Published online Oct 26, 2015. doi: 10.4330/wjc.v7.i10.633
Peer-review started: June 4, 2015
First decision: July 6, 2015
Revised: September 7, 2015
Accepted: September 16, 2015
Article in press: September 18, 2015
Published online: October 26, 2015
Processing time: 153 Days and 19.7 Hours
After the successful introduction of highly active antiretroviral agents the survival of patients infected with the human immunodeficiency virus (HIV) in developed countries has increased substantially. This has allowed the surfacing of several chronic diseases among which cardiovascular disease (CVD) is prominent. The pathogenesis of CVD in HIV is complex and involves a combination of traditional and HIV related factors. An accurate assessment of risk of CVD in these patients is still elusive and as a consequence the most appropriate preventive and therapeutic interventions remain controversial.
Core tip: Infection with the human immunodeficiency virus (HIV) was initially universally lethal but with the introduction of highly active antiretroviral therapies (HAART) the life span of HIV infected patients has drastically increased. Along with the lengthening of life span chronic diseases such as non-acquired immunodeficiency syndrome related cancers and cardiovascular diseases surfaced. Currently cardiovascular disease is the primary cause of death among HIV infected patients in industrialized countries and its pathogenesis is very complex. A combination of direct virion injury, chronic low-grade inflammation, adverse cardiometabolic effects of HAART and high burden of traditional risk factors contribute to this new epidemic.
- Citation: Shahbaz S, Manicardi M, Guaraldi G, Raggi P. Cardiovascular disease in human immunodeficiency virus infected patients: A true or perceived risk? World J Cardiol 2015; 7(10): 633-644
- URL: https://www.wjgnet.com/1949-8462/full/v7/i10/633.htm
- DOI: https://dx.doi.org/10.4330/wjc.v7.i10.633
In the era of highly active antiretroviral therapy (HAART), the prognosis for human immunodeficiency positive (HIV+) patients in developed countries has dramatically improved[1,2]. As a consequence HIV infected patients live longer[3] and the medical care for this population is becoming more focused on the management of non- acquired immunodeficiency syndrome (AIDS) related morbidities, including cardiovascular diseases (CVDs)[4].
Although CVD is a leading cause of mortality and morbidity in HIV+ patients[5,6], there remains some controversy as to whether the disease is accelerated (promoted by traditional and non-traditional risk factors for atherosclerosis) or accentuated (greater prevalence of traditional cardiovascular risk factors) in these patients[7,8]. The prevention and treatment may vary considerably according to which mechanism is the prevailing one. In this review, we address the epidemiology of CVD in HIV+ patients, we discuss the impact of traditional and HIV-related risk factors; we review risk assessment for CVD in HIV and provide a brief overview of future therapeutic approaches to prevention of CVD in patients infected with HIV.
| Ref. | Size | Follow-up | Findings |
| Freiberg et al[6] | 82459 27350 HIV+ 55109 HIV- | 5.9 yr | Increased risk of MI among HIV+ patients VACS-VS study[6] (HR: 1.48; 95%CI: 1.27-1.72) |
| Womack et al[9] VACS-VS study | 2187 women 32% HIV+ | 6 yr | Increased risk of CVD in HIV+ women compared to uninfected women (HR: 2.8; 95%CI: 1.7-4.6) |
| Paisible et al[10] VACS-VS study | 81322 33% HIV+ | 5.9 yr | HIV+ veterans without major CVD risk factors had a 2-fold increased risk of MI compared with HIV- veterans without CVD risk factors (HR: 2.0; 95%CI: 1.0- 3.9) |
| Triant et al[11] | 3851 HIV+ 1044589 HIV- | 8 yr | Increased risk of MI among HIV+ patients (RR: 1.75; P < 0.0001) |
| Silverberg et al[12] | 22081 HIV+ 23069 HIV- | 13 yr | Higher risk of MI among HIV+ patients with a low recent or nadir CD4 cells (< 200) compared with HIV- subjects (RR, 1.76; 95%CI: 1.31-2.37 for low recent CD4 RR, 1.74; 95%CI: 1.47-2.06 for low nadir CD4) |
| Lang et al[13] FHDH-ANRS CO4 | 74958 HIV+ | 6 yr | The risk of MI was higher in both HIV+ men and women compared with the general population Standardized mortality ratio: 1.4 (95%CI: 1.3-1.6) for HIV+ men and 2.7 (95%CI: 1.8 -3.9) for HIV+ women compared with the general population |
| Hsue et al[14] | 148 HIV+ 63 HIV- | 1 yr | Higher baseline carotid IMT of HIV+ patients (P = 0.0001) and faster progression (P = 0.002) |
| Currier et al[17] | 133 subjects in 45 triads1 | 144 wk | HIV infection and PI use did not contribute to the rate of carotid IMT progression. The median paired difference in IMT change between the PI and non-PI subjects was not statistically significant (P = 0.19). When the HIV+ groups were combined and compared with the HIV-negative group, the difference in progression was also not significant (P = 0.71) |
| Post et al[18] | 618HIV+ 383HIV- men cross-sectional study | HIV-infected men had a greater prevalence of CAC [PR: 1.21 (95%CI: 1.08); P = 0.001] and any plaque [PR: 1.14 (CI: 1.05- 1.24); P = 0.001], including non-calcified [PR: 1.28 (CI: 1.13-1.45); P < 0.001) and mixed [PR: 1.35 (CI: 1.10-1.65); P = 0.004) plaque, than uninfected men | |
| Klein et al[19] VACS-VS study | 95687 31% HIV+ | 15 yr | Decline in adjusted MI rate ratio for HIV status over time, reaching 1 (95%CI: 7-1.4) in 2010-2011 |
A large body of evidence supports the notion that the burden of both clinical and sub-clinical CVD is increased in HIV infected patients. Investigators in the Veterans Aging Cohort Study Virtual Cohort (VACS-VC) analyzed data collected in 82459 veterans followed for an average of 5.9 years. The 27350 veterans infected with HIV had a significantly higher risk of myocardial infarction (AMI) compared with uninfected veterans (HR, 1.48; 95%CI: 1.27-1.72)[6]. The highest AMI risk was recorded among patients with HIV-RNA levels of at least 500 copies/mL and CD4 cell (CD4+) count less than 200 cell/mL; the risk remained elevated among patients who achieved HIV-RNA levels less than 500 copies/mL over time, suggesting that HAART may have contributed to some of the AMI risk[6]. The increased risk of CVD was noted both in HIV infected men and women[9]. When the VACS-VC participants were categorized according to the presence or absence of standard risk factors (diabetes mellitus, current smoking, total cholesterol, blood pressure, statins and antihypertensive medications use), HIV-infected veterans without major CVD risk factors had a 2-fold greater risk of AMI compared with uninfected veterans and the risk increased rapidly with each additional risk factor added[10]. In a cohort of 3851 HIV-infected patients examined at two Boston health care facilities the rate of AMI was significantly higher than in 1044589 controls after adjustment for age, sex, race, hypertension, and dyslipidemia (RR, 1.75; 95%CI: 1.51-2.02; P < 0.0001). Importantly, race appeared to have a different influence on the rate of AMI, with risk being higher in African-Americans and in Hispanics compared to Caucasians[11]. Although the prevalence of hypertension, diabetes mellitus and dyslipidemia was higher in HIV patients, this study further suggested that traditional risk factors cannot fully account for the increased risk of CVD in HIV[11]. Other studies performed outside of the US confirmed an increased risk of CVD in infected individuals[12,13].
The evidence of an increased risk of CVD in HIV extends to studies of subclinical atherosclerosis. Hsue et al[14] measured carotid artery intima-media thickness (IMT), an independent predictor of AMI and stroke[15,16], in 148 HIV+ patients and 63 age and sex matched controls. They reported that the mean carotid IMT of HIV+ patients was significantly greater and progressed faster in HIV+ patients than in controls. Of note, HIV infection was a predictor of carotid IMT independent of all other risk factors such as age, sex, smoking, HTN, lipid abnormalities and diabetes mellitus; a nadir CD4 count < 200 cells/mL was associated with IMT progression. However, Currier et al[17] failed to show any association between HIV infection and rate of carotid IMT progression.
Coronary computed tomography with and without intravascular iodinated contrast provides information about coronary artery calcium and non-calcified plaques both measures of subclinical atherosclerosis. Post et al[18] showed that HIV-infected men had a greater prevalence of coronary artery plaques compared to uninfected men and more extensive non-calcified plaques. On the contrary, the extent of coronary calcification was similar in the two groups.
