Published online Feb 28, 2021. doi: 10.4329/wjr.v13.i2.40
Peer-review started: November 24, 2020
First decision: December 21, 2020
Revised: December 27, 2020
Accepted: January 28, 2021
Article in press: January 28, 2021
Published online: February 28, 2021
Processing time: 93 Days and 16.2 Hours
In growing patients with skeletal discrepancies, early assessment of functional factors can be vital for the restoration of normal craniofacial growth.
To compare airway volumes in patients with mandibular retrognathism with the normal anteroposterior skeletal relationship, thereby assessing the association between cephalometric variables and airway morphology.
Cone-beam computed tomography volume scans, and lateral cephalograms, 3-dimensional airway volume and cross-sectional areas of 120 healthy children (54 boys and 66 girls mean age 15.19 ± 1.28) which were done for orthodontic assessment were evaluated. The subjects were divided into 2 groups based on the angle formed between point A, Nasion and point B (ANB) values and cephalometric variables (such as anterior and posterior facial height, gonial angle etc.) airway volumes, and cross-sectional measurements were compared using independent t tests. Pearson’s correlation coefficient test was used to detect any relationship of different parts of the airway and between airway volume and 2-dimensional cephalometric variables.
Means and standard deviations for cephalometric, cross-sectional, and volumetric variables were compared. ANB, mandibular body length and facial convexity were statistically highly significant (P < 0.01) whereas condylion to point A, nasal airway and total airway volume (P < 0.05) were statistically significant. The nasal airway volume and the superior pharyngeal airway volume had a positive correlation (P < 0.01), nasal airway was correlated to middle (P < 0.05) and total airway superior had a relation with middle (P < 0.05), inferior and total airway (P < 0.05), middle was related to all other airways; inferior was also related to all the airways except nasal. Lateral cephalometric values were positively correlated with the airway volume with Frankfurt Mandibular Plane Angle and facial convexity showed significant correlations with total airway volume (P < 0.05). Additionally, ANB angle was significantly correlated with total airway volume and superior airway (P < 0.05).
The mean total airway volume in patients with retrognathic mandible was significantly smaller than that of patients with a normal mandible.
Core Tip: With the advent of cone beam computed tomography, analysis of airway has become possible. Patients who present with retrognathic jaw or anterior-posterior skeletal discrepancy have been contemplated to have reduced pharyngeal airway. When comparing the airway volumes of 120 healthy individuals with mandibular retrognathism and normal anteroposterior skeletal relationship, the mean total airway volume of patients with the angle formed between point A, Nasion and point B (ANB) more than 4 was significantly smaller than that of patients with ANB less than 4. The sub-volumes in the pharyngeal airway showed a positive correlation with each other. Frankfurt Mandibular Plane Angle and facial convexity and mandibular body length also had a significant interrelationship with total volume of airway.
- Citation: Kochhar AS, Sidhu MS, Bhasin R, Kochhar GK, Dadlani H, Sandhu J, Virk B. Cone beam computed tomographic evaluation of pharyngeal airway in North Indian children with different skeletal patterns. World J Radiol 2021; 13(2): 40-52
- URL: https://www.wjgnet.com/1949-8470/full/v13/i2/40.htm
- DOI: https://dx.doi.org/10.4329/wjr.v13.i2.40
Respiratory function plays a substantial role in orthodontic diagnosis and treatment planning. An association between the respiratory mode and facial morphology has been observed in various studies utilizing cephalograms[1]. Furthermore, a link between Class II Division 1 malocclusion and upper pharyngeal airway obstruction as well as mouth breathing, was demonstrated by Angle[2] in 1907. Various authors have presented characteristics related to obstructed breathing[1]. Primary clinical features of respiratory obstruction syndrome have been identified by Ricketts[3] as tonsil and adenoid enlargement, narrow nostrils, open bite, cross bite, and tongue thrusting.
The role of the upper anatomy in the craniofacial complex development is usually considered substantial[4]. Impaired breathing can be a result of narrow pharyngeal airway, which can further lead to diminished levels of growth hormone in growing children or obstructive sleep apnea in mature individuals. Diminished airway associated with obstructive sleep apnea tends to be typical in patients with Angle class II malocclusion, displaying retrognathic mandible and sagittal discrepancy[5,6].
Early diagnosis and evaluation of the functional factors in growing children with skeletal discrepancy and features of adenoid hypertrophy (adenoid faces) might be pivotal to restore proper craniofacial growth and treatment outcome stability. Pharyngeal airway measurements have usually been conducted by landmark identification followed by measurements of different lengths and areas in the pharyngeal region[7-9].
Although there is an avalanche of studies regarding airway morphology and its effects on craniofacial growth, most studies have used 2-dimensional (2D) techniques, frontal or lateral cephalograms, with inadequate assessment of length and areas. A technique for 3-dimensional (3D) visualization, utilized frequently is computed tomography[10]. However, a huge impediment to its use is the large radiation dose[11]. The radiation dose can be minimized to one-fifth, while not compromising on quality, with modern cone beam computed tomography (CBCT), due to which it is becoming increasingly popular[12].
Volumetric measurements of the pharyngeal airway space (PAS) and, narrowing or obstruction can be localized utilizing CBCT[13]. As narrowing or obstruction of the pharyngeal airway can be present in patients with altered maxillo-mandibular relationship and can be associated with sleep, as well as Obstructive Sleep Apnoea Syndrome, this analysis can be beneficial in the orthodontic diagnosis and planning orthognathic surgery[14]. Therefore, the aim of the present study was to compare the pharyngeal airway volumes in children with varying anteroposterior maxillo-mandibular relationships (ANB angles that is the angle formed between point A, Nasion and Point B) and study the possible correlations between different cephalometric variables and the airway morphology in these children.
