Published online Jan 28, 2022. doi: 10.4329/wjr.v14.i1.13
Peer-review started: March 30, 2021
First decision: October 17, 2021
Revised: November 16, 2021
Accepted: December 28, 2021
Article in press: December 28, 2021
Published online: January 28, 2022
Processing time: 297 Days and 10.8 Hours
The pandemic of novel coronavirus disease 2019 (COVID-19) is caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Diabetes mellitus is a risk factor for developing severe illness and a leading cause of death in patients with COVID-19. Diabetes can precipitate hyperglycaemic emergencies and cause prolonged hospital admissions. Insulin resistance is thought to cause endothelial dysfunction, alveolar capillary micro-angiopathy and interstitial lung fibrosis through pro-inflammatory pathways. Autopsy studies have also demonstrated the presence of microvascular thrombi in affected sections of lung, which may be associated with diabetes. Chest imaging using x-ray (CXR) and computed tomography (CT) of chest is used to diagnose, assess disease progression and severity in COVID-19. This article reviews current literature regarding chest imaging findings in patients with diabetes affected by COVID-19. A literature search was performed on PubMed. Patients with diabetes infected with SARS-CoV-2 are likely to have more severe infective changes on CXR and CT chest imaging. Severity of airspace consolidation on CXR is associated with higher mortality, particularly in the presence of co-morbidities such as ischaemic heart disease. Poorly controlled diabetes is associated with more severe acute lung injury on CT. However, no association has been identified between poorly-controlled diabetes and the incidence of pulmonary thromboembolism in patients with COVID-19.
Core Tip: COVID-19 infection can present as multifocal peripheral airspace changes on chest imaging using x-ray (CXR). Ground-glass opacities are the most common computed tomography finding in coronavirus disease 2019 (COVID-19). Post admission daily bloody glucose readings are a strong predictor for COVID-19 CXR changes that indicate poorer outcomes. Poorly controlled diabetes is associated with increased volumes of ground-glass opacity and consolidation. Diabetes is also linked with endothelial dysfunction and hypercoagulability, which may result in the formation of microvascular thrombi in peripheral segments of lung.
- Citation: Gangadharan S, Parker S, Ahmed FW. Chest radiological finding of COVID-19 in patients with and without diabetes mellitus: Differences in imaging finding. World J Radiol 2022; 14(1): 13-18
- URL: https://www.wjgnet.com/1949-8470/full/v14/i1/13.htm
- DOI: https://dx.doi.org/10.4329/wjr.v14.i1.13
The world is currently undergoing a significant healthcare crisis due to the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) pandemic. In March 2020, World Health Organisation declared a pandemic caused by SARs-CoV-2. SARS-CoV-2 was named novel coronavirus disease 2019 (COVID-19). Hospitals in different countries have been overwhelmed with patients suffering from COVID-19. So far, 2.78 million people have died as of 29th March 2021[1].
Diabetes mellitus (DM) is a risk factor associated with severe illness in SARS-CoV-2 infection, precipitating hyperglycaemic emergencies such as diabetic ketoacidosis (DKA) and hyperosmolar hyperglycaemic state (HHS)[2]. A third of deaths in England up to May 2020 related to COVID-19 occurred in people with DM[3]. Patients with DM are more likely to stay longer in hospital[4]. DM can cause a deregulated immune system predisposing to infection; the endothelial angiotensin-converting enzyme 2 (ACE2) receptor responsible for SARS-CoV-2 invasion in human cells has reduced expression in patients of DM, possibly due to glycosylation[5]. Insulin resistance and altered glucose homeostasis have been thought to cause alveolar capillary micro-angiopathy and interstitial fibrosis via over-inflammation[6].
A normal chest radiograph does not exclude COVID-19 pneumonia, and no single feature on a radiograph is diagnostic[7]. However, a combination of multifocal peripheral airspace changes often found bilaterally may be present in COVID-19. Due to limited PCR testing capacity in the early d of the pandemic, in addition to its low sensitivity and waiting period of up to 2 d, many clinicians turned to chest computed tomography (CT) for early detection of COVID-19.