In contrast with the above reported increased prevalence of CVD, Klein et al[19] recently reported a decline in incidence of AMI and CVD in HIV-patients. They reviewed data collected among the members of Kaiser Permanente Southern California and Northern California health plans between 1996 and 2011 (24768 HIV-infected patients and 257600 controls). The unadjusted relative risk of AMI for HIV+ patients decreased from 2.0 in 1996-1999 to 1.2 in 2010-2011, and the adjusted RR declined from 1.8 in 1996-1999 to 1.0 in 2010-2011 (Table 2). The decreased incidence of AMI in HIV+ patients may be due to the use of more lipid-friendly HAART medications, earlier initiation of HAART, resulting in lower incidence of severe immunodeficiency, and better control of CVD risk factors. The latter notion was supported by the finding that the Framingham risk scores were lower in HIV+ patients in more recent years compared to the late 90’s[19].
| Year | 1996-2011 | 1996-1999 | 2000-2003 | 2004-2007 | 2008-2009 | 2010-2011 |
| Crude | 1.6 (1.5, 1.8) | 2.0 (1.5, 2.8) | 2.0(1.6, 2.5) | 1.5 (1.2, 1.9) | 1.5 (1.1-2.0) | 1.2 (0.9-1.6) |
| Adjusted | 1.4 (1.2, 1.6) | 1.8 (1.3, 2.6) | 1.7(1.4, 2.1) | 1.3 (1.0, 1.6) | 1.3 (0.9, 1.7) | 1.0 (0.7, 1.4) |
In summary, a considerable body of literature shows a greater incidence of clinical CVD and a greater prevalence of subclinical atherosclerosis in HIV infected patients compared to the general population, supporting the notion that HIV is an independent risk factor for CVD. Whether the recently reported trend reversal is due to a greater awareness and more effective implementation of preventive measures in HIV+ patients remains to be demonstrated in different geographical areas and health delivery settings.
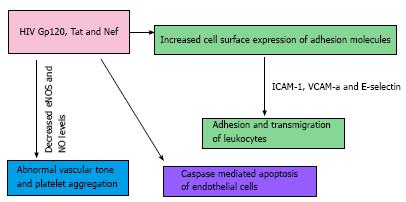
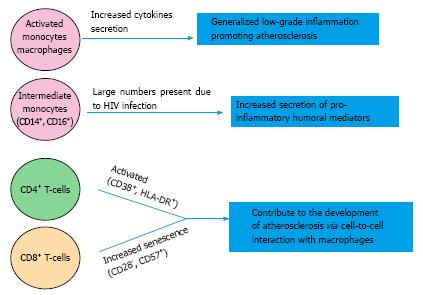
Infection related factors: The increased CVD risk in HIV may be dependent on a direct role of the HIV virus and on the immunological dysregulations caused by chronic HIV infection (Figures 1 and 2); data from observational studies provide evidence that both of these elements are involved[6,10,11].
The importance of continuous suppression of viral replication was investigated in the strategies for management of antiretroviral therapy (SMART) study[20]. The investigators compared the risk of all-cause death and cardiovascular, renal or hepatic complications in 2720 HIV+ patients receiving intermittent antiretroviral therapy and 2752 HIV+ patients receiving continuous antiretroviral therapy[20]. After a mean follow-up of 16 mo, the risk of death from any cause and for major cardiovascular, renal and hepatic diseases was significantly higher in patients who received intermittent compared to those who received continuous anti-retroviral therapy (HR for all-cause death: 2.6; 95%CI: 1.9-3.7; P < 0.001, HR for major CVD, renal and hepatic disease: 1.7; 95%CI: 1.1-2.5; P = 0.009). The authors suggested that the increased CV risk may be the consequence of alternating low and high CD4 cell numbers and viral loads experienced by the patients while receiving intermittent antiretroviral therapy, hence the importance of early and vigorous control of HIV replication and immune dysfunction[20].
In a cohort study of 22081 HIV+ and 230069 HIV-adult patients Silverberg et al[12] showed that the risk of AMI was 44% higher in HIV+ subjects compared with HIV- controls after adjustment for traditional risk factors. HIV+ patients with a nadir CD4 cell count < 200/mcl had a greater risk of AMI compared to controls[12]. However, the AMI rate of HIV+ subjects with a recent or nadir CD4 cell count ≥ 500 /mcl and that of HIV- subjects was the same. In a case-control study of 289 HIV+ patients and 884 HIV-infected controls[21], a viral RNA level > 50 copies/mL, a low CD4 nadir and a high current CD8 count (> 1150/mm3) were significantly associated with an increased risk of AMI. The ratio of nadir CD4/current CD8 count was the best predictor of an event[21].
In summary it would appear that a tight control of the HIV reproduction and maintenance of a good CD4 count may be protective against the risk of CV events. However, there exists conflicting evidence as to the actual association between CD4 cell count and the risk of AMI[22,23], as well as the HIV RNA levels and CV risk. Some investigators reported a direct association[21,22], while others were not able to identify one[24-26]; therefore this aspect of the CV pathogenicity of HIV needs to be clarified further.
The HIV can penetrate into endothelial cells utilizing CD4 receptors, galactosyl-ceramide receptors or chemokine receptors pathway[27-29], and different components of the HIV may have a role in the pathogenesis of CVD. Gp120 is a glycoprotein exposed on the surface of the HIV envelope and can also be found in the circulation from viral turn over[30,31]. It mediates HIV-1 entry into human cells by interacting with the HIV receptor for CD4 and co-receptors CXCR4 or CCR5[32]. Jiang et al[33] showed that Gp120 and tumor necrosis-alpha (TNF-α) synergistically decrease endothelial nitric oxide synthase (eNOS) and nitric oxide (NO) levels in both porcine and human coronary artery endothelial cells. NO is an important factor in the regulation of vascular tone and inhibition of platelet adhesion and aggregation[34]. HIV infection is a chronic inflammatory state, characterized by elevated serum levels of factors such as TNF-α and TNF-β, interferon gamma (IFN-γ) and monocyte chemo-attractant protein-1 (MCP-1)[32,35,36] and Gp120 can magnify the pro-atherosclerotic effects of these mediators. Gp120 can also induce apoptosis by interacting with CXCR4, a chemokine receptor[37,38], which is also expressed on vascular endothelium[39,40].
The trans-activator of transcription (Tat) protein is a regulatory protein that enhances the efficiency of viral transcription[41]. In a study on the role of Tat on the expression of adhesion molecules in human endothelial cells, Tat was shown to induce the expression of intercellular adhesion molecule-1 (ICAM-1), vascular cell adhesion molecule-1 (VCAM-1), and E-selectin[42]. In the early phases of atherosclerosis, leukocytes adhere on the surface of endothelial cells and subsequently transmigrate between vascular endothelial cells into the intima layer of the vessel wall. While E-selectin is involved in the initial rolling of leukocytes on the endothelial cells[40], ICAM-1 and VCAM-1 induce firm adhesion and transmigration of leukocytes across the vascular endothelium[43]. High levels of soluble ICAM-1, VCAM-1 and E-selectin are associated with and increased risk of AMI in healthy men and women[44-46]. The levels of ICAM-1, VCAM-1 and E-selectin are elevated in HIV+ patients and there is a correlation between ICAM-1 concentration and the progression of HIV disease as well as the reduction of CD4 count[47,48]. Similarly to Gp120, Tat can also decrease endothelium dependent vasorelaxation and eNOS secretion in porcine coronary arteries[49].
Negative factor (Nef) protein is an HIV regulatory protein with an important role in cell apoptosis and enhancement of viral infectivity[50]. Nef blocks the ATP-binding cassette transporter A1 (ABCA1) pathway, leading to impaired cholesterol efflux from HIV infected macrophages to HDL particles[51]. As a result, HIV-infected macrophages accumulate lipids turning into foam cells, a step that may contribute to atherosclerosis formation[51]. Like Gp120 and Tat protein, Nef decreases endothelium-dependent vasorelaxation in porcine pulmonary arteries and reduces eNOS expression in both porcine pulmonary artery and human pulmonary artery endothelial cells[52]. In the same model Nef increased the levels of reactive oxygen species (ROS)[52], with an attendant decrease in NO bioavailability[53]. In endothelial cells, Nef can induce monocyte chemoattractant protein-1 (MCP-1) expression and apoptosis through NF-κB signaling and ROS-dependent mechanisms, respectively[54].