Following the ethical clearance from the institutional review board, records of 150 children who visited the outpatient Department of Orthodontics were examined. Of this, CBCT scans of 120 healthy North Indian children (54 boys and 66 girls mean age 15.19 ± 1.28) were selected, after the following exclusion criteria was applied: History of any upper respiratory infection, pharyngeal pathology (like adenoid hypertrophy and tonsillitis) or a history of adenoid or tonsil removal (Table 1).
| Group IANB < 4 | Group II ANB > 4 | Total | |||
| Male | Female | Male | Female | ||
| Subjects (n) | 26 | 30 | 28 | 36 | 120 |
| Age (yr) | 13-17 | 13-17 | 13-17 | 13-17 | 15.19 ± 1.28 |
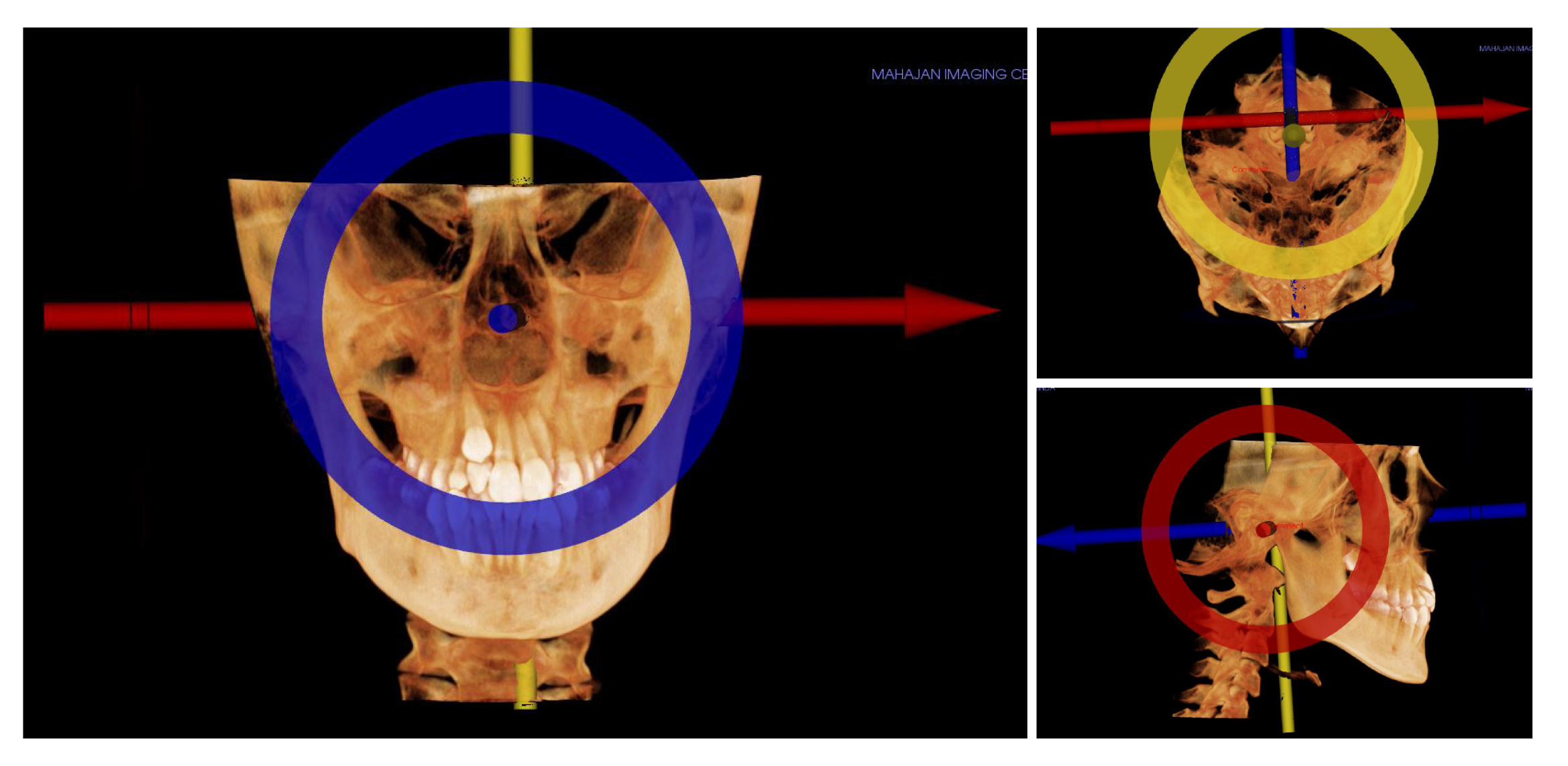
CBCT volume scans of all subjects were obtained by using the I-Cat CBCT unit (Imaging Sciences Hatfield, PA, United States), and the imaging protocol used a 17 cm × 21 cm field of view to include the entire craniofacial anatomy. The images were standardized with the subject seated in a chair, machine settings of 120 kV-5 mA-0.25 mm voxel, and scan time of 20 s. Patients, following the standard protocol of acquiring the scans in a natural head position, and their jaws in maximum intercuspation with the lips and tongue in resting position were used. For volume evaluation/measure-ment and cephalometric analysis, the axial images were transferred to InVivo Dental software (Anatomage, San Jose, CA, United States) The 3D images were reoriented using the Frankfort horizontal (FH) plane as the reference plane for uniformity and to reduce errors. A line joining the right and left portions, located in the most latero-superior point of the external auditory meatus, to the right orbitale was constructed as the FH plane (Figure 1).
