Published online Apr 26, 2017. doi: 10.4330/wjc.v9.i4.355
Peer-review started: August 25, 2016
First decision: October 8, 2016
Revised: January 10, 2017
Accepted: February 8, 2017
Article in press: February 13, 2017
Published online: April 26, 2017
Processing time: 247 Days and 17.4 Hours
To investigate the rates and determinants of success of repeat percutaneous coronary intervention (PCI) following an initial failed attempt at recanalising the chronic total occlusions (CTO) percutaneously.
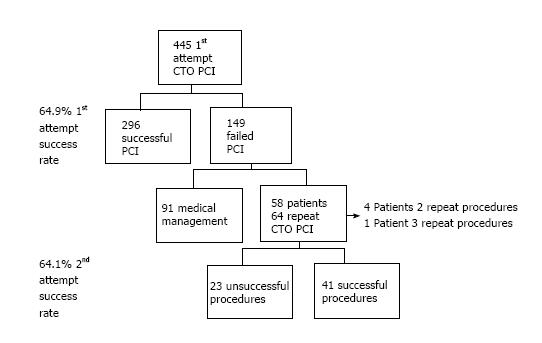
In 445 consecutive first attempt CTO-PCI procedures in our institution, procedural failure occurred in 149 (33.5%). Sixty-four re-PCI procedures were performed in 58 patients (39%) all had a single CTO. Procedural and outcome data in the re-PCI population was entered into the institutional database. A retrospective analysis of clinical, angiographic and procedural data was performed.
Procedural success was achieved in 41 (64%) procedures. Univariate analysis of clinical and angiographic characteristics showed that re-PCI success was associated with intravascular ultrasound (IVUS) guidance (19.5% vs 0%, P = 0.042), while failure was associated with severe calcification (30.4% vs 9.7%, P = 0.047) and a JCTO score > 3 (56.5% vs 17.1% P = 0.003). Following multiple regression analysis the degree of lesion complexity (J-CTO score > 3), IVUS use, involvement of an experienced CTO operator and LAD CTO location were significant predictors of successful re-PCI. Overall the complication rate was low, with the only MACCE two periprocedural MI’s neither of which required intervention.
Re-PCI substantially increases the overall success rate of CTO revascularization. Predictors of re-PCI success included the use of IVUS, the involvement of an experienced CTO operator in the repeat attempt and the location of the CTO.
Core tip: Failed percutaneous recanalization of chronic total occlusions (CTO) constitutes a clinical conundrum. While percutaneous treatment is often abandoned in favour of medical therapy, CTO-percutaneous coronary intervention (PCI) expertise and alternative techniques may contribute to improve procedural success. This study shows that with careful pre-procedural planning reattempt PCI in CTO’s is both safe and efficacious.
- Citation: Cuevas C, Ryan N, Quirós A, Del Angel JG, Gonzalo N, Salinas P, Jiménez-Quevedo P, Nombela-Franco L, Nuñez-Gil I, Fernandez-Ortiz A, Macaya C, Escaned J. Determinants of percutaneous coronary intervention success in repeat chronic total occlusion procedures following an initial failed attempt. World J Cardiol 2017; 9(4): 355-362
- URL: https://www.wjgnet.com/1949-8462/full/v9/i4/355.htm
- DOI: https://dx.doi.org/10.4330/wjc.v9.i4.355
Revascularization of chronic total occlusions (CTO) is a well accepted technique, albeit one of the most challenging procedures in interventional cardiology with the presence of a CTO a strong predictor against percutaneous recanalisation[1,2]. There is growing evidence that CTO recanalization confers benefit to patients[3-11], however the success rate of CTO percutaneous coronary intervention (PCI) is significantly lower than in non-CTO lesions, ranging from 51% to > 80% in different series[3,8,12,13]. Several attempts have been made to rate procedural difficulty in CTOs, with the JCTO score[14], the most commonly used score, identifying prior CTO failure as one of the five key determinants of PCI success. The introduction of novel techniques including parallel wiring, CART/reverse CART, hybrid procedures and bilateral injections have increased the success of the procedure[15-17], with several studies promoting the use of intravascular ultrasound (IVUS) in guiding wiring of the true lumen and optimizing CTO-PCI outcomes[17-19]. Despite the potential benefits of CTO recanalization a significant proportion of patients are managed medically rather than reattempting CTO-PCI[5], perhaps because, when compared to initial attempts at CTO-PCI, the predictors of and success rates in re-PCI are largely unknown. The purpose of this study was to evaluate the success rate of re-PCI, as well as to identify predictors of success.