In addition to the humoral effects described above, cellular immune activation may play a role in the increased incidence of CVD in infected patients[35]. Monocytes are readily infected by HIV[55]; they adhere to the endothelial surface and eventually penetrate in the subendothelial space and intima. Monocytes, especially intermediate monocytes expressing CD14++ and CD16+, are prone to a greater pro-inflammatory activity once infected with HIV[56]. Furthermore, Hearps et al[57] showed that infected monocytes and macrophages of HIV+ patients have a reduced phagocytic activity and demonstrate telomere shortening a marker of premature ageing. High levels of monocyte activation markers, such as soluble CD163, CD14 and MCP-1 have been associated with subclinical coronary artery atherosclerosis, after adjustment for traditional CVD risk factors, in a large cohort of HIV-infected men[58]. T-lymphocytes are also activated in HIV infection[59]. In the Women Interagency HIV study, Kaplan et al[59] showed that HIV infection was associated with significantly elevated levels of activated [CD38+ human leukocyte antigen (HLA)-DR+] peripheral CD4+ and CD8+ cells and CD8+ senescent cells (CD28-CD57+). The trend was reduced but not totally reversed after effective viral suppression with HAART. After adjustment for multiple confounders, CD4+ and CD8+ cell activation and CD8+ senescent cells were associated with subclinical carotid artery lesions detected by 2D ultrasound[59].
Traditional risk factors in HIV: Traditional risk factors are more prevalent in HIV infected patients and likely represent a major driver for CVD in HIV.
(1) Cigarette smoking: The prevalence of cigarette smoking in HIV infected patients has been reported to be higher than in the general population. Analyzing data from 4217 infected (who participated in the Medical Monitoring Project) and 27731 non infected adults (who participated in the National Health Interview Survey in 2009), Mdodo et al[60] reported that 42.4% of HIV patients (95%CI: 39.7%-45.1%) were current cigarette smokers, while 20.3% (CI: 18.6%-22.1%) were former smokers, and 37.3% (CI: 34.9%-39.6%) had never smoked. Compared with the US adult population, in which an estimated 20.6% of adults smoked cigarettes in 2009, adults with HIV were nearly twice as likely to smoke [adjusted prevalence difference, 17.0% (CI: 14.0%-20.1%)], but were less likely to quit smoking (quit ratio, 32.4% vs 51.7%). A higher prevalence of smoking was also described in the SMART trial[61] and in the D:A:D study[62], where the rates of smoking were 40.5% and 51.5% respectively in HIV infected patients. Social and psychological factors such as ethnicity, lower educational level, poverty, illicit drug use, depression are likely contributing to the tobacco epidemic in HIV[60]. The noxious effects of smoking may be enhanced in HIV patients. Recently Helleberg et al[63] reported a greater number of life-years lost due to smoking in HIV infected patients compared to smoking controls [12.3 years (95%CI: 11.5-13.0) vs 3.6 years (95%CI: 3.1-4.0), respectively].
(2) Diabetes mellitus: Using data from the Multicenter AIDS Cohort Study (MACS), Brown et al[64] reported that the incidence of diabetes mellitus in HIV-infected men with HAART exposure was four fold higher than that of HIV-seronegative men. Subsequently De Wit et al[65] reported an incidence rate of 5.72 per 1000 patient follow-up year (95%CI: 5.31-6.13). The incidence of diabetes increased with cumulative exposure to HAART, and the association remained significant after adjustment for confounding factors. Besides the possible influence of HAART, some investigators reported a higher incidence of insulin resistance and diabetes mellitus in patients co-infected with hepatitis C virus (HCV). The impact of the directly active agents used in the therapy of HCV to reduce the incidence of diabetes mellitus is currently unknown[66,67].
(3) Dyslipidemia: The dyslipidemia that develops during HAART is characterized by an increase in total and LDL cholesterol, and triglycerides levels. The effect varies according to the different HAART classes and within each class with different drugs. While this drug-induced toxicity was very common with the older antiretroviral drugs, it has become much less problematic with second generation nucleoside reverse transcriptase inhibitors (NRTI), (namely Rilpivirine) and with integrase inhibitors (Dolutegravir, Elvitegravir and Raltegravir). The HIV is a metabolically active virus capable to alter reverse cholesterol transport via modification of the HDL particles functionality and/or impairment of cellular cholesterol efflux. As discussed above, in vitro experiments have demonstrated that the HIV-related Nef protein can impair cellular cholesterol efflux through down-regulation of ABCA1[68,69]. ABCA1 plays a crucial role in stimulating cholesterol export from macrophages. Recently Lo et al[68] showed that, in the acute phase of HIV infection, HAART can restore the HDL-mediated cholesterol efflux capacity primarily through the suppression of viremia, providing additional evidence that prompt HAART initiation can potentially reduce atherosclerotic risk.
(4) Systemic hypertension: Among HIV infected patients the prevalence and incidence of systemic hypertension ranges from 20%-40% in high-income countries[70-72] to 11%-28% in low and middle income countries[73,74]. These trends may reflect the distribution of risk in the general populations of the same regions of the world. As a result, it is currently unclear whether hypertension is more prevalent in HIV infected patients than the general population.
Several studies failed to show a correlation between blood pressure levels and CD4 cell count and between viral load and hypertension. There are sparse and conflicting data on the role of antiretroviral therapy in the pathogenesis of hypertension[75,76], and the association remains inconclusive.
Impact of antiretroviral therapy on CVD: The first cases of myocardial infarction in HIV-infected patients receiving protease inhibitor were described in the late 1990s; since then several epidemiological studies have examined the association between HIV infection, HAART and the risk of CVD. In 2003 the investigators of the D:A:D study (Data Collection on Adverse Events of Anti-HIV Drugs) reported for the first time an increased incidence of myocardial infarction with longer exposure to combination antiretroviral therapy [adjusted risk rate per year of exposure, 1.26 (95%CI: 1.12-1.41); P < 0.001]. Patients with no exposure to therapy had a lower incidence of myocardial infarction than any of the treated groups[24]. Several years later the same investigators showed that cumulative exposure to some of the protease inhibitors (Indinavir, Lopinavir-Ritonavir) was associated with an increased risk of myocardial infarction (relative rate per year, 1.12 and 1.13, respectively) after adjustment for the impact of these drugs on lipid metabolism[77]. This was the first report that suggested that HAART might be responsible for an increased incidence of cardiovascular events independent of their lipid effects. The case of Abacavir remains paradigmatic in this setting. Several observational cohorts and a few randomized clinical trials reported an association between current, but not cumulative exposure of Abacavir and myocardial infarction. Abacavir, as a guanosine analogue, inhibits soluble guanylyl cyclase leading to enhanced platelet adhesion and, ultimately, to increased risk of myocardial infarction[78].
Numerous other studies implicated Abacavir and protease inhibitors in the development of cardiovascular events, however none of the reports provided conclusive evidence. The adverse effects of these drugs may be particularly harmful in patients with pre-existing high cardiovascular risk, and they should therefore be avoided in those patients.
In spite of early observations reporting an increased risk of CVD in patients receiving HAART, particularly protease inhibitors, more recent evidence suggests a safer cardio-metabolic profile of some categories of HAART[20,79]. Current strategies to decrease the risk of CVD in HIV infected patients include early initiation of HAART regimens known to be associated with the fewest metabolic adverse effects and careful management of traditional CV risk factors during HAART treatment.
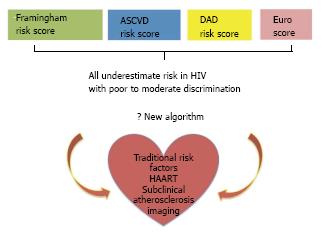
Cardiovascular risk assessment in HIV infected patients has traditionally been based on recommendations drafted for the general population. In 2010 Friis-Møller et al[80] reported on the performance of a new model (derived from the D.A.D. cohort) that included exposure to HAART along with traditional risk factors. Although the new model estimated outcomes more accurately than the Framingham Risk Score (FRS) in the HIV population, it has not been widely adopted.
A new risk prediction algorithm for the general population [atherosclerotic cardiovascular disease (ASCVD)] was introduced in 2013 by the American College of Cardiology/American Heart Association[81]. In preliminary analyses it appeared that the ASCVD might overestimate CVD risk in the general population[82]. However the situation is probably the opposite in HIV disease. In a cohort of 2270 HIV infected patients, Regan et al[83] recently reported that the ASCVD algorithm classified a larger proportion of HIV patients as high-risk compared to the FRS (25% vs 10%). However, comparing the 5-year observed-vs-predicted event rates, both models underestimated the actual CVD risk in HIV. Similarly, Thompson-Paul et al[84] compared ASCVD[81], FRS[85], the European algorithm known as SCORE[86] and the DAD score[80] to predict events in 2392 ambulatory HIV+ patients. After a median follow-up of 6.5 years they recorded 204 events; all models underestimated the actual risk of events. The FRS, ASCVD, and DAD equations showed moderate discrimination (C-statistic range, 0.68 to 0.72), while SCORE showed poor discrimination (C-statistic = 0.59).
Imaging of subclinical atherosclerosis has been suggested as a method to improve risk prediction and implementation of preventive therapies in the general population with variable success. Zanni et al[87] described a disproportion in HIV infected individuals between presence of subclinical atherosclerosis and statins recommendation. One third of 108 HIV infected patients without known CVD who underwent computed tomography angiography hosted coronary artery plaques with features of high vulnerability. Although a larger number of patients would require statins according to the new ASCVD algorithm compared to the FRS recommendations (26% vs 10%), the majority (74%) of HIV infected patients with subclinical coronary atherosclerosis did not have an indication to receive treatment even with the new algorithm.
