Published online Aug 28, 2020. doi: 10.4329/wjr.v12.i8.142
Peer-review started: June 3, 2020
First decision: July 4, 2020
Revised: July 6, 2020
Accepted: August 16, 2020
Article in press: August 16, 2020
Published online: August 28, 2020
Processing time: 81 Days and 19.1 Hours
The purpose of this study is to review the published literature for the range of radiographic findings present in patients suffering from coronavirus disease 2019 infection. This novel corona virus is currently the cause of a worldwide pandemic. Pulmonary symptoms and signs dominate the clinical picture and radiologists are called upon to evaluate chest radiographs (CXR) and computed tomography (CT) images to assess for infiltrates and to define their extent, distribution and progression. Multiple studies attempt to characterize the disease course by looking at the timing of imaging relative to the onset of symptoms. In general, plain CXR show bilateral disease with a tendency toward the lung periphery and have an appearance most consistent with viral pneumonia. Chest CT images are most notable for showing bilateral and peripheral ground glass and consolidated opacities and are marked by an absence of concomitant pulmonary nodules, cavitation, adenopathy and pleural effusions. Published literature mentioning organ systems aside from pulmonary manifestations are relatively less common, yet present and are addressed in this review. Similarly, publications focusing on imaging modalities aside from CXR and chest CT are sparse in this evolving crisis and are likewise addressed in this review. The role of imaging is examined as it is currently being debated in the medical community, which is not at all surprising considering the highly infectious nature of Severe Acute Respiratory Syndrome coronavirus 2.
Core tip: The world is presently confronting a global pandemic caused by a novel beta coronavirus. In hospitalized patients, coronavirus disease 2019 causes clinical lung disease with a range of severity. Plain chest radiography and chest computed tomography dominate the imaging landscape. Radiologists must be prepared to recognize the radiographic findings that often include bilateral and peripheral infiltrates on chest radiographs and bilateral and peripheral ground glass and consolidated opacities on computed tomography among other findings. The role of imaging as it relates to this disease is also covered.
- Citation: Kaufman AE, Naidu S, Ramachandran S, Kaufman DS, Fayad ZA, Mani V. Review of radiographic findings in COVID-19. World J Radiol 2020; 12(8): 142-155
- URL: https://www.wjgnet.com/1949-8470/full/v12/i8/142.htm
- DOI: https://dx.doi.org/10.4329/wjr.v12.i8.142
The World Health Organization (WHO) announced a disease outbreak on January 5, 2020, reporting that their China Country Office received notification on December 31, 2019 of multiple cases of pneumonia of undetermined etiology in Wuhan City in Hubei Province, China[1]. This outbreak has since ballooned into a worldwide pandemic. Patterns of radiographic findings have now been described in this illness. The intention of this study is to review published literature for the range of radiographic findings in these patients and review the overall role of imaging.
The WHO’s original report mentioned 44 cases diagnosed as of January 3, 2020 with 11 (25%) of those stricken being severely ill. Some of the patients shared a common work location at the Huanan Seafood market[1]. The seafood market being a common link suggests the possibility of animal to human transmission. A betacoronavirus has been identified as the cause of the illness and is similar to the viruses that cause severe acute respiratory syndrome (SARS) and Middle East Respiratory Syndrome[2]. Based upon comparative genomic analysis, this novel coronavirus likely emerged from an animal reservoir of a bat, with a Malayan pangolin potentially serving as an intermediate host[3].
The novel virus was officially named “Severe Acute Respiratory Syndrome coronavirus 2 (SARS-CoV-2)” by the International Committee on Taxonomy of Viruses[2]. On February 11, 2020 the WHO named the disease caused by the virus “coronavirus disease 2019 (COVID-19)”[4]. In rapid course over the last few months, the virus has spread worldwide causing a global pandemic. As of April 18, 2020, there have been 2160207 confirmed cases and 146088 deaths reported globally[5]. Also as of April 18, 2020, the United States has 690714 confirmed cases and 35443 deaths[6].
The presentation of COVID-19 is most remarkable for pulmonary symptoms that if become serious can quickly turn deadly. In a series of 41 patients, the clinical symptoms of COVID-19 commonly included fever (98%), cough (76%), and myalgia (44%)[7]. Dyspnea was present in 22 of 40 patients (55%) in the same study and became apparent a mean of 8.0 d after onset of symptoms. Respiratory symptoms progressed to acute respiratory distress syndrome (ARDS) in 29% of all cases. Another study focused on 21 moderately ill hospitalized patients who did not require oxygen therapy and found, among this group, fever (86%) and cough (57%) to be the most common presenting symptoms[8]. According to the Centers for Disease Control and Prevention, pulmonary disease is considered severe in 14% of cases with symptoms including dyspnea, hypoxia or > 50% lung involvement on imaging and is considered critical in 5% of cases with symptoms including respiratory failure, shock or multi-organ failure[9]. With the accumulation of data on hospitalized patients, it is now possible to examine characteristics and comorbidities related to COVID-19. A large study of 5700 patients in the New York City area showed hypertension (56.6%), obesity (41.7%) and diabetes (33.8%) to be the most common comorbidities[10].
Patterns of histopathology have been described to a limited extent. According to Xu et al[11] biopsy and autopsy reports are “barely accessible” in this fast-moving crisis. This group published a pathology case report of postmortem biopsy specimens from a patient that died of a severe case of COVID-19. The pulmonary specimens showed desquamated pneumocytes and hyaline membrane formation consistent with ARDS in the right lung, and pulmonary edema and hyaline membrane formation consistent with early ARDS in the left lung. Bilateral interstitial infiltrates, predominantly lymphocytic in nature, were also seen on histopathologic evaluation. Additionally, there were viral cytopathic-type changes in pneumocytes within the alveolar spaces. This single case study did not identify any viral inclusions within the pathology specimens. A more recent report of three cases where histopathology was obtained showed direct evidence on electron microscopy of viral inclusions within endothelial cells in one patient’s transplanted kidney[12]. Using light microscopy and immunohistochemical staining, the group also found endothelial cell inflammation and apoptosis in many organs including the heart, kidney, liver and small bowel. Laboratory findings of bloodwork on COVID-19 patients generally show a picture of viral infection with low lymphocyte counts. In a study of 99 patients, most subjects had a decreased absolute value of lymphocytes in the peripheral blood[13]. Another study of 34 patients found 50% had a decreased lymphocyte count[14]. This finding may be secondary to the virus causing an immune response in which chemokines recruit specific immune cells such as lymphocytes to the site of infection[13,15]. In addition, studies have found increased prothrombin time and D-dimer in COVID-19 patients admitted to the intensive care unit[16,17]. Other laboratory findings include elevations in C-reactive protein and erythrocyte sedimentation rate[13].
This study reviews the published literature for the range of radiographic findings evident in patients suffering from SARS-CoV-2 infection. Towards this end, we searched PubMed for the following terms on April 6, 2020 without imposing a starting date limit: “Covid”, “19”, “Radiology”, “Radiography”, “Computed Tomography (CT)”, “Imaging”, “Chest Radiographs (CXR)”, “X-Ray”, “Positron Emission Tomography (PET)”, “Ultrasound”, “Sonography”, “Magnetic Resonance Imaging”, and “Angiography”. English language, peer reviewed articles were looked at exclusively. After excluding articles that were outside the realm of investigation, a total of 133 articles were systematically reviewed to extract any radiographic data, both objective and subjective. In light of the swift development of new and increasing data, the study also included a look at preprinted manuscripts available on medRxiv.org and covered those that appeared most relevant with an indication of the unpublished status to date. The data within each article were organized in groups into the following categories: Study design (case study or series or letter etc.); Number of cases; Location (Country where imaged); Imaging Modality; Biological Sex; Age; COVID-19 lab diagnosis; Radiographic findings. The subtopics for radiographic findings include disease distribution, progression and focal and generalized patterns of disease.