Between January 2008 and September 2012, 445 patients had first time native vessel CTO PCI procedures in our institution. In 149 patients the initial procedure was unsuccessful with re-PCI planned in 58 patients (39%) who underwent 64 further procedures, four patients had two re-PCI attempts while one patient had three re-PCI attempts (Figure 1). Procedural and outcomes data in this re-PCI population was entered into the institutional database.
Data was collected in relation to factors that may affect procedural success, including fluoroscopy time, use of stiffer polymer coated CTO guide-wires and ad-hoc PCIs. Re-PCI data collected including change of strategy, IVUS guidance and involvement of an experienced operator. The patients were divided into subgroups according to procedural outcome (successful/failure) for analysis. Post-procedural data including evidence of peri-procedural MI, renal impairment and death was obtained from the institutional database.
CTO was defined as a TIMI (thrombolysis in myocardial infarction) grade 0 flow in the target segment, with a duration > 3 mo, determined based on clinical symptoms or prior angiography when available[12]. Angiographic success was defined as a residual stenosis < 30% with TIMI grade flow ≥ 2. The EuroCTO club definition of an operator with a success rate of at least 80% in CTO PCI was used to identify experienced operators[1] , all other operators were considered non-experienced operators. IVUS guidance included two techniques (IVUS-guided wiring of the CTO stump, and IVUS-guided penetration from the subintimal space). The lesion complexity was classified using the J-CTO score with lesions scored as 0-5 dependent on the presence of one or more of the following features: Blunt stump, length > 20 mm, severe calcification, > 45° tortuosity and previous failed attempt[14].
Categorical and continuous variables are expressed as counts (%) and mean ± SD, respectively. The angiographic, clinical and procedural factors were analyzed as possible determinants of success in a new attempt at recanalization and were compared between patient groups.
Categorical variables were compared with the Fisher’s exact test and continuous variables were compared with a t test. All indices with a P-value < 0.1 in the univariate analysis were included in a multiple logistic regression analysis and the final model was selected by Akaike’s Information Criterion[20]. Fitting a classical logistic regression model with this dataset leads to a non-identifiable problem, as some variables induce a separation. In order to obtain stable logistic regression coefficients, we use Bayesian inference[21]. The computations required to estimate the coefficients of the model are implemented in the arm package for applied regression and multilevel modelling in R v.3.2.2 software.
The success rate for CTO re-PCI was 64.1%. The baseline clinical characteristics of the patients are shown in Table 1. The mean age was 59.5 ± 11.5 years, and 86.2% were male. There were no significant differences between the successful and failed re-PCI groups. Baseline angiographic and procedural characteristics are shown in Tables 2 and 3. The left anterior descending artery (LAD) and the right coronary artery (RCA) were the most commonly affected vessel in the successful and failed groups respectively. Of the individual components of the JCTO score only calcification was significant with more severely calcified lesions in the failed group (30.4% vs 9.7%, P < 0.047). The successful group had a lower average J-CTO score (2.73 ± 0.84 vs 3.2 ± 0.99, P < 0.010), with fewer lesions with a J-CTO score ≥ 4.