Studies have reported the negative predictive value of using CT to be above 90%[8,9]. Chest CT was used to detect subtle radiological changes consistent with COVID-19 in patients where the chest radiograph was reported to be normal or indeterminate. Typical CT findings seen in patients with COVID-19 include peripheral ground-glass opacities (GGO), which progresses to consolidation and interstitial thickening within GGO areas known as ‘crazy paving pattern’[10,11]. These non-specific imaging findings of acute lung injury are indistinguishable from other types of viral pneu
This article reviews current literature regarding chest imaging changes in patients with DM affected by COVID-19.
A literature search was conducted on PubMed using the keywords of COVID-19 or Coronavirus; CXR or x-ray or radiograph; CT chest; CTPA or pulmonary embolism or PE; and diabetes mellitus or diabetes within the title or abstract.
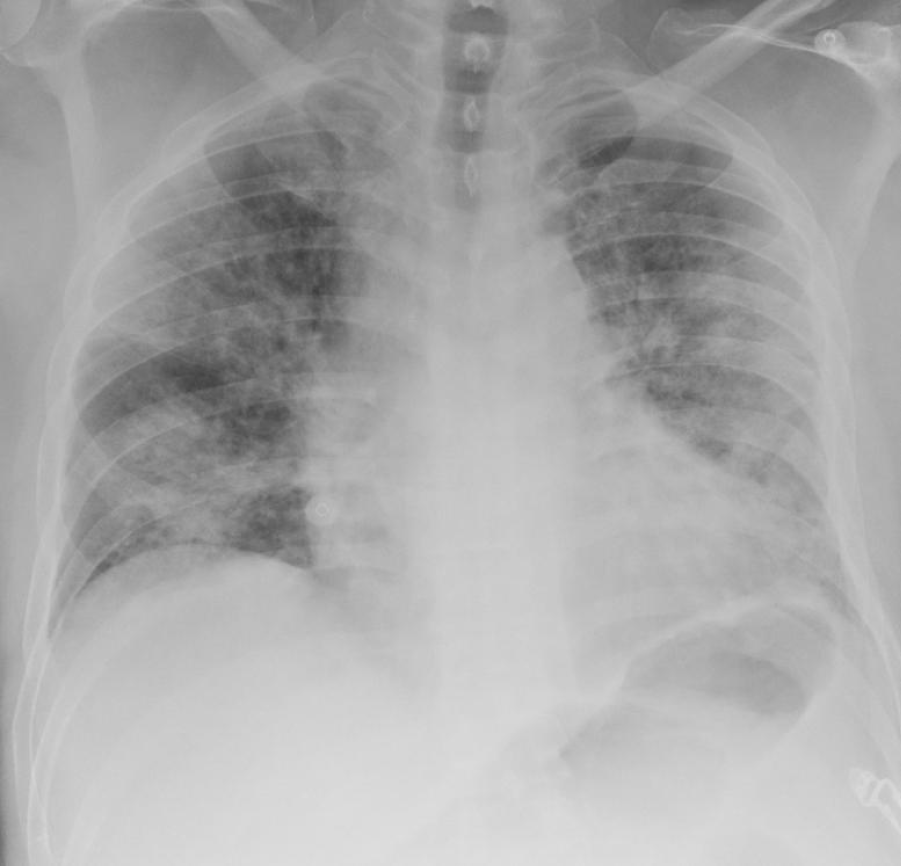
Studies have shown chest radiographs of patients with DM to have increased bilateral airspace consolidation compared to patients without DM[12,13]. The severity of chest radiograph changes in patients with DM has indicated a significant correlation with mortality, as evidenced in multivariate analysis by Cellina et al[14]. Patients with bilateral peripheral alveolar disease (Figure 1) often present at a later stage and have a worse outcome. However, some patients with COVID-19 have preserved lung comp
In some studies, DM alone was not associated with an increased risk of intensive care unit admission or death. Still, it was associated with cardiovascular disease as a driver of poorer outcomes. Izzi-Engbeaya et al[16] studied 889 patients admitted to London hospitals with COVID-19, and their outcomes found patients with DM were found to have a 33% increased risk of death or ICU admission if they also have ischaemic heart disease. Surprisingly, a similar severity of CXR changes was demonstrated for patients with and without DM. Mozzini et al[17] (2021) studied 50 Italian patients with COVID-19, 32% of which had DM. Patients with hypertension or DM had 8 times greater risk of having more severe CXR changes.