Coronary artery calcium (CAC) has been proven to be incremental to risk factors for the prediction of events in the general population[88,89], but to date it has not been shown to be as useful in HIV infected patients. In a metanalysis Hulten et al[90] described a similar prevalence of CAC between HIV-positive and uninfected patients (OR: 0.95, P = 0.851). In the MACS[91], among 615 HIV infected men and 332 controls, the adjusted odds ratio for CAC was 1.35 (95%CI: 0.7-2.61). No outcome study has yet been published demonstrating the utility of CAC as an incremental prognostic factor in HIV.
Inflammation plays a central role in the pathophysiology of atherosclerosis and 18-fluoredeoxyglucose positron emission tomography can identify activated macrophages infiltrating the arterial wall. Subramanian et al[92] compared 27 HIV positive patients receiving HAART without prior CVD, with two non-HIV control groups: one group was matched for age, sex and FRS (non-HIV, FRS-matched controls), while the second one was sex-matched but had known atherosclerotic CVD (non-HIV, atherosclerotic controls). Inflammation in the aortic wall (measured as target to background signal ratio, TBR) was similar between HIV infected patients and non-HIV atherosclerotic controls (2.23; 95%CI: 2.07-2.40 vs 2.13; 95%CI: 2.03-2.23; P = 0.29), but was higher than in the non-HIV FRS-matched controls (2.23; 95%CI: 2.07-2.40 vs 1.89; 95%CI: 1.80-1.97; P = 0.001).
In HIV infected patients the aortic TBR was associated with the serum concentration of soluble CD163, a novel marker of activated macrophages (P = 0.04)[93], but not with C-reactive protein (P = 0.65) or D-dimer (P = 0.08) levels.
More recently, Tawakol et al[94] performed 18-FDG-PET imaging in 41 HIV+ patients on stable HAART regimen without prior CVD, but with coronary plaques on coronary CT angiography. A higher tracer uptake was correlated with the presence of low-attenuation plaques (P = 0.02) and positive remodeling (P = 0.04), both features of plaque instability.
Despite these encouraging results, no algorithm to date has incorporated imaging information in risk models for HIV patients. Therefore risk assessment remains reliant on the use of traditional risk factors despite their demonstrated limitations, although there is hope that imaging may add useful information in the future (Figure 3).
In view of the information discussed so far it appears true that the CV risk of HIV infected patients is greater than that of the general population; a combination of higher prevalence of traditional risk factors and HIV specific factors likely predispose patients to such increased risk. Four questions emerge regarding risk reduction management in HIV patients: (1) should HIV infected patients be treated more aggressively than the general population for traditional risk factors? (2) As a corollary of the former, should a lower risk threshold be used to initiate treatment? (3) What HAART should be chosen to minimize CV risk in HIV? and (4) Finally, should therapy for HIV infected patients be guided by imaging and/or non-imaging biomarkers?
To date no study has addressed the impact of more aggressive therapy of traditional risk factors in HIV patients to reduce the attendant CV risk. While it would appear that there is a trend toward a relative reduction of CV morbidity and mortality in the past 10-15 years[95], it is unclear whether this is due to greater physicians’ awareness and increased preventive efforts in HIV patients or other as yet unknown factors. The current recommendations for risk assessment and risk reduction in HIV patients mimic those of the general population. However, as discussed above, in spite of a slightly better performance of newer versus older risk assessment algorithms, the majority of patients at risk are not identified as high-risk and are therefore not receiving potentially life saving therapies. The newest algorithm (ASCVD) lowered the threshold for identification of high-risk patients to 7.5% from 20% as recommended in the ATP-III guidelines[96], but it continues to under perform in HIV patients. In fact, despite the greater proportion of patients at risk identified by the ASCVD, as many as 40% of the patients suffering an acute cardiovascular event would not have met the requirement for prescription of statins prior to the event according to two recent studies[83,97]. Hence, a mere lowering of the threshold for initiation of preventive therapies may be insufficient to effectively lower risk of events in HIV patients. In view of the difficulties highlighted above, a new study was launched[98] that aims to show that early statin treatment in asymptomatic HIV infected patients will delay the development of inflamed atherosclerotic plaques and reduce cardiovascular events (death, myocardial infarction, angina, stroke and revascularizations).
The choice of HAART and timing of therapy initiation likely carry a significant weight in risk reduction, as some of these drugs appear to have both direct and indirect cardiovascular toxicity while others may lower CV risk. Since the initial observation from the SMART study[20] reporting a reduced incidence of CV events in patients reaching a stable viral suppression, newer antiretroviral agents have been introduced with improved cardiometabolic toxicity. However, there are currently no long-term studies to demonstrate the safety and effectiveness of new anti-retroviral agents such as integrase inhibitors, although the safety profile of drugs such as atanazavir (a protease inhibitor) and tenofovir (member of the NRTI family) has been established. To address the paucity of prospective follow-up data, the D.A.D. registry has been tasked with the collection of safety data on drugs with a minimum of 30000 person-year follow-up[99].
Surrogate markers of atherosclerosis and serological biomarkers may provide some insight into the effectiveness of a novel therapeutic agent. In a preliminary communication, Stein et al[79] reported the effect of various antiretroviral combinations on the progression of the intima-medial thickness (IMT) of the carotid artery bulb in 234 HIV+ patients followed for a maximum of 144 mo from randomization. Atanazavir (protease inhibitor) was associated with less IMT progression than darunavir (integrase inhibitor), but the progression was similar for atanazavir and raltegravir (first generation integrase inhibitor) despite the better lipid profile of patients receiving raltegravir. Hence, it would appear that only part of the pro-atherogenic effect of HAART may be dependent upon lipid metabolism alterations. Additionally, in a recent publication the authors reported that various HAART regimen did not differ as far as expression of markers of inflammation and immune activation, confirming the notion that the anti- or pro-atherogenic effects of HAART cannot be gauged by their effect on serological biomarkers at the current state of research[100]. The lack of effect on biomarkers and a not markedly different effect on measures of atherosclerosis suggests that despite a reduction of viral load with HAART, there are residual immunological perturbations and reservoirs of viral replication that may induce atherosclerosis progression. Timing of HAART initiation has recently received renewed interest with the publication of the INSIGHT-START trial results[101]. In this trial 4685 HIV infected patients were randomized to receive HAART (mainly based on tenofovir, emtricitabine and efavirenz) with a CD4+ cell count > 500/cc (median CD4+ at initiation 651/cc) or only once the CD4+ cell count dropped below 350/cc. The primary end point was a composite of death from all causes, and any serious AIDS related and non-AIDS related events (mostly cardiovascular). After a mean follow-up of 3 years the patient group that received early HAART demonstrated a significantly reduced event rate (HR: 0.43; CI: 0.30-0.62, P < 0.001) compared to the delayed treatment group. These results fuelled an intense debate as to the pros- and cons of early HAART initiation, an approach that needs to be carefully considered in view of the potential side effects of some HAART discussed above.
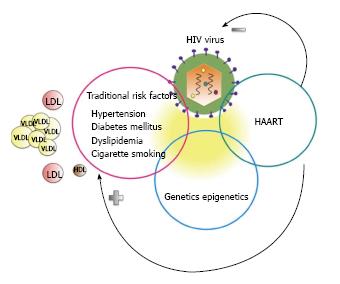
The etio-pathogenesis of CVD in HIV is very complex, with contributions from the retrovirus and the attendant immunologic perturbations, HAART, highly prevalent traditional risk factors and genetics (Figure 4). Therefore a multifaceted approach will be necessary to effectively prevent its development and progression. Since atherosclerosis in HIV patients is characterized by immune activation in association with highly inflamed, non-calcified, potentially vulnerable plaques, these may become the targets of choice in future clinical trials to test the effectiveness of therapy.