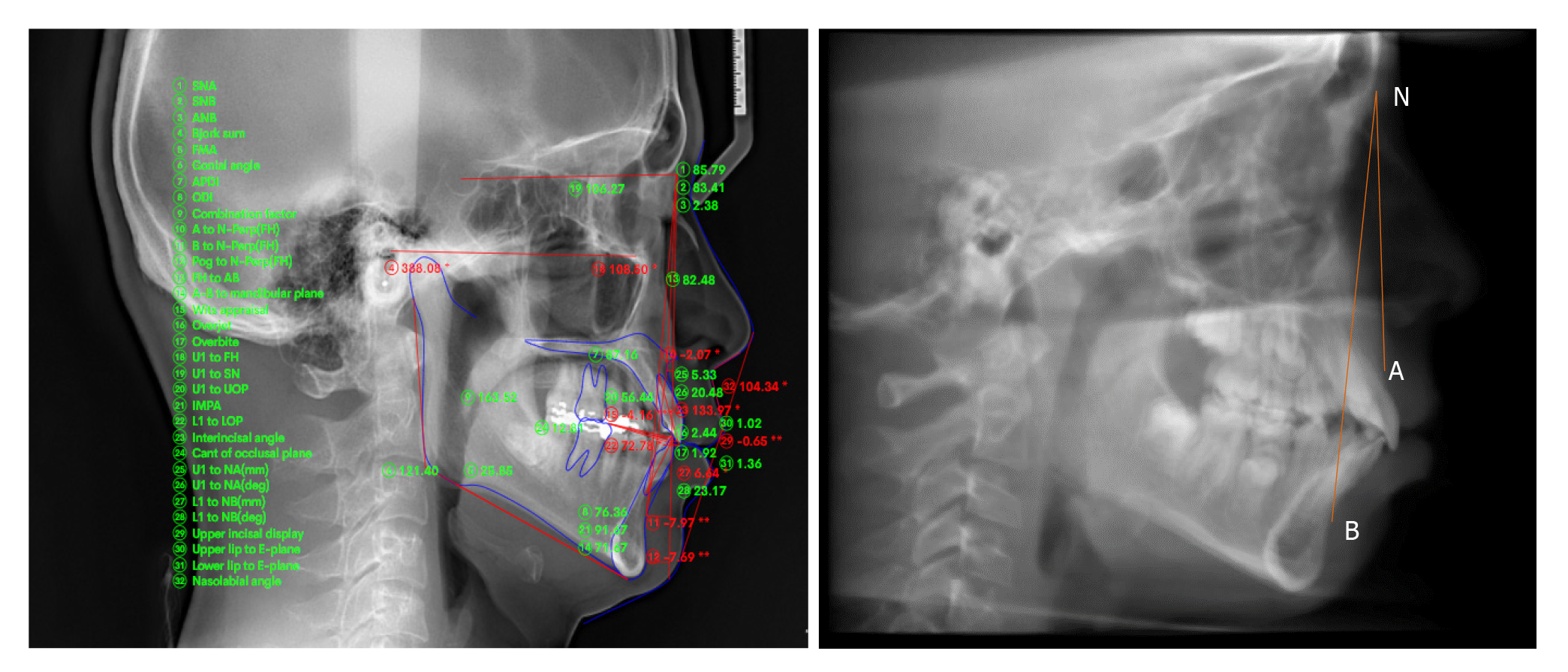
2D cephalometric images were derived from the CBCT scans by using SUPER CEPH feature of the software In Vivo Dental, and the images were imported into Nemoceph® (Dental Studio NX 2006 version 6.0) (Figure 2). Landmark identifications and physical measurements were performed by the same investigator. Using the software Downs, Steiner, Jarabak, Mc Namara and Tweed Merrifield analysis were done in order to classify patients (Tables 1 and 2).
| Two-dimensional cephalometric variables | |
| (1) Gonial angle: Angle formed between line drawn tangent to the lower border of the mandible and another line tangent to the distal border of the ascending ramus and the condyle on both sides; (2) Anterior facial height (AFH): Distance between the Nasion and Menton (Me); (3) Posterior facial height (PFH): Distance between Sella (S) to Gonion(Go); (4) PFH/AFH: Ratio of AFH and the PFH; (5) FMA: Frankfurt Mandibular Plane Angle formed by the intersection of the Frankfort horizontal plane and the mandibular plane; (6) ANB: The angle formed between point A, Nasion and Point B; (7) Facial convexity: Formed by the intersection of line from Nasion to point A, to point A to pogonion(Po); (8) Condylion to point A (Co-PtA); (9) Condylion to gnathion (Co-Gn); and (10) Mandibular body length (Mand-BL): Distance from gonion to pogonion | |
| Cross-sectional planes and volumes of the 3D pharyngeal airway | |
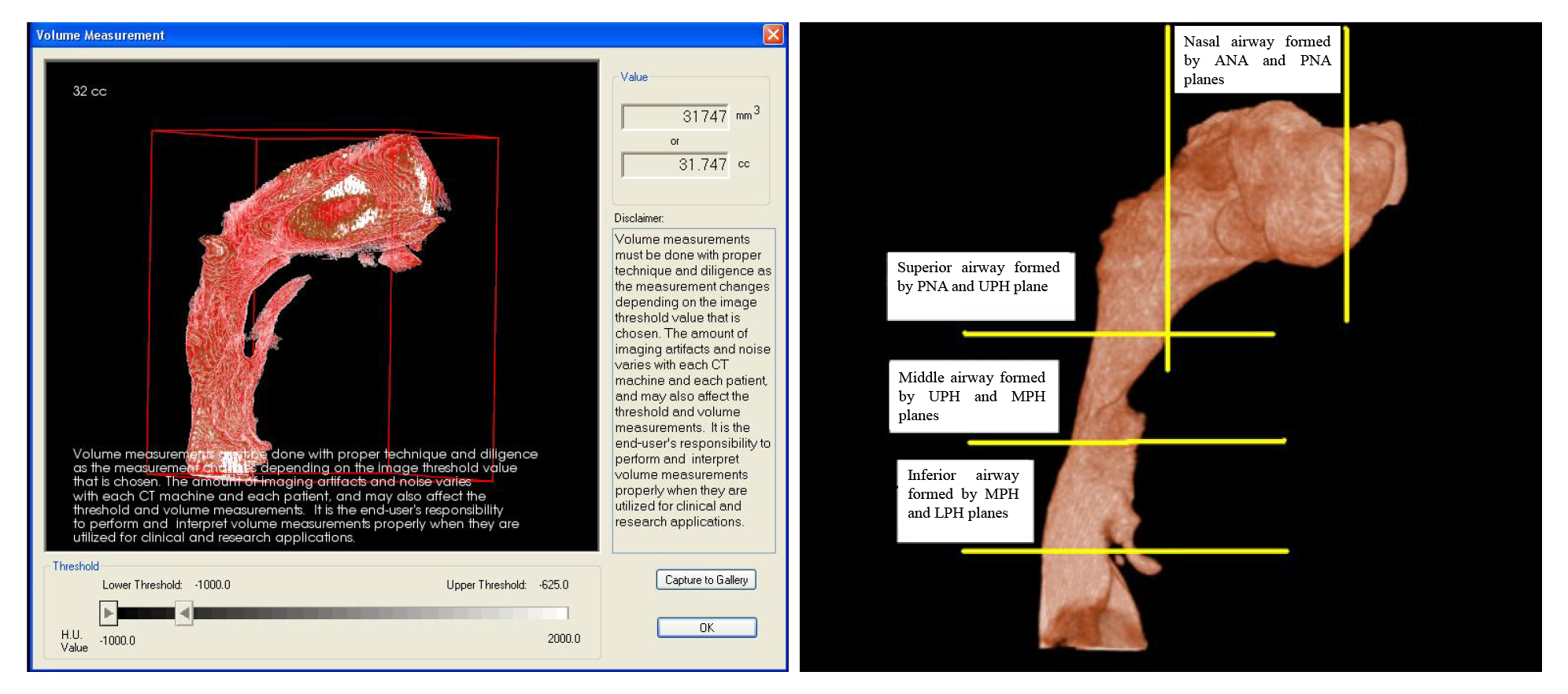
| Anterior nasal plane (Ana plane) | Plane passing through anterior nasal spine (ANS) and perpendicular to FH |
| Posterior nasal plane (Pna plane) | Plane passing through posterior nasal spine (PNS)and perpendicular to FH |
| Upper pharyngeal plane (Uph plane) | Plane passing through PNS parallel to FH |