| Patient demographics | Overall (n = 58) | Success (n = 40) | Failure (n = 18) | P value |
| Age, yr | 59.2 ± 11.6 | 59.2 ± 11.6 | 60.2 ± 11.7 | 0.78 |
| Male | 50 (86.2%) | 33 (82.5%) | 17 (94.4%) | 0.41 |
| Obesity | 20 (34.4%) | 14 (35%) | 6 (33.3%) | 1 |
| Hypertension | 39 (67.2%) | 24 (60%) | 15 (83.3%) | 0.15 |
| Dyslipidaemia | 41 (70.6%) | 27 (67.5%) | 14 (77.8%) | 0.63 |
| Diabetes | 23 (39.6%) | 15 (37.5%) | 8 (44.4%) | 0.84 |
| Smoking | 38 (65.5%) | 26 (65%) | 12 (66.7%) | 1 |
| Previous MI | 22 (37.9%) | 15 (37.5%) | 7 (38.9%) | 1 |
| Previous PCI | 27 (46.6%) | 17 (42.5%) | 10 (55.6%) | 0.52 |
| Previous CABG | 2 (3.4%) | 1 (2.5%) | 1 (5.6%) | 0.52 |
| LVEF < 45% | 22 (37.9%) | 15 (37.5%) | 7 (38.9%) | 1 |
| CKD IV | 3 (5.2%) | 3 (7.5%) | 0 (0%) | 0.55 |
| Angiographic characteristics | Overall (n = 64) | Success (n = 41) | Failure (n = 23) | P value |
| CTO Site | 0.0045 | |||
| LAD | 27 (42.2%) | 21 (51.5%) | 6 (26.1%) | |
| RCA | 31 (48.4%) | 16 (39.0%) | 15 (65.2%) | |
| LCx | 6 (9.4%) | 4 (9.7%) | 2 (8.7%) | |
| Blunt stump | 19 (29.7%) | 10 (24.3%) | 9 (39.1%) | 0.34 |
| Tortuous vessel | 23 (35.9%) | 12 (29.2%) | 11 (47.8%) | 0.225 |
| Calcified lesion | 11 (17.2%) | 4 (9.7%) | 7 (30.4%) | 0.047 |
| Lesion length > 20 mm | 49 (93.8%) | 30 (73.1%) | 19 (82.6%) | 0.58 |
| J-CTO Score | 2.9 ± 0.92 | 2.73 ± 0.84 | 3.2 ± 0.99 | 0.0063 |
| J-CTO 1 | 4 (6.25%) | 3 (7.3%) | 1 (21.4%) | 1 |
| J-CTO 2 | 18 (28.1%) | 12 (29.3%) | 6 (26.1%) | 1 |
| J-CTO 3 | 22 (34.4%) | 19 (46.3%) | 3 (39.1%) | 0.015 |
| J-CTO 4 | 20 (31.2%) | 7 (17.1%) | 13 (56.5%) | 0.0028 |
| Rentrop class 3 | 43 (67.2%) | 26 (48.8%) | 17 (73.9%) | 0.56 |
| Segment | ||||
| Distal | 4 (6.2%) | 2 (4.9%) | 2 (8.7%) | 0.61 |
| Mid | 27 (42.2%) | 16 (39.0%) | 11 (47.8%) | 0.67 |
| Proximal | 33 (51.6%) | 23 (56.1%) | 10 (43.5%) | 0.47 |
| Segment length | 26.95 ± 12.2 | 25.4 ± 10.3 | 29.7 ± 14.9 | 0.23 |
| Presence of proximal disease | 15 (23.4%) | 9 (21.9%) | 6 (26.1%) | 0.95 |
| Procedural characteristics | Overall (n = 64) | Success (n = 41) | Failure (n = 23) | P value |
| Planned initial attempt | 45 (70.3%) | 29 (70.7%) | 16 (69.6%) | 1 |
| Retrograde approach | 9 (14.1%) | 6 (14.6%) | 3 (13.0%) | 1 |
| Contralateral injection | 36 (56.3%) | 22 (53.6%) | 14 (60.9%) | 0.76 |
| Parallell wire | 16 (25%) | 10 (24.3%) | 6 (26.1%) | 1 |
| Intravascular ultrasound | 8 (12.5%) | 8 (19.5%) | 0 (0%) | 0.042 |
| Rotablator | 5 (7.8%) | 5 (12.2%) | 0 (0%) | 0.15 |
| Change of operator | 39 (60.9%) | 27 (65.9%) | 12 (52.2%) | 0.41 |
| Experienced operator | 36 (56.3%) | 27 (65.9%) | 9 (39.1%) | 0.065 |
| Change in guide catheter | 11 (17.2%) | 7 (17.1%) | 4 (17.4%) | 1 |
| Change of wire | 38 (59.4%) | 23 (56.1%) | 15 (65.2%) | 0.65 |
| Microcatheter use | 46 (71.9%) | 28 (68.3%) | 18 (78.3%) | 0.57 |
| Procedure time | 127.8 ± 44.3 | 129.1 ± 51.3 | 125.3 ± 32.8 | 0.71 |
| Fluroscopy time | 45.44 ± 21 | 43.3 ± 21.5 | 49.2 ± 20.0 | 0.27 |
| Contrast (mL) | 337.5 ± 127.5 | 355.5 ± 127.2 | 305.4 ± 124.3 | 0.13 |
Inability to cross the lesion with a guidewire is the most common reason for failure in CTO-PCI. We analyzed factors associated with the initial failed attempt that may reflect the effort invested in the initial attempt and therefore could have an indirectly proportional relationship with success in re-PCI. These included fluoroscopy time, use of dedicated guidewires and a planned initial attempt vs ad-hoc CTO-PCI. There were no significant differences in any of these variables. In the patients who underwent a reattempt 30% of the initial failed procedures were ad-hoc CTO PCI attempts at the time of diagnostic angiography. The group who underwent initial ad-hoc PCI had a higher success rate than those who underwent an initial planned attempt (89% vs 48.8%, P = 1) however this did not reach statistical significance.
