverse over the image. CNNs downsample and summarize features by alternating convolutional layers with pooling layers. Their computational requirements are much lower because they operate on a small subset of an image[4,5].
Generative Adversarial Networks are made up of two networks, a generator, and a discriminator, that are in a zero-sum game. Generators generate fake input data to minimize the difference between counterfeits and real inputs. The discriminator classifies the real and counterfeit inputs, attempting to maximize efficiency. Over time, the generator will be good at generating input data and the discriminator will be good at classification[5].
AI advancements in the last decade have improved AI’s application in medical imaging. The myriad of applications of AI in nuclear medicine includes all steps of a typical medical imaging workflow: planning, image acquisition, and interpretation. In the future, even patient admission and payment could be included[7-9].
For medical imaging planning, AI will automatically check for specific contraindications, such as allergies and drug interference, or eliminate needless repetition of exams by evaluating past examinations before any examination is done on a patient[10,11].
In nuclear medicine, attenuation maps and scatter correction remain relevant topics for image scanning, thus AI research focuses on these topics intensively. Hwang et al[10] generated attenuation maps for whole-body positron emission tomography/magnetic resonance imaging (PET/MRI) using a modified U-Net, a specialized convolutional network architecture for biomedical image segmentation. They compared the CT-derived attenuation map to the Dixon-based 4-segment technique[10,11].
Another hot topic for research is the enhancement of image quality; Hong et al[12] improved the picture resolution and noise properties of PET scanners using large pixelated crystals with a deep residual convolutional neural network[12,13]. Kim et al[14] demonstrated that Iterative PET recon
For the interpretation of images, studies on an AI-based triage system for identifying artifacts have been published recently[15]. In the near future, similar systems will be able to detect directly using raw data, such as sinograms, and issue alarms throughout the scanning process, even before reconstruction, so that technicians can adjust or prolong the scheduled scan procedure to accommodate an unexpected discovery[16]. Automated identification of pathologies provides additional intriguing potential in identifying overlooked results and secondary discoveries, saving time and effort[17].
Despite the improvements that the field of AI brings to nuclear medicine, there are drawbacks. Ethical considerations, data protection, legal regulations, privacy, and education are among these problems. According to Hagendorf, the ethical concerns of AI in healthcare can be summarized in the “fairness, accountability, and transparency paradigm of AI ethics”[18,19]. Moreover, AI requires considerable sensitive data in healthcare, thus standards for data protection and privacy raise issues that must be dealt with. Furthermore, for AI to generalize large numbers, large amounts of data with variability are needed. This raises more questions about consent, data anonymization, and de-identification[19]. There are promising techniques being developed on top of DL algorithms such as federative learning that might mitigate some of these issues[20]. Additionally, traditional regulatory pathways are lagging behind the recent advancements, creating difficulties regarding regulations and laws. Lastly, insufficient education about AI both from patients, physicians, and academia causes mistrust of AI applications in healthcare. Physicians and academia need familiarity with AI and the rudimentary knowledge necessary to provide patients with the necessary information[19].
The integration of AI into clinical practice will transform the medical profession and nuclear medicine imaging in particular. New abilities, such as clinical data science, computer science, and ML will be considered a necessity when AI is applied to medical imaging workflow and protocols. This could provide the opportunity to decrease the error rate of physicians and eventually lead to improved patient management.