| Middle pharyngeal plane (Mph plane) | Plane passing through lower margin of the soft palate and parallel to FH |
| Lower pharyngeal plane (Lph plane) | Plane passing through superior margin of the epiglottis and parallel to FH |
| Volume | |
| Nasal airway | Airway formed by the planes between Ana and Pna |
| Superior pharyngeal airway | Airway formed by the planes between Pna and Uph |
| Middle pharyngeal airway | Airway formed by the planes between Uph and Mph |
| Inferior pharyngeal airway | Airway formed by the planes between Mph and Lph planes |
| Total airway | Airway extending between Ana plane to Lph plane |
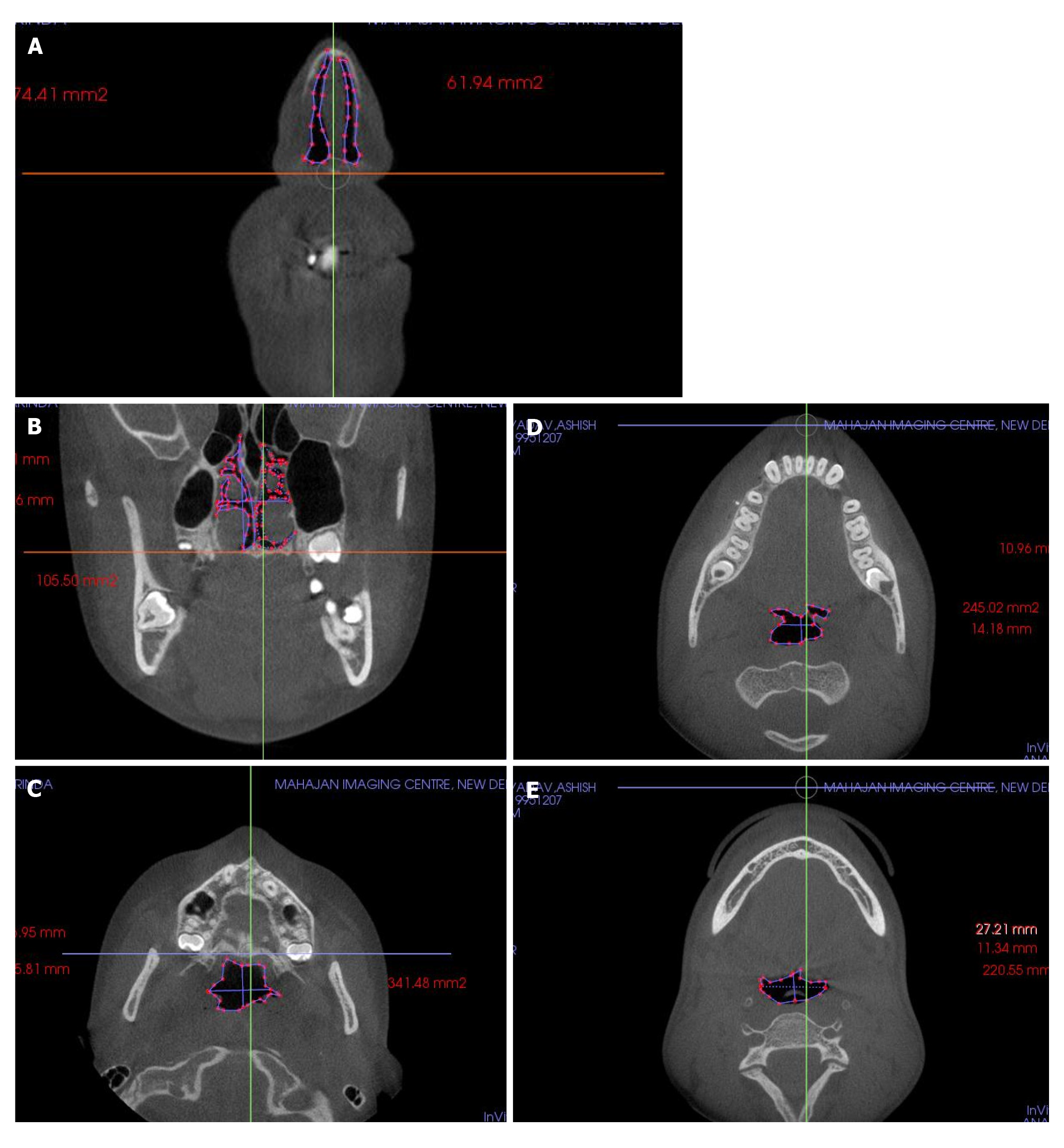
Cross-sectional views of the pharyngeal airway in the 5 planes: a, represents the length (axial slice) or height (frontal slice) of the airway defined by the greatest distance in the anteroposterior or vertical direction of the airway cross-section; b is the width of the airway defined by the greatest distance in the right and left directions of the airway cross-section and 5 volumes A, right lateral view and B, frontal view of volume rendered images. a, Anterior nasal plane; b, posterior nasal plane; c, upper pharyngeal plane; d, middle pharyngeal plane; and e, lower pharyngeal plane (Table 3 and Figures 3 and 4). Cross-sectional planes of the nasal cavity were perpendicular to the FH plane, whereas the pharyngeal cross-sections are parallel to the FH plane. Although these cross-sections are not directly perpendicular to the long axis of the airway, the FH plane was used as a reference plane to standardize the plane orientation and minimize error in identifying the studied cross-sectional planes. Cross-sectional measurements, that is width and length, were computed in frontal and axial views to provide linear accuracy.