Study design was represented by 39 case reports and 78 case series reports. These were all retrospective review studies. Relevant letters to journals and review articles were also reviewed for background. The single case reports have a male:female ratio of 23:15, while the series reports have a male to female ratio of 1982:2016. The range of number of participants within each series report shows very small series of 2-10 cases being the most common (28/78) and representing over one third of these studies (35.9%). Table 1 shows the breakdown of number of participants per study.
| Participants (range) | Papers |
| 2-10 | 28 |
| 11-20 | 4 |
| 21-30 | 9 |
| 31-40 | 3 |
| 41-50 | 4 |
| 51-60 | 6 |
| 61-70 | 4 |
| 71-80 | 4 |
| 81-90 | 4 |
| 91-100 | 2 |
| 101+ | 10 |
The case distribution by country where the imaging was performed in this data set is as follows: China 99; Italy 8; United States 5; Korea 4; Taiwan 3; Germany 2; Japan 2; Singapore 2; Turkey 2; Brazil 1; Thailand 1; and Vietnam 1. The age range distribution reveals single case reports have an age range of 27-79, with a mean age of 47.1 years old (with standard deviation of 17.4), while series reports have an age range of 2 mo-95 years old. It is difficult to assess the number of laboratory proven cases in our literature review, however the vast majority of studies mention that the cases were laboratory confirmed mostly using real-time reverse transcription polymerase chain reaction (RT-PCR) technique on samples obtained via bronchoalveolar lavage, endotracheal aspirate, nasopharyngeal swab or oropharyngeal swab.
The number of instances in which an imaging modality was utilized and focused upon in the various studies is as follows: CT 121, plain CXR 8, PET 4, and Sonography 4. Due to the significant and often fulminant pulmonary pathology present with SARS-CoV-2 infection, radiographic imaging has concentrated mainly on CXR and CT. An overall review of the published data shows pulmonary findings on both CXR and chest CT with infiltrates nearly universally bilateral, peripheral and often multi-lobar with adjacent reactive pleural changes. Ground glass opacities (GGO) were frequently present either with or without concurrent consolidation, crazy paving (ground glass density superimposed with thickening of the interlobular septa and intralobular lines) and rounded morphology. Pleural effusions, lymphadenopathy and pneumothorax are seldom mentioned. Other imaging modalities noted in our review included rare mention of 18F labeled fluorodeoxyglucose positron emission tomography (18F-FDG-PET), sonography and magnetic resonance (MR).
CXR are described in the literature but the data are relatively scant as compared to CT. In a series of two female patients, the CXR for each subject showed bilateral consolidation in the lower lung fields with follow up showing patchy consolidation[18]. Another case study included chest radiography, the first described in Taiwan, and showed bilateral, ill-defined and patchy opacities consistent with pneumonia[17]. Another study of 21 patients which focused on chest CT also included 5 patients with CXR[19]. In this study the CXR failed to demonstrate early GGO in two patients and missed the peripheral nature of the infiltrates in another patient. This suggests that CXR performed early in disease course may lack the sensitivity needed to detect COVID-19 related lung disease. Such studies led to the use of chest CT to diagnose COVID-19 lung disease at earlier stages, particularly in the earlier months of the outbreak.
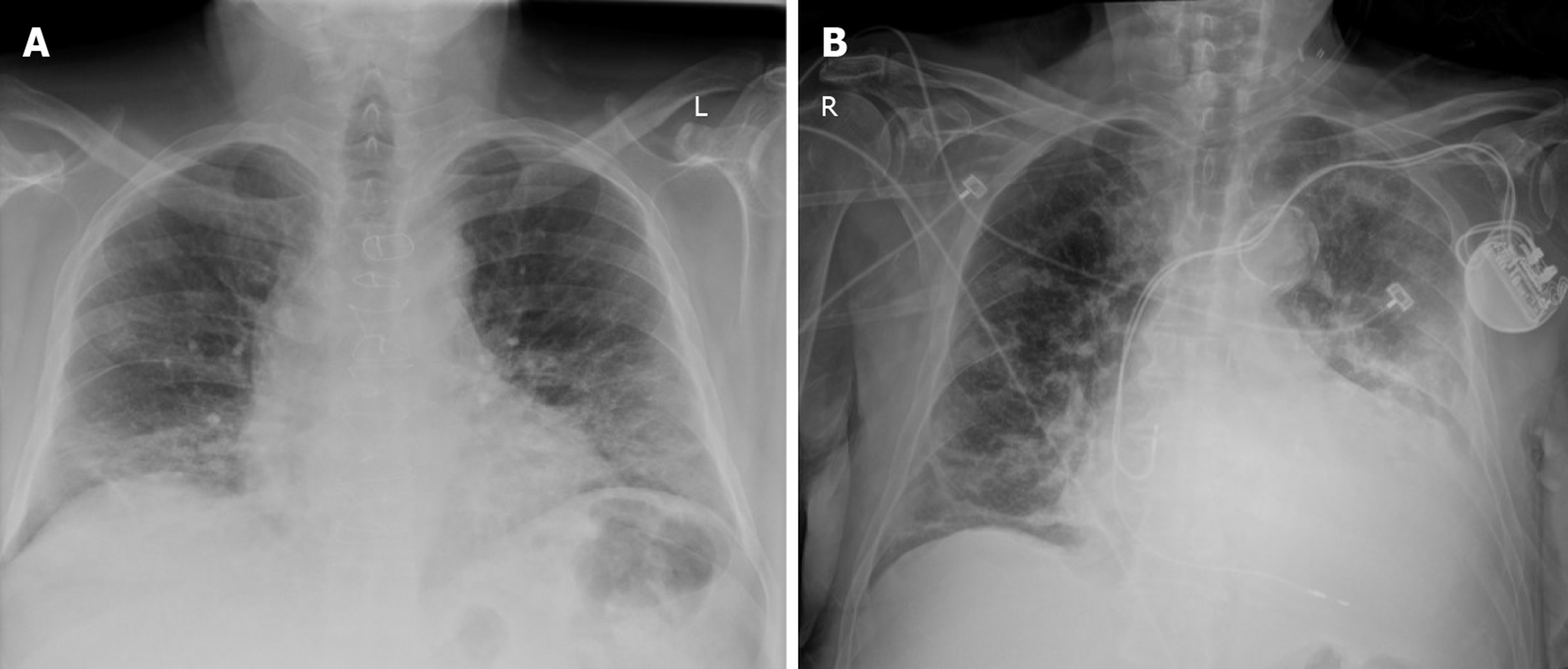
A larger study out of Hong Kong examines the usefulness of CXR[20]. They studied 64 RT-PCR positive cases of COVID-19 and determined consolidation to be the most common CXR finding in 30/64 (47%). Also commonly noted were GGO 21/64 (33%), peripheral infiltrates 26/64 (41%), lower lung zone involvement 32/64 (50%) and bilaterality 32/64 (50%). Pleural effusion was uncommonly present in 2/64 (3%). They found peak severity on CXR at 10-12 d from the onset of symptoms. They concluded that CXR depicts similar findings as CT. Their study found RT-PCR to be more sensitive 58/64 (91%) to early disease than the CXR 44/64 (69%). Similarly, a recent study out of Italy retrospectively analyzed 240 RT-PCR positive cases to determine the most common lung alterations found using CXR with respect to time since symptom onset[21]. Abnormalities most frequently occurred bilaterally and peripherally, with reticular alteration being the most common finding in early phases of the disease. GGO became predominant in later phases and consolidation, while occurring less frequently, also increased with time. CXR showed abnormalities in 75% of the RT-PCR confirmed cases. Therefore, while it has not generally been recommended due to low sensitivity, these observations confirm recent suggestions that CXR should be considered as a feasible imaging technique in diagnosing COVID pneumonia. Figure 1 depicts original CXR in laboratory positive cases of COVID-19 from our institution.
CT has been widely used to evaluate lung pathology associated with COVID-19. During the time period reviewed, the larger series studies in which CT was performed reveal a range of pulmonary findings. Han et al[22] in a study of 108 COVID-19 patients found 70 (65%) had infiltrates in two or more lobes with almost all the infiltrates (97%) peripherally located. When only present in a single lobe, the right lower lobe was the most common in 30/38 (79%). Overall, the findings commonly included patchy GGO (86%) and GGO with consolidation (41%), vascular thickening (80%), crazy paving (40%), air bronchograms (48%), and a halo sign (an area of GGO surrounded by consolidation, 64%). Lymph node enlargement, effusion and pleural thickening were absent.
Liu et al[23] in a study of 73 COVID-19 patients grouped laboratory positive patients by disease severity in order to assess for differences in CT appearance. In mild illness (6 patients), 8% had bilateral infiltrates, 50% showed no lung changes, and 50% had an enlarged hilus and thickened lung texture. In moderate illness (43 patients), 100% of cases showed GGO bilaterally and peripherally with GGO as the single manifestation in the lungs in 12/43 (28%). Other common findings in this group were paving stone sign (35%), inter and intralobular septal thickening (27%) and air bronchogram (7%). In severe disease (21 patients), 76% had bilateral, extensive GGO, 24% had consolidation and 67% had peribronchial thickening. Critical disease (3 patients) showed confluent lesions, multi-lobe involvement, pulmonary fibrosis and white lung. Short term follow-up CT scans show dramatic improvement in 12 cases with only four patients having residual linear densities. Overall these larger studies highlight the prevalence of GGO in the lung periphery often bilaterally, that with severe disease tend to coalesce into more dense consolidation.
