Published online May 28, 2019. doi: 10.4329/wjr.v11.i5.62
Peer-review started: February 18, 2019
First decision: March 15, 2019
Revised: April 5, 2019
Accepted: May 21, 2019
Article in press: May 22, 2019
Published online: May 28, 2019
Processing time: 102 Days and 6.8 Hours
Chronic cocaine use is associated with stroke, coronary artery disease and myocardial infarction, resulting in severe impairments or sudden mortality. In the absence of clear cardiovascular symptoms, individuals with cocaine use disorder (iCUD) seeking addiction treatment receive mostly psychotherapy and psychiatric pharmacotherapy, with no attention to vascular disease (i.e., atherosclerosis). Little is known about the pre-clinical signs of cardiovascular risk in iCUD and early signs of vascular disease are undetected in this underserved population.
To assess inflammation, plaque burden and plaque composition in iCUD aiming to detect markers of atherosclerosis and vascular disease.
The bilateral carotid arteries were imaged with positron emission tomography/magnetic resonance imaging (PET/MRI) in iCUD asymptomatic for cardiovascular disease, healthy controls, and individuals with cardiovascular risk. PET with 18F-fluorodeoxyglucose (18F-FDG) evaluated vascular inflammation and 3-D dark-blood MRI assessed plaque burden including wall area and thickness. Drug use and severity of addiction were assessed with standardized instruments.
The majority of iCUD and controls had carotid FDG-PET signal greater than 1.6 but lower than 3, indicating the presence of mild to moderate inflammation. However, the MRI measure of wall structure was thicker in iCUD as compared to the controls and cardiovascular risk group, indicating greater carotid plaque burden. iCUD had larger wall area as compared to the healthy controls but not as compared to the cardiovascular risk group, indicating structural wall similarities between the non-control study groups. In iCUD, wall area correlated with greater cocaine withdrawal and craving.
These preliminary results show markers of carotid artery disease burden in cardiovascular disease-asymptomatic iCUD. Broader trials are warranted to develop protocols for early detection of cardiovascular risk and preventive intervention in iCUD.
Core tip: Despite undetected clinical signs, cocaine use increases risk of stroke, coronary artery disease and myocardial infarction. Simultaneous carotid positron emission tomography/magnetic resonance imaging can effectively evaluate vascular inflammation and plaque burden in individuals with cocaine use disorder. Cocaine users had increased wall area, comparable to individuals with cardiovascular risk and significantly higher than healthy controls. Wall area in cocaine users positively correlated with greater cocaine withdrawal and craving. Broader trials are warranted to develop protocols for early detection of cardiovascular risk and preventive intervention in individuals with cocaine use disorder.
- Citation: Bachi K, Mani V, Kaufman AE, Alie N, Goldstein RZ, Fayad ZA, Alia-Klein N. Imaging plaque inflammation in asymptomatic cocaine addicted individuals with simultaneous positron emission tomography/magnetic resonance imaging. World J Radiol 2019; 11(5): 62-73
- URL: https://www.wjgnet.com/1949-8470/full/v11/i5/62.htm
- DOI: https://dx.doi.org/10.4329/wjr.v11.i5.62
Cocaine use disorder (CUD), chronic brain disease, imparts multiple cardiovascular effects. The phenomenology of cocaine addiction involves decades of chronic cocaine and other drug use as well as an unhealthy lifestyle (e.g., poor sleep and nutrition) that affect cardiovascular health. Furthermore, cocaine’s main vasoactive metabolite benzoylmethylecgonine, a tropane alkaloid, is associated with hematological effects on the vessel and the loss of the endothelium’s protective functions[1-3]. Cocaine creates an elevated immune system inflammatory state with increased pro-inflammatory cytokines, and brain-derived neurotrophic factor levels, all contributing to vascular disease[4,5]. These effects are expressed by activation of cells in the endothelium (interior surface of blood vessels) leading to macrophage proliferation and vascular inflammation, with subsequent formation of complex plaque that manifests as structural abnormalities and progresses to atherosclerotic disease[6,7]. Atherosclerosis reflects a long-term inflammatory process, where, in medium to large arteries (e.g., the carotid arteries), it may be present even before it becomes susceptible to rupture, without overt clinical symptoms[8]. However, once symptoms occur, the artery is severely damaged and cerebral ischemia can ensue, a common fatal outcome in CUD[1,9].
Significant advances in multi-modal imaging for early detection of atherosclerosis in asymptomatic populations who are at increased risk for vascular disease (e.g., individuals with high cholesterol, Type II diabetes mellitus) have proven efficacy for preventive treatment[10,11]. Thus, characterizing the atherosclerotic cascade with magnetic resonance imaging (MRI) and positron emission tomography (PET) in asymptomatic individuals at cardiovascular risk can help delineate disease stage and inform on medication choices and follow-up[10,11]. The presence of inflammation captured by PET- with 18F-fluorodeoxyglucose (18F-FDG) is an important indicator of early stage disease progression and validation that the cause of vascular pathology is indeed atherosclerosis. For the purpose of imaging vascular inflammation, FDG is internalized (but not metabolized as in brain FDG) by tissues with active anaerobic metabolism, such as inflamed areas. 18F-FDG PET can quantify inflammation in atherosclerotic plaques[12-14] and has been correlated consistently with plaque macrophage content (white blood cells that increase inflammation and stimulate the immune system) in atherosclerotic rabbits[15] and patients[12,16,17]. An important indication of atherosclerosis overall burden is assessed using MR, an excellent modality for evaluating the blood vessel wall. The MR sequence uses black (or dark) blood techniques, in which the blood appears black and the arterial wall can be seen, accurately depicting plaque presence, size, and morphology with sub-millimeter resolution and high reproducibility, providing new indices of atherosclerotic burden that can be applied in large scale studies to varied populations[6].
Thus, PET with FDG can detect early disease stages and simultaneous MR is used to quantify atherosclerosis burden. Such simultaneous PET/MRI[10,11] has never been used for early detection of vascular pathology in asymptomatic drug addicted individuals. Targeting this population for early detection is of urgency now that the “Crack generation” of the mid 1980s is aging[18]. Owing to decades of cocaine and comorbid tobacco and alcohol use, these individuals with CUD (iCUD) are at particularly high risk for vascular disease and atherosclerosis. Hence, the characterization of atherosclerosis by multimodal imaging can help to detect early signs of disease and inform treatment trials with non-invasive end-points. We applied imaging protocols with PET/MRI of the bilateral carotids for measuring markers of cardiovascular risk for the first time in iCUD. We hypothesize that iCUD will have elevated inflammation and carotid plaque burden as compared to non-addicted controls and even as compared to non-addicted individuals with established cardiovascular risk who are a decade older.