All IVUS guided procedures were successful (P = 0.020). There were no significant differences observed with the use of individual pieces of equipment such as guide catheters, guidewire type or microcatheters; or implementation of new strategies such as retrograde access, contralateral injection or parallel wiring.
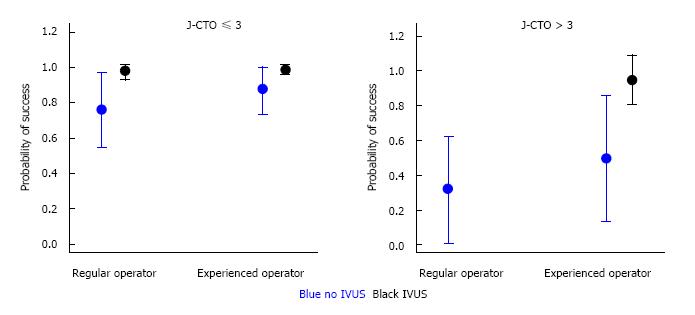
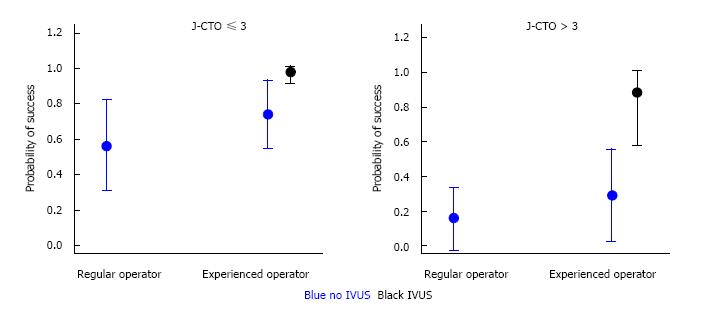
Multiple logistic regression analysis identified the degree of lesion complexity (J-CTO score ≤ 3 and >3), IVUS use, involvement of an experienced CTO operator in the repeat PCI attempt, and LAD location of the CTO as independent predictors of procedural success/failure (Table 4). A model for predicting probability of procedural success was developed with logistic regression analysis that combined these angiographic and technical variables (Figures 2 and 3). As seen in Figures 2 and 3, IVUS use in combination with an experienced CTO operator increases the probability of success particularly when the J-CTO score is > 3.
| Variable | Coefficient (b) | SD (b) | 95%CI | P (b ≠ 0 | data) | P value |
| J-CTO ≤ 3 | 0.26 | 0.52 | -2.04 | 0.69 | 0.31 |
| J-CTO > 3 | -1.67 | 0.67 | -3.34 | 0.99 | 0.01 |
| LAD | 0.9 | 0.6 | -2.36 | 0.93 | 0.07 |
| IVUS use | 2.96 | 1.58 | -6.2 | 0.97 | 0.03 |
| Experienced operator | 0.78 | 0.58 | -2.28 | 0.91 | 0.09 |
Overall, the complication rate was low with 2 periprocedural MI’s one occurring in a successful procedure and one in a failed procedure, both were characterized by minimal elevation in cardiac enzymes post procedure and neither required further intervention. There were no deaths in the population and no contrast induced nephropathy.
The main conclusion of our study is that re-PCI in CTO after a failed attempt is associated with a good success rate. Adequate pre procedural evaluation and planning is crucial. In complex lesions factors such as IVUS-guidance and experienced CTO operators increase the chances of success. Less complex lesions, particularly those in the LAD, may be attempted by non-experienced CTO operators with a good success rate.