| 1. | May MT, Sterne JA, Costagliola D, Sabin CA, Phillips AN, Justice AC, Dabis F, Gill J, Lundgren J, Hogg RS. HIV treatment response and prognosis in Europe and North America in the first decade of highly active antiretroviral therapy: a collaborative analysis. Lancet. 2006;368:451-458. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 179] [Cited by in RCA: 180] [Article Influence: 9.0] [Reference Citation Analysis (0)] |
| 2. | Wada N, Jacobson LP, Cohen M, French A, Phair J, Muñoz A. Cause-specific life expectancies after 35 years of age for human immunodeficiency syndrome-infected and human immunodeficiency syndrome-negative individuals followed simultaneously in long-term cohort studies, 1984-2008. Am J Epidemiol. 2013;177:116-125. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 147] [Cited by in RCA: 165] [Article Influence: 12.7] [Reference Citation Analysis (0)] |
| 3. | van Sighem AI, Gras LA, Reiss P, Brinkman K, de Wolf F. Life expectancy of recently diagnosed asymptomatic HIV-infected patients approaches that of uninfected individuals. AIDS. 2010;24:1527-1535. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 299] [Cited by in RCA: 316] [Article Influence: 19.8] [Reference Citation Analysis (0)] |
| 4. | Schwarcz SK, Vu A, Hsu LC, Hessol NA. Changes in causes of death among persons with AIDS: San Francisco, California, 1996-2011. AIDS Patient Care STDS. 2014;28:517-523. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 47] [Article Influence: 3.9] [Reference Citation Analysis (0)] |
| 5. | Mocroft A, Reiss P, Gasiorowski J, Ledergerber B, Kowalska J, Chiesi A, Gatell J, Rakhmanova A, Johnson M, Kirk O. Serious fatal and nonfatal non-AIDS-defining illnesses in Europe. J Acquir Immune Defic Syndr. 2010;55:262-270. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 193] [Cited by in RCA: 214] [Article Influence: 13.4] [Reference Citation Analysis (0)] |
| 6. | Freiberg MS, Chang CC, Kuller LH, Skanderson M, Lowy E, Kraemer KL, Butt AA, Bidwell Goetz M, Leaf D, Oursler KA. HIV infection and the risk of acute myocardial infarction. JAMA Intern Med. 2013;173:614-622. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 886] [Cited by in RCA: 1072] [Article Influence: 82.5] [Reference Citation Analysis (0)] |
| 7. | Savès M, Chêne G, Ducimetière P, Leport C, Le Moal G, Amouyel P, Arveiler D, Ruidavets JB, Reynes J, Bingham A. Risk factors for coronary heart disease in patients treated for human immunodeficiency virus infection compared with the general population. Clin Infect Dis. 2003;37:292-298. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 296] [Cited by in RCA: 289] [Article Influence: 12.6] [Reference Citation Analysis (0)] |
| 8. | Sklar P, Masur H. HIV infection and cardiovascular disease-is there really a link? N Engl J Med. 2003;349:2065-2067. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 34] [Cited by in RCA: 28] [Article Influence: 1.2] [Reference Citation Analysis (0)] |
| 9. | Womack JA, Chang CC, So-Armah KA, Alcorn C, Baker JV, Brown ST, Budoff M, Butt AA, Gibert C, Goetz MB. HIV infection and cardiovascular disease in women. J Am Heart Assoc. 2014;3:e001035. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 98] [Cited by in RCA: 127] [Article Influence: 10.6] [Reference Citation Analysis (0)] |
| 10. | Paisible AL, Chang CC, So-Armah KA, Butt AA, Leaf DA, Budoff M, Rimland D, Bedimo R, Goetz MB, Rodriguez-Barradas MC. HIV infection, cardiovascular disease risk factor profile, and risk for acute myocardial infarction. J Acquir Immune Defic Syndr. 2015;68:209-216. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 141] [Cited by in RCA: 180] [Article Influence: 16.4] [Reference Citation Analysis (0)] |
| 11. | Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab. 2007;92:2506-2512. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 1257] [Cited by in RCA: 1277] [Article Influence: 67.2] [Reference Citation Analysis (0)] |
| 12. | Silverberg MJ, Leyden WA, Xu L, Horberg MA, Chao CR, Towner WJ, Hurley LB, Quesenberry CP, Klein DB. Immunodeficiency and risk of myocardial infarction among HIV-positive individuals with access to care. J Acquir Immune Defic Syndr. 2014;65:160-166. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 116] [Cited by in RCA: 139] [Article Influence: 11.6] [Reference Citation Analysis (0)] |
| 13. | Lang S, Mary-Krause M, Cotte L, Gilquin J, Partisani M, Simon A, Boccara F, Bingham A, Costagliola D. Increased risk of myocardial infarction in HIV-infected patients in France, relative to the general population. AIDS. 2010;24:1228-1230. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 230] [Cited by in RCA: 230] [Article Influence: 14.4] [Reference Citation Analysis (0)] |
| 14. | Hsue PY, Lo JC, Franklin A, Bolger AF, Martin JN, Deeks SG, Waters DD. Progression of atherosclerosis as assessed by carotid intima-media thickness in patients with HIV infection. Circulation. 2004;109:1603-1608. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 420] [Cited by in RCA: 427] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 15. | Craven TE, Ryu JE, Espeland MA, Kahl FR, McKinney WM, Toole JF, McMahan MR, Thompson CJ, Heiss G, Crouse JR. Evaluation of the associations between carotid artery atherosclerosis and coronary artery stenosis. A case-control study. Circulation. 1990;82:1230-1242. [PubMed] |
| 16. | Hodis HN, Mack WJ, LaBree L, Selzer RH, Liu CR, Liu CH, Azen SP. The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med. 1998;128:262-269. [PubMed] |
| 17. | Currier JS, Kendall MA, Henry WK, Alston-Smith B, Torriani FJ, Tebas P, Li Y, Hodis HN. Progression of carotid artery intima-media thickening in HIV-infected and uninfected adults. AIDS. 2007;21:1137-1145. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 102] [Cited by in RCA: 113] [Article Influence: 5.9] [Reference Citation Analysis (0)] |
| 18. | Post WS, Budoff M, Kingsley L, Palella FJ, Witt MD, Li X, George RT, Brown TT, Jacobson LP. Associations between HIV infection and subclinical coronary atherosclerosis. Ann Intern Med. 2014;160:458-467. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 260] [Cited by in RCA: 284] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 19. | Klein DB, Leyden WA, Xu L, Chao CR, Horberg MA, Towner WJ, Hurley LB, Marcus JL, Quesenberry CP, Silverberg MJ. Declining relative risk for myocardial infarction among HIV-positive compared with HIV-negative individuals with access to care. Clin Infect Dis. 2015;60:1278-1280. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 89] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 20. | El-Sadr WM, Lundgren J, Neaton JD, Gordin F, Abrams D, Arduino RC, Babiker A, Burman W, Clumeck N, Cohen CJ. CD4+ count-guided interruption of antiretroviral treatment. N Engl J Med. 2006;355:2283-2296. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1779] [Cited by in RCA: 1703] [Article Influence: 85.2] [Reference Citation Analysis (0)] |
| 21. | Lang S, Mary-Krause M, Simon A, Partisani M, Gilquin J, Cotte L, Boccara F, Costagliola D. HIV replication and immune status are independent predictors of the risk of myocardial infarction in HIV-infected individuals. Clin Infect Dis. 2012;55:600-607. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 125] [Article Influence: 8.9] [Reference Citation Analysis (0)] |
| 22. | Marin B, Thiébaut R, Bucher HC, Rondeau V, Costagliola D, Dorrucci M, Hamouda O, Prins M, Walker S, Porter K. Non-AIDS-defining deaths and immunodeficiency in the era of combination antiretroviral therapy. AIDS. 2009;23:1743-1753. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 185] [Cited by in RCA: 189] [Article Influence: 11.1] [Reference Citation Analysis (0)] |
| 23. | Baker JV, Peng G, Rapkin J, Abrams DI, Silverberg MJ, MacArthur RD, Cavert WP, Henry WK, Neaton JD. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008;22:841-848. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 270] [Cited by in RCA: 298] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 24. | Friis-Møller N, Sabin CA, Weber R, d’Arminio Monforte A, El-Sadr WM, Reiss P, Thiébaut R, Morfeldt L, De Wit S, Pradier C. Combination antiretroviral therapy and the risk of myocardial infarction. N Engl J Med. 2003;349:1993-2003. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1212] [Cited by in RCA: 1235] [Article Influence: 53.7] [Reference Citation Analysis (0)] |