We studied a group of iCUD (n = 14), a group of non-addicted healthy controls (n = 10), and a group of non-addicted individuals with cardiovascular risk (n = 62). Individuals with CUD and non-addicted healthy controls were recruited using advertisement in websites, local newspapers, bulletin boards, and by word-of-mouth with calls for imaging in individuals with cocaine problems or healthy controls. Subjects were given a complete physical examination that included electrocardi-ography and laboratory tests of renal, hepatic, pancreatic, hematopoietic, and thyroid functions to ensure good physical health. Drug use was assessed with urine tests in all subjects on screening day and pregnancy was tested in women on screening as well as on imaging visits. In addition, on screening day alcohol use was measured with a breathalyzer and tobacco use was measured by levels of nicotine and cotinine in blood. An in-depth interview included the following instruments for assessing inclusion/exclusion criteria: The Structured Clinical Interview for the Diagnostic and Statistical Manual-IV of Axis I Disorders (research version[19,20]) for psychiatric diagnostics. Addiction Severity Index[21], a semi-structured interview provided an estimate of the years of drug/alcohol and severity of use and a detailed assessment for recent and lifetime history of use of various drugs including alcohol. We supplement this interview with brief, well-validated, instruments of addiction severity to assess potential covariates: Cocaine Selective Severity Assessment Scale[22] evaluated cocaine withdrawal symptoms occurring over the past 24 h, Cocaine Craving Questionnaire assessed cocaine craving symptoms over the past 24 h[23], and Severity of Dependence Scale[24,25] examined the severity of addiction during the past 12 mo.
Inclusion criteria: (1) Ability to understand and give informed consent; (2) age 35-65 years; (3) Primary current diagnosis of CUD for the iCUD group; diagnoses for tobacco and alcohol use disorders were allowed; (4) Framingham score of < 10%-20% in iCUD and controls; and (5) right-handed. Exclusion criteria: (1) Urine positive for any psychoactive drugs (except cocaine in iCUD) or their metabolites tested on the day of screening; (2) Psychiatric disorders with psychosis and pervasive developmental disorders such as autism; (3) Head trauma with loss of consciousness > 30 min; (4) Present or past history of neurological disease of central origin (including seizures); (5) Any cardiovascular disease or abnormal vital signs; (6) Any other medical condition (e.g., diabetes mellitus) that may alter cerebral function, endocrinological, oncological or autoimmune diseases; (7) Pregnant or breast feeding; and (8) Counter-indications to PET scanning and metal implants or other counter-indicators to MRI.
In addition to our healthy control comparison group, MRI values in iCUD were compared with values of existing data[11] from 62 non-addicted individuals (age 64.6 ± 7.8, 83% males), with the following inclusion criteria: (1) Ability to understand and give informed consent; (2) Men and women aged 18–75 years; (3) Previous known coronary heart disease or at high risk of coronary heart disease (diabetes or a 10-year risk of coronary heart disease events > 20% by Framingham Risk scoring), triglyceride concentrations of 400 mg/dL or lower (≤ 4.5 mmol/L), and carotid or aortic arterial wall (target) to background (blood) ratio (TBR) of 1.6 or higher, as identified by 18F-FDG uptake measured by PET/CT during the screening period; and (4) Clinically stable and receiving appropriate and stable treatment with a statin or other low-density lipoprotein (LDL)-C lowering drugs with LDL-C concentrations of 100 mg/dL or lower (< 2.6 mmol/L) unless receiving maximum tolerated doses of therapy or intolerant to statins. Exclusion criteria included: (1) Concomitant treatment with fibrates or nicotinic acid; (2) Presence of uncontrolled blood pressure or diabetes (HbA1c >10%); and (3) Recent (< 3 mo) clinically significant coronary or cerebral vascular event, diagnosis of familial hypercholesterolaemia, or a glomerular filtration rate lower than 30 mL/min. Other reasons for exclusion were standard for this type of trial, as previously described[11].
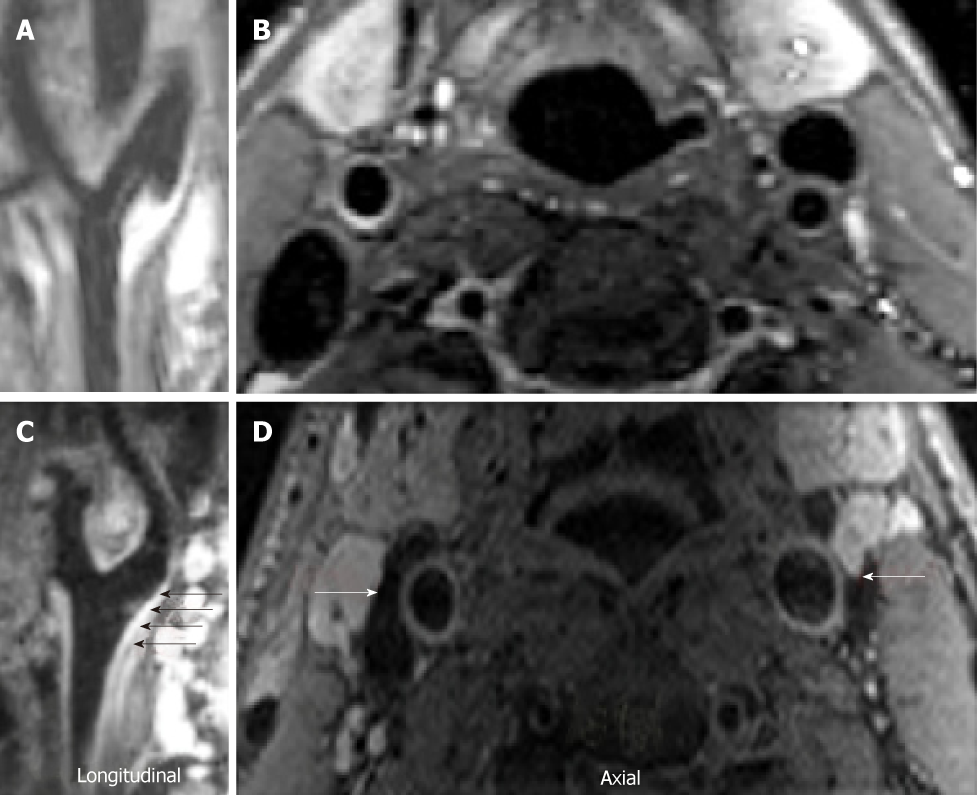
Carotid PET/MR image acquisition:18F-FDG PET was used to evaluate arterial inflammation within the right and left carotid of the subjects[10,11,26]. Participants were imaged at rest in supine position 90 min after injection of 10mCi of 18-FDG[13]. MRI sequences for PET attenuation correction were acquired while the FDG was still circulating. 3-D dark-blood MRI imaging of the internal carotid arteries extending 3 cm below and above the carotid bifurcations using a 4-channel carotid coil was conducted. After localization with gradient echo sequences, time-of-flight images were acquired to delineate vessel lumen (interior of the vessel). Then, dark blood images were obtained using 3D SPACE with multiple contrast weightings. Proton density weighted, T1 and T2 weighted images were acquired[27-29], during free breathing[30,31], un-triggered with fat suppression, with template based attenuation correction as previously validated[26]. PET data for one subject, right and left carotid MRI data of one subject, and right carotid MRI data of a third subject were not analyzable for iCUD.