| Nasal airway | Superior airway | Middle airway | Inferior airway | Totalairway | |
| Nasal airway | |||||
| Pearson correlation | 1 | 0.085 | 0.471a | 0.386 | 0.879b |
| Sig.(2-tailed) | 0.722 | 0.036 | 0.093 | 0 | |
| n | 120 | 120 | 120 | 120 | 120 |
| Superior airway | |||||
| Pearson correlation | 0.85 | 1 | 0.494a | 0.651b | 0.4623a |
| Sig.(2-tailed) | 0.722 | - | 0.027 | 0.002 | 0.04 |
| n | 120 | 120 | 120 | 120 | 120 |
| Middle airway | |||||
| Pearson correlation | 0.471a | 0.494a | 1 | 0.763b | 0.779b |
| Sig.(2-tailed) | 0.036 | 0.027 | - | 0 | 0 |
| n | 120 | 120 | 120 | 120 | 120 |
| Inferior airway | |||||
| Pearson correlation | 0.386 | 0.651b | 0.763b | 1 | 0.744b |
| Sig.(2-tailed) | 0.093 | 0.002 | 0 | - | 0 |
| n | 120 | 120 | 120 | 120 | 120 |
| Totalairway | |||||
| Pearson correlation | 0.879b | 0.4623a | 0.779b | 0.744b | 1 |
| Sig.(2-tailed) | 0 | 0.04 | 0 | 0 | - |
| n | 120 | 120 | 120 | 120 | 120 |
Volumetric renderings of the subjects’ CBCT scans were acquired with the In Vivo Dental software, and we proceeded with volumetric analysis of the defined airways. 3D image inversion to convert negative image to a positive value was done, which is required as the airway is a void space. This process removes the hard and soft tissues of the image around the airway and embodies the airway spaces of the craniofacial region including the paranasal sinuses and other empty spaces. Furthermore, to isolate the required airway section and remove structures that were not necessary, sculpting was performed which was an inherent feature of the software. Threshold values were thereafter altered to remove the artifacts and enhance the selected region of airway. Lastly, designated airway volume was computed in cubic millimeters.
Descriptive statistics including the mean and standard deviation for each group were calculated by using SPSS for Windows software (version 20). Differences between groups were tested by using independent t tests. Pearson’s correlation coefficient test was used to detect any relationship of different parts of the airway and between airway volume and 2D cephalometric variables.
Means and standard deviations for cephalometric, cross-sectional, and volumetric variables were compared. Table 4 gives the comparison results of groups I and II. ANB, mandibular body length, facial convexity were statistically highly significant (P < 0.01) whereas condylion to point A, nasal airway and total airway volume (P < 0.05) were statistically significant. Cross-sectional and volumetric measurements at different levels when compared were statistically insignificant. However, total airway volume was significantly greater in group I (P < 0.05).
Table 3 show the correlations among the studied variables. The nasal airway volume and the superior pharyngeal airway volume had a positive correlation (P < 0.01), nasal airway was correlated to middle (P < 0.05) and total airway superior had a relation with middle (P < 0.05), inferior and total airway (P < 0.05), middle was related to all other airways, inferior was also related to all the airways except nasal (Table 5). Lateral cephalometric values were positively correlated with the airway volume with Frankfurt Mandibular Plane Angle (FMA) and facial convexity showed significant correlations with total airway volume (P < 0.05). Additionally, ANB angle was significantly correlated with total airway volume and superior airway (P < 0.05).
| Group | Group I ANB < 4 | Group II ANB > 4 | P value | ||
| mean | SD | mean | SD | ||
| Ana height | 29.63 | 4.69 | 30.06 | 6.73 | 0.871 |
| Ana width | 13.81 | 1.98 | 15.24 | 3.61 | 0.273 |
| Ana C. area | 194.41 | 13.93 | 218.14 | 52.41 | 0.22 |
| Pna height | 29.95 | 7.85 | 28.73 | 8.70 | 0.744 |
| Pna width | 23.88 | 3.06 | 24.14 | 4.84 | 0.884 |
| Pna C. area | 257.85 | 74.25 | 284.32 | 78.01 | 0.448 |
| Uph length | 19.28 | 6.61 | 17.54 | 4.19 | 0.503 |
| Uph width | 26.01 | 4.81 | 24.14 | 7.87 | 0.522 |
| Uph C. area | 293.37 | 71.56 | 314.97 | 99.76 | 0.58 |
| Mph length | 11.67 | 3.47 | 11.54 | 3.60 | 0.935 |
| Mph width | 22.66 | 5.87 | 20.16 | 8.26 | 0.439 |
| Mph C. area | 226.32 | 85.90 | 213.25 | 102.48 | 0.76 |
| Lph length | 14.77 | 7.93 | 11.88 | 3.07 | 0.286 |
| Lph width | 24.44 | 5.19 | 28.90 | 6.76 | 0.111 |
| Lph C. area | 231.31 | 83.23 | 199.84 | 78.36 | 0.399 |
| Gonial angle | 126.37 | 7.79 | 125.24 | 7.55 | 0.748 |
| AFH | 108.38 | 6.08 | 112.12 | 4.86 | 0.152 |
| PFH | 73.06 | 5.42 | 71.81 | 7.58 | 0.672 |
| PFH/AFH, % | 67.47 | 4.49 | 64.02 | 5.83 | 0.152 |
| FMA | 25.06 | 3.61 | 27.48 | 6.02 | 0.281 |
| ANB | 2.85 | 1.56 | 6.14 | 1.02 | < 0.001b |
| MAND-BL | 66.94 | 3.74 | 61.61 | 4.27 | 0.008b |
| Facial convexity | 5.17 | 3.57 | 10.76 | 4.56 | 0.007b |
| Co-pt A | 82.10 | 6.09 | 82.68 | 3.44 | 0.802 |
| Co-pt GN | 109.83 | 5.54 | 103.72 | 6.42 | 0.035a |
| Nasal airway | 36407.36 | 2526.59 | 30446.00 | 7060.88 | 0.037a |
| Superior airway | 5563.27 | 1350.80 | 4559.67 | 1263.62 | 0.106 |
| Middle airway | 5322.45 | 2124.81 | 4213.89 | 1291.90 | 0.188 |
| Inferior airway | 5487.82 | 2018.25 | 5077.67 | 1521.36 | 0.621 |
| Total airway | 52780.91 | 6435.84 | 44297.22 | 8662.49 | 0.022a |
| Nasal airway | Superior airway | Middle airway | Inferior airway | Total airway | |
| Gonial angle | |||||
| Pearson correlation | -0.021 | 0.135 | 0.098 | -0.019 | 0.024 |
| Sig.(2-tailed) | 0.928 | 0.571 | 0.681 | 0.937 | 0.918 |
| n | 120 | 120 | 120 | 120 | 120 |
| AFH | |||||
| Pearson correlation | 0.055 | 0.201 | 0.057 | 0.342 | 0.154 |
| Sig.(2-tailed) | 0.818 | 0.395 | 0.811 | 0.14 | 0.517 |