In our study population, the success rate for CTO-re-PCI was 64.1%, this compares favorably with the Japanese CTO registry where re-PCI attempts had a procedural success rate of 68.5%[11]. Involvement of experienced operators and used of IVUS is associated with improved success, particularly in CTOs in the LAD location. Compared to the Japanese CTO data where success rates in re-PCI cases were significantly lower than initial attempts (68.5% vs 86.6% respectively), in our group, we found a similar overall success rate in the re-PCI group (64.1% vs 66.5%). This is likely explained by a less aggressive initial approach to CTO-PCI in our population during the study period. Patients who underwent initial ad-hoc PCI had a higher re-PCI success rates than those who underwent an initial planned attempt (89% vs 48.8%, P = 1), probably reflecting a less dedicated attempt, with difficulties easily overcome in a second, more aggressive procedure. The success rate in re-PCI contributes to a significant increase in per patient success of PCI in this complex anatomical scenario.
There have been several attempts to rate CTO procedural difficulty. The J-CTO score, the most popular of the CTO procedural difficulty scores, was developed by Morino et al[14] in their large multicenter registry to classify the difficulty of antegrade lesion crossing, and identified prior CTO failure as a one of the five key determinants of PCI success. In our population, we found only one of the traditional predictors of procedural difficulty in initial attempt CTO-PCI, severe calcification to be significantly associated with procedural success re-PCI. Nombela-Franco et al[22] validated the predictive value of the J-CTO score in successful anterograde crossing of the lesion within thirty minutes however they failed to show an ability to predict procedural success. Although not validated for predicting success the J-CTO score remains a useful tool in stratifying lesion complexity with significantly more patients with a J-CTO score > 4 in the failed group. Similar to data from Thompson et al[23] showing a significantly higher PCI success rate with experienced operators (75.2% vs 58.9%; P < 0.001), we found an experienced operator an important predictor of success in re-PCI attempts.
Data from the EuroCTO club puts IVUS use at 1.5% amongst its members in 2010. This is likely due to economic constraints and differs from Japan and the US where imaging techniques play a much larger role in CTO revascularization[24]. In our population, IVUS was used in 20% of all successful re-PCIs, in 6 (15%) cases a second wire was introduced into the true lumen via IVUS guidance after visualizing the first wire in false lumen and in 2 (5%) cases IVUS was used for ostial wiring. Furthermore, in these cases IVUS aided vessel sizing prior to stent implantation suggesting IVUS can be used to enhance the safety of CTO-PCI and optimize final results.
The CTO PCI reattempt rate remains relatively low, approximately 38% in this study, perhaps due to lack of large randomized clinical trials demonstrating benefit with CTO revascularisation. A large meta-analysis from Joyal et al[25] in 2010 comparing successful to failed CTO recanalization showed a 44% reduction in mortality, 78% reduction in subsequent CABG and a 55% reduction in residual angina in successfully recanalised CTO’s. However there was significant heterogeneity amongst the clinical outcomes and successful recanalization did not impact on MI or MACE. Other studies have shown successful percutaneous coronary intervention in a chronic total occlusion (CTO-PCI) to be beneficial in terms of recurrent myocardial, infarction, all-cause death, recurrent angina pectoris, subsequent CABG and cumulative survival rate compared to conservative management after failed PCI attempts however these are small heterogeneous populations[3-8]. In the context of acute myocardial infarctions it has been shown that the presence of a CTO increases long term mortality[9], with CTO an independent predictor of mortality in STEMI with cardiogenic shock[10]. The high success rate, low procedural complication and in-hospital MACE rates observed in this study suggest that after failed attempt a reattempt a CTO-PCI should be considered.
Finally, combination of the angiographic and procedural factors identified by multiple regression analysis as predictive of success or failure (degree of lesion complexity, IVUS use and an experienced CTO operator) yielded anticipated success rates ranging from 16% to 99%. It was observed that the implementation of procedural factors such as IVUS-guidance and experienced CTO operators are crucial when it comes to complex lesions (J-CTO score > 3), increasing in the probability of success from 16% to 99%. In comparison in less complex lesions (J-CTO score < 3), technical factors play a lesser role and these lesions even when attempted by non-experienced operators using IVUS have a high probability of success. These factors should be considered when planning a CTO-PCI strategy.