Analysis of inflammation by PET: Image analysis of PET/MRI data was performed using OsiriX MD (Pixmeo, Geneva, Switzerland). T2 TSE MRI images of the head and neck were fused with PET images of the same region and analyzed in the axial plane. The technique employed has been previously described in other studies10,32. The common carotid artery was assessed where it was well delineated from its most caudal extent up to the level of the carotid bifurcation. Using the closed polygon drawing tool, the common carotid artery was traced on the fused images. MRI signal differences between the target and adjacent tissue were used as guidelines to best mark the region of interest (ROI). The right and left carotid arteries were analyzed separately, as the bifurcation is often not at the same level when comparing the two sides. The mean and maximum standardized uptake values (SUV) of the target vessel were measured for each slice.
Background was measured within the jugular veins using an oval drawing tool to acquire five measurements of at least 10 mm2 on both the right and left sides for a total of 10 measurements. The lowest fused image SUVmean-slice within each slice of the jugular vein was chosen for background ROI placement. The SUVmean-background represents the average of the SUVmean-slice values acquired from the 10 background slices. TBR mean and maximum were then calculated by dividing respectively the target SUVmean of a slice and the target SUVmax of a slice by the SUVmean-background. The TBRmean-overall and TBRmax-overall represent the average of the metric’s values when considering all slices evaluated for each artery. The most diseased segment (MDS) is defined as the highest TBRmax-slice and that of its two adjacent slices and the TBR of the MDS (TBR-MDS) is the average of the TBRmax-slice of this three level segment. Calculations were made using Excel (Microsoft, Washington, USA).
Analysis of atherosclerotic burden by MRI: 3D-SPACE MRI images of the neck were obtained and reformatted into the axial plane prior to analysis. Using these reformatted ‘black blood’ MRI images, the carotid arteries were analyzed at a dedicated workstation running the software program VesselMASS, (VesselMASS, Division of Image Processing, Department of Radiology, Leiden University Medical Center, Leiden, Netherlands). The technique used has been previously described in other studies[33,34]. As with the MR/PET analysis, the common carotid artery was assessed separately and bilaterally in the slices where each vessel was well delineated, from its most caudal extent up to the level of the carotid bifurcation. The metrics acquired for each vessel included: lumen area, wall area, total vessel area, wall thickness and wall thickness SD. A normalized wall index was also calculated to account for arterial wall size differences that are found within each subject.
Statistical analysis was conducted in SPSS (IBM Corp., Version 23.0. Armonk, NY) to compare between the iCUD and the healthy control group on demographics and drug use by a two samples t-tests (two-tailed). Comparisons of PET/MR measurements between the iCUD and the healthy controls groups were conducted by univariate analysis of covariance (ANCOVA) while controlling for age. Comparisons of MRI measurements between the iCUD and the group of individuals with cardiovascular risk were conducted by one sample t-tests (two-tailed) using the mean values of the group of individuals with cardiovascular risk (since only mean values were available for this group). Associations between the findings that differed between the iCUD and the healthy control group and drug use measures were examined by partial correlations with age and nicotine lifetime use (which differed significantly between the groups) as covariates. A familywise correction for multiple correlations at significance level of P = 0.05 was applied.
Cocaine addicted individuals were slightly older than non-addicted healthy controls and about a decade younger than those with cardiovascular risk. The race distribution was unequal, with more African Americans in the iCUD group. There were no differences between the iCUD and non-addicted healthy controls in gender, education, body mass index, and resting heart rate. Framingham risk scores were available only for a limited number of participants (3 iCUD scored 8.7 ± 3.6 vs 6 healthy controls 2.7 ± 2.1, P < 0.05). iCUD were chronic users with 21.9 ± 7.9 years of cocaine use, 20.8 ± 11.8 years of alcohol use, and 9.1 ± 10.5 years of cannabis use; 64% were current smokers whereas in the healthy controls 10.0% were current and 20.0% were past smokers (groups differences on lifetime use of cocaine, cannabis, and nicotine smoking, P < 0.001; alcohol lifetime use did not differ between the iCUD and healthy control groups) (Table 1).
| Group 1: Cardiovascular risk[15] (n = 62) | Group 2: Healthy controls (n = 10) | Group 3: Cocaine users (n = 14) | |
| Demographics | |||
| Race | 62 white (94%); 4 other | 5 black (50%); 4 white; 1 other | 13 black (93%); 1 white |
| Gender | 55 men (83%) | 8 men (80%) | 10 men (71%) |
| Agead | 64.6 ± 7.8 | 46.2 ± 5.3 | 50.8 ± 4.1 |
| Education | NA | 15.0 ± 2.0 | 13.6 ± 1.8 |
| Cardiovascular risk | |||
| BMI | NA | 29.1 ± 5.0 | 28.3 ± 3.71 |
| Heart rate | NA | 74.9 ± 11.9 | 79.1 ± 10.9 |
| Total cholesterol | NA | 182.7 ± 28.22 | 163.3 ± 28.93 |
| HDL cholesterol | NA | 55.8 ± 16.14 | 42.3 ± 9.15 |
| Drug use | |||
| Alcohol lifetime | NA | 18.9 ± 13.4 | 20.8 ± 11.8 |
| Cocaine lifetime | NA | NA | 21.9 ± 7.9 |
| Nicotine lifetimef | 12% current | 10.0% current; 20.0% past; 70.0% never; 3.5 ± 8.1 | 64.3% current; 28.6% past; 7.1% never; 26.4 ± 10.1 |
| THC lifetimee | NA | 0.5 ± 1.3 | 9.1 ± 10.5 |
| Cocaine withdrawal[22] | NA | NA | 18.6 ± 11.9 |
| Cocaine craving[23] | NA | NA | 14.7 ± 14.5 |
| Severity of drug dependence[24] | NA | NA | 3.2 ± 3.6 |
According to norms established in clinical research studies of risk detection[35,36], TBR ≥ 1.6 is indicative of inflamed plaque. The PET FDG results showed that both iCUD (85%) and the healthy controls (90%) had slightly inflamed plaque in one or both carotid arteries. There were no significant differences in plaque inflammation between the iCUD and the non-addicted healthy controls measured by maximum target-to-background ratios and measures of most diseased segment (Table 2).