| n | 120 | 120 | 120 | 120 | 120 |
| PFH | |||||
| Pearson correlation | 0.159 | 0.172 | 0.124 | 0.319 | 0.23 |
| Sig.(2-tailed) | 0.503 | 0.47 | 0.602 | 0.171 | 0.329 |
| n | 120 | 120 | 120 | 120 | 120 |
| PFH/AFH, % | |||||
| Pearson correlation | 0.133 | 0.057 | 0.086 | 0.113 | 0.142 |
| Sig.(2-tailed) | 0.576 | 0.813 | 0.717 | 0.636 | 0.55 |
| n | 120 | 120 | 120 | 120 | 120 |
| FMA | |||||
| Pearson correlation | -0.372 | -0.314 | -0.377 | -0.411 | -0.473a |
| Sig.(2-tailed) | 0.106 | 0.178 | 0.102 | 0.072 | 0.035 |
| n | 120 | 120 | 120 | 120 | 120 |
| ANB | |||||
| Pearson correlation | -0.364 | -0.408 | -0.197 | -0.152 | -0.389 |
| Sig.(2-tailed) | 0.115 | 0.034a | 0.405 | 0.522 | 0.22a |
| n | 120 | 120 | 120 | 120 | 120 |
| Mand-BL | |||||
| Pearson correlation | 0.136 | 0.523a | 0.038 | 0.184 | 0.225 |
| Sig.(2-tailed) | 0.567 | 0.018 | 0.874 | 0.436 | 0.341 |
| n | 120 | 120 | 120 | 120 | 120 |
| Facial convexity | |||||
| Pearson correlation | -0.362 | -0.306 | -0.221 | -0.22 | -0.391 |
| Sig.(2-tailed) | 0.116 | 0.189 | 0.349 | 0.351 | 0.088 |
| n | 120 | 120 | 120 | 120 | 120 |
| Co-pt A | |||||
| Pearson correlation | 0.127 | 0.324 | 0.289 | 0.469a | 0.3 |
| Sig.(2-tailed) | 0.594 | 0.163 | 0.217 | 0.037 | 0.199 |
| n | 120 | 120 | 120 | 120 | 120 |
| Co-pt GN | |||||
| Pearson correlation | 0.301 | 0.296 | 0.012 | 0.225 | 0.303 |
| Sig.(2-tailed) | 0.197 | 0.204 | 0.959 | 0.34 | 0.194 |
| n | 120 | 120 | 120 | 120 | 120 |
In the last few decades’ airway assessment has been done using nasal resistance and airflow tests, nasoendoscopy and lateral cephalograms[15]. In the current study, CBCT produced anatomically precise images, sans magnification or distortion were reconstructed 3 dimensionally to completely understand the pharyngeal airway anatomy of growing children in all dimensions (sagittal, transverse and frontal)[11,14]. Generally, a requisite for 3D imaging such as conventional CT or magnetic resonance imaging is for the patients to be supine. However, due to the effect of gravity on the soft tissues enveloping the oropharyngeal cavity, there are substantial anatomical changes in the airway[16].
Hsu et al[17] further found that the minimum of PAS and linear distance along perpendicular changes from the most upper anterior point of the hyoid bone to mandibular plane, as the position of body is changed from upright to supine[17]. Nevertheless, in recent times, advancements in CBCT have permitted axial CT images to be acquired in upright sitting posture, which is more valid for our study.
Owing to this study’s retrospective design, direct examination of the nasopharyngeal functions of the patients was not possible and previous clinical charts and diagnoses for orthodontic treatment were used to select subjects. Nonetheless, a study by Laine-Alava et al[18] stated that there is no effect of a history or symptoms of upper respiratory disease on variables related to naso-respiratory function when the measurements are made during an asymptomatic period, which justifies the retrospective format of our study[18].
2D lateral cephalometric images were created from the CBCT scans to allocate the subjects to the 2 groups, and to assess correlations among the cephalometric parameters and the pharyngeal airway volumes. Linear accuracy of the CBCT-derived lateral cephalometric images has been studied in the past[19,20]. The classification of the subjects based on their anteroposterior skeletal relationships, was done utilizing north Indian standards for the ANB angle[21].Additionally, previously it has been demonstrated that the prepubertal ANB angle and the angle of convexity measured have high prediction accuracy for postpubertal anteroposterior jaw relationships[19,22]. In the current study, the anteroposterior analyses displayed statistically significant differences further confirming that the ANB angle, which was used to classify our subjects, was a reliable parameter[22].
Previous studies have presented excellent intra-rater reliability values of InVivo 5 software, hence in the present study the InVivo 5 software was used to analyse the pharyngeal volume[14,23]. In the current study, no sexual dimorphism in any cross-sectional and volumetric measurements was observed between the two sexes. These findings were in agreement with the study by Ceylan et al[24] and de Freitas et al[25]. Similarly, in a study by Xu et al[26] in 2019, no significant difference was observed in patient sexas well as age.
In groups I and II ANB, mandibular body length and facial convexity were statistically highly significant (P < 0.01) whereas condylion to point A, nasal airway and total airway volume (P < 0.05) were statistically significant. Although group I demonstrated greater cross-sectional areas and volumetric measurements of the sub-regions of the pharyngeal airway, this was statistically insignificant signifying lack of correlation between segmental airway capacities and mandibular deficiencies. This was in accordance with Di Carlo et al[27] who did not find a direct correlation between individual skeletal patterns, and overall upper airway anatomy. Moreover, former 2D studies also asserted a lack of relationship between airway dimensions and malocclusion class[24,25]. Ceylan et al[24] stated that despite skeletal anteroposterior relationship changes, the airway dimensions remain constant, owing to postural changes in the pharyngeal structures. However, certain authors emphasized that upper airway dimensions vary according to different skeletal classes, developmental ages, and gender[1].