| 25. | Lichtenstein KA, Armon C, Buchacz K, Chmiel JS, Buckner K, Tedaldi EM, Wood K, Holmberg SD, Brooks JT. Low CD4+ T cell count is a risk factor for cardiovascular disease events in the HIV outpatient study. Clin Infect Dis. 2010;51:435-447. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 209] [Cited by in RCA: 221] [Article Influence: 13.8] [Reference Citation Analysis (0)] |
| 26. | Phillips AN, Carr A, Neuhaus J, Visnegarwala F, Prineas R, Burman WJ, Williams I, Drummond F, Duprez D, Belloso WH. Interruption of antiretroviral therapy and risk of cardiovascular disease in persons with HIV-1 infection: exploratory analyses from the SMART trial. Antivir Ther. 2008;13:177-187. [PubMed] |
| 27. | Banks WA, Akerstrom V, Kastin AJ. Adsorptive endocytosis mediates the passage of HIV-1 across the blood-brain barrier: evidence for a post-internalization coreceptor. J Cell Sci. 1998;111:533-540. [PubMed] |
| 28. | Cohen OJ, Kinter A, Fauci AS. Host factors in the pathogenesis of HIV disease. Immunol Rev. 1997;159:31-48. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 151] [Cited by in RCA: 138] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 29. | Shieh JT, Albright AV, Sharron M, Gartner S, Strizki J, Doms RW, González-Scarano F. Chemokine receptor utilization by human immunodeficiency virus type 1 isolates that replicate in microglia. J Virol. 1998;72:4243-4249. [PubMed] |
| 30. | Pegu A, Yang ZY, Boyington JC, Wu L, Ko SY, Schmidt SD, McKee K, Kong WP, Shi W, Chen X. Neutralizing antibodies to HIV-1 envelope protect more effectively in vivo than those to the CD4 receptor. Sci Transl Med. 2014;6:243ra88. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 199] [Cited by in RCA: 218] [Article Influence: 19.8] [Reference Citation Analysis (1)] |
| 31. | Oh SK, Cruikshank WW, Raina J, Blanchard GC, Adler WH, Walker J, Kornfeld H. Identification of HIV-1 envelope glycoprotein in the serum of AIDS and ARC patients. J Acquir Immune Defic Syndr. 1992;5:251-256. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 95] [Cited by in RCA: 103] [Article Influence: 3.0] [Reference Citation Analysis (0)] |
| 32. | Hogan CM, Hammer SM. Host determinants in HIV infection and disease. Part 2: genetic factors and implications for antiretroviral therapeutics. Ann Intern Med. 2001;134:978-996. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 100] [Cited by in RCA: 87] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 33. | Jiang J, Fu W, Wang X, Lin PH, Yao Q, Chen C. HIV gp120 induces endothelial dysfunction in tumour necrosis factor-alpha-activated porcine and human endothelial cells. Cardiovasc Res. 2010;87:366-374. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 37] [Cited by in RCA: 41] [Article Influence: 2.6] [Reference Citation Analysis (0)] |
| 34. | Chatterjee A, Black SM, Catravas JD. Endothelial nitric oxide (NO) and its pathophysiologic regulation. Vascul Pharmacol. 2008;49:134-140. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 175] [Cited by in RCA: 169] [Article Influence: 9.4] [Reference Citation Analysis (0)] |
| 35. | Shikuma CM, Barbour JD, Ndhlovu LC, Keating SM, Norris PJ, Budoff M, Parikh N, Seto T, Gangcuangco LM, Ogata-Arakaki D. Plasma monocyte chemoattractant protein-1 and tumor necrosis factor-α levels predict the presence of coronary artery calcium in HIV-infected individuals independent of traditional cardiovascular risk factors. AIDS Res Hum Retroviruses. 2014;30:142-146. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 20] [Cited by in RCA: 20] [Article Influence: 1.7] [Reference Citation Analysis (0)] |
| 36. | Pober JS, Gimbrone MA, Lapierre LA, Mendrick DL, Fiers W, Rothlein R, Springer TA. Overlapping patterns of activation of human endothelial cells by interleukin 1, tumor necrosis factor, and immune interferon. J Immunol. 1986;137:1893-1896. [PubMed] |
| 37. | Hesselgesser J, Taub D, Baskar P, Greenberg M, Hoxie J, Kolson DL, Horuk R. Neuronal apoptosis induced by HIV-1 gp120 and the chemokine SDF-1 alpha is mediated by the chemokine receptor CXCR4. Curr Biol. 1998;8:595-598. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 321] [Article Influence: 11.5] [Reference Citation Analysis (0)] |
| 38. | Herbein G, Mahlknecht U, Batliwalla F, Gregersen P, Pappas T, Butler J, O’Brien WA, Verdin E. Apoptosis of CD8+ T cells is mediated by macrophages through interaction of HIV gp120 with chemokine receptor CXCR4. Nature. 1998;395:189-194. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 324] [Cited by in RCA: 315] [Article Influence: 11.3] [Reference Citation Analysis (0)] |
| 39. | Ridker PM, Buring JE, Rifai N. Soluble P-selectin and the risk of future cardiovascular events. Circulation. 2001;103:491-495. [PubMed] |
| 40. | Davies MJ, Gordon JL, Gearing AJ, Pigott R, Woolf N, Katz D, Kyriakopoulos A. The expression of the adhesion molecules ICAM-1, VCAM-1, PECAM, and E-selectin in human atherosclerosis. J Pathol. 1993;171:223-229. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 550] [Cited by in RCA: 548] [Article Influence: 16.6] [Reference Citation Analysis (0)] |
| 41. | Debaisieux S, Rayne F, Yezid H, Beaumelle B. The ins and outs of HIV-1 Tat. Traffic. 2012;13:355-363. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 164] [Cited by in RCA: 184] [Article Influence: 12.3] [Reference Citation Analysis (0)] |
| 42. | Dhawan S, Puri RK, Kumar A, Duplan H, Masson JM, Aggarwal BB. Human immunodeficiency virus-1-tat protein induces the cell surface expression of endothelial leukocyte adhesion molecule-1, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 in human endothelial cells. Blood. 1997;90:1535-1544. [PubMed] |
| 43. | Cerletti C, Evangelista V, de Gaetano G. P-selectin-beta 2-integrin cross-talk: a molecular mechanism for polymorphonuclear leukocyte recruitment at the site of vascular damage. Thromb Haemost. 1999;82:787-793. [PubMed] |
| 44. | Blankenberg S, Rupprecht HJ, Bickel C, Peetz D, Hafner G, Tiret L, Meyer J. Circulating cell adhesion molecules and death in patients with coronary artery disease. Circulation. 2001;104:1336-1342. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 395] [Cited by in RCA: 419] [Article Influence: 16.8] [Reference Citation Analysis (0)] |
| 45. | Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836-843. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 4096] [Cited by in RCA: 4190] [Article Influence: 161.2] [Reference Citation Analysis (7)] |
| 46. | Hwang SJ, Ballantyne CM, Sharrett AR, Smith LC, Davis CE, Gotto AM, Boerwinkle E. Circulating adhesion molecules VCAM-1, ICAM-1, and E-selectin in carotid atherosclerosis and incident coronary heart disease cases: the Atherosclerosis Risk In Communities (ARIC) study. Circulation. 1997;96:4219-4225. [PubMed] |
| 47. | Puppo F, Brenci S, Scudeletti M, Lanza L, Bosco O, Indiveri F. Elevated serum levels of circulating intercellular adhesion molecule-1 in HIV infection. AIDS. 1993;7:593-594. [PubMed] |
| 48. | Zietz C, Hotz B, Stürzl M, Rauch E, Penning R, Löhrs U. Aortic endothelium in HIV-1 infection: chronic injury, activation, and increased leukocyte adherence. Am J Pathol. 1996;149:1887-1898. [PubMed] |
| 49. | Paladugu R, Fu W, Conklin BS, Lin PH, Lumsden AB, Yao Q, Chen C. Hiv Tat protein causes endothelial dysfunction in porcine coronary arteries. J Vasc Surg. 2003;38:549-555; discussion 555-556. [PubMed] |
| 50. | Abraham L, Fackler OT. HIV-1 Nef: a multifaceted modulator of T cell receptor signaling. Cell Commun Signal. 2012;10:39. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 58] [Cited by in RCA: 68] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 51. | Asztalos BF, Mujawar Z, Morrow MP, Grant A, Pushkarsky T, Wanke C, Shannon R, Geyer M, Kirchhoff F, Sviridov D. Circulating Nef induces dyslipidemia in simian immunodeficiency virus-infected macaques by suppressing cholesterol efflux. J Infect Dis. 2010;202:614-623. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 47] [Cited by in RCA: 46] [Article Influence: 2.9] [Reference Citation Analysis (0)] |
| 52. | Duffy P, Wang X, Lin PH, Yao Q, Chen C. HIV Nef protein causes endothelial dysfunction in porcine pulmonary arteries and human pulmonary artery endothelial cells. J Surg Res. 2009;156:257-264. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 59] [Cited by in RCA: 60] [Article Influence: 3.5] [Reference Citation Analysis (0)] |