While the majority of the articles we reviewed were imaged in Asia, recent studies also suggest similar radiographic findings in other areas of the world. For example, in one study from Italy, GGO were the most prominent abnormality noted on CT in RT-PCR positive COVID-19 patients where they were present in 58/58 (100%) of cases[24]. Commonly noted findings in this study were: Multi-lobe involvement (93%), bilateral infiltrates (91%), consolidation (72%), crazy paving pattern (39%), rounded morphology (32%) and linear opacities (27%), while pleural effusion (3%) and cavitation (0%) were uncommonly seen. Additional interesting findings from this Italian study include perilesional sub-segmental vessels enlargement (> 3 mm, mean of 3.9 mm ± 0.6) in 89% of cases. In our review, vessel enlargement in COVID-19 was not often mentioned, but was described by Han et al[22] (80%), Bai et al[25] (59%), and Zhao et al[26] (71%). The notable contrast in this Italian study was the presence of mediastinal lymphadenopathy that was noted in 58% of cases.
Disease progression through CT imaging is documented by Shi et al[27] in a case study followed over three weeks with four CT scans[27]. This patient was SARS-CoV-2 positive and was treated with anti-viral and anti-inflammatory medications and supportive care. On day eight from onset of symptoms, there are bilateral peripheral multifocal GGO. A day 15 scan shows mixed GGO and consolidation. A day 19 scan shows similar yet resolving findings and is followed by a day 31 scan showing complete healing. A larger series of moderately ill hospitalized patients demonstrated a peak in severity of CT findings approximately 10 d after symptom onset[8]. Ling et al[28] described their experience with 295 RT-PCR positive COVID-19 cases. They found 49 cases had negative initial CT scans and 15/49 developed findings after 3-6 d, while 34/49 CT scans remained negative. They suggest that since clinical symptoms and CT findings are not always present in COVID-19 careful screening and isolation practices are critical.
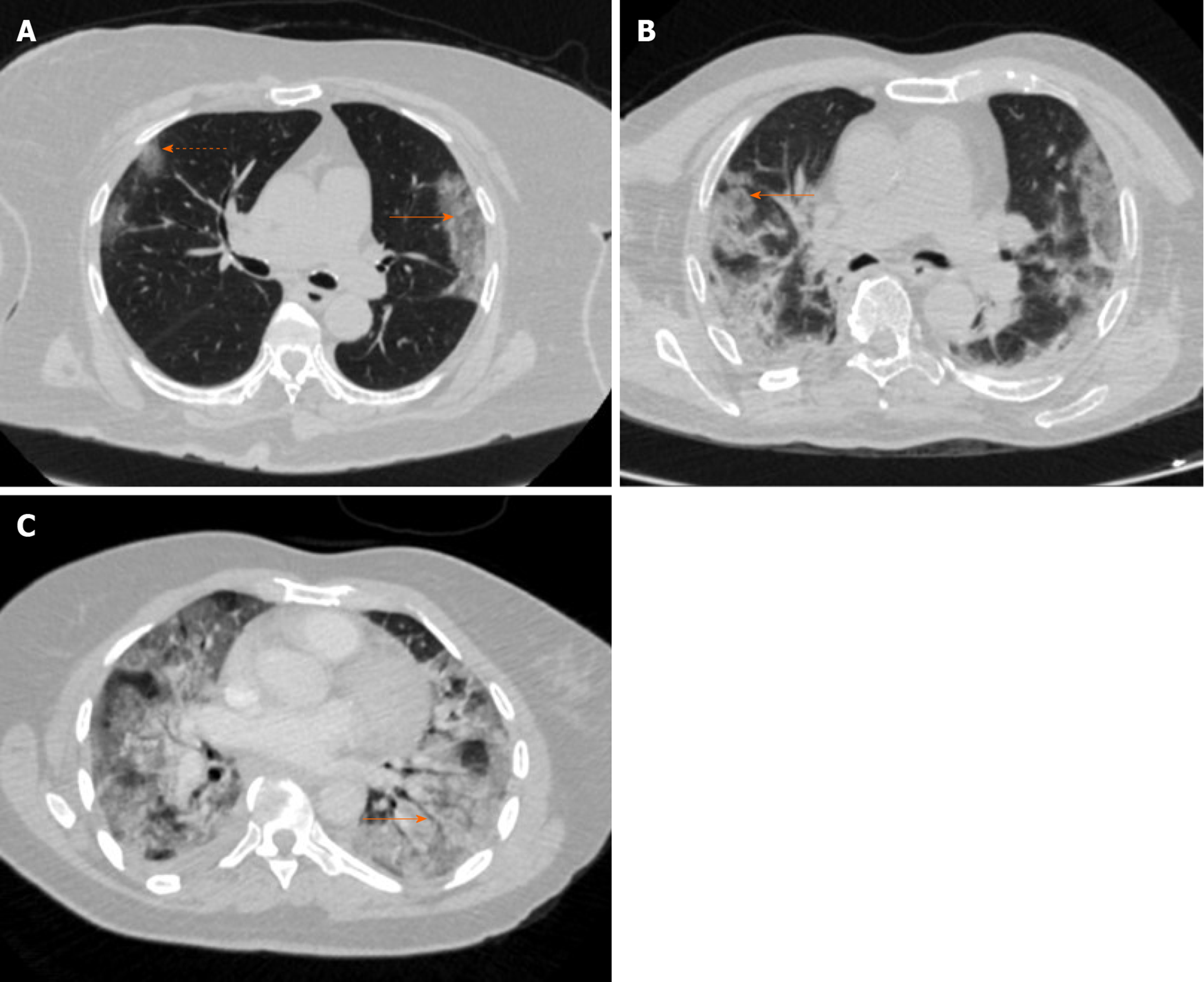
Bernheim et al[29] give another perspective on disease progression by looking at CT appearance relative to duration of symptoms in their review of 121 patients from China. Bilaterality increased in incidence when patients were scanned later relative to symptom onset: Early (0-2 d, 28%), intermediate (3-5 d, 76%) and late (6-12 d, 88%). Additionally, the group found the following increasing over time: GGO and consolidation both bilaterally and peripherally, total lung involvement, linear opacities, crazy paving pattern and reverse halo sign (central ground glass density with surrounding consolidation). A rounded morphology of the infiltrates was seen in 54% of all the patients and was most prevalent in the intermediate group (67%). Figure 2 depicts original chest CT images in laboratory positive cases of SARS-CoV-2 from our institution.
Regarding distribution of pulmonary findings in COVID-19, two studies showed a predominance of multilobar involvement. Five lobes were involved in 27% of 121 cases and 44.4% of 63 cases[29,30]. This is consistent with an imaging picture of viral pneumonia rather than bacterial lobar pneumonia. Regarding central versus peripheral distribution of infiltrates, Bernheim’s group found none of the 121 cases with a central peribronchovascular distribution[29]. Conversely, peripheral pathology was present in 52% of all cases, and became more prominent as a finding later in the disease course (64% in intermediate time-point scans, 72% in late scans).
A few studies evaluated COVID-19 in a pediatric population. In a series of five confirmed positive cases of (age range of 10 mo – 6 years), three pediatric patients had modest patchy GGO. The other two cases showed normal findings on CT, and none of these patients had CXR[31]. Another group that evaluated 4 pediatric cases found the CT scans to be nonspecific and mild[32]. These small samples suggest milder disease in a pediatric population.
A more recent study includes radiographic findings from nine COVID-19 infected pediatric patients[33]. Compared to adults, these patients also presented with less severe symptoms. Ground glass opacities, patchy, high-density shadows, and parenchymal bands were the most common CT findings, however, some of these infected pediatric patients showed no abnormal CT features.
A few investigations looked at COVID-19 in pregnancy. One study followed 15 confirmed COVID-19 positive pregnant women ranging from gestational week 12-38[34]. CT findings were reported to be similar to non-pregnant patients with GGO early in the time course, and further consolidation and crazy paving pattern with progression. Delivery itself did not exacerbate the pneumonia in 10 cesarean sections and one vaginal delivery. Additionally, this group found no infections in the neonates (n = 11). Another study of COVID-19 positive patients (41 pregnant; 14 non-pregnant) examined pulmonary findings on CT and showed that consolidation was statistically more common in pregnant patients than non-pregnant patients, and conversely, GGO with reticulation was significantly less common in pregnant patients than non-pregnant patients[23]. These differences may relate to the physiological and anatomic changes of pregnancy.