COVID-19 infection in patients with DM leads to hyperglycaemia, and in some cases leads to DKA and/or HHS[2]. It has been shown that there is a positive correlation between daily average blood glucose readings and CXR findings. Similarly, post-admission day-1 hyperglycaemia was found to be the strongest independent predictor for COVID-19 CXR changes. This was a stronger predictor than age, body mass index, and temperature[18].
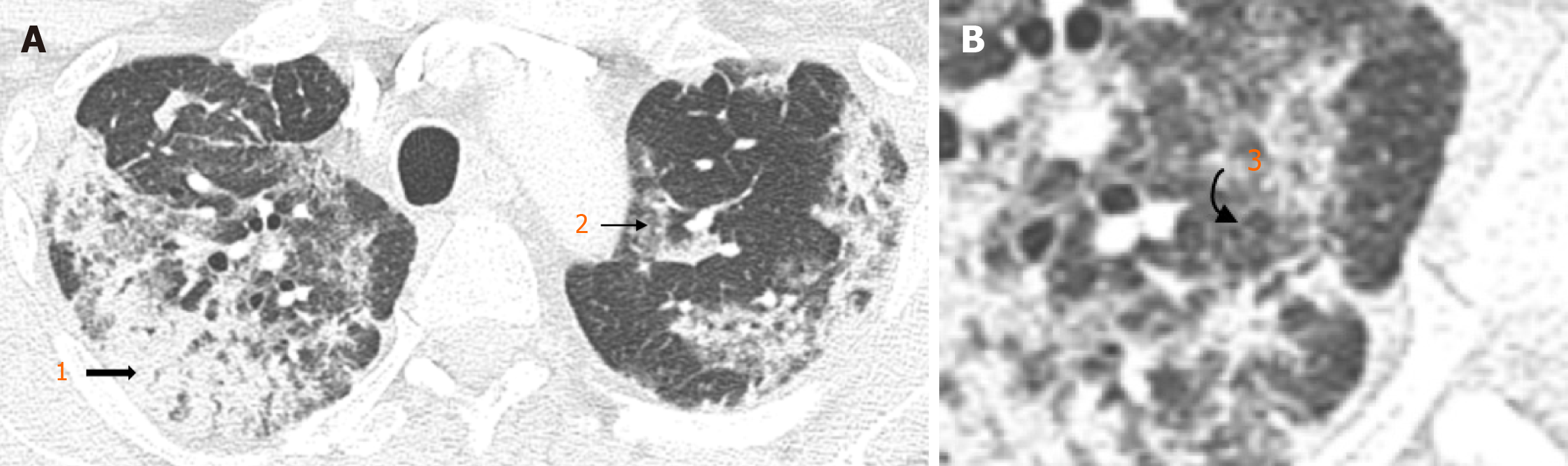
Earlier studies employed semi-quantitative methods to analyse chest computed tomography (CT) findings (Figure 2) in patients with COVID-19[19,20]. This involved a single, or multiple experienced radiologists blinded to clinical parameters and assigning a score based on the severity of findings. Higher chest CT scores have been found in patients with DM, suggesting more severe COVID-19 pneumonia when compared with patients without DM[19]. Findings by Iacobellis et al[18] suggested day-1 hyperglycaemia as a predictor of COVID-19 severity on CXR were confirmed on CT.