| Group 1: Cardiovascular risk[15] (n = 62) | Group 2: Cocaine users (n = 13) | Group 3: Healthy controls (n = 10) | Group 2 and 3 difference [Sig. (ANCOVA)] | ||
| PET results | |||||
| Target-to-Background ratios (TBR max) | |||||
| Left | NA | 1.77 ± 0.10 | 1.77 ± 0.07 | F (1, 20) = 0.3, P = 0.619 | |
| Right | NA | 1.93 ± 0.09 | 1.76 ± 0.04 | F (1, 20) = 1.9, P = 0.178 | |
| R+L | NA | 1.85 ± 0.09 | 1.76 ± 0.05 | F (1, 20)= -0.2, P = 0.687 | |
| Most diseased segment | |||||
| Left | NA | 2.05 ± 0.15 | 1.99 ± 0.09 | F (1, 20) = 0.0, P = 0.914 | |
| Right | NA | 2.10 ± 0.10 | 1.93 ± 0.07 | F (1, 20) = 1.3, P = 0.259 | |
| R+L | NA | 2.07 ± 0.11 | 1.96 ± 0.08 | F (1, 20) = 0.4, P = 0.560 | |
| MR results | |||||
| Wall thickness (mm; mean, SE) | |||||
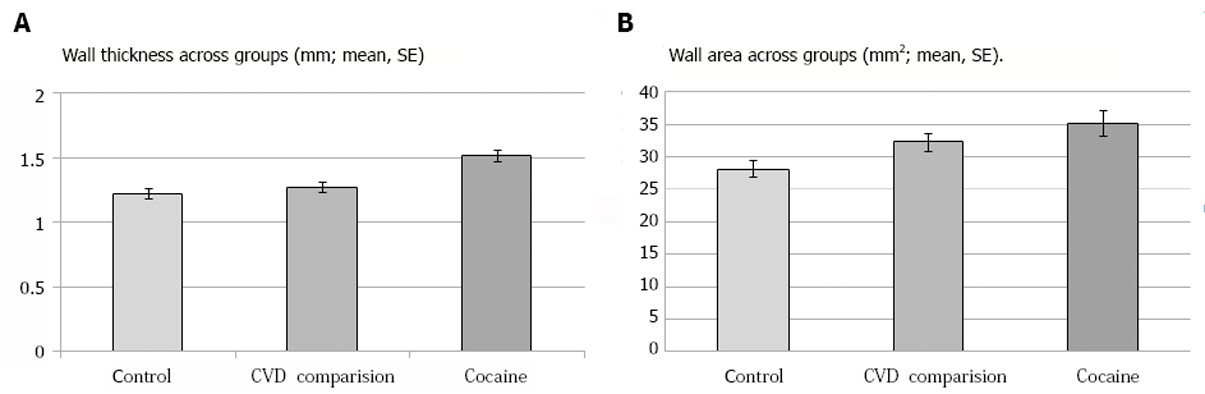
| Left | NA | 1.53 ± 0.06 | 1.25 ± 0.04 | F (1, 20) = 7.6, P = 0.012 | |
| Right | NA | 1.50 ± 0.07 1 | 1.20 ± 0.05 | F (1, 19) = 8.3, P = 0.009 | |
| R+L | 1.27 ± 0.04; t(12)2 = 4.12, P = 0.001 | 1.51 ± 0.062 | 1.22 ± 0.04 3 | F (1, 20) = 100, P = 0.005 | |
| Wall area (mm2) | |||||
| Left | NA | 35.67 ± 2.25 | 29.02 ± 1.54 | F (1, 20) = 3.3, P = 0.086 | |
| Right | NA | 34.51 ± 2.061 | 27.37 ± 1.43 | F (1, 19) = 4.9, P = 0.039 | |
| R + L | 32.28 ± 1.43; t(9)4 = -3.13, P = 0.012 | 35.18 ± 1.95 5 | 28.19 ± 1.314 | F (1, 20) = 4.9, P = 0.039 | |
The MRI measures demonstrated that the iCUD had significantly elevated carotid plaque burden as compared to the non-addicted healthy controls and the group of individuals with cardiovascular risk (Figure 1 and Figure 2, Table 2). The ANCOVA results showed that, as compared to the healthy controls, the iCUD group had significantly increased wall thickness and wall area. Notably, in one sample t-tests using the individuals with cardiovascular risk comparison group’s mean values, a similar pattern of elevated plaque in iCUD was observed as follows: iCUD had significantly thicker wall, whereas the cardiovascular risk group and healthy controls did not differ on this measure indicating the presence of more plaque and worse structural disease state in the carotids of iCUD than the much older symptomatic comparison sample, who has been identified for risk for cardiovascular events. Using the cardiovascular risk comparison group’s mean values for wall area, significant differences were detected when compared with healthy controls but differences did not reach significance when compared with iCUD.