Although CT and CXR predominate the imaging landscape in COVID-19, there are a few studies of other modalities. Qin et al[35] studied 4 presumptively positive cases of COVID-19 that underwent 18F-FDG PET/CT. According to the group, the nuclear scans were performed before the infectivity of the virus was apparent and this was the first PET study on presumptive positive patients in Wuhan, China. They found uptake in ground glass and/or consolidated opacities peripherally located in more than two lobes of the lung. Of note, they found no disseminated lesions suggesting tropism of the disease for the lungs. Also, in three of four cases, they found 18F-FDG uptake in nearby (hilar, mediastinal and subclavian) non-enlarged lymph nodes suggesting lymphadenitis. There was also a single case report in the literature of 18F-FDG PET/CT imaging in a COVID-19 confirmed positive case that shows avid uptake of the tracer in areas of consolidation bilaterally and uptake in hilar and mediastinal lymph nodes as well[36]. The risk to perform nuclear scans in COVID-19 patients is noted, as the high infectivity of the virus and the extended length of time the patient stays in the radiology suite for PET imaging is much lengthier than most other tests[37].
There is a published case report and a 20 case series report describing the use of sonography in COVID-19, in a point-of-care setting[38,39]. The lung sonograms were performed using a portable device at the bedside and in both studies demonstrated evidence of irregular pleural lines, sub-pleural consolidation, areas of white thick lungs and thick irregular vertical artifacts suggesting interstitial-alveolar damage. The authors highlight the advantage of reducing infectious exposure to other patients and healthcare workers by performing the study at the bedside. In addition, they believe it to be easily repeatable and widely available particularly in a low resource setting.
A more recently published paper by Soldati et al[40] is a proposed guide to standardize the performance of lung ultrasound exams in patients with COVID-19. The proposal covers acquisition protocol, reviews anatomical landmarks, highlights salient findings and proposes a scoring mechanism. The group presents an important point that because sonography is more portable and available in a wider range of settings throughout the world, as compared to other imaging modalities, it is more accessible to low and middle income countries and will, thus, improve the disparity often present in trials with imaging endpoints. Regarding specificity of lung ultrasound, a separate study showed that in the not-very-early stages of COVID-19, lung ultrasound can provide relative disease specificity when bilateral and patchy artifactual findings and signs of multifocal white lung are present[41]. In addition, there is a more recently published study of lung ultrasound (LUS) in a pediatric population with confirmed COVID-19[42]. This was an observational study of 10 consecutive symptomatic pediatric patients admitted to two tertiary medical centers in Rome. In these cases, LUS was performed using the Soldati et al[40] acquisition protocol referenced above. A LUS was performed via a portable device at the bedside while awaiting RT-PCR results. Abnormal LUS findings were noted in every case. Vertical artifacts (70%) and pleural irregularities (60%) were the most common abnormalities detected. Pleural effusions were notably absent in all 10 patients. The group argues LUS has a distinct advantage in following COVID-19 in children because it does not use ionizing radiation.
A panel of international experts in sonography reviewed its use in COVID-19 and cover a multitude of important topics[43]. They discuss the need to establish appropriate use of sonography by thoroughly evaluating both the sensitivity of the technique in varying degrees of COVID-19 lung involvement and the positive and negative predictive values under varied conditions. The group highlights the potential benefits of sonography to evaluate for the following: Progression of pulmonary involvement, post-procedure changes, presence of pneumothorax, ventricular function, fluid status and presence and drainage of pleural effusions. The challenges of using sonography are described and include prolonged exposure to the operator, the need for scanner boards and transducers that can be disinfected thoroughly and the corollary of the need for scanner covers that protect the machine itself without overheating it.
There is a report of MR being used to evaluate a single SARS-CoV-2 patient showing mild pulmonary symptoms but with a severe progressive encephalopathy[44]. A gadolinium enhanced MR scan of the brain was performed but revealed no pathology. A more recent case report compares lung findings in a chest CT to those found in a cardiac MR performed two days later to rule out myocarditis[45]. The black-blood single shot fast spin echo sequence showed pulmonary infiltrates and consolidation closely matching those in the CT.
As emphasized by the overwhelming dominance of pulmonary symptoms and the PET/CT finding of tropism for the lungs, it is not surprising that there is little written regarding radiographic findings in other organs systems. The literature review revealed cardiac injury, defined by elevation in serum cardiac biomarkers, is common among patients hospitalized with COVID-19. In a study from Wuhan, China of 416 consecutive hospitalized patients, cardiac injury was found in 82 (19.7%) cases and was statistically associated with older age, comorbidities such as hypertension, a tendency to require mechanical ventilation and higher in-hospital mortality[46]. This same study mentioned renal injury in 8/416 (1.9%) and coagulopathy in 12/416 (2.9%). Another study in preprint found acute myocardial injury in 6/53 (11.3%) cases[47]. Neurologic issues have also been reported in the literature. In a different study from Wuhan, China, neurologic manifestations were found in 78 of 214 (36.4%) consecutive hospitalized patients[48]. Symptoms included headache, loss of taste or smell, dizziness, impaired consciousness, myopathy and stroke. Gastrointestinal symptoms associated with COVID-19 have been described as well. For example, one study found 5 of 34 (14.7%) patients complained of diarrhea upon hospital admission[17].
The use of imaging in COVID-19 is debated within the radiology community. Many of the major radiology professional societies have recently come out with statements on this subject. The American College of Radiology states: Viral testing is the only specific method of diagnosis as recommended by the Centers for Disease Control; generally, chest imaging findings are not specific; and chest CT is considered “Usually Not Appropriate” in standard acute respiratory illness[49]. Because of these considerations, joined with additional complex issues relating to infection control, the American College of Radiology says performing CT is not advised for screening for COVID-19 infection. They advise radiologists to become acquainted with the CT appearance of the disease so it can be recognized in patients imaged for a concurrent medical issue. Similarly, the Society for Cardiovascular Magnetic Resonance states cardiovascular MR should not be performed in COVID-19 patients unless absolutely clinically necessary[50]. Our medical center follows these guidelines and reserves advanced imaging for when it is critical to clinical care, rather than using it as a first-line diagnostic tool in COVID-19 patients.
To understand the debate it is important to know that the current diagnostic gold standard for SARS-CoV-2 infection is laboratory RT-PCR testing to identify viral ribonucleic acid from the aforementioned biological samples. This process, in many settings, takes significantly longer than performing a CT scan and has been shown to have a lower sensitivity (60%) than chest CT (97%)[51]. Similarly, another group showed a lower sensitivity of RT-PCR (71%) as compared to chest CT (98%)[52]. Thus, diagnostic testing via chest CT was recommended in various studies and has been used clinically, mostly outside the United States. For example, an Italian preprint study supports using chest CT because it is known as the gold standard for interstitial pneumonia[53]. They found CT to have a sensitivity of 95.48%. The group also points out that diagnostic accuracy is influenced by the prevalence of the disease, and thus, during a pandemic the predictive value gives a more precise measure of CT reliability. Their study showed a high negative predictive value of > 90% and positive predictive value of 69%-84%. Therefore, they argue CT is fast, reliable and good for first-line triage where the virus is widespread.
In a region of Japan with lower disease incidence, Himoto et al[54] studied the diagnostic performance of typical CT features of COVID-19 when imaged more than three days following symptom onset. The group concluded CT has an important supplemental role in identifying COVID-19 pneumonia and differentiating it from other forms of pneumonia, which can be clinically useful while awaiting the RT-PCR results. Specifically, of the 21 patients they studied, six were RT-PCR positive for COVID-19 and 15 suffered from pneumonia of other origin. Of the six positive cases, 100% showed GGO with or without consolidation bilaterally with peripheral predominance. Rounded morphology and a higher number of lobes affected were significantly higher in COVID-19 infection as compared to other pneumoniae. Their radiologists showed high sensitivity (100% and 83%) in detecting COVID-19 pneumonia. They found the highest specificity (93% and 80%) when using criteria of bilateral GGO, peripheral predominance and absence of any airway abnormalities, nodules, mediastinal adenopathy and pleural effusion.