Patients with poorly-controlled DM are likely to have more severe COVID-19 pneumonia. A recent study by Lu et al[21] using a quantitative artificial intelligence algorithm found parameters including the percentage of ground glass volume (PGV) and percentage of consolidation volume (PCV), positively correlated with fasting blood glucose and HbA1c. Unlike semi-quantitative methods, results using this approach were not affected by inter- and intra-observer variability. Raoufi et al[20] used a semi-quantitative method to study 117 patients with DM in Iran and found no significant difference in patients with well-controlled (defined as maintaining glycaemic variability between 3.9-10 mmol/L) and poorly-controlled DM. However, the poorly-controlled group contained almost 4 times the number of patients (93 vs 24). Furthermore, the median age of patients in the well-controlled group were older (75 vs 62 years) which may have been a confounding factor for this negative result[20].
Studies have shown mortality rates to be higher among patients with poorly-controlled DM and COVID-19 than the general population with COVID-19[22,23]. In particular, high HbA1c levels have been linked with inflammation and hypercoagulability, resulting in an increased mortality rate in patients with DM suffering from COVID-19[24]. However, the accuracy of these results may be influenced by other co-morbidities such as ischaemic heart disease and stroke. No large-scale studies have yet shown an association between worse CT findings and mortality in DM.
A high incidence of venous and arterial thrombotic complications in critically ill patients with COVID-19 has been reported previously[25]. Recent literature based on autopsy studies shows that the origin of thrombotic lesions in COVID-19 is largely unknown. Lung histopathological analysis found multiple thrombi in small to medium pulmonary arteries giving rise to the theory of COVID-19 associated immu
The radiological finding of subsegmental or segmental thrombi in peripheral segments of lung affected by acute lung injury and the absence of deep vein thrombosis (DVT) in patients with COVID-19 infection, assumes the theory of immunothrombosis[27]. Monfardini et al[29] found 76% of patients with a moderate-high pre-test probability of PE and positive D-dimer level (a fibrin degradation product measured to help diagnose thrombosis), had positive CTPA findings. Nevertheless, only 15% of these patients were associated with ultrasound detected lower limb DVT[29], suggesting the remainder probably represented immunothrombosis. A meta-analysis of twenty-seven studies by Suh et al[30] revealed DVT was only found in 42% of patients with PE.
As yet, no large-scale studies have reported a link between pulmonary thromboembolism and DM in patients with COVID-19. Kaminetzky et al[31] found patients with DM were significantly less frequently observed to have CTPA examinations. Of 23 patients identified to have PE in this study, only 3 had DM; however, this finding may be attributed to the small sample size.
DM predisposes to immune deregulation and reduced expression of the ACE2 receptor, leading to severe acute lung injury[5,6]. Studies have proven a link between DM and more severe airspace consolidation based on chest x-ray findings[12,13]. Furthermore, CXR evidence suggests DM is associated with higher mortality in COVID-19. The exact pathogenesis of this is unclear but may be related to microvascular immunothrombosis[26,28].
There is now quantitative evidence to suggest poorly controlled DM is associated with more severe lung injury on CT[21]. However, no large-scale studies have investigated a direct link between CT findings and mortality in DM. Although the incidence of PE is greater in critically ill patients with COVID-19[25], no link has been established between poorly controlled DM and the risk of PE.
As new research into COVID-19 is produced and evidence emerges from autopsy studies, the understanding of pathobiology of the disease has evolved. However, there remains scope for future research; particularly whether small pulmonary thromboses represent venous thromboembolism, immunothrombosis, or a combination of both. Furthermore, a direct link between DM and immunothrombosis may help to guide future management strategies.