| 53. | Nedeljkovic ZS, Gokce N, Loscalzo J. Mechanisms of oxidative stress and vascular dysfunction. Postgrad Med J. 2003;79:195-199; quiz 198-200. [PubMed] |
| 54. | Wang T, Green LA, Gupta SK, Kim C, Wang L, Almodovar S, Flores SC, Prudovsky IA, Jolicoeur P, Liu Z. Transfer of intracellular HIV Nef to endothelium causes endothelial dysfunction. PLoS One. 2014;9:e91063. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 41] [Cited by in RCA: 58] [Article Influence: 4.8] [Reference Citation Analysis (0)] |
| 55. | Kedzierska K, Crowe SM. The role of monocytes and macrophages in the pathogenesis of HIV-1 infection. Curr Med Chem. 2002;9:1893-1903. [PubMed] |
| 56. | Palmer CS, Anzinger JJ, Zhou J, Gouillou M, Landay A, Jaworowski A, McCune JM, Crowe SM. Glucose transporter 1-expressing proinflammatory monocytes are elevated in combination antiretroviral therapy-treated and untreated HIV+ subjects. J Immunol. 2014;193:5595-5603. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 77] [Article Influence: 6.4] [Reference Citation Analysis (0)] |
| 57. | Hearps AC, Maisa A, Cheng WJ, Angelovich TA, Lichtfuss GF, Palmer CS, Landay AL, Jaworowski A, Crowe SM. HIV infection induces age-related changes to monocytes and innate immune activation in young men that persist despite combination antiretroviral therapy. AIDS. 2012;26:843-853. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 120] [Cited by in RCA: 139] [Article Influence: 9.9] [Reference Citation Analysis (0)] |
| 58. | McKibben RA, Margolick JB, Grinspoon S, Li X, Palella FJ, Kingsley LA, Witt MD, George RT, Jacobson LP, Budoff M. Elevated levels of monocyte activation markers are associated with subclinical atherosclerosis in men with and those without HIV infection. J Infect Dis. 2015;211:1219-1228. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 132] [Cited by in RCA: 164] [Article Influence: 13.7] [Reference Citation Analysis (0)] |
| 59. | Kaplan RC, Sinclair E, Landay AL, Lurain N, Sharrett AR, Gange SJ, Xue X, Hunt P, Karim R, Kern DM. T cell activation and senescence predict subclinical carotid artery disease in HIV-infected women. J Infect Dis. 2011;203:452-463. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 227] [Cited by in RCA: 246] [Article Influence: 16.4] [Reference Citation Analysis (1)] |
| 60. | Mdodo R, Frazier EL, Dube SR, Mattson CL, Sutton MY, Brooks JT, Skarbinski J. Cigarette smoking prevalence among adults with HIV compared with the general adult population in the United States: cross-sectional surveys. Ann Intern Med. 2015;162:335-344. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 309] [Cited by in RCA: 380] [Article Influence: 34.5] [Reference Citation Analysis (0)] |
| 61. | Lifson AR, Neuhaus J, Arribas JR, van den Berg-Wolf M, Labriola AM, Read TR. Smoking-related health risks among persons with HIV in the Strategies for Management of Antiretroviral Therapy clinical trial. Am J Public Health. 2010;100:1896-1903. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 221] [Cited by in RCA: 234] [Article Influence: 14.6] [Reference Citation Analysis (0)] |
| 62. | Friis-Møller N, Weber R, Reiss P, Thiébaut R, Kirk O, d’Arminio Monforte A, Pradier C, Morfeldt L, Mateu S, Law M. Cardiovascular disease risk factors in HIV patients--association with antiretroviral therapy. Results from the DAD study. AIDS. 2003;17:1179-1193. [RCA] [PubMed] [DOI] [Full Text] [Cited by in RCA: 294] [Reference Citation Analysis (0)] |
| 63. | Helleberg M, Afzal S, Kronborg G, Larsen CS, Pedersen G, Pedersen C, Gerstoft J, Nordestgaard BG, Obel N. Mortality attributable to smoking among HIV-1-infected individuals: a nationwide, population-based cohort study. Clin Infect Dis. 2013;56:727-734. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 310] [Cited by in RCA: 368] [Article Influence: 26.3] [Reference Citation Analysis (0)] |
| 64. | Brown TT, Cole SR, Li X, Kingsley LA, Palella FJ, Riddler SA, Visscher BR, Margolick JB, Dobs AS. Antiretroviral therapy and the prevalence and incidence of diabetes mellitus in the multicenter AIDS cohort study. Arch Intern Med. 2005;165:1179-1184. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 571] [Cited by in RCA: 626] [Article Influence: 29.8] [Reference Citation Analysis (0)] |
| 65. | De Wit S, Sabin CA, Weber R, Worm SW, Reiss P, Cazanave C, El-Sadr W, Monforte Ad, Fontas E, Law MG. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D: A: D) study. Diabetes Care. 2008;31:1224-1229. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 384] [Cited by in RCA: 377] [Article Influence: 20.9] [Reference Citation Analysis (0)] |
| 66. | Mehta SH, Moore RD, Thomas DL, Chaisson RE, Sulkowski MS. The effect of HAART and HCV infection on the development of hyperglycemia among HIV-infected persons. J Acquir Immune Defic Syndr. 2003;33:577-584. [PubMed] |
| 67. | Duong M, Petit JM, Piroth L, Grappin M, Buisson M, Chavanet P, Hillon P, Portier H. Association between insulin resistance and hepatitis C virus chronic infection in HIV-hepatitis C virus-coinfected patients undergoing antiretroviral therapy. J Acquir Immune Defic Syndr. 2001;27:245-250. [PubMed] |
| 68. | Lo J, Rosenberg ES, Fitzgerald ML, Bazner SB, Ihenachor EJ, Hawxhurst V, Borkowska AH, Wei J, Zimmerman CO, Burdo TH. High-density lipoprotein-mediated cholesterol efflux capacity is improved by treatment with antiretroviral therapy in acute human immunodeficiency virus infection. Open Forum Infect Dis. 2014;1:ofu108. [PubMed] |
| 69. | Mujawar Z, Rose H, Morrow MP, Pushkarsky T, Dubrovsky L, Mukhamedova N, Fu Y, Dart A, Orenstein JM, Bobryshev YV. Human immunodeficiency virus impairs reverse cholesterol transport from macrophages. PLoS Biol. 2006;4:e365. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 224] [Cited by in RCA: 264] [Article Influence: 13.9] [Reference Citation Analysis (0)] |
| 70. | Krauskopf K, Van Natta ML, Danis RP, Gangaputra S, Ackatz L, Addessi A, Federman AD, Branch AD, Meinert CL, Jabs DA; Studies of the Ocular Complications of AIDS Research Group. Correlates of hypertension in patients with AIDS in the era of highly active antiretroviral therapy. J Int Assoc Provid AIDS Care. 2013;12:325-333. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 22] [Cited by in RCA: 29] [Article Influence: 2.2] [Reference Citation Analysis (0)] |
| 71. | Chu C, Umanski G, Blank A, Meissner P, Grossberg R, Selwyn PA. Comorbidity-related treatment outcomes among HIV-infected adults in the Bronx, NY. J Urban Health. 2011;88:507-516. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 39] [Cited by in RCA: 47] [Article Influence: 3.1] [Reference Citation Analysis (0)] |
| 72. | Balderson BH, Grothaus L, Harrison RG, McCoy K, Mahoney C, Catz S. Chronic illness burden and quality of life in an aging HIV population. AIDS Care. 2013;25:451-458. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 110] [Cited by in RCA: 140] [Article Influence: 10.0] [Reference Citation Analysis (0)] |
| 73. | Bloomfield GS, Hogan JW, Keter A, Sang E, Carter EJ, Velazquez EJ, Kimaiyo S. Hypertension and obesity as cardiovascular risk factors among HIV seropositive patients in Western Kenya. PLoS One. 2011;6:e22288. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 84] [Cited by in RCA: 99] [Article Influence: 6.6] [Reference Citation Analysis (0)] |
| 74. | Mateen FJ, Kanters S, Kalyesubula R, Mukasa B, Kawuma E, Kengne AP, Mills EJ. Hypertension prevalence and Framingham risk score stratification in a large HIV-positive cohort in Uganda. J Hypertens. 2013;31:1372-1378; discussion 1378. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 55] [Cited by in RCA: 67] [Article Influence: 5.6] [Reference Citation Analysis (0)] |
| 75. | Medina-Torne S, Ganesan A, Barahona I, Crum-Cianflone NF. Hypertension is common among HIV-infected persons, but not associated with HAART. J Int Assoc Physicians AIDS Care (Chic). 2012;11:20-25. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 35] [Cited by in RCA: 48] [Article Influence: 3.2] [Reference Citation Analysis (0)] |
| 76. | Crane HM, Van Rompaey SE, Kitahata MM. Antiretroviral medications associated with elevated blood pressure among patients receiving highly active antiretroviral therapy. AIDS. 2006;20:1019-1026. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 113] [Cited by in RCA: 124] [Article Influence: 6.2] [Reference Citation Analysis (1)] |