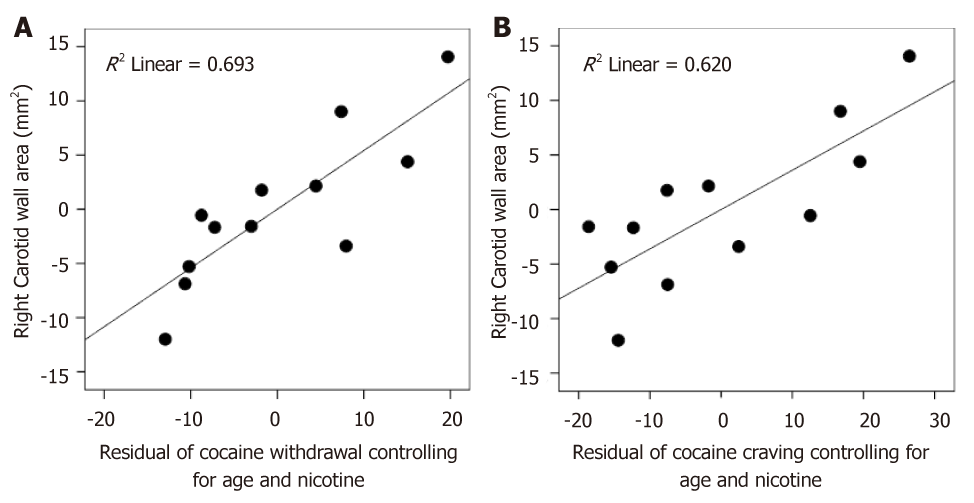
Testing whether these elevated inflammation markers in iCUD correlated with addiction symptoms, we found that plaque burden (wall area) was positively associated with the degree of cocaine withdrawal and craving even after controlling for age and nicotine use and also familywise error correcting for multiple analyses. The greater the cocaine withdrawal symptoms (r = 0.838, Puncorr = 0.003, Pcorr. = 0.021) and the greater the cocaine craving (r = 0.787, Puncorr. = 0.007, Pcorr. = 0.049) the larger the wall area in iCUD (Figure 3). No correlations with PET inflammation markers were found.
In this study, we conducted noninvasive vascular PET/MR imaging of the bilateral carotid arteries in iCUD and two control groups. Elevated markers of carotid artery atherosclerotic disease burden were found in iCUD as compared to non-addicted healthy controls and even as compared to older non-addicted individuals with high risk for cardiovascular disease. Specifically, the MRI measure of carotid wall structure showed higher thickness in the iCUD as compared to the healthy controls and cardiovascular risk group, indicating greater carotid plaque burden. The iCUD also had larger wall area as compared to the healthy controls (a difference that did not reach significance when compared to the cardiovascular risk group), indicating structural wall abnormalities that reached levels of those in the cardiovascular risk group. These elevated cardiovascular disease markers were associated with elevated degree of cocaine withdrawal and craving in iCUD, indicating a relationship between the extent of substance use disorder and the development of atherosclerosis.
The carotid FDG-PET images indicating the presence of inflammation did not differ between the iCUD and non-addicted healthy controls, as most of these individuals had inflammatory presence in one side or bilaterally in the carotid arteries. This result may indicate the beginning of an atherosclerosis process in all subjects with inflammation levels (i.e., TBR) over 1.6[35,36], yet, overall the detected inflammatory levels in both samples were mild to moderate.
A most intriguing aspect of this study is the comparison with the cardiovascular risk group, whereby the iCUD group showed the most severe elevations in wall thickness (with similar results that did not reach significance also for the wall area). The thickening of the arterial wall to form an atherosclerotic plaque is a process in which cholesterol deposition, inflammation, extracellular-matrix formation and thrombosis have important roles[6,37]. Thus, although many of the healthy control participants showed some inflammation in the carotids (PET results), only iCUD showed a statistically significant elevation as compared with the cardiovascular risk group in the indices of plaque burden. Atherosclerosis and progression to cardiovascular disease are characterized by a slow and “silent” disease accumulation that occurs over decades and progress from a chronic inflammatory condition that can be converted into an acute clinical event by plaque rupture and thrombosis[38]. Since iCUD in this study had over 20 years of lifetime cocaine use as well as nicotine and alcohol it is possible that they passed the inflammatory disease stage and have progressed into an atherosclerosis disease state with a clear vascular structural impact (i.e., the formation of plaques). Interestingly, iCUD who had increased carotid plaque burden also had greater withdrawal and craving, which have been implicated with negative outcomes of cocaine dependence[22,23].
These preliminary results should be considered in light of several caveats which limit the generalizability of the findings, including small sample size, the limited number of women, and the absence of a match on race. Race is very important for cardiovascular disease with African-American individuals showing greater progression of coronary atherosclerosis as compared to Caucasians[39]. Notably, among African-American men, cocaine was the largest contributor to overdose deaths[40]. Therefore, close matching on race in similar future studies could reduce potential bias in results. Despite considerable efforts, recruitment of healthy control individuals who match the iCUD group on years of nicotine smoking was also a challenge. While nicotine smoking, which is part of the phenomenology of CUD (frequently concomitant with multiple substance use), was accounted for in analyses, matching between groups on nicotine use could provide a better approximation of the vascular effects of cocaine use. Data for PET-18FDG in the cardiovascular risk group and data for calculating Framingham Risk Scores for the full sample were not available. The cross sectional design of the study further limited tracking of disease progression as should be done in future studies. Thus, examining iCUD with less years of lifetime cocaine use and those in earlier stages of the addiction disease could provide opportunities for further stratification of the progression of atherosclerosis disease, even prior to structural narrowing of the arteries. In addition, longitudinal studies should explore whether preventive cardiovascular measures will combat disease progression and may also reduce addiction symptoms. Early detection and preventive intervention protocols will thus await the results of a broader trial.
Given the known vascular toxicity induced by cocaine[1,41] and the progressing age of the crack generation, there is a public health imperative for early detection of the preclinical markers of atherosclerosis in iCUD[42-44]. Once pathology is identified, and especially if identified at an early stage, timely intervention can be deployed to prevent the progression into severe impairments, emergency cardiovascular events and premature mortality.
Cocaine is one of the most commonly illicit drugs involved in emergency department visits, amounting to a vast social and economic burden. Cocaine use disorder (CUD), a chronic relapsing condition, frequently leads to life-threatening vascular disease including stroke, coronary artery disease and myocardial infarction. Cocaine’s main vasoactive metabolite benzoylmethylecgonine, a tropane alkaloid, is associated with hematological effects on the vessel and the loss of the endothelium’s protective functions leading to elevated immune state including macrophage proliferation, atherosclerosis, and ischemic vascular disease. The life-style associated with chronic cocaine use (poor sleep and nutrition) further affects cardiovascular health.
Despite the known vascular toxicity associated with cocaine use, individuals with (iCUD) seeking addiction treatment receive mostly psychotherapy and psychiatric pharmacotherapy with no attention to vascular disease in the absence of clear symptoms. Little is known about the pre-clinical signs of cardiovascular risk in iCUD and early signs of vascular disease are undetected in this underserved population.