We will like to thank Kirresh OZ for providing radiographs.
| 1. | CCSE Dashboard [Internet]. COVID-19 Dashboard by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University (JHU). [cited 20 February 2021]. Available from: https://www.arcgis.com/apps/opsdashboard/index.html#/bda7594740fd40299423467b48e9ecf6. |
| 2. | Rafique S, Ahmed FW. A Case of Combined Diabetic Ketoacidosis and Hyperosmolar Hyperglycemic State in a Patient With COVID-19. Cureus. 2020;12:e8965. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 3. | Barron E, Bakhai C, Kar P, Weaver A, Bradley D, Ismail H, Knighton P, Holman N, Khunti K, Sattar N, Wareham NJ, Young B, Valabhji J. Associations of type 1 and type 2 diabetes with COVID-19-related mortality in England: a whole-population study. Lancet Diabetes Endocrinol. 2020;8:813-822. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 634] [Cited by in RCA: 678] [Article Influence: 113.0] [Reference Citation Analysis (0)] |
| 4. | Ahmed FW, Kirresh OZ, Robinson AV. A Retrospective Study Assessing the Effect of Diabetes on Mortality in Patients With COVID-19 at a Teaching Hospital in the United Kingdom [Internet]. 2021. [cited 20 February 2021]. Available from: https://www.cureus.com/articles/54241. |
| 5. | Sartore G, Ragazzi E, Faccin L, Lapolla A. A role of glycation and methylation for SARS-CoV-2 infection in diabetes? Med Hypotheses. 2020;144:110247. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 6. | Sardu C, Gargiulo G, Esposito G, Paolisso G, Marfella R. Impact of diabetes mellitus on clinical outcomes in patients affected by Covid-19. Cardiovasc Diabetol. 2020;19:76. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 55] [Cited by in RCA: 80] [Article Influence: 13.3] [Reference Citation Analysis (0)] |
| 7. | Cleverley J, Piper J, Jones MM. The role of chest radiography in confirming covid-19 pneumonia. BMJ. 2020;370:m2426. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 125] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (2)] |
| 8. | Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, Tao Q, Sun Z, Xia L. Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020;296:E32-E40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3614] [Cited by in RCA: 3297] [Article Influence: 549.5] [Reference Citation Analysis (2)] |
| 9. | Herpe G, Lederlin M, Naudin M, Ohana M, Chaumoitre K, Gregory J, Vilgrain V, Freitag CA, De Margerie-Mellon C, Flory V, Ludwig M, Mondot L, Fitton I, Jacquier ARR, Ardilouze P, Petit I, Gervaise A, Bayle O, Crombe A, Mekuko Sokeng M, Thomas C, Henry G, Bliah V, Le Tat T, Guillot MS, Gendrin P, Garetier M, Bertolle E, Montagne C, Langlet B, Kalaaji A, Kayayan H, Desmots F, Dhaene B, Saulnier PJ, Guillevin R, Bartoli JM, Beregi JP, Tasu JP. Efficacy of Chest CT for COVID-19 Pneumonia Diagnosis in France. Radiology. 2021;298:E81-E87. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 53] [Cited by in RCA: 50] [Article Influence: 10.0] [Reference Citation Analysis (1)] |
| 10. | Ufuk F, Savaş R. Chest CT features of the novel coronavirus disease (COVID-19). Turk J Med Sci. 2020;50:664-678. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 38] [Cited by in RCA: 32] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 11. | Ye Z, Zhang Y, Wang Y, Huang Z, Song B. Chest CT manifestations of new coronavirus disease 2019 (COVID-19): a pictorial review. Eur Radiol. 2020;30:4381-4389. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 914] [Cited by in RCA: 796] [Article Influence: 132.7] [Reference Citation Analysis (0)] |