Dangis et al[55] in a report in press, found low-dose submillisievert chest CT to show a high degree of sensitivity, specificity, positive predictive value, negative predictive value and accuracy overall (86,7%, 93.6%, 91.1%, 90.3%, and 90.2%, respectively) and after 48 h of symptoms (95.6%, 93.2%, 91.5%, 96.5%, and 94.4%, respectively). They also relay the advantage of finding concurrent bacterial pneumonia in COVID-19 true positive cases (7 of 72 cases, 9.7%) and finding alternative diagnoses in true negative cases (18 of 102 cases, 17.6%). As described early on in this crisis by Kim[56], the role of the radiologist includes detecting lung abnormalities at the earliest time point in the disease course, gauging the severity and progress of disease, and identifying radiographic findings that suggest concomitant bacterial co-infections. Overall, in the current state of affairs when testing is readily and quickly available, CT is reserved for complicated cases and to rule out concomitant pathology. Alternatively, where RT-PCR testing is slower, CT imaging can be used to characterize and diagnose COVID-19 pneumonia.
The American College of Radiology emphasizes in boldface type on their website, “... knowledge of this new condition is rapidly evolving, and not all of the published and publicly available information is complete or up-to-date”[49]. The medical community needs to be circumspect in decision-making based upon the available literature. In a recent report by Wynants et al[57] a critical assessment is made of published and preprint reports of prediction models of COVID-19 disease. The group found all 31 of the analyzed prediction models to have a high risk of bias relating to a multitude of reasons including using non-representative controls, excluding cases that had not experienced the target event by the end of the study and performing inadequate statistical analyses. They recommend that the medical community proceed with caution in using the prediction models available. Clinical radiologists should stay abreast of updated professional guidelines and, as always, read current literature with a critical eye. During a pandemic, there is value in reading review articles, such as Sun et al[58] to remain aware of accepted science in the field.
Recent additions from professional societies include comprehensive consensus statements that provide critically important guidance to radiologists. A notable example was released by the Canadian Society of Thoracic Radiology in conjunction with the Canadian Association of Radiologists covering thoracic imaging in suspected and confirmed COVID-19 cases[59]. As this particular consensus statement covers a multitude of key practical issues, clinical radiologists should be familiar with its contents and refer to it as needed.
Considering the contagion, the world faces, caution has clear benefits. Imaging at point-of-care is safer. However, if thoughtful and viable institutional policies are in place to create a decontaminated and safe radiology suite, then advanced imaging should be instituted to better understand the manifestation and progression of this deadly disease. The risk of being too prudent in investigating COVID-19 is that the medical community does not know enough and needs to know more. SARS-CoV-2 infections could spread further and wider over time before we fully understand its mechanism of action. Clearly, we need to be vigilant about safety, but we need to be equally vigilant about elucidating the pathology at hand. Collecting robust data via advanced imaging in a safe environment should also be a priority.
For radiologists, another pertinent issue is overall quality in reported findings. In a recent article, Salehi et al[60] systematically reviewed 37 published studies on diagnostic findings in CT of the chest in COVID-19 disease[60]. They found wide variability in the terminology used in imaging reporting which can lead to confusion for healthcare providers. This is the first proposal of a reporting and data system for COVID-19. The group proposed a comprehensive lexicon to be used for typical and atypical findings in CT of COVID-19 patients with a grading system corresponding to the suspicion for lung involvement. Radiology practices should strongly consider implementing a structured reporting system for consistency and optimization in quality of patient care.
As more is learned about COVID-19, there likely will be an expanded role for imaging and radiology research. For example, mentioned above in relatively few publications was enlargement of pulmonary vessels[22,24-26]. This finding potentially could have been present in other studies but was perhaps missed because vascular enlargement is most commonly measured subjectively in clinical radiology. As was noted in laboratory findings, these patients can have increased prothrombin time and D-dimer levels suggesting a hypercoagulable state[17]. Thus, CT angiography may become more prominent in evaluation of thromboembolic complications. Qanadli and Rotzinger[61] promote this idea as they write in support of structured CT reporting for COVID-19, while strongly advocating for the inclusion of vascular abnormalities therein[61]. Objective analysis of pulmonary segmental and sub-segmental arterial branches on CT could also prove to be an interesting way to evaluate COVID-19 perfusion abnormalities. Dual energy CT, not regularly used in the clinical domain for this purpose, could be researched to measure lung perfusion in COVID-19 patients.
Other imaging modalities may also have a larger role in future investigations of COVID-19. With a better understanding of the pathophysiology of SARS-CoV-2 infection, PET-CT has the potential to delineate involvement of the hematopoietic and other organ systems using 18F-FDG and other, novel radiotracers. MR will be useful to better define cardiovascular, neurologic, gastrointestinal and renal sequelae. Sonography will likewise be useful in characterizing sequelae in multiple organ systems.
An entirely different angle of research involves combining imaging with artificial intelligence (AI). AI is often cited as an important method to compile large volumes of data to assist health experts. The SARS-CoV-2 pandemic has overwhelmed health care systems. Putting AI to work as intelligence augmentation needs to be a priority in order to assist physicians and public health experts during this and future crises. For radiologists the overarching goal of AI is to read imaging studies expeditiously and accurately, preferably in a triaged fashion. Augmented intelligence can integrate data from disparate sources to develop predictive models in diagnostics[62]. By integrating imaging data, radiology reports and clinical information, AI will be a powerful tool for rapidly cataloguing information to assist radiologists and clinicians to best care for patients[63].
As described by Rijn et al[64], following an initial time investment by a radiologist to train a deep learning system, tasks such as image segmentation can be performed with speed and accuracy[64]. Articles in preprint archives describe training and testing of convolutional neural networks on CXR[65,66]. Deep learning algorithms based upon 2D and 3D learning models on non-contrast chest CT are being developed to aid in detection, quantification and analysis of progression in COVID-19 and are also found in print and in preprint repositories[67-69]. An additional recent CT study tested an AI model that combines CT image data with multiple clinical factors and performed well compared to an experienced thoracic radiologist[70]. A final and important addition to the conversation is the potential for AI-derived radiographic imaging biomarkers to enhance predictive population health[71,72]. Utilizing AI in this manner could literally change the course of a multitude of diseases, including the current pandemic.
This review study has some limitations. First, the majority of studies reviewed have a small number of subjects. Of our 117 case and series studies combined, 67 (57.3%) of these had 1-10 subjects with more than half of these, 39 in total, being single case studies. The lack of many larger series studies represents the novelty of COVID-19 and over time larger studies will surface in the literature. Another limitation is the possibility that some cases and series reports are represented more than once within the dataset. Also, this review did not attempt to identify image acquisition parameters or image quality. Finally, these early case and series reports are chiefly representative of hospitalized patients. Therefore, they do not represent the community-based experience of COVID-19.
The radiology literature reveals general hallmarks of SARS-CoV-2 infections. These include CXR findings of bilateral pulmonary infiltrates with a tendency toward the lung periphery and chest CT findings of bilateral and peripheral ground glass and consolidated opacities, with an absence of concomitant pulmonary nodules, cavitation, adenopathy and pleural effusions. Data from more recently affected countries are now emerging. The cutoff for this study was April 6, 2020 – since then, multiple studies have been published with results that mirror those discussed in this review. Radiologists will need to follow accumulating published data of radiographic findings in COVID-19 to confirm and reassess these earliest reported studies and to continue to address best radiographic practices.