We aim to assess inflammation composition and plaque burden in individuals with cocaine use disorder aiming to quantify markers of atherosclerosis and vascular disease. The characterization of vascular disease in iCUD with no pre-clinical cardiovascular symptoms can inform development of future preventive and treatment protocols.
Advancements in multi-modal imaging technologies have been efficacious in early detection of atherosclerosis in asymptomatic populations who are at heightened risk for vascular disease. Simultaneous magnetic resonance imaging (MRI) and positron emission tomography (PET) allows for the precise quantification of inflammatory composition and plaque burden during a single non-operator dependent scan.
The bilateral carotid arteries were imaged with PET/MRI in iCUD asymptomatic for cardiovascular disease, healthy controls, and MRI in individuals with cardiovascular risk. PET with 18F-fluorodeoxyglucose evaluated vascular inflammation and 3-D dark-blood MRI assessed plaque burden including wall area and thickness. Addiction questionnaires assessed drug use and severity of addiction.
The MRI measure of wall structure was thicker in iCUD as compared to the controls and even as compared with the cardiovascular risk group, indicating greater carotid plaque burden. iCUD had also statistically significant larger wall area as compared to the healthy controls but not as compared to the cardiovascular risk group (the later results did not reach significance). These findings indicate structural wall similarities between the iCUD and cardiovascular risk study groups.
The majority of iCUD and controls had carotid FDG-PET signal greater than Target-to-Background ratios (TBR max) 1.6, indicating the presence of inflammation, yet, overall the observed inflammatory levels in both groups were mild (TBR max level under 3). In iCUD, wall area correlated with greater cocaine withdrawal and craving.
For the first time in cocaine addiction, this preliminary study used noninvasive simulations PET/MRI vascular imaging of the bilateral carotid arteries in cardiovascular disease-asymptomatic iCUD and two control groups, including healthy individuals and those with cardiovascular disease risk. Aligned with study hypothesis, we observed markers of elevated carotid artery plaque burden in iCUD, reaching similar (wall area) and even exceeding (wall thickness) levels of those in cardiovascular risk group. This plaque burden in iCUD was positively associated with extent of cocaine withdrawal and craving symptoms, indicative of a relationship between the severity of addiction and vascular disease state.
Several caveats limit generalizability of findings, including a small sample size, the limited number of women, and variance between groups in race and nicotine smoking. These factors were covaried in the current analyses, nonetheless, matching between groups in future studies would provide a better approximation of cardiovascular disease in iCUD.
This PET/MRI investigation showed that markers of cardiovascular disease abnormalities were detected in iCUD with no presenting clinical symptoms. Expanding this line of research to examination of iCUD with fewer years of lifetime cocaine use could provide further stratification of cardiovascular disease progression in this population. Broader trials are warranted to develop protocols for early detection of cardiovascular risk and preventive intervention in individuals with cocaine use disorder.
| 1. | Schwartz BG, Rezkalla S, Kloner RA. Cardiovascular effects of cocaine. Circulation. 2010;122:2558-2569. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 238] [Cited by in RCA: 264] [Article Influence: 17.6] [Reference Citation Analysis (0)] |
| 2. | Afonso L, Mohammad T, Thatai D. Crack whips the heart: a review of the cardiovascular toxicity of cocaine. Am J Cardiol. 2007;100:1040-1043. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 95] [Article Influence: 5.0] [Reference Citation Analysis (0)] |
| 3. | Bachi K, Mani V, Jeyachandran D, Fayad ZA, Goldstein RZ, Alia-Klein N. Vascular disease in cocaine addiction. Atherosclerosis. 2017;262:154-162. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 93] [Cited by in RCA: 103] [Article Influence: 11.4] [Reference Citation Analysis (0)] |
| 4. | Narvaez JC, Magalhães PV, Fries GR, Colpo GD, Czepielewski LS, Vianna P, Chies JA, Rosa AR, Von Diemen L, Vieta E, Pechansky F, Kapczinski F. Peripheral toxicity in crack cocaine use disorders. Neurosci Lett. 2013;544:80-84. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 51] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Fox HC, D'Sa C, Kimmerling A, Siedlarz KM, Tuit KL, Stowe R, Sinha R. Immune system inflammation in cocaine dependent individuals: implications for medications development. Hum Psychopharmacol. 2012;27:156-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 127] [Article Influence: 9.1] [Reference Citation Analysis (0)] |
| 6. | Sanz J, Fayad ZA. Imaging of atherosclerotic cardiovascular disease. Nature. 2008;451:953-957. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 402] [Cited by in RCA: 405] [Article Influence: 22.5] [Reference Citation Analysis (0)] |
| 7. | Shirai T, Hilhorst M, Harrison DG, Goronzy JJ, Weyand CM. Macrophages in vascular inflammation--From atherosclerosis to vasculitis. Autoimmunity. 2015;48:139-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 83] [Cited by in RCA: 124] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 8. | Fleg JL, Stone GW, Fayad ZA, Granada JF, Hatsukami TS, Kolodgie FD, Ohayon J, Pettigrew R, Sabatine MS, Tearney GJ, Waxman S, Domanski MJ, Srinivas PR, Narula J. Detection of high-risk atherosclerotic plaque: report of the NHLBI Working Group on current status and future directions. JACC Cardiovasc Imaging. 2012;5:941-955. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 180] [Article Influence: 12.9] [Reference Citation Analysis (0)] |
| 9. | De Giorgi A, Fabbian F, Pala M, Bonetti F, Babini I, Bagnaresi I, Manfredini F, Portaluppi F, Mikhailidis DP, Manfredini R. Cocaine and acute vascular diseases. Curr Drug Abuse Rev. 2012;5:129-134. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 21] [Article Influence: 1.5] [Reference Citation Analysis (0)] |