There are a number of limitations to our study. First, it is a descriptive and retrospective study designed only to look at the angiographic success rates and immediate in hospital outcomes of reattempt PCI. Long-term clinical and angiographic outcomes require evaluation in large-scale prospective clinical trials. Second, the angiographic characteristics of the CTOs were evaluated retrospectively. Third, this is a small sample from a single centre therefore one must be cautious when interpreting these results.
In conclusion, our findings suggest that re-PCI increased substantially the overall success rate of CTO revascularization. Predictors of re-PCI success included the use of IVUS, the involvement of an experienced CTO operator in the repeat attempt and the location of the CTO. The high success rate, low procedural complication and in-hospital MACE rates observed in this study suggest that after failed attempt a carefully planned reattempt at CTO-PCI should be considered.
Failed percutaneous recanalization of chronic total occlusions (CTO) constitutes a clinical conundrum. While percutaneous treatment is often abandoned in favour of medical therapy, CTO PCI expertise and alternative techniques may contribute to improve procedural success. There is growing evidence that CTO recanalization confers benefit to patients, however the success rate of CTO PCI is significantly lower than in non-CTO lesions, ranging from 51% to > 80% in different series. In this study the authors evaluated the success rates and predictors of success in reattempt PCI in CTO’s.
Recanalising CTO’s with viable myocardium appears to be beneficial to patients. Few prior studies have evaluated the benefit of reattempting PCI after an initial failed attempt.
Re-PCI in CTO after a failed attempt is associated with a good success rate. Adequate pre procedural evaluation and planning is crucial. In complex lesions factors such as IVUS-guidance and experienced CTO operators increase the chances of success. Less complex lesions, particularly those in the LAD, may be attempted by non-experienced CTO operators with a good success rate.
The results of this study can assist operators in adequate pre-procedural planning in CTO’s.
CTO: Chronic total occlusion an artery that has been occluded for longer than three months. IVUS: Intra-vascular ultrasound a technique that can be used to assist in visualizing the stump of a CTO, identifying wire position peri-procedurally and optimizing stenting. PCI: Percutaneous coronary intervention a transcatheter technique used to revascularise a coronary territory.
This study has value as it emphasis the need for pre-procedural evaluation of lesion complexity and therefore complex lesions must be faced by experienced operators through an IVUS guided CTO-PCI approach.
| 1. | Sianos G, Werner GS, Galassi AR, Papafaklis MI, Escaned J, Hildick-Smith D, Christiansen EH, Gershlick A, Carlino M, Karlas A. Recanalisation of chronic total coronary occlusions: 2012 consensus document from the EuroCTO club. EuroIntervention. 2012;8:139-145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 273] [Article Influence: 19.5] [Reference Citation Analysis (0)] |
| 2. | Christofferson RD, Lehmann KG, Martin GV, Every N, Caldwell JH, Kapadia SR. Effect of chronic total coronary occlusion on treatment strategy. Am J Cardiol. 2005;95:1088-1091. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 288] [Cited by in RCA: 299] [Article Influence: 14.2] [Reference Citation Analysis (0)] |
| 3. | Suero JA, Marso SP, Jones PG, Laster SB, Huber KC, Giorgi LV, Johnson WL, Rutherford BD. Procedural outcomes and long-term survival among patients undergoing percutaneous coronary intervention of a chronic total occlusion in native coronary arteries: a 20-year experience. J Am Coll Cardiol. 2001;38:409-414. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 482] [Cited by in RCA: 464] [Article Influence: 18.6] [Reference Citation Analysis (0)] |
| 4. | Khan MF, Wendel CS, Thai HM, Movahed MR. Effects of percutaneous revascularization of chronic total occlusions on clinical outcomes: a meta-analysis comparing successful versus failed percutaneous intervention for chronic total occlusion. Catheter Cardiovasc Interv. 2013;82:95-107. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 94] [Cited by in RCA: 95] [Article Influence: 7.3] [Reference Citation Analysis (0)] |