Nasal airway was positively correlated with middle and total airways. This may be justified by the location of the 2 sections, that are just superior to hard palate and not anatomically adjacent, yet there is direct correlation of their volumetric dimensions. The sections superior airway with middle, inferior and total airway and inferior airway with superior, middle and total airway display significant correlations. According to Ricketts[3] and Dunn et al[28], a restricted nasopharyngeal airway width is associated with mouth breathing, because it is readily obstructed by adenoid enlargement. Total airway was positively correlated with all superior, middle and inferior airways in our study.
The negative correlation of the ANB angle and the total airway can be explained by group I (ANB less than 4) having significantly greater airway volume than group II (ANB more than 4). Mandibular body length and total airway volume were both significantly greater in group I, demonstrating a positive correlation. Total airway volume had significant association with ANB angle and mandibular body length (anterior-posterior discriminants) supporting the intergroup comparison of different anterior-posterior skeletal patterns in the study. Similar results were observed by Lopatienė et al[29], where statistically significantly narrower airways were found in patients with ANB more than 4.
Alhammadi et al[30] and Xu et al[26] also concluded that patients with skeletal Class II presented with reduced glossopharyngeal airway volume, larger total minimum constricted area in average faces and more nasal minimum constricted area in long faces. Hwang et al[31] reported that a constricted nasopharyngeal airway is associated with retruded mandible and maxilla.
A significant correlation exists between the skeletal facial pattern and upper airway dimensions according to a study done by Shokri et al[32], who concluded that the total airway volume and the mean airway area of class III patients were larger than those in class II patients.
In the present study we did not evaluate class III malocclusion patients, also all the patients were scanned in a sitting upright position so conclusion about obstructive sleep apnea cannot be derived. But there is strong evidence from the large sample size that mandible backward position is correlated with reduced airway.
The mean total airway volume of patients with ANB more than 4 was significantly smaller than that of patients with ANB less than 4. The sub volumes in the pharyngeal airway showed a positive correlation with each other. FMA and facial convexity and mandibular body length also had a significant interrelationship with total volume of airway.
Diminished airway associated with obstructive sleep apnea tends to be typical in patients with Angle class II malocclusion, displaying retrognathic mandible and sagittal discrepancy. Early diagnosis and evaluation of the functional factors in growing children with skeletal discrepancy and features of adenoid hypertrophy (adenoid faces) might be pivotal to restore proper craniofacial growth and treatment outcome stability.
A lot of data has been published related to the identification of airway in the general population, even comparing different cone beam computed tomography machines for the same. However, there is a paucity of data on tomographic evaluation of airways in different skeletal patterns, which is often challenging due to their morphology and plays a vital role in their treatment planning.
Comparing the airway volumes in patients with mandibular retrognathism and those with the normal anteroposterior skeletal relationship.
Cone-beam computed tomography volume scans, and lateral cephalograms, 3-dimensional airway volume and cross-sectional areas of 120 healthy children which were done for orthodontic assessment was evaluated. The subjects were divided into 2 groups based on the angle formed between point A, Nasion and Point B (ANB) values and cephalometric variables (such as anterior and posterior facial height, gonial angle etc.) airway volumes, and cross-sectional measurements were compared using independent t tests. Pearson’s correlation coefficient test was used to detect any relationship of different parts of the airway and between airway volume and 2-dimensional cephalometric variables.
Means and standard deviations for cephalometric, cross-sectional, and volumetric variables were compared. ANB, mandibular body length, facial convexity was statistically highly significant whereas condylion to point A, nasal airway and total airway volume were statistically significant. The nasal airway volume and the superior pharyngeal airway volume had a positive correlation, nasal airway was correlated to middle and total airway superior had a relation with middle, inferior and total airway, middle was related to all other airways, inferior was also related to all the airways except nasal. Lateral cephalometric values were positively correlated with the airway volume with Frankfurt Mandibular Plane Angle and facial convexity showed significant correlations with total airway volume. Additionally, ANB angle was significantly correlated with total airway volume and superior airway.
Position of the mandible has positive correlation with the airway volume. Retrognathic mandible showed decreased overall airway in patients. Facial convexity and length of the mandible also influence the airway.
The current study gives direction for future research on a larger cohort related to mandibular position and airway, linking the two for timely maxillo-facial orthopedic treatment interventions.
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: India
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Mani V, Xie Q S-Editor: Gao CC L-Editor: A P-Editor: Wang LYT
| 1. | Gholinia F, Habibi L, Amrollahi Boyouki M. Cephalometric Evaluation of the Upper Airway in Different Skeletal Classifications of Jaws. J Craniofac Surg. 2019;30:e469-e474. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6] [Cited by in RCA: 12] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 2. | Angle E. Treatment of malocclusion of the teeth. Philadelphia: SS White Manufacturing Company. 1907. |
| 3. | Ricketts RM. Respiratory obstruction syndrome. Am J Orthod. 1968;54:495-507. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 181] [Cited by in RCA: 154] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 4. | Martin O, Muelas L, Viñas MJ. Nasopharyngeal cephalometric study of ideal occlusions. Am J Orthod Dentofacial Orthop 2006; 130: 436.e1-436. e9. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 39] [Article Influence: 2.0] [Reference Citation Analysis (0)] |
| 5. | Uslu-Akcam O. Pharyngeal airway dimensions in skeletal class II: A cephalometric growth study. Imaging Sci Dent. 2017;47:1-9. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 11] [Cited by in RCA: 11] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 6. | Bollhalder J, Hänggi MP, Schätzle M, Markic G, Roos M, Peltomäki TA. Dentofacial and upper airway characteristics of mild and severe class II division 1 subjects. Eur J Orthod. 2013;35:447-453. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 16] [Cited by in RCA: 23] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 7. | Arun T, Isik F, Sayinsu K. Vertical growth changes after adenoidectomy. Angle Orthod. 2003;73:146-150. [PubMed] |