| 1. | World Health Organization. Emergencies Preparedness, response: Pneumonia of unknown cause – China, 2020. Available from: https://www.who.int/csr/don/05-january-2020-pneumonia-of-unkown-cause-china/en/. |
| 2. | Centers for Disease Control and Prevention. Coronavirus Disease 2019 (Covid-19): Cases and Latest Updates, 2020. Available from: http://www.cdc.gov. |
| 3. | Andersen KG, Rambaut A, Lipkin WI, Holmes EC, Garry RF. The proximal origin of SARS-CoV-2. Nat Med. 2020;26:450-452. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3581] [Cited by in RCA: 2894] [Article Influence: 482.3] [Reference Citation Analysis (0)] |
| 4. | World Health Organization. Technical Guidance: Naming the coronavirus disease (COVID-19) and the virus that causes it, 2020. Available from: https://www.who.int/emergencies/diseases/novel-coronavirus-2019/technical-guidance/naming-the-coronavirus-disease-(covid-2019)-and-the-virus-that-causes-it. |
| 5. | World Health Organization. Coronavirus disease 2019: Situation report – 72. 2020. Available from: https://www.who.int/docs/default-source/coronaviruse/situation-reports/20200401-sitrep-72-covid-19.pdf?sfvrsn=3dd8971b_2. |
| 6. | Centers for Disease Control and Prevention. Coronavirus Disease 2019 (Covid-19): Cases & Latest Updates: Cases in U.S. 2020. Available from: http://www.cdc.gov. |
| 7. | Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, Zhang L, Fan G, Xu J, Gu X, Cheng Z, Yu T, Xia J, Wei Y, Wu W, Xie X, Yin W, Li H, Liu M, Xiao Y, Gao H, Guo L, Xie J, Wang G, Jiang R, Gao Z, Jin Q, Wang J, Cao B. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497-506. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 35178] [Cited by in RCA: 30483] [Article Influence: 5080.5] [Reference Citation Analysis (13)] |
| 8. | Pan F, Ye T, Sun P, Gui S, Liang B, Li L, Zheng D, Wang J, Hesketh RL, Yang L, Zheng C. Time Course of Lung Changes at Chest CT during Recovery from Coronavirus Disease 2019 (COVID-19). Radiology. 2020;295:715-721. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1617] [Cited by in RCA: 1780] [Article Influence: 296.7] [Reference Citation Analysis (1)] |
| 9. | Centers for Disease Control and Prevention. Coronavirus Disease 2019 (Covid-19): Healthcare Professionals: Interim Clinical Guidance for Management of Patients with Confirmed Coronovirus Disease (COVID-19). 2020. Available from: http://www.cdc.gov. |
| 10. | Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; and the Northwell COVID-19 Research Consortium, Barnaby DP, Becker LB, Chelico JD, Cohen SL, Cookingham J, Coppa K, Diefenbach MA, Dominello AJ, Duer-Hefele J, Falzon L, Gitlin J, Hajizadeh N, Harvin TG, Hirschwerk DA, Kim EJ, Kozel ZM, Marrast LM, Mogavero JN, Osorio GA, Qiu M, Zanos TP. Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area. JAMA. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 6024] [Cited by in RCA: 6580] [Article Influence: 1096.7] [Reference Citation Analysis (0)] |
| 11. | Xu Z, Shi L, Wang Y, Zhang J, Huang L, Zhang C, Liu S, Zhao P, Liu H, Zhu L, Tai Y, Bai C, Gao T, Song J, Xia P, Dong J, Zhao J, Wang FS. Pathological findings of COVID-19 associated with acute respiratory distress syndrome. Lancet Respir Med. 2020;8:420-422. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 5228] [Cited by in RCA: 5833] [Article Influence: 972.2] [Reference Citation Analysis (3)] |
| 12. | Varga Z, Flammer AJ, Steiger P, Haberecker M, Andermatt R, Zinkernagel AS, Mehra MR, Schuepbach RA, Ruschitzka F, Moch H. Endothelial cell infection and endotheliitis in COVID-19. Lancet. 2020;395:1417-1418. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 4227] [Cited by in RCA: 4667] [Article Influence: 777.8] [Reference Citation Analysis (2)] |
| 13. | Chen N, Zhou M, Dong X, Qu J, Gong F, Han Y, Qiu Y, Wang J, Liu Y, Wei Y, Xia J, Yu T, Zhang X, Zhang L. Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet. 2020;395:507-513. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 14869] [Cited by in RCA: 13064] [Article Influence: 2177.3] [Reference Citation Analysis (4)] |
| 14. | Huang Y, Tu M, Wang S, Chen S, Zhou W, Chen D, Zhou L, Wang M, Zhao Y, Zeng W, Huang Q, Xu H, Liu Z, Guo L. Clinical characteristics of laboratory confirmed positive cases of SARS-CoV-2 infection in Wuhan, China: A retrospective single center analysis. Travel Med Infect Dis. 2020;101606. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 122] [Cited by in RCA: 147] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 15. | Tisoncik JR, Korth MJ, Simmons CP, Farrar J, Martin TR, Katze MG. Into the eye of the cytokine storm. Microbiol Mol Biol Rev. 2012;76:16-32. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1171] [Cited by in RCA: 1356] [Article Influence: 96.9] [Reference Citation Analysis (1)] |
| 16. | Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. 2020;18:844-847. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3992] [Cited by in RCA: 4077] [Article Influence: 679.5] [Reference Citation Analysis (0)] |
| 17. | Cheng SC, Chang YC, Fan Chiang YL, Chien YC, Cheng M, Yang CH, Huang CH, Hsu YN. First case of Coronavirus Disease 2019 (COVID-19) pneumonia in Taiwan. J Formos Med Assoc. 2020;119:747-751. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 129] [Cited by in RCA: 134] [Article Influence: 22.3] [Reference Citation Analysis (0)] |
| 18. | Huang WH, Teng LC, Yeh TK, Chen YJ, Lo WJ, Wu MJ, Chin CS, Tsan YT, Lin TC, Chai JW, Lin CF, Tseng CH, Liu CW, Wu CM, Chen PY, Shi ZY, Liu PY. 2019 novel coronavirus disease (COVID-19) in Taiwan: Reports of two cases from Wuhan, China. J Microbiol Immunol Infect. 2020;53:481-484. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 43] [Cited by in RCA: 45] [Article Influence: 7.5] [Reference Citation Analysis (0)] |
| 19. | Ng MY, Lee EY, Yang J, Yang F, Li X, Wang H, Lui MM-s, Lo CS-Y, Leung B, Khong P-L, Hui CK-M, Yuen K-y, Kuo MD. Imaging Profile of the COVID-19 Infection: Radiologic Findings and Literature Review. Radiology: Cardiothoracic Imaging. 2020; 2: e200034. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 604] [Cited by in RCA: 480] [Article Influence: 80.0] [Reference Citation Analysis (1)] |
| 20. | Wong HYF, Lam HYS, Fong AH, Leung ST, Chin TW, Lo CSY, Lui MM, Lee JCY, Chiu KW, Chung TW, Lee EYP, Wan EYF, Hung IFN, Lam TPW, Kuo MD, Ng MY. Frequency and Distribution of Chest Radiographic Findings in Patients Positive for COVID-19. Radiology. 2020;296:E72-E78. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 943] [Cited by in RCA: 829] [Article Influence: 138.2] [Reference Citation Analysis (3)] |
| 21. | Vancheri SG, Savietto G, Ballati F, Maggi A, Canino C, Bortolotto C, Valentini A, Dore R, Stella GM, Corsico AG, Iotti GA, Mojoli F, Perlini S, Bruno R, Preda L. Radiographic findings in 240 patients with COVID-19 pneumonia: time-dependence after the onset of symptoms. Eur Radiol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 83] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 22. | Han R, Huang L, Jiang H, Dong J, Peng H, Zhang D. Early Clinical and CT Manifestations of Coronavirus Disease 2019 (COVID-19) Pneumonia. AJR Am J Roentgenol. 2020;1-6. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 188] [Cited by in RCA: 221] [Article Influence: 36.8] [Reference Citation Analysis (1)] |
| 23. | Liu KC, Xu P, Lv WF, Qiu XH, Yao JL, Gu JF, Wei W. CT manifestations of coronavirus disease-2019: A retrospective analysis of 73 cases by disease severity. Eur J Radiol. 2020;126:108941. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 169] [Cited by in RCA: 146] [Article Influence: 24.3] [Reference Citation Analysis (0)] |
| 24. | Caruso D, Zerunian M, Polici M, Pucciarelli F, Polidori T, Rucci C, Guido G, Bracci B, De Dominicis C, Laghi A. Chest CT Features of COVID-19 in Rome, Italy. Radiology. 2020;296:E79-E85. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 398] [Cited by in RCA: 384] [Article Influence: 64.0] [Reference Citation Analysis (0)] |