| 5. | Syrseloudis D, Secco GG, Barrero EA, Lindsay AC, Ghione M, Kilickesmez K, Foin N, Martos R, Di Mario C. Increase in J-CTO lesion complexity score explains the disparity between recanalisation success and evolution of chronic total occlusion strategies: insights from a single-centre 10-year experience. Heart. 2013;99:474-479. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 42] [Cited by in RCA: 52] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 6. | Aziz S, Stables RH, Grayson AD, Perry RA, Ramsdale DR. Percutaneous coronary intervention for chronic total occlusions: improved survival for patients with successful revascularization compared to a failed procedure. Catheter Cardiovasc Interv. 2007;70:15-20. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 91] [Cited by in RCA: 88] [Article Influence: 4.6] [Reference Citation Analysis (0)] |
| 7. | Hoye A, van Domburg RT, Sonnenschein K, Serruys PW. Percutaneous coronary intervention for chronic total occlusions: the Thoraxcenter experience 1992-2002. Eur Heart J. 2005;26:2630-2636. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 267] [Cited by in RCA: 254] [Article Influence: 12.1] [Reference Citation Analysis (0)] |
| 8. | Olivari Z, Rubartelli P, Piscione F, Ettori F, Fontanelli A, Salemme L, Giachero C, Di Mario C, Gabrielli G, Spedicato L. Immediate results and one-year clinical outcome after percutaneous coronary interventions in chronic total occlusions: data from a multicenter, prospective, observational study (TOAST-GISE). J Am Coll Cardiol. 2003;41:1672-1678. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 369] [Cited by in RCA: 357] [Article Influence: 15.5] [Reference Citation Analysis (0)] |
| 9. | van der Schaaf RJ, Vis MM, Sjauw KD, Koch KT, Baan J, Tijssen JG, de Winter RJ, Piek JJ, Henriques JP. Impact of multivessel coronary disease on long-term mortality in patients with ST-elevation myocardial infarction is due to the presence of a chronic total occlusion. Am J Cardiol. 2006;98:1165-1169. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 98] [Cited by in RCA: 105] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 10. | van der Schaaf RJ, Claessen BE, Vis MM, Hoebers LP, Koch KT, Baan J, Meuwissen M, Engstrom AE, Kikkert WJ, Tijssen JG. Effect of multivessel coronary disease with or without concurrent chronic total occlusion on one-year mortality in patients treated with primary percutaneous coronary intervention for cardiogenic shock. Am J Cardiol. 2010;105:955-959. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 86] [Cited by in RCA: 88] [Article Influence: 5.5] [Reference Citation Analysis (0)] |
| 11. | Morino Y, Kimura T, Hayashi Y, Muramatsu T, Ochiai M, Noguchi Y, Kato K, Shibata Y, Hiasa Y, Doi O. In-hospital outcomes of contemporary percutaneous coronary intervention in patients with chronic total occlusion insights from the J-CTO Registry (Multicenter CTO Registry in Japan). JACC Cardiovasc Interv. 2010;3:143-151. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 210] [Cited by in RCA: 208] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 12. | Stone GW, Colombo A, Teirstein PS, Moses JW, Leon MB, Reifart NJ, Mintz GS, Hoye A, Cox DA, Baim DS. Percutaneous recanalization of chronically occluded coronary arteries: procedural techniques, devices, and results. Catheter Cardiovasc Interv. 2005;66:217-236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 93] [Article Influence: 4.7] [Reference Citation Analysis (0)] |
| 13. | Noguchi T, Miyazaki MD S, Morii I, Daikoku S, Goto Y, Nonogi H. Percutaneous transluminal coronary angioplasty of chronic total occlusions. Determinants of primary success and long-term clinical outcome. Catheter Cardiovasc Interv. 2000;49:258-264. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 1] [Reference Citation Analysis (0)] |
| 14. | Morino Y, Abe M, Morimoto T, Kimura T, Hayashi Y, Muramatsu T, Ochiai M, Noguchi Y, Kato K, Shibata Y. Predicting successful guidewire crossing through chronic total occlusion of native coronary lesions within 30 minutes: the J-CTO (Multicenter CTO Registry in Japan) score as a difficulty grading and time assessment tool. JACC Cardiovasc Interv. 2011;4:213-221. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 506] [Cited by in RCA: 690] [Article Influence: 46.0] [Reference Citation Analysis (0)] |