| 8. | Kirjavainen M, Kirjavainen T. Upper airway dimensions in Class II malocclusion. Effects of headgear treatment. Angle Orthod. 2007;77:1046-1053. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 52] [Cited by in RCA: 72] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 9. | Joy A, Park J, Chambers DW, Oh H. Airway and cephalometric changes in adult orthodontic patients after premolar extractions. Angle Orthod. 2020;90:39-46. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 8] [Cited by in RCA: 20] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 10. | Barrera JE, Pau CY, Forest VI, Holbrook AB, Popelka GR. Anatomic measures of upper airway structures in obstructive sleep apnea. World J Otorhinolaryngol Head Neck Surg. 2017;3:85-91. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 31] [Cited by in RCA: 53] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 11. | Ayoub N, Eble P, Kniha K, Peters F, Möhlhenrich SC, Goloborodko E, Hölzle F, Modabber A. Three-dimensional evaluation of the posterior airway space: differences in computed tomography and cone beam computed tomography. Clin Oral Investig. 2019;23:603-609. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 19] [Article Influence: 2.4] [Reference Citation Analysis (0)] |
| 12. | Hofmann E, Schmid M, Lell M, Hirschfelder U. Cone beam computed tomography and low-dose multislice computed tomography in orthodontics and dentistry: a comparative evaluation on image quality and radiation exposure. J Orofac Orthop. 2014;75:384-398. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 19] [Article Influence: 1.6] [Reference Citation Analysis (0)] |
| 13. | Kim KB. How has our interest in the airway changed over 100 years? Am J Orthod Dentofacial Orthop. 2015;148:740-747. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 13] [Cited by in RCA: 15] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 14. | Torres HM, Evangelista K, Torres EM, Estrela C, Leite AF, Valladares-Neto J, Silva MAG. Reliability and validity of two software systems used to measure the pharyngeal airway space in three-dimensional analysis. Int J Oral Maxillofac Surg. 2020;49:602-613. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4] [Cited by in RCA: 8] [Article Influence: 1.1] [Reference Citation Analysis (0)] |
| 15. | Major MP, Flores-Mir C, Major PW. Assessment of lateral cephalometric diagnosis of adenoid hypertrophy and posterior upper airway obstruction: a systematic review. Am J Orthod Dentofacial Orthop. 2006;130:700-708. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 122] [Cited by in RCA: 121] [Article Influence: 6.1] [Reference Citation Analysis (0)] |
| 16. | Neelapu BC, Kharbanda OP, Sardana HK, Balachandran R, Sardana V, Kapoor P, Gupta A, Vasamsetti S. Craniofacial and upper airway morphology in adult obstructive sleep apnea patients: A systematic review and meta-analysis of cephalometric studies. Sleep Med Rev. 2017;31:79-90. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 262] [Article Influence: 26.2] [Reference Citation Analysis (0)] |
| 17. | Hsu WE, Wu TY. Comparison of upper airway measurement by lateral cephalogram in upright position and CBCT in supine position. J Dent Sci. 2019;14:185-191. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 9] [Cited by in RCA: 19] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 18. | Laine-Alava MT, Minkkinen UK. Should a history of nasal symptoms be considered when estimating nasal patency? Angle Orthod. 1999;69:126-132. [PubMed] |
| 19. | Sam A, Currie K, Oh H, Flores-Mir C, Lagravére-Vich M. Reliability of different three-dimensional cephalometric landmarks in cone-beam computed tomography : A systematic review. Angle Orthod. 2019;89:317-332. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 25] [Cited by in RCA: 50] [Article Influence: 6.3] [Reference Citation Analysis (0)] |
| 20. | Park J, Baumrind S, Curry S, Carlson SK, Boyd RL, Oh H. Reliability of 3D dental and skeletal landmarks on CBCT images. Angle Orthod. 2019;89:758-767. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 11] [Cited by in RCA: 29] [Article Influence: 4.1] [Reference Citation Analysis (0)] |
| 21. | Grewal H, Sidhu SS, Kharbanda OP. A cephalometric appraisal of dento-facial and soft tissue pattern in Indo-Aryans. J Pierre Fauchard Acad. 1994;8:87-96. [PubMed] |
| 22. | Li L, Liu H, Cheng H, Han Y, Wang C, Chen Y, Song J, Liu D. CBCT evaluation of the upper airway morphological changes in growing patients of class II division 1 malocclusion with mandibular retrusion using twin block appliance: a comparative research. PLoS One. 2014;9:e94378. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 44] [Cited by in RCA: 57] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 23. | Kamaruddin N, Daud F, Yusof A, Aziz ME, Rajion ZA. Comparison of automatic airway analysis function of Invivo5 and Romexis software. PeerJ. 2019;7:e6319. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 8] [Cited by in RCA: 9] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 24. | Ceylan I, Oktay H. A study on the pharyngeal size in different skeletal patterns. Am J Orthod Dentofacial Orthop. 1995;108:69-75. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 111] [Article Influence: 3.6] [Reference Citation Analysis (0)] |
| 25. | de Freitas MR, Alcazar NM, Janson G, de Freitas KM, Henriques JF. Upper and lower pharyngeal airways in subjects with Class I and Class II malocclusions and different growth patterns. Am J Orthod Dentofacial Orthop. 2006;130:742-745. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 81] [Cited by in RCA: 92] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 26. | Xu J, Sun R, Wang L, Hu X. Cone-beam evaluation of pharyngeal airway space in adult skeletal Class II patients with different condylar positions. Angle Orthod. 2019;89:312-316. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 4] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 27. | Di Carlo G, Polimeni A, Melsen B, Cattaneo PM. The relationship between upper airways and craniofacial morphology studied in 3D. A CBCT study. Orthod Craniofac Res. 2015;18:1-11. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 56] [Cited by in RCA: 71] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 28. | Dunn GF, Green LJ, Cunat JJ. Relationships between variation of mandibular morphology and variation of nasopharyngeal airway size in monozygotic twins. Angle Orthod. 1973;43:129-135. [PubMed] |
| 29. | Lopatienė K, Dabkutė A, Juškevičiūtė V. Vertical and sagittal morphology of the facial skeleton and the pharyngeal airway. Stomatologija. 2016;18:21-25. [PubMed] |
| 30. | Alhammadi MS, Almashraqi AA, Helboub E, Almahdi S, Jali T, Atafi A, Alomar F. Pharyngeal airway spaces in different skeletal malocclusions: a CBCT 3D assessment. Cranio. 2019: 1-10. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 10] [Cited by in RCA: 26] [Article Influence: 3.7] [Reference Citation Analysis (1)] |
| 31. |
Hwang YI, Lee KH, Lee KJ, Kim SC, Cho HJ, Cheon SH, et al Effect of airway and tongue in facial morphology of prepubertal Class I, II children.
|
| 32. | Shokri A, Mollabashi V, Zahedi F, Tapak L. Position of the hyoid bone and its correlation with airway dimensions in different classes of skeletal malocclusion using cone-beam computed tomography. Imaging Sci Dent. 2020;50:105-115. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4] [Cited by in RCA: 10] [Article Influence: 1.7] [Reference Citation Analysis (0)] |