| 10. | Fayad ZA, Mani V, Woodward M, Kallend D, Bansilal S, Pozza J, Burgess T, Fuster V, Rudd JH, Tawakol A, Farkouh ME. Rationale and design of dal-PLAQUE: a study assessing efficacy and safety of dalcetrapib on progression or regression of atherosclerosis using magnetic resonance imaging and 18F-fluorodeoxyglucose positron emission tomography/computed tomography. Am Heart J. 2011;162:214-221.e2. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 46] [Cited by in RCA: 43] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 11. | Fayad ZA, Mani V, Woodward M, Kallend D, Abt M, Burgess T, Fuster V, Ballantyne CM, Stein EA, Tardif JC, Rudd JH, Farkouh ME, Tawakol A; dal-PLAQUE Investigators. Safety and efficacy of dalcetrapib on atherosclerotic disease using novel non-invasive multimodality imaging (dal-PLAQUE): a randomised clinical trial. Lancet. 2011;378:1547-1559. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 416] [Cited by in RCA: 426] [Article Influence: 28.4] [Reference Citation Analysis (0)] |
| 12. | Davies JR, Izquierdo-Garcia D, Rudd JH, Figg N, Richards HK, Bird JL, Aigbirhio FI, Davenport AP, Weissberg PL, Fryer TD, Warburton EA. FDG-PET can distinguish inflamed from non-inflamed plaque in an animal model of atherosclerosis. Int J Cardiovasc Imaging. 2010;26:41-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 44] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 13. | Rudd JH, Elkhawad M, Fayad ZA. Vascular imaging with 18F-FDG PET/CT: optimal 18F-FDG circulation time? J Nucl Med. 2009;50:1560; author reply 1560-1560; author reply 1561. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 12] [Cited by in RCA: 13] [Article Influence: 0.8] [Reference Citation Analysis (0)] |
| 14. | Rudd JH, Myers KS, Bansilal S, Machac J, Pinto CA, Tong C, Rafique A, Hargeaves R, Farkouh M, Fuster V, Fayad ZA. Atherosclerosis inflammation imaging with 18F-FDG PET: carotid, iliac, and femoral uptake reproducibility, quantification methods, and recommendations. J Nucl Med. 2008;49:871-878. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 362] [Cited by in RCA: 351] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 15. | Zhang Z, Machac J, Helft G, Worthley SG, Tang C, Zaman AG, Rodriguez OJ, Buchsbaum MS, Fuster V, Badimon JJ. Non-invasive imaging of atherosclerotic plaque macrophage in a rabbit model with F-18 FDG PET: a histopathological correlation. BMC Nucl Med. 2006;6:3. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 99] [Cited by in RCA: 98] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 16. | Rudd JH, Myers KS, Bansilal S, Machac J, Rafique A, Farkouh M, Fuster V, Fayad ZA. (18)Fluorodeoxyglucose positron emission tomography imaging of atherosclerotic plaque inflammation is highly reproducible: implications for atherosclerosis therapy trials. J Am Coll Cardiol. 2007;50:892-896. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 345] [Cited by in RCA: 351] [Article Influence: 18.5] [Reference Citation Analysis (0)] |
| 17. | Rudd JH, Narula J, Strauss HW, Virmani R, Machac J, Klimas M, Tahara N, Fuster V, Warburton EA, Fayad ZA, Tawakol AA. Imaging atherosclerotic plaque inflammation by fluorodeoxyglucose with positron emission tomography: ready for prime time? J Am Coll Cardiol. 2010;55:2527-2535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 184] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 18. | Bachi K, Sierra S, Volkow ND, Goldstein RZ, Alia-Klein N. Is biological aging accelerated in drug addiction? Curr Opin Behav Sci. 2017;13:34-39. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 69] [Cited by in RCA: 77] [Article Influence: 8.6] [Reference Citation Analysis (0)] |
| 19. | First MB, Gibbon M, Williams J. Structured Clinical Interview for DSM-IV Axis I disorders - Patient Edition (SCID-I/P, Version 2.0). New York: Biometrics Research Department, New York State Psychiatric Institute 1996; . |
| 20. | Ventura J, Liberman RP, Green MF, Shaner A, Mintz J. Training and quality assurance with the Structured Clinical Interview for DSM-IV (SCID-I/P). Psychiatry Res. 1998;79:163-173. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 424] [Cited by in RCA: 438] [Article Influence: 15.6] [Reference Citation Analysis (0)] |
| 21. | McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, Pettinati H, Argeriou M. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9:199-213. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 2978] [Cited by in RCA: 3134] [Article Influence: 92.2] [Reference Citation Analysis (0)] |
| 22. | Kampman KM, Volpicelli JR, McGinnis DE, Alterman AI, Weinrieb RM, D'Angelo L, Epperson LE. Reliability and validity of the Cocaine Selective Severity Assessment. Addict Behav. 1998;23:449-461. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 172] [Cited by in RCA: 183] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 23. | Tiffany ST, Singleton E, Haertzen CA, Henningfield JE. The development of a cocaine craving questionnaire. Drug Alcohol Depend. 1993;34:19-28. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 255] [Cited by in RCA: 286] [Article Influence: 8.7] [Reference Citation Analysis (0)] |
| 24. | Gossop M, Griffiths P, Powis B, Strang J. Severity of dependence and route of administration of heroin, cocaine and amphetamines. Br J Addict. 1992;87:1527-1536. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 227] [Cited by in RCA: 238] [Article Influence: 7.0] [Reference Citation Analysis (0)] |
| 25. | Gossop M, Darke S, Griffiths P, Hando J, Powis B, Hall W, Strang J. The Severity of Dependence Scale (SDS): psychometric properties of the SDS in English and Australian samples of heroin, cocaine and amphetamine users. Addiction. 1995;90:607-614. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 430] [Cited by in RCA: 638] [Article Influence: 20.6] [Reference Citation Analysis (0)] |
| 26. | Zaidi H, Ojha N, Morich M, Griesmer J, Hu Z, Maniawski P, Ratib O, Izquierdo-Garcia D, Fayad ZA, Shao L. Design and performance evaluation of a whole-body Ingenuity TF PET-MRI system. Phys Med Biol. 2011;56:3091-3106. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 323] [Cited by in RCA: 273] [Article Influence: 18.2] [Reference Citation Analysis (0)] |
| 27. | Itskovich VV, Samber DD, Mani V, Aguinaldo JG, Fallon JT, Tang CY, Fuster V, Fayad ZA. Quantification of human atherosclerotic plaques using spatially enhanced cluster analysis of multicontrast-weighted magnetic resonance images. Magn Reson Med. 2004;52:515-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 68] [Cited by in RCA: 59] [Article Influence: 2.7] [Reference Citation Analysis (0)] |