| 1. | National Human Genome Research Institute. Personalized Medicine. Available from: https://www.genome.gov/genetics-glossary/Personalized-Medicine. |
| 2. | Chartrand G, Cheng PM, Vorontsov E, Drozdzal M, Turcotte S, Pal CJ, Kadoury S, Tang A. Deep Learning: A Primer for Radiologists. Radiographics. 2017;37:2113-2131. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 511] [Cited by in RCA: 718] [Article Influence: 89.8] [Reference Citation Analysis (0)] |
| 3. | Currie G, Rohren E. Intelligent Imaging in Nuclear Medicine: the Principles of Artificial Intelligence, Machine Learning and Deep Learning. Semin Nucl Med. 2021;51:102-111. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 17] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 4. | Herskovits EH. Artificial intelligence in molecular imaging. Ann Transl Med. 2021;9:824. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5] [Cited by in RCA: 10] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Castiglioni I, Rundo L, Codari M, Di Leo G, Salvatore C, Interlenghi M, Gallivanone F, Cozzi A, D'Amico NC, Sardanelli F. AI applications to medical images: From machine learning to deep learning. Phys Med. 2021;83:9-24. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 74] [Cited by in RCA: 254] [Article Influence: 50.8] [Reference Citation Analysis (0)] |
| 6. | Machine Learning. Available from: https://www.ibm.com/cloud/Learn/machine-learning. |
| 7. | Visvikis D, Cheze Le Rest C, Jaouen V, Hatt M. Artificial intelligence, machine (deep) learning and radio(geno)mics: definitions and nuclear medicine imaging applications. Eur J Nucl Med Mol Imaging. 2019;46:2630-2637. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 95] [Article Influence: 13.6] [Reference Citation Analysis (0)] |
| 8. | Currie GM. Intelligent Imaging: Artificial Intelligence Augmented Nuclear Medicine. J Nucl Med Technol. 2019;47:217-222. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 35] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 9. | Currie G, Hawk KE, Rohren E, Vial A, Klein R. Machine Learning and Deep Learning in Medical Imaging: Intelligent Imaging. J Med Imaging Radiat Sci. 2019;50:477-487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 235] [Article Influence: 33.6] [Reference Citation Analysis (0)] |
| 10. | Hwang D, Kang SK, Kim KY, Seo S, Paeng JC, Lee DS, Lee JS. Generation of PET Attenuation Map for Whole-Body Time-of-Flight 18F-FDG PET/MRI Using a Deep Neural Network Trained with Simultaneously Reconstructed Activity and Attenuation Maps. J Nucl Med. 2019;60:1183-1189. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 75] [Cited by in RCA: 98] [Article Influence: 14.0] [Reference Citation Analysis (0)] |
| 11. | Ronneberger O, Fischer P, Brox T. U-Net: convolutional networks for biomedical image segmentation. In: Navab N, Hornegger J, Wells WM, Frangi AF, eds. mMedical Image Computing and Computer-Assisted Intervention–MICCAI 2015.Cha, Switzerland: Springer International Publishing; 2015:234-241. |
| 12. | Hong X, Zan Y, Weng F, Tao W, Peng Q, Huang Q. Enhancing the Image Quality via Transferred Deep Residual Learning of Coarse PET Sinograms. IEEE Trans Med Imaging. 2018;37:2322-2332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 40] [Cited by in RCA: 38] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 13. | Orlhac F, Boughdad S, Philippe C, Stalla-Bourdillon H, Nioche C, Champion L, Soussan M, Frouin F, Frouin V, Buvat I. A Postreconstruction Harmonization Method for Multicenter Radiomic Studies in PET. J Nucl Med. 2018;59:1321-1328. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 169] [Cited by in RCA: 272] [Article Influence: 34.0] [Reference Citation Analysis (0)] |
| 14. | Kim K, Wu D, Gong K, Dutta J, Kim JH, Son YD, Kim HK, El Fakhri G, Li Q. Penalized PET Reconstruction Using Deep Learning Prior and Local Linear Fitting. IEEE Trans Med Imaging. 2018;37:1478-1487. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 119] [Cited by in RCA: 115] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 15. | Li W, Liu H, Cheng F, Li Y, Li S, Yan J. Artificial intelligence applications for oncological positron emission tomography imaging. Eur J Radiol. 2021;134:109448. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 16. | Noortman WA, Vriens D, Mooij CDY, Slump CH, Aarntzen EH, van Berkel A, Timmers HJLM, Bussink J, Meijer TWH, de Geus-Oei LF, van Velden FHP. The Influence of the Exclusion of Central Necrosis on [18F]FDG PET Radiomic Analysis. Diagnostics (Basel). 2021;11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 11] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 17. | Nensa F, Demircioglu A, Rischpler C. Artificial Intelligence in Nuclear Medicine. J Nucl Med. 2019;60:29S-37S. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 47] [Cited by in RCA: 112] [Article Influence: 16.0] [Reference Citation Analysis (0)] |
| 18. | Hagendorff T. The Ethics of AI Ethics: An Evaluation of Guidelines. Minds & Machines. 2020;30: 99-120. [RCA] [DOI] [Full Text] [Cited by in Crossref: 294] [Cited by in RCA: 219] [Article Influence: 36.5] [Reference Citation Analysis (0)] |
| 19. | Aktolun C. Artificial intelligence and radiomics in nuclear medicine: potentials and challenges. Eur J Nucl Med Mol Imaging. 2019;46:2731-2736. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (1)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: Turkey
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Liu J, China; Morilla I, France; Tanabe S, Japan A-Editor: Yao QG, China S-Editor: Liu JH L-Editor: A P-Editor: Liu JH