| 12. | Elemam NM, Hannawi H, Salmi IA, Naeem KB, Alokaily F, Hannawi S. Diabetes mellitus as a comorbidity in COVID-19 infection in the United Arab Emirates. Saudi Med J. 2021;42:170-180. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 18] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 13. | Bhandari S, Rankawat G, Singh A, Gupta V, Kakkar S. Impact of glycemic control in diabetes mellitus on management of COVID-19 infection. Int J Diabetes Dev Ctries. 2020;1-6. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 16] [Cited by in RCA: 21] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 14. | Cellina M, Gibelli D, Valenti Pittino C, Toluian T, Marino P, Oliva G. Risk Factors of Fatal Outcome in Patients With COVID-19 Pneumonia. Disaster Med Public Health Prep. 2020;1-8. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 9] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 15. | Gattinoni L, Coppola S, Cressoni M, Busana M, Rossi S, Chiumello D. COVID-19 Does Not Lead to a "Typical" Acute Respiratory Distress Syndrome. Am J Respir Crit Care Med. 2020;201:1299-1300. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1087] [Cited by in RCA: 997] [Article Influence: 166.2] [Reference Citation Analysis (0)] |
| 16. | Izzi-Engbeaya C, Distaso W, Amin A, Yang W, Idowu O, Kenkre JS, Shah RJ, Woin E, Shi C, Alavi N, Bedri H, Brady N, Blackburn S, Leczycka M, Patel S, Sokol E, Toke-Bjolgerud E, Qayum A, Abdel-Malek M, Hope DCD, Oliver NS, Bravis V, Misra S, Tan TM, Hill NE, Salem V. Adverse outcomes in COVID-19 and diabetes: a retrospective cohort study from three London teaching hospitals. BMJ Open Diabetes Res Care. 2021;9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 42] [Cited by in RCA: 44] [Article Influence: 8.8] [Reference Citation Analysis (0)] |
| 17. | Mozzini C, Cicco S, Setti A, Racanelli V, Vacca A, Calciano L, Pesce G, Girelli D. Spotlight on Cardiovascular Scoring Systems in Covid-19: Severity Correlations in Real-world Setting. Curr Probl Cardiol. 2021;46:100819. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 7] [Cited by in RCA: 7] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 18. | Iacobellis G, Penaherrera CA, Bermudez LE, Bernal Mizrachi E. Admission hyperglycemia and radiological findings of SARS-CoV2 in patients with and without diabetes. Diabetes Res Clin Pract. 2020;164:108185. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 89] [Cited by in RCA: 88] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 19. | Guo W, Li M, Dong Y, Zhou H, Zhang Z, Tian C, Qin R, Wang H, Shen Y, Du K, Zhao L, Fan H, Luo S, Hu D. Diabetes is a risk factor for the progression and prognosis of COVID-19. Diabetes Metab Res Rev. 2020;e3319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 884] [Cited by in RCA: 914] [Article Influence: 152.3] [Reference Citation Analysis (1)] |
| 20. | Raoufi M, Khalili S, Mansouri M, Mahdavi A, Khalili N. Well-controlled vs poorly-controlled diabetes in patients with COVID-19: Are there any differences in outcomes and imaging findings? Diabetes Res Clin Pract. 2020;166:108286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 22] [Cited by in RCA: 28] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 21. | Lu X, Cui Z, Pan F, Li L, Liang B, Yang L, Zheng C. Glycemic status affects the severity of coronavirus disease 2019 in patients with diabetes mellitus: an observational study of CT radiological manifestations using an artificial intelligence algorithm. Acta Diabetol. 2021;58:575-586. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 10] [Cited by in RCA: 13] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 22. | Wu ZH, Tang Y, Cheng Q. Diabetes increases the mortality of patients with COVID-19: a meta-analysis. Acta Diabetol. 2021;58:139-144. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 98] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 23. | Zhu L, She ZG, Cheng X, Qin JJ, Zhang XJ, Cai J, Lei F, Wang H, Xie J, Wang W, Li H, Zhang P, Song X, Chen X, Xiang M, Zhang C, Bai L, Xiang D, Chen MM, Liu Y, Yan Y, Liu M, Mao W, Zou J, Liu L, Chen G, Luo P, Xiao B, Zhang Z, Lu Z, Wang J, Lu H, Xia X, Wang D, Liao X, Peng G, Ye P, Yang J, Yuan Y, Huang X, Guo J, Zhang BH. Association of Blood Glucose Control and Outcomes in Patients with COVID-19 and Pre-existing Type 2 Diabetes. Cell Metab. 2020;31:1068-1077.e3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1198] [Cited by in RCA: 1123] [Article Influence: 187.2] [Reference Citation Analysis (0)] |