| 25. | Bai HX, Hsieh B, Xiong Z, Halsey K, Choi JW, Tran TML, Pan I, Shi LB, Wang DC, Mei J, Jiang XL, Zeng QH, Egglin TK, Hu PF, Agarwal S, Xie FF, Li S, Healey T, Atalay MK, Liao WH. Performance of Radiologists in Differentiating COVID-19 from Non-COVID-19 Viral Pneumonia at Chest CT. Radiology. 2020;296:E46-E54. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 726] [Cited by in RCA: 729] [Article Influence: 121.5] [Reference Citation Analysis (1)] |
| 26. | Zhao W, Zhong Z, Xie X, Yu Q, Liu J. Relation Between Chest CT Findings and Clinical Conditions of Coronavirus Disease (COVID-19) Pneumonia: A Multicenter Study. AJR Am J Roentgenol. 2020;214:1072-1077. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 665] [Cited by in RCA: 700] [Article Influence: 116.7] [Reference Citation Analysis (0)] |
| 27. | Shi H, Han X, Zheng C. Evolution of CT Manifestations in a Patient Recovered from 2019 Novel Coronavirus (2019-nCoV) Pneumonia in Wuhan, China. Radiology. 2020;295:20. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 145] [Cited by in RCA: 149] [Article Influence: 24.8] [Reference Citation Analysis (0)] |
| 28. | Ling Z, Xu X, Gan Q, Zhang L, Luo L, Tang X, Liu J. Asymptomatic SARS-CoV-2 infected patients with persistent negative CT findings. Eur J Radiol. 2020;126:108956. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 49] [Cited by in RCA: 49] [Article Influence: 8.2] [Reference Citation Analysis (0)] |
| 29. | Bernheim A, Mei X, Huang M, Yang Y, Fayad ZA, Zhang N, Diao K, Lin B, Zhu X, Li K, Li S, Shan H, Jacobi A, Chung M. Chest CT Findings in Coronavirus Disease-19 (COVID-19): Relationship to Duration of Infection. Radiology. 2020;295:200463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1728] [Cited by in RCA: 1608] [Article Influence: 268.0] [Reference Citation Analysis (3)] |
| 30. | Pan Y, Guan H, Zhou S, Wang Y, Li Q, Zhu T, Hu Q, Xia L. Initial CT findings and temporal changes in patients with the novel coronavirus pneumonia (2019-nCoV): a study of 63 patients in Wuhan, China. Eur Radiol. 2020;30:3306-3309. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 564] [Cited by in RCA: 634] [Article Influence: 105.7] [Reference Citation Analysis (0)] |
| 31. | Li W, Cui H, Li K, Fang Y, Li S. Chest computed tomography in children with COVID-19 respiratory infection. Pediatr Radiol. 2020;50:796-799. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 141] [Cited by in RCA: 153] [Article Influence: 25.5] [Reference Citation Analysis (0)] |
| 32. | Liu H, Liu F, Li J, Zhang T, Wang D, Lan W. Clinical and CT imaging features of the COVID-19 pneumonia: Focus on pregnant women and children. J Infect. 2020;80:e7-e13. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 263] [Article Influence: 43.8] [Reference Citation Analysis (0)] |
| 33. | Lu Y, Wen H, Rong D, Zhou Z, Liu H. Clinical characteristics and radiological features of children infected with the 2019 novel coronavirus. Clin Radiol. 2020;75:520-525. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 26] [Cited by in RCA: 29] [Article Influence: 4.8] [Reference Citation Analysis (1)] |
| 34. | Liu D, Li L, Wu X, Zheng D, Wang J, Yang L, Zheng C. Pregnancy and Perinatal Outcomes of Women With Coronavirus Disease (COVID-19) Pneumonia: A Preliminary Analysis. AJR Am J Roentgenol. 2020;215:127-132. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 247] [Cited by in RCA: 270] [Article Influence: 45.0] [Reference Citation Analysis (0)] |
| 35. | Qin C, Liu F, Yen TC, Lan X. 18F-FDG PET/CT findings of COVID-19: a series of four highly suspected cases. Eur J Nucl Med Mol Imaging. 2020;47:1281-1286. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 178] [Cited by in RCA: 205] [Article Influence: 34.2] [Reference Citation Analysis (5)] |
| 36. | Zou S, Zhu X. FDG PET/CT of COVID-19. Radiology. 2020;296:E118. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 68] [Cited by in RCA: 79] [Article Influence: 13.2] [Reference Citation Analysis (0)] |
| 37. | Joob B, Wiwanitkit V. 18F-FDG PET/CT and COVID-19. Eur J Nucl Med Mol Imaging. 2020;47:1348. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 28] [Cited by in RCA: 25] [Article Influence: 4.2] [Reference Citation Analysis (0)] |
| 38. | Buonsenso D, Piano A, Raffaelli F, Bonadia N, de Gaetano Donati K, Franceschi F. Point-of-Care Lung Ultrasound findings in novel coronavirus disease-19 pnemoniae: a case report and potential applications during COVID-19 outbreak. Eur Rev Med Pharmacol Sci. 2020;24:2776-2780. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 91] [Reference Citation Analysis (0)] |
| 39. | Peng QY, Wang XT, Zhang LN; Chinese Critical Care Ultrasound Study Group (CCUSG). Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med. 2020;46:849-850. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 469] [Cited by in RCA: 512] [Article Influence: 85.3] [Reference Citation Analysis (1)] |
| 40. | Soldati G, Smargiassi A, Inchingolo R, Buonsenso D, Perrone T, Briganti DF, Perlini S, Torri E, Mariani A, Mossolani EE, Tursi F, Mento F, Demi L. Proposal for International Standardization of the Use of Lung Ultrasound for Patients With COVID-19: A Simple, Quantitative, Reproducible Method. J Ultrasound Med. 2020;39:1413-1419. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 380] [Cited by in RCA: 434] [Article Influence: 72.3] [Reference Citation Analysis (1)] |
| 41. | Soldati G, Smargiassi A, Inchingolo R, Buonsenso D, Perrone T, Briganti DF, Perlini S, Torri E, Mariani A, Mossolani EE, Tursi F, Mento F, Demi L. On Lung Ultrasound Patterns Specificity in the Management of COVID-19 Patients. J Ultrasound Med. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 19] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 42. | Musolino AM, Supino MC, Buonsenso D, Ferro V, Valentini P, Magistrelli A, Lombardi MH, Romani L, D'Argenio P, Campana A; Roman Lung Ultrasound Study Team for Pediatric COVID-19 (ROMULUS COVID Team). Lung Ultrasound in Children with COVID-19: Preliminary Findings. Ultrasound Med Biol. 2020;46:2094-2098. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 94] [Article Influence: 15.7] [Reference Citation Analysis (0)] |
| 43. | Piscaglia F, Stefanini F, Cantisani V, Sidhu PS, Barr R, Berzigotti A, Chammas MC, Correas JM, Dietrich CF, Feinstein S, Huang P, Jenssen C, Kono Y, Kudo M, Liang P, Lyshchik A, Nolsøe C, Xie X, Tovoli F. Benefits, Open questions and Challenges of the use of Ultrasound in the COVID-19 pandemic era. The views of a panel of worldwide international experts. Ultraschall Med. 2020;41:228-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 40] [Article Influence: 6.7] [Reference Citation Analysis (0)] |
| 44. | Pilotto A, Odolini S, Masciocchi S, Comelli A, Volonghi I, Gazzina S, Nocivelli S, Pezzini A, Focà E, Caruso A, Leonardi M, Pasolini MP, Gasparotti R, Castelli F, Ashton NJ, Blennow K, Zetterberg H, Padovani A. Steroid-Responsive Encephalitis in Coronavirus Disease 2019. Ann Neurol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 156] [Cited by in RCA: 203] [Article Influence: 33.8] [Reference Citation Analysis (0)] |
| 45. | Fonseca EKUN, Chate RC, Neto RS, Ishikawa WY, Silva MMdA, Yokoo P, Szarf G. Findings of COVID-19 on Magnetic Resonance Imaging. Radiology: Cardiothoracic Imaging 2020; e200193. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3] [Cited by in RCA: 3] [Article Influence: 0.5] [Reference Citation Analysis (0)] |
| 46. | Shi S, Qin M, Shen B, Cai Y, Liu T, Yang F, Gong W, Liu X, Liang J, Zhao Q, Huang H, Yang B, Huang C. Association of Cardiac Injury With Mortality in Hospitalized Patients With COVID-19 in Wuhan, China. JAMA Cardiol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2428] [Cited by in RCA: 3038] [Article Influence: 506.3] [Reference Citation Analysis (2)] |
| 47. | Xu H, Hou K, Xu H, Li Z, Chen H, Zhang N, Xu R, Fu H, Sun R, Wen L, Xie L, Liu H, Zhang K, Selvanayagam JB, Fu C, Zhao S, Yang Z, Yang M, Guo Y. Acute Myocardial Injury of Patients with Coronavirus Disease 2019. [Preprint]. In press 2020. |
| 48. | Mao L, Jin H, Wang M, Hu Y, Chen S, He Q, Chang J, Hong C, Zhou Y, Wang D, Miao X, Li Y, Hu B. Neurologic Manifestations of Hospitalized Patients With Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4761] [Cited by in RCA: 4766] [Article Influence: 794.3] [Reference Citation Analysis (1)] |