| 77. | Worm SW, Sabin C, Weber R, Reiss P, El-Sadr W, Dabis F, De Wit S, Law M, Monforte AD, Friis-Møller N. Risk of myocardial infarction in patients with HIV infection exposed to specific individual antiretroviral drugs from the 3 major drug classes: the data collection on adverse events of anti-HIV drugs (D: A: D) study. J Infect Dis. 2010;201:318-330. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 459] [Cited by in RCA: 489] [Article Influence: 30.6] [Reference Citation Analysis (0)] |
| 78. | Baum PD, Sullam PM, Stoddart CA, McCune JM. Abacavir increases platelet reactivity via competitive inhibition of soluble guanylyl cyclase. AIDS. 2011;25:2243-2248. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 60] [Article Influence: 4.0] [Reference Citation Analysis (0)] |
| 79. | Stein JH, Hodis H, Brown T, Ribaudo HJ, Tran TTT, Yan M, Lauer-Brodell E, McComsey G, Dube MP, Murphy RL. Prospective randomized clinical trial of the effects of three modern antiretroviral therapies on carotid intima-media thickness in HIV-infected individuals (AIDS clinical trials group study A5260S). J Am Coll Cardiol. 2014;63:A1322. [RCA] [DOI] [Full Text] [Cited by in Crossref: 2] [Cited by in RCA: 3] [Article Influence: 0.3] [Reference Citation Analysis (0)] |
| 80. | Friis-Møller N, Thiébaut R, Reiss P, Weber R, Monforte AD, De Wit S, El-Sadr W, Fontas E, Worm S, Kirk O. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17:491-501. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 256] [Cited by in RCA: 294] [Article Influence: 19.6] [Reference Citation Analysis (0)] |
| 81. | Goff DC, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S49-S73. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1958] [Cited by in RCA: 2719] [Article Influence: 226.6] [Reference Citation Analysis (1)] |
| 82. | Ridker PM, Cook NR. Statins: new American guidelines for prevention of cardiovascular disease. Lancet. 2013;382:1762-1765. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 341] [Cited by in RCA: 356] [Article Influence: 27.4] [Reference Citation Analysis (0)] |
| 83. | Regan S, Meigs JB, Massaro J, Triant V. Evaluation of the ACC/AHA CVD risk prediction algorithm among HIV-infected patients. Conference on Retroviruses and Opportunistic Infections. Available from: http://www.croiconference.org/sessions/evaluation-accaha-cvd-risk-prediction-algorithm-among-hiv-infected-patients. |
| 84. | Thompson-Paul AM, Lichtenstein KA, Armon C. Cardiovascular disease risk prediction in the HIV Outpatient Study (HOPS). Conference onRetroviruses and Opportunistic Infections. Available from: http://www.croiconference.org/sessions/cardiovascular-disease-risk-prediction-hiv-outpatient-study-hops. |
| 85. | National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) final report. Circulation. 2002;106:3143-3421. [PubMed] |
| 86. | Perk J, De Backer G, Gohlke H, Graham I, Reiner Z, Verschuren WM, Albus C, Benlian P, Boysen G, Cifkova R. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012): The Fifth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of nine societies and by invited experts). Atherosclerosis. 2012;223:1-68. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 263] [Cited by in RCA: 271] [Article Influence: 19.4] [Reference Citation Analysis (0)] |
| 87. | Zanni MV, Fitch KV, Feldpausch M, Han A, Lee H, Lu MT, Abbara S, Ribaudo H, Douglas PS, Hoffmann U. 2013 American College of Cardiology/American Heart Association and 2004 Adult Treatment Panel III cholesterol guidelines applied to HIV-infected patients with/without subclinical high-risk coronary plaque. AIDS. 2014;28:2061-2070. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 58] [Cited by in RCA: 64] [Article Influence: 5.3] [Reference Citation Analysis (0)] |
| 88. | Greenland P, Alpert JS, Beller GA, Benjamin EJ, Budoff MJ, Fayad ZA, Foster E, Hlatky MA, Hodgson JM, Kushner FG. 2010 ACCF/AHA guideline for assessment of cardiovascular risk in asymptomatic adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2010;56:e50-103. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 935] [Cited by in RCA: 1035] [Article Influence: 69.0] [Reference Citation Analysis (0)] |
| 89. | Hecht HS. Coronary artery calcium scanning: past, present, and future. JACC Cardiovasc Imaging. 2015;8:579-596. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 187] [Cited by in RCA: 237] [Article Influence: 23.7] [Reference Citation Analysis (0)] |
| 90. | Hulten E, Mitchell J, Scally J, Gibbs B, Villines TC. HIV positivity, protease inhibitor exposure and subclinical atherosclerosis: a systematic review and meta-analysis of observational studies. Heart. 2009;95:1826-1835. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 79] [Cited by in RCA: 84] [Article Influence: 4.9] [Reference Citation Analysis (0)] |
| 91. | Kingsley LA, Cuervo-Rojas J, Muñoz A, Palella FJ, Post W, Witt MD, Budoff M, Kuller L. Subclinical coronary atherosclerosis, HIV infection and antiretroviral therapy: Multicenter AIDS Cohort Study. AIDS. 2008;22:1589-1599. [RCA] [PubMed] [DOI] [Full Text] [Full Text (PDF)] [Cited by in Crossref: 87] [Cited by in RCA: 92] [Article Influence: 5.1] [Reference Citation Analysis (0)] |
| 92. | Subramanian S, Tawakol A, Burdo TH, Abbara S, Wei J, Vijayakumar J, Corsini E, Abdelbaky A, Zanni MV, Hoffmann U. Arterial inflammation in patients with HIV. JAMA. 2012;308:379-386. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 347] [Cited by in RCA: 393] [Article Influence: 28.1] [Reference Citation Analysis (0)] |
| 93. | Burdo TH, Lo J, Abbara S, Wei J, DeLelys ME, Preffer F, Rosenberg ES, Williams KC, Grinspoon S. Soluble CD163, a novel marker of activated macrophages, is elevated and associated with noncalcified coronary plaque in HIV-infected patients. J Infect Dis. 2011;204:1227-1236. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 321] [Cited by in RCA: 368] [Article Influence: 24.5] [Reference Citation Analysis (0)] |
| 94. | Tawakol A, Lo J, Zanni MV, Marmarelis E, Ihenachor EJ, MacNabb M, Wai B, Hoffmann U, Abbara S, Grinspoon S. Increased arterial inflammation relates to high-risk coronary plaque morphology in HIV-infected patients. J Acquir Immune Defic Syndr. 2014;66:164-171. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 48] [Cited by in RCA: 65] [Article Influence: 5.4] [Reference Citation Analysis (0)] |
| 95. | Cardiovascular disease mortality among HIV-infected persons, New York City, 2001-2012 . Conference on Retroviruses and Opportunistic Infections [Internet]. Available from: http://www.croiconference.org/sessions/cardiovascular-disease-mortality-among-hiv-infected-persons-new-york-city-2001-2012. |
| 96. | Grundy SM, Cleeman JI, Merz CN, Brewer HB, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III Guidelines. J Am Coll Cardiol. 2004;44:720-732. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 992] [Cited by in RCA: 1006] [Article Influence: 45.7] [Reference Citation Analysis (0)] |
| 97. | Available from: http: //www.croiconference.org/sessions/hiv-infected-veterans-and-new-accaha-cholesterol-guidelines-got-statins. |
| 98. | Available from: https://depts.washington.edu/actu/website-for-reprieve-study/. |
| 99. | Monforte Ad, Reiss P, Ryom L, El-Sadr W, Dabis F, De Wit S, Worm SW, Law MG, Weber R, Kirk O. Atazanavir is not associated with an increased risk of cardio- or cerebrovascular disease events. AIDS. 2013;27:407-415. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 82] [Cited by in RCA: 81] [Article Influence: 6.2] [Reference Citation Analysis (0)] |
| 100. | Kelesidis T, Tran TT, Stein JH, Brown TT, Moser C, Ribaudo HJ, Dube MP, Murphy R, Yang OO, Currier JS. Changes in Inflammation and Immune Activation With Atazanavir-, Raltegravir-, Darunavir-Based Initial Antiviral Therapy: ACTG 5260s. Clin Infect Dis. 2015;61:651-660. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 88] [Cited by in RCA: 106] [Article Influence: 9.6] [Reference Citation Analysis (0)] |
| 101. | Lundgren JD, Babiker AG, Gordin F, Emery S, Grund B, Sharma S, Avihingsanon A, Cooper DA, Fätkenheuer G, Llibre JM. Initiation of Antiretroviral Therapy in Early Asymptomatic HIV Infection. N Engl J Med. 2015;373:795-807. [RCA] [PubMed] [DOI] [Full Text] [Cited by in Crossref: 1887] [Cited by in RCA: 2167] [Article Influence: 197.0] [Reference Citation Analysis (0)] |
P- Reviewer: Diamantidis MD, Falconi M, Skobel E S- Editor: Gong XM L- Editor: A E- Editor: Lu YJ
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/