| 24. | Wang Z, Du Z, Zhu F. Glycosylated hemoglobin is associated with systemic inflammation, hypercoagulability, and prognosis of COVID-19 patients. Diabetes Res Clin Pract. 2020;164:108214. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 116] [Cited by in RCA: 110] [Article Influence: 18.3] [Reference Citation Analysis (0)] |
| 25. | Klok FA, Kruip MJHA, van der Meer NJM, Arbous MS, Gommers D, Kant KM, Kaptein FHJ, van Paassen J, Stals MAM, Huisman MV, Endeman H. Confirmation of the high cumulative incidence of thrombotic complications in critically ill ICU patients with COVID-19: An updated analysis. Thromb Res. 2020;191:148-150. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1017] [Cited by in RCA: 1198] [Article Influence: 199.7] [Reference Citation Analysis (0)] |
| 26. | Patel BV, Arachchillage DJ, Ridge CA, Bianchi P, Doyle JF, Garfield B, Ledot S, Morgan C, Passariello M, Price S, Singh S, Thakuria L, Trenfield S, Trimlett R, Weaver C, Wort SJ, Xu T, Padley SPG, Devaraj A, Desai SR. Pulmonary Angiopathy in Severe COVID-19: Physiologic, Imaging, and Hematologic Observations. Am J Respir Crit Care Med. 2020;202:690-699. [PubMed] |
| 27. | van Dam LF, Kroft LJM, van der Wal LI, Cannegieter SC, Eikenboom J, de Jonge E, Huisman MV, Klok FA. Clinical and computed tomography characteristics of COVID-19 associated acute pulmonary embolism: A different phenotype of thrombotic disease? Thromb Res. 2020;193:86-89. [PubMed] |
| 28. | Loo J, Spittle DA, Newnham M. COVID-19, immunothrombosis and venous thromboembolism: biological mechanisms. Thorax. 2021;76:412-420. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 211] [Article Influence: 42.2] [Reference Citation Analysis (0)] |
| 29. | Monfardini L, Morassi M, Botti P, Stellini R, Bettari L, Pezzotti S, Alì M, Monaco CG, Magni V, Cozzi A, Schiaffino S, Bnà C. Pulmonary thromboembolism in hospitalised COVID-19 patients at moderate to high risk by Wells score: a report from Lombardy, Italy. Br J Radiol. 2020;93:20200407. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14] [Cited by in RCA: 18] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 30. | Suh YJ, Hong H, Ohana M, Bompard F, Revel MP, Valle C, Gervaise A, Poissy J, Susen S, Hékimian G, Artifoni M, Periard D, Contou D, Delaloye J, Sanchez B, Fang C, Garzillo G, Robbie H, Yoon SH. Pulmonary Embolism and Deep Vein Thrombosis in COVID-19: A Systematic Review and Meta-Analysis. Radiology. 2021;298:E70-E80. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 301] [Cited by in RCA: 326] [Article Influence: 65.2] [Reference Citation Analysis (0)] |
| 31. | Kaminetzky M, Moore W, Fansiwala K, Babb JS, Kaminetzky D, Horwitz LI, McGuinness G, Knoll A, Ko JP. Pulmonary Embolism at CT Pulmonary Angiography in Patients with COVID-19. Radiol Cardiothorac Imaging. 2020;2:e200308. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 52] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: https://creativecommons.org/Licenses/by-nc/4.0/
Provenance and peer review: Invited article; Externally peer reviewed.
Peer-review model: Single blind
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: United Kingdom
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Liu D S-Editor: Wang LL L-Editor: A P-Editor: Wang LL