| 15. | Kimura M, Katoh O, Tsuchikane E, Nasu K, Kinoshita Y, Ehara M, Terashima M, Matsuo H, Matsubara T, Asakura K. The efficacy of a bilateral approach for treating lesions with chronic total occlusions the CART (controlled antegrade and retrograde subintimal tracking) registry. JACC Cardiovasc Interv. 2009;2:1135-1141. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 115] [Cited by in RCA: 114] [Article Influence: 7.1] [Reference Citation Analysis (0)] |
| 16. | Ozawa N. A new understanding of chronic total occlusion from a novel PCI technique that involves a retrograde approach to the right coronary artery via a septal branch and passing of the guidewire to a guiding catheter on the other side of the lesion. Catheter Cardiovasc Interv. 2006;68:907-913. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 64] [Cited by in RCA: 58] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 17. | Rathore S, Katoh O, Tuschikane E, Oida A, Suzuki T, Takase S. A novel modification of the retrograde approach for the recanalization of chronic total occlusion of the coronary arteries intravascular ultrasound-guided reverse controlled antegrade and retrograde tracking. JACC Cardiovasc Interv. 2010;3:155-164. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 124] [Cited by in RCA: 115] [Article Influence: 7.7] [Reference Citation Analysis (0)] |
| 18. | Tian NL, Gami SK, Ye F, Zhang JJ, Liu ZZ, Lin S, Ge Z, Shan SJ, You W, Chen L. Angiographic and clinical comparisons of intravascular ultrasound- versus angiography-guided drug-eluting stent implantation for patients with chronic total occlusion lesions: two-year results from a randomised AIR-CTO study. EuroIntervention. 2015;10:1409-1417. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 153] [Article Influence: 15.3] [Reference Citation Analysis (0)] |
| 19. | Kim BK, Shin DH, Hong MK, Park HS, Rha SW, Mintz GS, Kim JS, Kim JS, Lee SJ, Kim HY. Clinical Impact of Intravascular Ultrasound-Guided Chronic Total Occlusion Intervention With Zotarolimus-Eluting Versus Biolimus-Eluting Stent Implantation: Randomized Study. Circ Cardiovasc Interv. 2015;8:e002592. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 150] [Cited by in RCA: 236] [Article Influence: 23.6] [Reference Citation Analysis (0)] |
| 20. | Sakamoto Y, Ishiguro M, Kitagawa G. Akaike Information Criterion Statistics. D. China: Reidel Publishing Company 1986; . |
| 21. | Gelman A, Jakulin A, Pittau MG, Su YS. A weakly informative default prior distribution for logistic and other regression models. Ann Appl Stat. 2008;2:1360-1383. [RCA] [DOI] [Full Text] [Cited by in Crossref: 1037] [Cited by in RCA: 1060] [Article Influence: 58.9] [Reference Citation Analysis (0)] |
| 22. | Nombela-Franco L, Urena M, Jerez-Valero M, Nguyen CM, Ribeiro HB, Bataille Y, Rodés-Cabau J, Rinfret S. Validation of the J-chronic total occlusion score for chronic total occlusion percutaneous coronary intervention in an independent contemporary cohort. Circ Cardiovasc Interv. 2013;6:635-643. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 89] [Article Influence: 6.8] [Reference Citation Analysis (0)] |
| 23. | Thompson CA, Jayne JE, Robb JF, Friedman BJ, Kaplan AV, Hettleman BD, Niles NW, Lombardi WL. Retrograde techniques and the impact of operator volume on percutaneous intervention for coronary chronic total occlusions an early U.S. experience. JACC Cardiovasc Interv. 2009;2:834-842. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 171] [Cited by in RCA: 178] [Article Influence: 10.5] [Reference Citation Analysis (0)] |
| 24. | Reifart N. Percutaneous revascularization of coronary chronic total occlusion. Minerva Med. 2011;102:391-397. [PubMed] |
| 25. | Joyal D, Afilalo J, Rinfret S. Effectiveness of recanalization of chronic total occlusions: a systematic review and meta-analysis. Am Heart J. 2010;160:179-187. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 315] [Cited by in RCA: 305] [Article Influence: 19.1] [Reference Citation Analysis (0)] |
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Manuscript source: Invited manuscript
Specialty type: Cardiac and cardiovascular systems
Country of origin: Spain
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
P- Reviewer: Bugiardini P, Sato A S- Editor: Kong JX L- Editor: A E- Editor: Lu YJ