| 28. | Yuan C, Hatsukami TS, Cai J. MRI plaque tissue characterization and assessment of plaque stability. Stud Health Technol Inform. 2005;113:55-74. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 29. | Yuan C, Mitsumori LM, Ferguson MS, Polissar NL, Echelard D, Ortiz G, Small R, Davies JW, Kerwin WS, Hatsukami TS. In vivo accuracy of multispectral magnetic resonance imaging for identifying lipid-rich necrotic cores and intraplaque hemorrhage in advanced human carotid plaques. Circulation. 2001;104:2051-2056. [PubMed] [DOI] [Full Text] |
| 30. | Mani V, Itskovich VV, Aguiar SH, Mizsei G, Aguinaldo JG, Samber DD, Macaluso FM, Fayad ZA. Comparison of gated and non-gated fast multislice black-blood carotid imaging using rapid extended coverage and inflow/outflow saturation techniques. J Magn Reson Imaging. 2005;22:628-633. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 36] [Cited by in RCA: 33] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 31. | Mani V, Itskovich VV, Szimtenings M, Aguinaldo JG, Samber DD, Mizsei G, Fayad ZA. Rapid extended coverage simultaneous multisection black-blood vessel wall MR imaging. Radiology. 2004;232:281-288. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 54] [Cited by in RCA: 49] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 32. | Mani V, Woodward M, Samber D, Bucerius J, Tawakol A, Kallend D, Rudd JH, Abt M, Fayad ZA. Predictors of change in carotid atherosclerotic plaque inflammation and burden as measured by 18-FDG-PET and MRI, respectively, in the dal-PLAQUE study. Int J Cardiovasc Imaging. 2014;30:571-582. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 21] [Cited by in RCA: 27] [Article Influence: 2.3] [Reference Citation Analysis (0)] |
| 33. | Mani V, Muntner P, Gidding SS, Aguiar SH, El Aidi H, Weinshelbaum KB, Taniguchi H, van der Geest R, Reiber JH, Bansilal S, Farkouh M, Fuster V, Postley JE, Woodward M, Fayad ZA. Cardiovascular magnetic resonance parameters of atherosclerotic plaque burden improve discrimination of prior major adverse cardiovascular events. J Cardiovasc Magn Reson. 2009;11:10. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 57] [Cited by in RCA: 54] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 34. | Wong SK, Mobolaji-Iawal M, Arama L, Cambe J, Biso S, Alie N, Fayad ZA, Mani V. Atherosclerosis imaging using 3D black blood TSE SPACE vs 2D TSE. World J Radiol. 2014;6:192-202. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in CrossRef: 14] [Cited by in RCA: 17] [Article Influence: 1.4] [Reference Citation Analysis (0)] |
| 35. | Abdelbaky A, Tawakol A. Noninvasive Positron Emission Tomography Imaging of Coronary Arterial Inflammation. Curr Cardiovasc Imaging Rep. 2011;4:41-49. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 12] [Cited by in RCA: 9] [Article Influence: 0.6] [Reference Citation Analysis (0)] |
| 36. | Tawakol A, Migrino RQ, Bashian GG, Bedri S, Vermylen D, Cury RC, Yates D, LaMuraglia GM, Furie K, Houser S, Gewirtz H, Muller JE, Brady TJ, Fischman AJ. In vivo 18F-fluorodeoxyglucose positron emission tomography imaging provides a noninvasive measure of carotid plaque inflammation in patients. J Am Coll Cardiol. 2006;48:1818-1824. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 710] [Cited by in RCA: 718] [Article Influence: 35.9] [Reference Citation Analysis (0)] |
| 37. | Fernández-Ortiz A, Jiménez-Borreguero LJ, Peñalvo JL, Ordovás JM, Mocoroa A, Fernández-Friera L, Laclaustra M, García L, Molina J, Mendiguren JM, López-Melgar B, de Vega VM, Alonso-Farto JC, Guallar E, Sillesen H, Rudd JH, Fayad ZA, Ibañez B, Sanz G, Fuster V. The Progression and Early detection of Subclinical Atherosclerosis (PESA) study: rationale and design. Am Heart J. 2013;166:990-998. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 66] [Cited by in RCA: 84] [Article Influence: 6.5] [Reference Citation Analysis (0)] |
| 38. | Lusis AJ. Atherosclerosis. Nature. 2000;407:233-241. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4039] [Cited by in RCA: 4204] [Article Influence: 161.7] [Reference Citation Analysis (0)] |
| 39. | Kataoka Y, Hsu A, Wolski K, Uno K, Puri R, Tuzcu EM, Nissen SE, Nicholls SJ. Progression of coronary atherosclerosis in African-American patients. Cardiovasc Diagn Ther. 2013;3:161-169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 6] [Reference Citation Analysis (0)] |
| 40. | Shiels MS, Freedman ND, Thomas D, Berrington de Gonzalez A. Trends in U.S. Drug Overdose Deaths in Non-Hispanic Black, Hispanic, and Non-Hispanic White Persons, 2000-2015. Ann Intern Med. 2018;168:453-455. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 103] [Cited by in RCA: 124] [Article Influence: 15.5] [Reference Citation Analysis (1)] |
| 41. | Finkel JB, Marhefka GD. Rethinking cocaine-associated chest pain and acute coronary syndromes. Mayo Clin Proc. 2011;86:1198-1207. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 46] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 42. | Aquaro GD, Gabutti A, Meini M, Prontera C, Pasanisi E, Passino C, Emdin M, Lombardi M. Silent myocardial damage in cocaine addicts. Heart. 2011;97:2056-2062. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 44] [Cited by in RCA: 45] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 43. | D'Agostino RB, Russell MW, Huse DM, Ellison RC, Silbershatz H, Wilson PW, Hartz SC. Primary and subsequent coronary risk appraisal: new results from the Framingham study. Am Heart J. 2000;139:272-281. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 381] [Article Influence: 14.7] [Reference Citation Analysis (0)] |
| 44. | Farooq MU, Bhatt A, Patel M. Neurotoxic and cardiotoxic effects of cocaine and ethanol. J Med Toxicol. 2009;5:134-138. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 38] [Cited by in RCA: 35] [Article Influence: 2.1] [Reference Citation Analysis (0)] |
STROBE Statement: The authors have read the STROBE Statement—checklist of items, and the manuscript was prepared and revised according to the STROBE Statement—checklist of items.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See:
Manuscript source: Invited manuscript
Specialty type: Radiology, nuclear medicine and medical imaging
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): D
Grade E (Poor): 0
P-Reviewer: Kwok WE, Nouh MR S-Editor: Wang JL L-Editor: A E-Editor: Xing YX