| 49. | American College of Radiology. ACR Position Statements: Recommendations for Chest Radiography and CT for Suspected COVID-19 Infection 2020. Available from: https://www.acr.org/Advocacy-and-Economics/ACR-Position-Statements/Recommendations-for-Chest-Radiography-and-CT-for-Suspected-COVID19-Infection. |
| 50. | Society for Cardiovascular Magnetic Resonance. SCMR’s COVID-19 Preparedness Toolkit: Clinical Service General Considerations 2020. Available from: https://www.scmr.org/page/COVID19. |
| 51. | Ai T, Yang Z, Hou H, Zhan C, Chen C, Lv W, Tao Q, Sun Z, Xia L. Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology. 2020;296:E32-E40. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 3614] [Cited by in RCA: 3297] [Article Influence: 549.5] [Reference Citation Analysis (2)] |
| 52. | Fang Y, Zhang H, Xie J, Lin M, Ying L, Pang P, Ji W. Sensitivity of Chest CT for COVID-19: Comparison to RT-PCR. Radiology. 2020;296:E115-E117. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 2088] [Cited by in RCA: 1879] [Article Influence: 313.2] [Reference Citation Analysis (3)] |
| 53. | Esposito A, Palmisano A, Scotti GM, Morelli MJ, Vignale D, De Cobelli F, Tonon G, Tacchetti C. Why is chest CT important for early diagnosis of COVID-19? Prevalence matters. [Preprint]. In press 2020. |
| 54. | Himoto Y, Sakata A, Kirita M, Hiroi T, Kobayashi KI, Kubo K, Kim H, Nishimoto A, Maeda C, Kawamura A, Komiya N, Umeoka S. Diagnostic performance of chest CT to differentiate COVID-19 pneumonia in non-high-epidemic area in Japan. Jpn J Radiol. 2020;38:400-406. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 56] [Article Influence: 9.3] [Reference Citation Analysis (0)] |
| 55. | Dangis A, Gieraerts C, Bruecker YD, Janssen L, Valgaeren H, Obbels D, Gillis M, Ranst MV, Frans J, Demeyere A, Symons R. Accuracy and reproducibility of low-dose submillisievert chest CT for the diagnosis of COVID-19. Radiology: Cardiothoracic Imaging. 2020;e200196. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 79] [Cited by in RCA: 81] [Article Influence: 13.5] [Reference Citation Analysis (0)] |
| 56. | Kim H. Outbreak of novel coronavirus (COVID-19): What is the role of radiologists? Eur Radiol. 2020;30:3266-3267. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 103] [Cited by in RCA: 114] [Article Influence: 19.0] [Reference Citation Analysis (0)] |
| 57. | Wynants L, Van Calster B, Collins GS, Riley RD, Heinze G, Schuit E, Bonten MMJ, Damen JAA, Debray TPA, De Vos M, Dhiman P, Haller MC, Harhay MO, Henckaerts L, Kreuzberger N, Lohman A, Luijken K, Ma J, Andaur CL, Reitsma JB, Sergeant JC, Shi C, Skoetz N, Smits LJM, Snell KIE, Sperrin M, Spijker R, Steyerberg EW, Takada T, van Kuijk SMJ, van Royen FS, Wallisch C, Hooft L, Moons KGM, van Smeden M. Prediction models for diagnosis and prognosis of covid-19 infection: systematic review and critical appraisal. BMJ. 2020;369:m1328. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1833] [Cited by in RCA: 1799] [Article Influence: 299.8] [Reference Citation Analysis (0)] |
| 58. | Sun Z, Zhang N, Li Y, Xu X. A systematic review of chest imaging findings in COVID-19. Quant Imaging Med Surg. 2020;10:1058-1079. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 87] [Article Influence: 14.5] [Reference Citation Analysis (0)] |
| 59. | Dennie C, Hague C, Lim RS, Manos D, Memauri BF, Nguyen ET, Taylor J. Canadian Society of Thoracic Radiology/Canadian Association of Radiologists Consensus Statement Regarding Chest Imaging in Suspected and Confirmed COVID-19. Can Assoc Radiol J. 2020;846537120924606. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 41] [Article Influence: 6.8] [Reference Citation Analysis (1)] |
| 60. | Salehi S, Abedi A, Balakrishnan S, Gholamrezanezhad A. Coronavirus disease 2019 (COVID-19) imaging reporting and data system (COVID-RADS) and common lexicon: a proposal based on the imaging data of 37 studies. Eur Radiol. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 86] [Cited by in RCA: 104] [Article Influence: 17.3] [Reference Citation Analysis (0)] |
| 61. | Qanadli SD, Rotzinger DC. Vascular Abnormalities as Part of Chest CT Findings in COVID-19. Radiology: Cardiothoracic Imaging. 2020;e200161. [RCA] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 6] [Cited by in RCA: 6] [Article Influence: 1.0] [Reference Citation Analysis (0)] |
| 62. | Long JB, Ehrenfeld JM. The Role of Augmented Intelligence (AI) in Detecting and Preventing the Spread of Novel Coronavirus. J Med Syst. 2020;44:59. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 71] [Cited by in RCA: 48] [Article Influence: 8.0] [Reference Citation Analysis (0)] |
| 63. | Li M, Lei P, Zeng B, Li Z, Yu P, Fan B, Wang C, Li Z, Zhou J, Hu S, Liu H. Coronavirus Disease (COVID-19): Spectrum of CT Findings and Temporal Progression of the Disease. Acad Radiol. 2020;27:603-608. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 192] [Cited by in RCA: 171] [Article Influence: 28.5] [Reference Citation Analysis (0)] |
| 64. | Rijn RRv, Luca AD. Three Reasons Why Artificial Intelligence Might Be the Radiologist’s Best Friend. Radiology [Internet]. 2020. Available from: https://pubs.rsna.org/doi/abs/10.1148/radiol.2020200855. |
| 65. | Castiglioni I, Ippolito D, Interlenghi M, Monti CB, Salvatore C, Schiaffino S, Polidori A, Gandola D, Messa C, Sardanelli F. Artificial intelligence applied on chest X-ray can aid in the diagnosis of COVID-19 infection: a first experience from Lombardy, Italy. [Preprint]. In press 2020. |
| 66. | Abbas A, Abdelsamea M, Gaber M. Classification of COVID-19 in chest X-ray images using DeTraC deep convolutional neural network. [Preprint]. In press 2020. |
| 67. | Gozes O, Frid-Adar M, Greenspan H, Browning PD, Zhang H, Ji W, Bernheim A, Siegel E. Rapid AI Development Cycle for the Coronavirus (COVID-19) Pandemic: Initial Results for Automated Detection & Patient Monitoring using Deep Learning CT Image Analysis. arXiv e-prints. [Preprint]. In press 2020. |
| 68. | Gozes O, Frid-Adar M, Sagie N, Zhang H, Ji W, Greenspan H. Coronavirus Detection and Analysis on Chest CT with Deep Learning. arXiv e-prints. [Preprint]. In press 2020. |
| 69. | Li L, Qin L, Xu Z, Yin Y, Wang X, Kong B, Bai J, Lu Y, Fang Z, Song Q, Cao K, Liu D, Wang G, Xu Q, Fang X, Zhang S, Xia J, Xia J. Using Artificial Intelligence to Detect COVID-19 and Community-acquired Pneumonia Based on Pulmonary CT: Evaluation of the Diagnostic Accuracy. Radiology. 2020;296:E65-E71. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1260] [Cited by in RCA: 945] [Article Influence: 157.5] [Reference Citation Analysis (13)] |
| 70. | Mei X, Lee HC, Diao KY, Huang M, Lin B, Liu C, Xie Z, Ma Y, Robson PM, Chung M, Bernheim A, Mani V, Calcagno C, Li K, Li S, Shan H, Lv J, Zhao T, Xia J, Long Q, Steinberger S, Jacobi A, Deyer T, Luksza M, Liu F, Little BP, Fayad ZA, Yang Y. Artificial intelligence-enabled rapid diagnosis of patients with COVID-19. Nat Med. 2020;. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 737] [Cited by in RCA: 572] [Article Influence: 95.3] [Reference Citation Analysis (0)] |
| 71. | Weiss J, Hoffmann U, Aerts HJWL. Artificial intelligence-derived imaging biomarkers to improve population health. Lancet Digit Health. 2020;2:e154-e5. [RCA] [DOI] [Full Text] [Cited by in Crossref: 3] [Cited by in RCA: 8] [Article Influence: 1.3] [Reference Citation Analysis (0)] |
| 72. | McCall B. COVID-19 and artificial intelligence: protecting health-care workers and curbing the spread. Lancet Digit Health. 2020;2:e166-e167. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 232] [Cited by in RCA: 172] [Article Influence: 28.7] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article that was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution NonCommercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country/Territory of origin: United States
Peer-review report’s scientific quality classification
Grade A (Excellent): A
Grade B (Very good): B
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Ferraioli G, Georgiev T, Tovoli F S-Editor: Zhang L L-Editor: A P-Editor: Liu JH